Abstract
Cryptococcus neoformans is a fungal pathogen that encounters various microenvironments during growth in the mammalian host, including intracellular vacuoles, blood, and cerebrospinal fluid (CSF). Because the CSF is isolated by the blood-brain barrier, we hypothesize that CSF presents unique stresses that C. neoformans must overcome to establish an infection. We assayed 1,201 mutants for survival defects in growth media, saline, and human CSF. We assessed CSF-specific mutants for (i) mutant survival in both human bronchoalveolar lavage (BAL) fluid and fetal bovine serum (FBS), (ii) survival in macrophages, and (iii) virulence using both Caenorhabditis elegans and rabbit models of cryptococcosis. Thirteen mutants exhibited significant survival defects unique to CSF. The mutations of three of these mutants were recreated in the clinical serotype A strain H99: deletions of the genes for a cation ATPase transporter (ena1Δ), a putative NEDD8 ubiquitin-like protein (rub1Δ), and a phosphatidylinositol 4-kinase (pik1Δ). Mutant survival rates in yeast media, saline, and BAL fluid were similar to those of the wild type; however, survival in FBS was reduced but not to the levels in CSF. These mutant strains also exhibited decreased intracellular survival in macrophages, various degrees of virulence in nematodes, and severe attenuation of survival in a rabbit meningitis model. We analyzed the CSF by mass spectrometry for candidate compounds responsible for the survival defect. Our findings indicate that the genes required for C. neoformans survival in CSF ex vivo are necessary for survival and infection in this unique host environment.
Cryptococcus neoformans is a basidiomycete fungal pathogen that frequently invades the central nervous system, causing meningoencephalitis in severely immunocompromised patients. Among these patients, such as those with HIV or solid-organ transplants, cryptococcosis results in one of the largest burdens of mortality and morbidity (10). There are now over 1 million cases of cryptococcosis worldwide, with estimated deaths of 700,000 per year (38). However, recent outbreaks of cryptococcosis by a newly identified genotype of Cryptococcus gattii (a sister species of C. neoformans) in the Pacific Northwest have emphasized that apparently immunocompetent individuals are susceptible to this disease as well (17, 18, 32, 48).
Although the pathobiology of cryptococcosis within each body site is not fully understood, the ability of C. neoformans to survive in the cerebrospinal fluid (CSF) is critical for its production of life-threatening disease in the central nervous system. CSF performs a variety of essential functions: cushions the brain from physical impact, transports nutrients and wastes to and from cells, and circulates various signaling molecules, such as regulatory peptides (29). Due to the relative isolation of the CSF by the blood-brain and blood-CSF barriers, recent proteomic and metabolomic analyses support the hypothesis that the CSF is a unique environment compared to other body fluids and sites, such as the alveolar spaces, intracellular vacuoles, and blood, that C. neoformans encounters in the course of an infection (55, 57, 60).
Three well-established virulence factors essential for cryptococcal disease have been identified, namely, the production of a polysaccharide capsule, melanin, and survival at 37°C. Many genes controlling these phenotypes have also been identified (40). The use of random insertion mutagenesis has led to the discovery of many essential virulence-associated genes, such as CNA1, VPH1, and CLC1 (14, 26, 27, 59), and site-directed techniques have revealed the importance of urease and phospholipase genes for virulence (12, 13). Furthermore, the recent completion of the C. neoformans var. grubii genome presents an exciting opportunity for accelerated discovery of genes encoding virulence factors through the use of targeted genetic screens. With these genomic tools, forward genetics can be a powerful strategy to better understand how C. neoformans copes with various microenvironments within the host.
We hypothesized that the CSF presents unique stresses to C. neoformans in the host and that determination of specific factors required for survival in CSF could shed light on novel disease-producing mechanisms. Using a library of signature-tagged, site-directed mutant strains, we employed a forward genetics screen to assay for mutant-cell survival defects in human CSF. A series of mutants that displayed significant deficiencies in survival when exposed to CSF were found. Extensive characterization of these mutants suggests that CSF presents a unique stress composite to this yeast, and the inability of certain C. neoformans mutants to survive CSF exposure in vitro correlates with attenuated virulence in the mammalian host.
MATERIALS AND METHODS
Strains and media.
The targeted deletion library of 1,201 mutants of the C. neoformans strain CM018 (a serotype A strain derived from H99) was obtained from the Fungal Genetics Stock Center, and its use in screens has been previously described (35). All other C. neoformans mutants were created in serotype A strain H99. C. neoformans strains were grown in either yeast extract-peptone-dextrose (YPD) broth (46), phosphate-buffered saline, pH 7.4 (PBS), or tissue culture medium (Dulbecco's modified Eagle's medium [DMEM] with or without 22 mM NaHCO3 buffer, 10% NCTC-109 medium (Invitrogen Corp., Carlsbad, CA), 10% FBS, 1% MEM nonessential amino acid solution, 1% penicillin-streptomycin). CSF and bronchoalveolar lavage (BAL) fluid were each pooled from Duke University Hospital patients; their inclusion was approved by required Duke University Internal Review Board protocols for determining exempt status. Both biological fluid pools were collected anonymously from populations of at least 20 and 5 patients for CSF and BAL samples, respectively, in order to ensure that the pools did not contain samples from a unique patient(s). Two to three additional CSF pools from at least 20 patients were used with selected mutants and controls, with similar results. All fluids were filter sterilized prior to use. These samples came from unidentified patients whose medical histories were unknown. The CSF was fully characterized for contaminating drugs by proteomics and mass spectrometry (MS) analysis; only vancomycin and pipotiazine sulfoxide were present. Three independent C. neoformans strains were recreated in the serotype A wild-type (WT) strain H99 after identification in the mutant strain library. The Saccharomyces cerevisiae BW31 strain (ena1-4Δ nh1Δ) was a generous gift from Hana Sychrova.
CSF survival assay.
For the initial assay screen, a library of 1,201 targeted mutants with known gene disruptions were replicated in 96-well plates with YPD broth and incubated at 30°C for 4 days until saturation (∼107 cells/ml). Strains were then diluted 1:10 into YPD, PBS, or CSF and exposed at 37°C to air for another 4 days. At days 1 and 4 of this incubation, strains were diluted 1:10 (1:100 overall) in PBS and plated 1:10 and 1:100 onto YPD plates. Plates were scored after 2 days of growth at 30°C.
For repeat CSF assays of all hits positive for the lack of survival in CSF, strains were grown in YPD broth for 1 to 2 days in a 30°C incubator at 225 rpm until saturation prior to CSF inoculation.
Recreation of the ena1Δ, rub1Δ, and pik1Δ mutants in H99.
Site-directed mutations in the CM018 strain were identified in the screen and then recreated in our serotype A H99 strain. The gene knockouts of interest in the CM018 background were PCR amplified for biolistic transformation into wild-type strain H99. The primers used were as follows: for the ena1 deletion, forward primer 1A10.F1 and reverse primer 1A10.R1; for the rub1 deletion, forward primer 7F4.F1 and reverse primer 7F4.R1; and for the pik1 deletion, forward primer 14A4.F1 and reverse primer 14A4.R1 (Table 1). These knockout constructs were amplified with a PCR of 5 min at 95°C and 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 4 min, with a final step of 72°C for 5 min with 1 μl of genomic CM018 mutant DNA from a standard genomic DNA preparation. The PCR product was then reduced to a 2-μl volume and adsorbed onto gold microparticles for biolistic transformation as previously described (51). Stable transformants were selected by plating cells onto YPD plates containing 100 μg/ml nourseothricin. Gene deletions were verified by PCR using a primer within the NATR cassette and a primer flanking the deletion construct for the gene of interest (Table 1).
TABLE 1.
Oligonucleotides used in this study
| Function | Primer | Sequence (5′ to 3′) |
|---|---|---|
| Creation of knockout genomic DNA for biolistics | 1A10.F1 | AAGGCGAAGAGGGAAAGAAG |
| transformation (flanking the gene of interest) | 1A10.R1 | GCAGTGTTGTCCACCAGAGA |
| 14A4.F1 | CTGTGAGCTATGAGTTTGACC | |
| 14A4.R1 | CAGTTGGGACAGTTTATTGC | |
| 7F4.F1 | GCAGATGATCGGGTGACTTT | |
| 7F4.R1 | ACACCTTTTTGTGCCTCGTC | |
| Analysis of proper homologous recombination of | NAT.F1 | ACCTCTGGCTGGAGGTCAC |
| gene construct | NAT.R1 | GTGACCTCCAGCCAGAGGT |
| 1A10.F2 | TGGGGATGACTCACATTATGG | |
| 7F4.F3 | CGTGCTAGGCTTCCTGATGT | |
| 14A4.R2 | GCTTGGGTTGTATGGATGGT | |
| Creation of gene probe for Southern analysis | NAT.probeF | ACTGGATGGGTCCTTCAC |
| NAT.probeR | GGGCATGCTCATGTAGAGC | |
| 1A10.probeF | GAAGTCGCTTTCCACAAAGA | |
| 1A10.probeR | GACATGGAAAACAACCTTCG | |
| 7F4.probeF | AGAACGGGTTGAAGAGAAGG | |
| 7F4.probeR | AGCAAGGACTAGATGGATGG | |
| 14A4.probeF | ACTCTTCCAAACCACCTTCC | |
| 14A4.probeR | TTTGACCTTGAGCTTGAACC |
The reconstituted ena1Δ::ENA1 strain (MATα) was made by biolistic transformation of the ena1Δ strain with a neomycin resistance marker (NEOR; amplified from pJAF1 [18]) and the native ENA1 gene, amplified with 1A10.F1 and 1A10.R2 from the WT. Transformants were selected on YPD-neomycin and YPD-nourseothricin plates. Three transformants did not grow on nourseothricin-containing plates but were isolated on neomycin-containing plates, indicating that there was a homologous-recombination event at the original ena1Δ locus. Reconstitution was confirmed by PCR, Southern hybridization, and reconstitution of the CSF survival phenotype.
Southern hybridization.
All mutants were reconfirmed using Southern hybridization analysis for gene replacement. C. neoformans genomic DNA (20 μg) was digested with the restriction endonucleases HindIII (for the ena1Δ mutant), StuI (for the rub1Δ mutant), and PstI (for the pik1Δ mutant) and separated on a 0.7% agarose gel. The DNA was transferred to a positively charged nylon membrane (Roche Applied Science, Indianapolis, IN) by following established protocols (45). Membranes were hybridized with ENA1, RUB1, PIK1, or pCH233 digoxigenin-labeled DNA probes overnight, as specified by the manufacturer (Roche Applied Science, Indianapolis, IN). Membranes were washed in an SDS-SSC solution at a final stringency of 0.1% SDS and 0.1% SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) before being incubated with anti-digoxigenin-alkaline phosphate Fab, followed by the addition of CDP-Star for chemiluminescent detection. DNA bands of interest were visualized on film following 3 to 10 min of exposure.
Capsule and melanin production.
Strains were incubated at 37°C in 5% CO2 for 2 days in tissue culture wells with 2 ml of Dulbecco's modified Eagle's medium for induction of capsule as previously described (42), and images were visualized at ×100 magnification. Melanin production was verified following induction of growth on Niger seed agar (NSA) for 2 to 5 days at 30°C to visually confirm dark colonies.
In vitro stress assays.
To determine whether the CSF defect of the mutants is pH sensitive, we grew the strains to saturation and then inoculated them into YPD broth adjusted to various pHs for 5 days before plating them onto YPD agar for 2 days. To test the cell wall integrity of the mutants, strains were grown to saturation and then inoculated onto YPD agar with 1 M sorbitol for 3 days. Because ENA1 encodes a cation transporter, we tested the ena1Δ mutant for survival under cationic stress. We inoculated saturated mutant cultures into YPD broth with either 1.5 M KCl or NaCl for 4 days. Strains were then plated onto YPD agar for visualization.
CSF protein exclusion assays.
We tested the CSF for proteins that may have roles in affecting the CSF-defective mutants as previously described (8). Briefly, to estimate the size of the CSF lethal factor(s), CSF samples were serially fractionated using 30-, 10-, and 3-kDa-cutoff Centricon filters according to the manufacturer's instructions (Millipore, Billerica, MA). Retentates and filtrates were reconstituted to their original volumes with sterile distilled water and tested for activity against the CSF survival-defective mutants in a CSF growth assay. In addition, the wild-type and CSF survival-defective mutants were tested in the CSF growth assay using boiled, clarified CSF. CSF samples were placed in a double boiling-water bath for 20 to 30 min. Any insoluble denatured protein was removed by centrifugation at 3,000 rpm. Sterile distilled water was added to reconstitute the solution to the original concentration. The clarified, boiled CSF was used in a liquid growth assay with wild-type and CSF-defective mutants. Lastly, we digested CSF proteins and peptides using 50 μg/ml proteinase K for 16 h at 30°C and tested the digested samples for activity against wild-type and CSF-defective mutants in a liquid growth assay. As a control, YPD was also treated with proteinase K to see if active proteinase K had any influence on strain growth.
Oxidation of CSF.
CSF was treated with a final concentration of 50 mM H2O2 at room temperature for 2 min and then quenched with 25,000 units of catalase at room temperature for 10 min. To remove the enzyme from the CSF, the sample was centrifuged through a 30-kDa-cutoff Centricon filter at 3,500 rpm for 20 min at 4°C. The filtrate was then tested for activity with the CSF survival assay.
Susceptibility of C. neoformans to vancomycin.
The MIC of vancomycin for C. neoformans was assessed via the Etest using a vancomycin Etest strip. Isolated colonies from a 3-day culture on YPD were mixed with PBS (pH 7.4) to obtain a 0.5 McFarland standard. The yeast suspension was then spread with a sterile cotton swab over the entire surface of a yeast nitrogen base (YNB) agar plate. After the plates were dry, the Etest strip was placed in the center of the plate. The plates were incubated at 30°C for 2 days and at 37°C for 3 days and then observed for survival inhibition.
Survival in macrophages.
We assessed ena1Δ, rub1Δ, and pik1Δ mutant strains for survival of these yeasts within macrophages. The WT and the ena1Δ, rub1Δ, and pik1Δ mutant strains were incubated overnight at 30°C at 150 rpm as inoculum for the macrophage survival assay, as previously described (11, 42).
Briefly, 105 gamma interferon- and lipopolysaccharide-activated J774A.1 macrophages were coincubated with 105 yeast cells of each yeast strain for 1 h at 37°C in 5% CO2 and DMEM to allow phagocytosis. Prior to coincubation, cells of each yeast strain were opsonized with the monoclonal antibody mAb18B7 (a gift from Arturo Casadevall) (56). Then, extracellular yeasts were washed away with PBS and the monolayers were incubated in DMEM overnight. A duplicate monolayer was stained at 1 h after the washes, and numbers of yeasts associated with macrophages were counted by microscopy for each strain as previously described (11, 16). Macrophages for the intracellular killing assay were then lysed with 0.5% SDS, and lysates were diluted and plated on YPD to count numbers of viable yeasts present following incubation at 30°C for 2 days.
Caenorhabditis elegans virulence assay.
C. neoformans strains were assayed for virulence in C. elegans as described previously (36). Briefly, a standard C. elegans strain was maintained at 20°C on a lawn of E. coli strain OP50. Prior to the feeding, C. neoformans strains were inoculated into 2 ml of YPD for 8 h, and 75 μl of the culture was spread on a 6-cm plate of brain heart infusion (BHI) agar with gentamicin (50 μg/ml). These plates of yeast lawns were allowed to grow overnight at 30°C. Fifty 1-day-old hermaphrodites were transferred from the lawn of E. coli to the lawn of yeasts, incubated at 25°C, and then examined every 12 h for the first 3 days, followed by every 24 h thereafter. Animals were considered dead by a lack of reflex when touched. Data from the survival assays were plotted using Kaplan-Meier survival curves and assessed by the log rank test with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Curve comparisons with P values of ≤0.05 were considered significantly different.
In vivo virulence assay.
Virulence of the C. neoformans mutant strains were evaluated in a rabbit meningitis model as previously described (43). In brief, C. neoformans strains were grown in YPD for 18 to 36 h and diluted to 3.3 × 108 cells/ml, with 0.3 ml inoculum introduced via intracisternal injection into cortisone-treated, male New Zealand White rabbits (n = 3). The CSF for each rabbit was withdrawn via intracisternal tap at the days indicated, and fungal burdens were determined by plating serial dilutions of CSF and assessing CFU growth. All assays were performed according to Duke University's Institutional Animal Care and Use Committee guidelines and approvals.
Mass spectrometry.
For each heated and nonheated biological condition, three separate CSF samples were prepared by the 3-kDa-cutoff Centricon fractionation protocol as described above. CSF fractions less than 3 kDa were acidified to pH 3.0 with neat formic acid and subjected to solid-phase extraction on a reversed-phase HLB Oasis cartridge (Waters Corporation, Milford, MA) by following the manufacturer's protocol. Analytes were eluted in 100 μl of 50% isopropanol-50% acetonitrile and brought to dryness using vacuum centrifugation. Samples were resuspended in 30 μl of 2% acetonitrile-0.1% trifluoroacetic acid (TFA; Thermo Scientific, Rockford, IL), and analyte quantities were normalized to 0.39 μg/μl with 2% acetonitrile, 0.1% TFA by following a micro-bicinchoninic acid (Pierce Biosciences, Rockford, IL) assay. To allow for a separate qualitative-factor-only liquid chromatography-tandem-MS (LC-MS/MS) analysis, a pooled aliquot of all three samples from the same condition was created. All samples were stored at 4°C until LC-MS/MS analysis.
Each sample was analyzed by injecting analyte onto a 75-μm by 250-mm bridged ethyl hybrid (BEH) C18 column (Waters) and separated using a linear gradient of 5 to 40% acetonitrile with 0.1% formic acid at a flow rate of 0.3 μl/min in 90 min on a nanoAcquity liquid chromatograph (Waters). Electrospray ionization was used to introduce the sample in real time to a Synapt HDMS mass spectrometer (Waters) operating in the positive-ion mode. The instrument was operated in a high-energy-/low-energy-switching (MSE) acquisition mode for each individual sample and in a data-dependent acquisition (DDA) mode for pooled samples.
To qualitatively identify peptide analytes in the mixture, DDA data were searched against the Swiss-Prot 57.10 database with Homo sapiens taxonomy (http://ca.expasy.org/sprot/) using Mascot v2.2 (Matrix Science, Inc.). Mascot search parameters were 20 ppm for precursor ions and 0.04 Da for product ions, with no enzyme specificity and with differential mass modification corresponding to oxidation on all Met residues. Mascot search results were imported and interpreted in Scaffold 2.6 (Proteome Software, Portland, OR). Label-free quantitation of analytes across all biological samples was accomplished using the Rosetta Elucidator v3.3 software package (Rosetta Biosoftware, Seattle, WA). Feature identification and chromatographic retention time alignment was performed using the PeakTeller algorithm. Visual scripting within Elucidator was used to extract peak intensities for features above 1,000 counts and present in 50% of the biological replicates. Monoisotopic masses of features with a minimum 5-fold increase in the nonheated samples and with P values less than 0.001 were searched against the Human Metabolomics Database (http://www.hmdb.ca/) and Metlin (http://metlin.scripps.edu/), with a mass tolerance of 50 ppm. Preliminary matches were manually verified for the presence of unique isotope distributions using ICR-2LS (PNNL Laboratories; http://omics.pnl.gov) and available high-energy fragmentation data from the Metlin database. The data associated with this paper may be downloaded from ProteomeCommons.org Tranche repository using the following hash: iC/qf/tZlLpRiGb3F4GpSNhoav52ia3mWjMTbp7D5EkD3TSaSANcOmggaJDlTRLndGWi0BOmljyinEsfIqPRZzNwChIAAAAAAAADVw = =. The hash may be used to prove exactly what files were published as part of this study's data set, and the hash may also be used to check that the data have not changed since publication.
Statistical analysis.
All statistical analyses, unless specifically noted below, were performed using JMP7 software (Cary, NC) and Student's t test with an α of 0.05. Rabbit experiments were done with 3 rabbits in each group. All in vitro data were collected at least in triplicate.
RESULTS
Identification of mutants with survival defects in CSF.
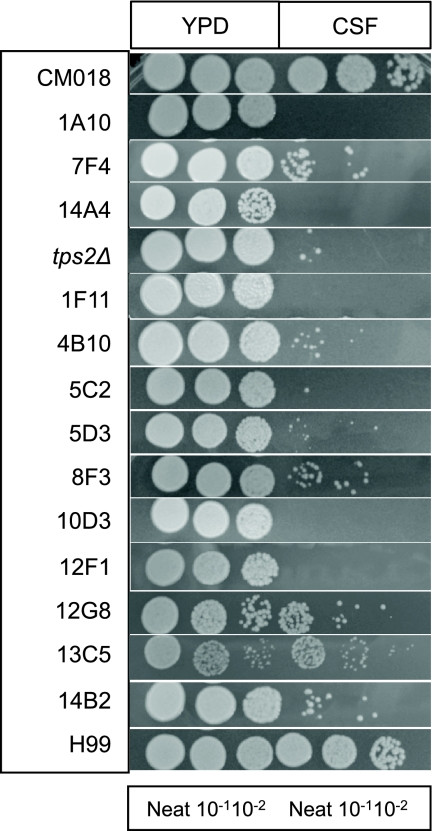
We assessed the ability of C. neoformans to survive in CSF due to its neurotropic nature and presence in clinical specimens. CSF is continuously made in the lateral ventricle of the brain and circulates throughout the subarachnoid space, and it is environmentally isolated by the blood-brain and blood-CSF barriers (29). Using a site-directed mutant library of 1,201 individual strains (35), we found 13 mutant strains that exhibit marked survival defects compared to parental strain CM018 when exposed to CSF at 37°C for 4 days (Fig. 1; Table 2). Survival defects were highly varied within these 13 strains, ranging from a 10-fold decrease in survival to complete death. The survival defect observed in CSF was not due to nutrient starvation or absolute high-temperature sensitivity, as these mutant strains showed normal survival in YPD medium and PBS.
FIG. 1.
CSF-defective ATCC mutants in a CSF survival assay. The strains were grown to saturation in YPD at 30°C and then diluted 1:10 in CSF or YPD and incubated at 37°C for 4 days. “Neat” represents the CFU concentration in CSF, and strains were diluted serially in 10-fold increments prior to being spotted onto YPD plates. CM018 is the background strain of the targeted mutant library, H99 is the clinical correlate, and the tps2Δ mutant (trehalose-6-phosphotase mutant), which does not survive in CSF at 37°C, serves as a positive control. (YPD spots are diluted from the saturated stock cultures.) Names of genes described in this study are listed in Table 2.
TABLE 2.
ATCC mutants with survival defects in CSF
| Functional group of protein | Knockout | Gene | Transcript name | Broad database annotationa | Description | Melanin | Capsule | Mutant phenotype(s)b |
|---|---|---|---|---|---|---|---|---|
| Transporter | 1A10 | ENA1 | ENA P-type ATPase 1 | CNAG_00531 | Cation ATPase transporter | + | + | Known to be |
| 1F11c | PXA2 | Adrenoleukodystrophy protein | CNAG_02764 | ABC transporter | + | + | hypovirulent | |
| Transcription | 5C2 | SRE1 | Conserved hypothetical protein | CNAG_04804 | Helix-loop-helix DNA-binding domain; ergosterol biosynthesis | + | + | Known to be hypovirulent |
| Protein sorting/protein degradation | 10D3 | VPS25 | Conserved hypothetical protein | CNAG_04863 | ESCRT-II complex subunit; endosomal protein sorting | + | +/− | |
| 7F4 | RUB1 | Ribosomal chaperone | CNAG_02827 | Ribosomal chaperone activity | − | + | Abnormal morphology; | |
| 5D3 | CSN1201 | Conserved hypothetical protein | CNAG_01697 | Transcription-associated recombination protein | +/− | + | slower growth | |
| 8F3 | HRD1 | Synoviolin | CNAG_05469 | Transmembrane ubiquitin ligase required for endoplasmic reticulum-associated degradation; Zinc finger domain | + | + | ||
| 12F1 | VAM6 | RabGEF | CNAG_05395 | Rab guanyl-nucleotide exchange factor activity | − | + | ||
| Signaling | 4B10c | NSR1 | RNA splicing factor Pad-1 | CNAG_02130 | Binds nuclear localization sequences for pre-rRNA processing/biogenesis | |||
| 13C5c | CKB1 | Casein kinase II beta chain | CNAG_03590 | Regulation of cell cycle; cellular ion homeostasis; flocculation via cell wall protein-carbohydrate interaction; response to DNA damage stimulus | − | + | ||
| 14A4c | PIK1 | 1-Phosphatidylinositol 4-kinase | CNAG_07744 | Inositol phosphate metabolism and phosphatidylinositol signaling system | ++ | + | ||
| 14B2c | SWE1 | MYT1 kinase | CNAG_03171 | Protein kinase in regulation of cell cycle | ++ | + | ||
| Cell wall protein | 12G8 | CPS1 | Cellubiuronic acid synthase | CNAG_04320 | Glycotransferase | − | − | Known to be hypovirulent |
Mutants defective for survival in CSF show various degrees of virulence in Caenorhabditis elegans.
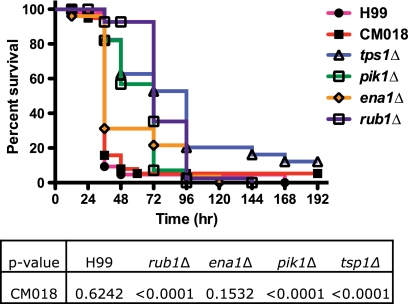
To assess whether the CSF survival defects resulted in an inability to respond to the host's innate immune response, we performed the C. elegans killing assay on our 13 CSF survival-defective mutant strains (36). Lacking an adaptive immune response and possessing tractable genetics, C. elegans has emerged as an efficient way to screen for candidate genes that mediate virulence through interactions with innate immunity (15, 36, 37). This model allows investigators to distinguish generally “sick” strains and appreciate possible differences in mutant susceptibility to host responses. Screening through our CSF survival-defective mutants revealed various degrees of virulence in the worm (Fig. 2; data not shown for all 13 strains): the ena1Δ mutant exhibited wild-type (WT) virulence (P = 0.1532), whereas the virulence of the rub1Δ and pik1Δ mutants was significantly attenuated (P < 0.0001). These results show that these mutants have some differences in response to the host but were not uniformly susceptible to any host challenge.
FIG. 2.
CSF-defective mutant strains show a wide range of virulence in a C. elegans killing assay. Fifty 1-day-old adult hermaphroditic nematodes were placed on BHI with 50 μg/ml gentamicin containing a lawn of wild-type or mutant C. neoformans. Worms were monitored for mortality twice daily. Dead nematodes were defined as nematodes that failed to respond to touch. The tps1Δ mutant is a trehalose-6-phosphate synthase mutant that exhibits virulence attenuation in C. elegans (41).
Phenotypes of CSF-specific-survival-defective mutants.
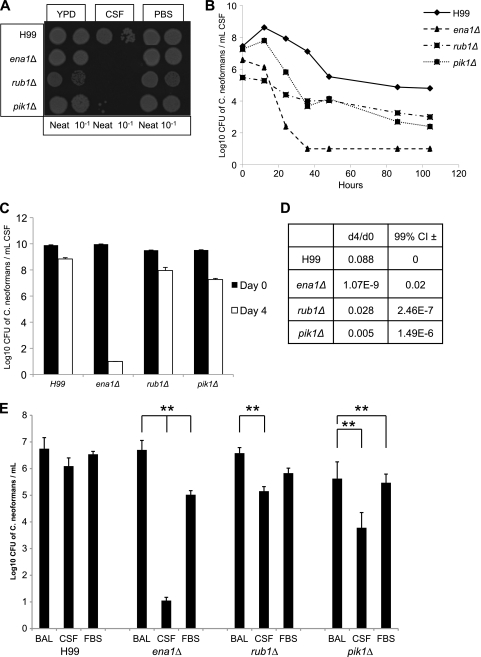
We chose 3 mutants from the 13 candidates for further study using the following criteria: (i) the predicted functions of their proteins in one of three major functional groups (transporter, protein sorting/degradation, or signaling), (ii) their reduced rates of survival in CSF (from a 10-fold reduction to complete inviability), and (iii) their overall vigor as determined by virulence in C. elegans survival assays, demonstrating that these strains are not generally sick and can cause disease. Three mutants in the CM018 background were recreated in the clinically relevant serotype A strain H99 background: ena1Δ, rub1Δ, and pik1Δ mutants (Table 3). The original CSF-specific survival defect for each strain was reconfirmed in the wild-type H99 background strain when grown in CSF at 37°C for 4 days (Fig. 3A). The ena1Δ strain showed complete cell death after 36 h of incubation, and the rub1Δ and pik1Δ strains also exhibited prominent survival defects in ex vivo CSF (Fig. 3B). Compared to the survival of the WT after 4 days, the ena1Δ mutant had no viable cells (a log10 CFU of 1 is considered sterile) (compared to the WT value, P was <0.01) (Fig. 3C), and the rub1Δ and pik1Δ mutants exhibited 89.6% and 99.5% decreases of cells, respectively (P < 0.05 and P < 0.01, respectively) (Fig. 3C). Because the WT also exhibited a significant decrease after 4 days of incubation in CSF, ratios of day 4 to day 0 CFU were determined to see if the mutants had significant differences in survival. Indeed, all three mutants showed significantly greater decreases in survival in CSF than the WT (P < 0.01) (Fig. 3D). The day 4 CFU/day 0 CFU ratio for H99 was 0.088, while the ena1Δ, rub1Δ, and pik1Δ mutants had ratios of 1.07E−9, 0.028, and 0.005, respectively. Interestingly, no survival defect was seen when these strains were grown in YPD, PBS (Fig. 3A), or BAL fluid (Fig. 3E). Mutant survival in fetal bovine serum (FBS) was improved in comparison with that in CSF but was not restored to WT survival levels for the ena1Δ mutant (P < 0.01) (Fig. 3E).
TABLE 3.
Summary of strains used in this study
| H99 genotypea | Corresponding ATCC mutantb | Broad gene identifier for serotype A |
|---|---|---|
| ena1Δ | 1A10 | CNAG_00531 |
| rub1Δ | 7F4 | CNAG_02827 |
| pik1Δ | 14A4 | CNAG_03535 |
| ena1Δ::ENA1 |
The ATCC mutants were recreated in the clinically relevant H99 background.
The mutants used in the screen were of the CM018 background and came from the ATCC.
FIG. 3.
Growth defects are specific to mutants in CSF. Selected H99 mutants were grown until saturation and then diluted 1:10 in YPD, CSF, or PBS, in which they were then grown at 37°C for 4 days. Then mutants were serially diluted in 10-fold increments and spotted onto YPD plates. (A) The survival-defective phenotype is seen specifically in CSF and is not the result of nutrient starvation. (B) The ena1Δ mutant exhibits complete cell death after 36 h of incubation in CSF. (C) The rub1Δ and pik1Δ mutants show approximately 10- to 20-fold cell loss compared to WT survival at day 4; the ena1Δ mutant shows complete cell death (P < 0.01). (D) All mutants have a significant decrease in CSF survival when a decrease in H99 levels (P < 0.01) is controlled for. d4/d0, day 4/day 0 ratio; 99% CI, 99% confidence interval. (E) At day 4, survival defects were greatest in CSF compared to in BAL fluid and FBS for all mutants (**, P < 0.01). Error bars represent standard errors.
Three critical factors influencing C. neoformans pathogenesis are capsule formation, melanin production, and the ability to grow at 37°C. When all three mutant strains were incubated under capsule-inducing conditions (DMEM containing 22 mM NaHCO3 at 37°C in 5% CO2), no defects in capsule formation were observed (data not shown). When grown on NSA, the ena1Δ and pik1Δ mutants exhibited normal development of melanin production, but the rub1Δ mutant remained white, suggesting impaired melanin production. It is interesting that the original pik1Δ mutant in the CM018 background showed an increase in melanin production (data not shown). The rub1Δ mutant also exhibited a lower basal rate of growth in YPD (a roughly 10-fold decrease) and a small-colony morphology compared to that of H99; at 37°C, these phenotypes were exaggerated. The other mutant strains exhibited normal growth rates at 37°C compared to H99.
The effect on survival in CSF is not mediated by osmotic or monovalent-cation stress.
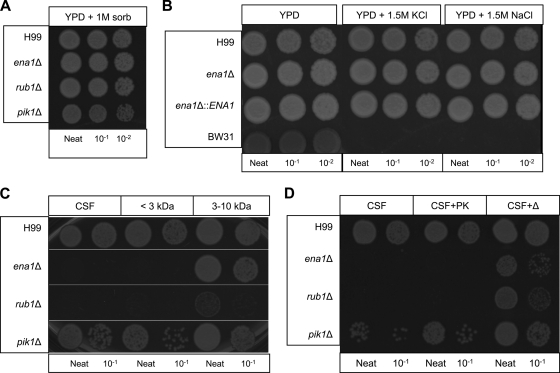
To assess what factors in the CSF cause the CSF-specific survival defects, we investigated whether these mutants showed any cell wall integrity defects during growth under osmotic stress. The mutant strains were grown at 37°C for 5 days on YPD agar plates containing 1 M sorbitol. All mutant strains showed no survival differences from the WT strain on this medium (Fig. 4A).
FIG. 4.
The survival factor in CSF is a small molecule. To determine the survival-inhibiting factor in CSF, we performed a series of assays to assess fungal viability. (A) Incubation in 1 M sorbitol (sorb) shows that osmotic stress is not the survival-inhibiting factor. (B) The ena1Δ mutants are not cation sensitive to 1.5 M KCl or NaCl. The BW31 strain (ena1Δ-ena4Δ nh1Δ) is an S. cerevisiae control strain. (C) Serial fractions of the CSF reveal that the survival-inhibiting factor is smaller than 3 kDa, suggesting a small peptide or unique ion composition. (D) Mutants were grown in proteinase K-treated or heat-inactivated CSF (to denature proteins in CSF). Proteinase K appeared to have no effect on the survival of the ena1Δ and rub1Δ mutants in CSF; however, heated CSF ablated the growth inhibition effect for all mutant strains.
Since ENA1 encodes a cation ATPase transporter and the Saccharomyces cerevisiae ena1Δ mutant is susceptible to cation stress, we examined whether cation stress could cause the CSF survival defect for the ena1Δ mutant. Using a cation-sensitive S. cerevisiae strain, BW31 (ena1-4Δ nh1Δ [6]), as a positive control, we found that the ena1Δ mutant does not appear to be sensitive to 1.5 M K+ or Na+ stress (Fig. 4B).
Characterization of the survival factor(s) in CSF.
We next attempted to isolate the CSF survival factor on the basis of molecular weight as described previously (8). CSF was size fractionated via centrifugation at 2,300 rpm for 20 min using Centricon filters with 30-, 10-, and 3-kDa cutoff values. Retentates and filtrates were reconstituted to an original volume with sterile water. Fractions with masses less than 3 kDa were found to retain the CSF-specific survival effect as seen in whole CSF (Fig. 4C). Thus, the survival effector in CSF is a molecule that is less than 3 kDa in size, such as a small peptide or metabolite. If the survival-altering factor of CSF is a peptide, protease digestion or heat denaturing should abolish the growth-inhibiting effect (8). Whole CSF was subjected to proteinase K treatment for 16 h at 37°C to digest any peptides. To control for potential effects of proteinase K on basal rates of growth, YPD was also treated with proteinase K. Proteinase K treatment did not have any effect on the viability of the mutant strains; furthermore, it did not abolish the effect of CSF on mutant survival compared to that of the WT (Fig. 4D). Given the broad cleavage specificity of proteinase K to peptide bonds adjacent to the carboxylic groups of aliphatic and aromatic amino acids, the survival effector(s) is likely not to be a small peptide. However, after boiling whole CSF in a double water bath for 30 min, we were able to restore CSF survival in the mutant strains, suggesting that the survival compound(s) is heat labile (Fig. 4D).
To determine if the survival effect is sensitive to oxidation, we treated CSF with 50 mM H2O2 and quenched the reaction with catalase. The enzyme was removed by filtering CSF through a 30-kDa-cutoff Centricon filter, and the filtrate was tested for activity against wild-type and mutant fungi by the CSF survival assay. There was no difference between mutants in H2O2-treated CSF and control CSF, indicating that the inhibition factor(s) is not sensitive to oxidation (data not shown).
To identify candidate compounds responsible for the decreased survival effect in CSF, an MS-based quantitative comparison between the <3-kDa fraction of heated and nonheated CSF was employed. A total of 4,318 signals were quantitated across triplicate preparations of heated CSF and nonheated CSF. To focus database searching on candidate compounds most likely responsible for decreased survival, signals were required to be at least 5-fold increased in the nonheated CSF samples compared to in heated samples, with P values less than 0.001, and to have a minimum signal intensity of 1,000. A total of 13 signals meeting these criteria were submitted to two independent databases, the Human Metabolite Database and Scripp's Metlin metabolite database, and were matched based on the accurate mass of the precursor ion to within 50 ppm of the theoretical mass (Table 4; see also Fig. S1 in the supplemental material).
TABLE 4.
Metlin and Human Metabolomics Database search results
| M + H value | Charge | P value | Fold changea | Retention time (min) | Database search resultb | Molecular formula | Mass error (ppm)c |
|---|---|---|---|---|---|---|---|
| 492.196 | 1 | 6.38E−05 | 51.0 | 60 | Pipotiazine sulfoxide | C24H33S2N3O4 | 4.1 |
| 1,448.431 | 2 | 3.80E−06 | 5.6 | 27 | Vancomycin | C66H75Cl2N9O24 | 5.0 |
| 144.103 | 1 | 4.90E−06 | 5.6 | 27 | Vancomycin fragment | NAd | NA |
| 1,305.349 | 1 | 1.96E−04 | 6.0 | 27 | Vancomycin fragment | NA | NA |
| 270.126 | 1 | 5.46E−04 | 6.7 | 81 | No matches | NA | NA |
| 314.233 | 1 | 8.64E−09 | 30.4 | 84 | No matches | NA | NA |
| 474.263 | 1 | 1.89E−04 | 6.6 | 43 | No matches | NA | NA |
| 522.216 | 1 | 6.69E−04 | 8.2 | 81 | No matches | NA | NA |
| 725.154 | 1 | 7.07E−10 | 34.2 | 80 | No matches | NA | NA |
| 795.225 | 2 | 2.44E−04 | 4.8 | 81 | No matches | NA | NA |
| 856.201 | 1 | 9.61E−05 | 7.1 | 81 | No matches | NA | NA |
| 1,047.325 | 2 | 7.46E−04 | 5.2 | 80 | No matches | NA | NA |
| 1,299.400 | 2 | 8.81E−05 | 5.1 | 80 | No matches | NA | NA |
Fold changes are averages of the three individual measurements and indicate fold increases in nonheated samples.
Search results from both the Metlin and Human Metabolomics databases are included.
Mass difference between the experimentally measured mass and the theoretical mass.
NA, not applicable.
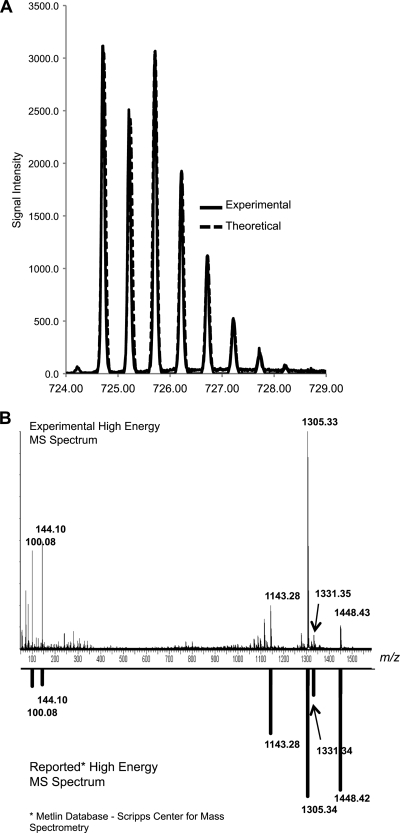
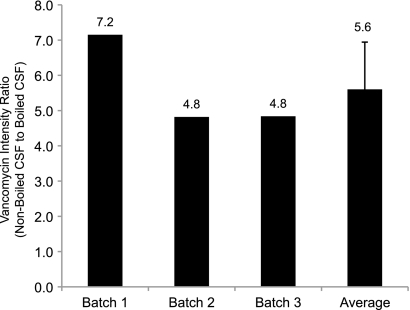
Metlin database searches revealed a putative identification for a compound at m/z 724.719 ([M + 2H]2+), which matched vancomycin at 5.0 ppm. Due to vancomycin's atypical isotope distribution from two Cl atoms within its structure (C66H75Cl2N9O24), the identification was validated by comparing the experimental isotope distribution of the precursor ion with the theoretical isotope distribution of a compound with the same molecular formula (Fig. 5A). The identification was further validated by manually verifying the presence of product ions in the available high-energy MS/MS spectrum by comparison with the experimentally acquired MS/MS spectrum (Fig. 5B). To quantitate relative levels of vancomycin between heated and nonheated CSF samples, peak intensities for the [M + 2H]2+ precursor ion were calculated across all samples (Fig. 6). An average 5.6-fold increase in vancomycin in nonheated samples was measured (P value = 0.03).
FIG. 5.
Qualitative identification of vancomycin. (A) The measured isotopic distribution of the m/z 724.7190 ([M + 2H]2+) signal was overlaid with the theoretical isotope distribution of C66H75Cl2N9O24 (vancomycin). (B) High-energy MS/MS spectrum of the m/z 724.7190 (M + 2H]2+) signal correlated with the reported MS/MS spectrum at a collision energy of 40 (reported directly from the Metlin database).
FIG. 6.
Vancomycin in heat-treated and nontreated samples. Vancomycin intensity ratios of nonboiled CSF to boiled CSF indicates an average 5.6-fold increase in nonboiled CSF, with a two-tailed t test significance value of 0.03. Intensity ratios are plotted relative to vancomycin levels in heated CSF.
To test if vancomycin acts synergistically with our mutants to cause the CSF survival defect, we tested for its activity against the CSF survival-defective C. neoformans mutants on YNB agar plates. The MIC was assessed via the Etest by using a vancomycin Etest strip. The antibiotic did not affect the growth or survival of these mutants, suggesting that vancomycin is not the compound that reduces mutant survival in CSF.
Effect of the ENA1, RUB1, and PIK1 deletions on survival in macrophages.
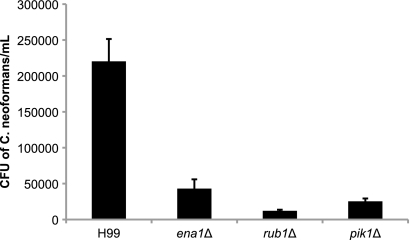
During the course of an infection, C. neoformans encounters many microenvironments within the host, including intracellular phagosomes within macrophages. Thus, we assessed the CSF survival-defective mutants for their ability to survive a potential cellular biological compartment in the CSF by coculture with activated macrophages. After 48 h of coincubation with activated J774A.1 macrophages, the mutant strains exhibited the following reductions in intracellular survival compared to that of WT H99: for the ena1Δ mutant, −81.8%; for the rub1Δ mutant, −94.4%; and for the pik1Δ mutant, −88.5% (P < 0.01) (Fig. 7). Since extracellular yeasts were removed after the first hour of coincubation, these results measured the intracellular survival of the C. neoformans mutants. In addition to the strains' demonstration of no survival defects after the first hour of coincubation and our detection of no differences in phagocytosis among all the strains, these mutants showed significant defects in intracellular survival within macrophages after 48 h.
FIG. 7.
Ena1, Rub1, and Pik1 mediate survival in macrophages. Activated J774A.1 macrophages were coincubated with the ena1Δ, rub1Δ, and pik1Δ mutants of C. neoformans for 60 min at 37°C in 5% CO2. Extracellular yeasts were then removed, and cocultures were incubated overnight under the same conditions as described above. Following coincubation, macrophages were lysed and viable C. neoformans colonies were incubated on YPD plates overnight at 30°C for 2 days for CFU quantification. Strains were assayed in triplicate (P < 0.01).
Effect of CSF-specific-survival-defective mutants on virulence in vivo.
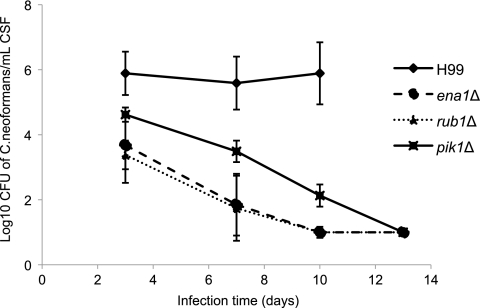
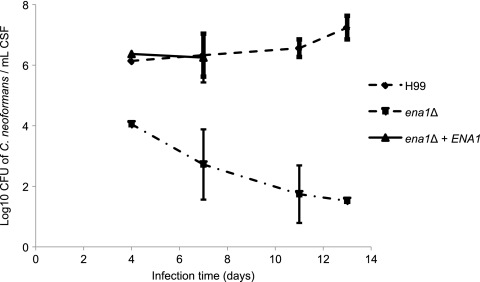
We next sought to ascertain if the CSF-specific survival defects in vitro would predict a reduced survival in the dynamic CSF of the host and translate into an attenuated virulence of the mutant strains. Using the rabbit model of meningitis, we inoculated equal numbers of the WT, ena1Δ, rub1Δ, and pik1Δ strains directly into the CSF of the subarachnoid space of New Zealand White rabbits. On specified days, CSF was withdrawn from each rabbit and colonies of C. neoformans were counted to assess the level of yeast survival. All strains that showed CSF-specific survival defects in vitro showed significantly attenuated virulence by day 7. On day 10, WT-infected rabbits had a fungal burden of log10 5.89, compared to log10 2.13 for the pik1Δ mutant and log10 1.00 (sterile CSF) for both the rub1Δ and the ena1Δ mutant (Fig. 8). Reconstitution of the ena1Δ mutant strain restored virulence, with ena1Δ::ENA1 strain-infected rabbits unable to survive past day 7 of infection (Fig. 9). On day 7, the number of CFU for the mutant-infected rabbit was log10 2.72 ± 0.95 (mean ± standard error of the mean [SEM]), compared to log10 6.25 ± 0.4 for the reconstituted strain and log10 6.33 ± 0.29 for the WT-infected rabbit. These results demonstrate that a functional Ena1 protein is essential for the survival of C. neoformans in the CSF within the subarachnoid space.
FIG. 8.
In vitro CSF-specific-survival-defective mutants exhibit an attenuation of virulence in a rabbit model of meningitis. CSF-sensitive mutants were each inoculated into the subarachnoid space of rabbits (n = 3) with 3.3 × 108 cells/ml. CSF was removed from each rabbit via spinal taps at the indicated times, and serial dilutions were plated onto YPD medium and incubated at 30°C for 2 days for CFU quantification. CFU was used as a measure of the burden of infection. Strains that exhibited growth defects in CSF in vitro also showed attenuated virulence in vivo. Note that no animals injected with H99 survived past 10 days of infection.
FIG. 9.
In vitro, the CSF-defective ena1Δ mutant shows severe virulence attenuation in a rabbit model of meningitis, and its reconstituted strain recovers full virulence. The ena1Δ mutant was reconstituted via a 2-step biolistic transformation with the original gene and a neoR cassette. Strains were then grown to saturation and inoculated into CSF for 4 days at 37°C prior to being plated on YPD medium for CFU quantification. The ena1Δ mutant shows significant attenuation of infection in a rabbit model of meningitis. The ena1Δ::ENA1 mutant recovers the virulence phenotype. All rabbits infected with the reconstituted strain died on day 7 (n = 3).
DISCUSSION
C. neoformans has a unique propensity for invading and surviving in the central nervous system, which is a highly dynamic microenvironment of neural tissue and surrounding fluid. CSF is continuously being produced and eliminated at a rate of 500 ml per day in healthy human adults, with a total volume of 135 ml at any one time. An array of transporters and channels line the basolateral and apical membranes of the choroid plexus epithelium at the lateral ventricles, facilitating the net transfer of ions, including Na+, Cl−, K+, and HCO3−, into CSF (4, 9, 24, 29). Furthermore, the CSF not only carries polypeptides that pass through the blood-brain barrier but also harbors neuro-peptides and proteins manufactured locally as a recipient of cell-shedding products from the brain and the surrounding epithelium (58, 60). Because of the CSF being compartmentalized from its surrounding microenvironments, we hypothesized that the CSF presents a unique stress environment for C. neoformans survival. For instance, it has been shown in human disease that over a million yeasts per ml of CSF can be grown in an HIV-infected patient. This ability to survive and proliferate to large numbers in human CSF is not shared by other pathogenic fungi.
Survival of C. neoformans mutants in human CSF.
Using a forward genetics approach, we identified 13 mutants from a library of 1,200 mutants that covers approximately one-fifth of the genome with survival defects specific to CSF (Table 2; Fig. 3). In the original, independent screen of this library, Liu et al. identified a number of novel mutants exhibiting virulence deficiencies in a murine inhalation model of cryptococcosis (35). Eight of our CSF survival-defective mutants also had infectivity deficiencies in that previous report: the ENA1, RUB1, SRE1, VPS25, CSN1201, HRD1, VAM6, and CPS1 mutants (Table 2). In our CSF survival assay, we identified 5 additional genes previously unassociated with yeast survival in vitro or in vivo: PXA2, NSR1, SWE1, CKB1, and PIK1 (Table 2; Fig. 1).
These newly identified genes encode a wide array of functions. PXA2 encodes Pxa2p, a heterodimeric subunit of an ABC transporter required for the transport of long fatty acids into the peroxisome for β-oxidation (52, 54); NSR1 encodes a nuclear localization-binding protein with two RNA binding domains, mediating the initial step of small nucleolar pre-rRNA processing and ribosome biogenesis (19, 33, 34, 53); Swe1p is a protein kinase that inhibits Cdc28p, delaying the G2/M transition until appropriate conditions are met (20, 22); and CKB1 encodes the β-regulatory subunit of casein kinase II, which plays wide roles in mediating stress responses, including DNA damage and cellular ion homeostasis, by directly upregulating ENA1 when the organism is under Na+ stress (1, 2, 50). Taken together, these genes describe an organism seeking to actively maintain basic growth and electrolyte homeostasis as it encounters the distinct microenvironment of CSF. As this yeast adjusts to CSF, our findings suggest that there is also tight regulation for proper protein sorting and degradation, transporter levels, and signaling (Table 2). In fact, using transcription profiling, we have found that C. neoformans induces the expression of a set of genes in CSF that is distinct from those induced in serum in these functional groups (25). Therefore, C. neoformans strains causing meningitis are put under unique stresses as they survive and multiply in the CSF of the host. In addition, we could not have predicted these genes' importance to CSF survival through classical in vitro phenotypes, such as capsule or melanin production. Clearly, C. neoformans uses several functional pathways and multigenic controls to survive in CSF.
CSF as a unique, hostile microenvironment.
Our analysis of the CSF showed that one feature impacting yeast survival in CSF is a small, heat-labile molecule less than 3 kDa. Recent proteomic studies have shown that CSF is a reservoir for up to 563 peptides from 91 precursor proteins, with an expanded proteome of 798 proteins (60). In a comparison with the Human Proteome Organization (HUPO) high-confidence plasma proteome data set of 889 proteins, Zougman et al. found that 586 proteins were unique to their CSF data set (76% of the CSF proteome). These proteomic studies of CSF have found endogenous antimicrobial peptides such as chitinase-3-like protein-1 precursors ranging from 7 to 23 amino acids (58, 60). However, our treatment with a broad-spectrum protease, proteinase K, did not alleviate the survival defect, suggesting that the factor(s) implicated with our mutants' survival defect is likely not a standard biological peptide (Fig. 4). In addition, the factor(s) presumably does not contain disulfide bonds frequently observed in antimicrobial peptides, because oxidation treatment of CSF did not mitigate the survival activity.
We further characterized the composition of our CSF by mass spectrometry analysis of heated and unheated CSF fractions less than 3 kDa. Because the survival factor(s) was heat labile and less than 3 kDa, we reasoned that retention peaks that were present in the unheated samples, but not in the heated samples, could represent possible candidates for a compound(s) responsible for the mutants' CSF survival phenotypes. This analysis revealed 13 compounds whose expression was at least 5-fold higher in the heated than in the nonheated samples (Table 4). One candidate compound was vancomycin (3). We tested vancomycin for activity against our CSF survival-defective mutants on YNB and were not able to reproduce the survival defects, suggesting that vancomycin is not the survival factor. Although our CSF was pooled from many patients whose medical histories were unknown to us, mass spectroscopy and proteomic analyses did not detect exogenous compounds other than pipotiazine sulfoxide and vancomycin, and this thus decreased the likelihood that our human CSF samples were contaminated with other administered drugs. In fact, several random human CSF pools produced similar results. Further investigations will be necessary to uncover which compound(s) is responsible for this survival defect, but we have compiled a list containing additional potential CSF survival effectors (Table 4).
Although the survival in CSF of our mutants was significantly decreased compared to that in BAL fluid and FBS, survival in FBS was also significantly different from that in BAL fluid for the ENA1Δ mutant (P < 0.01) (Fig. 3E). This finding may suggest that the survival-limiting effector(s) for the ena1Δ mutant may originate in the CSF and may escape into the serum through the blood-CSF barrier. Outside the CSF, the survival effector is “diluted out” by the increased serum volume and the high serum/CSF protein ratio of 250 (58). In fact, it has also previously been shown that blood has anticryptococcal components (47), although the yeast can survive in blood.
Ena1, Rub1, and Pik1 are required for C. neoformans pathogenesis.
The ena1Δ, rub1Δ, and pik1Δ mutants display severe attenuation of virulence in a rabbit model of cryptococcal meningitis and confirm the importance of those proteins to the CSF survival phenotype. Ena1 is a P-type ATPase cation transporter. In S. cerevisiae, it controls the efflux of multiple monovalent ions, including Na+, Li+, and possibly K+ ions (21). Furthermore, the Ena1 homolog in Schizosaccharomyces pombe, Cta3, is thought to transport Ca2+ as well as K+ (6). An intriguing explanation is that these ATPases developed additional ion specificities due to gene duplication and specialization as the fungi evolved to cope with different environments (6). For example, Sceloporus occidentalis has two Ena1-like ATPases, one for Na+ and the other for K+, at high pHs (5). In S. cerevisiae, the regulation of Ena1 has been extensively studied (44). Cna1, Rim101, Hog1, and Snf1 are all known to positively affect Ena1 transcription. Recently, Idnurm et al. observed that C. neoformans Ena1 was essential for virulence in a murine inhalation model and that it is sensitive to alkaline pHs (28). Since the pH of the CSF in vivo is tightly regulated at pH 7.4, the virulence attenuation and CSF survival defect of our ena1Δ mutant was likely not due to alkaline conditions. Our preliminary transcription profiling of yeast cells taken directly from the CSF of rabbits showed that ENA1 is highly upregulated in C. neoformans at days 1 and 7 postinfection in a rabbit model of meningitis, further demonstrating the importance of Ena1 for CSF persistence (unpublished data). Finally, Liu et al. also showed that an ena1Δ mutant was less fit than the WT in a competition assay in the lung (35).
Nedd8/Rub1 is a ubiquitin-like protein that posttranslationally modifies, or neddylates, cullin proteins, subunits of E3 ubiquitin protein ligases. Rub1 is currently known only to bind to cullins, notably CDC53 in budding yeast. Besides the importance of the Rub1/Cdc53 complex for E3 function, the precise function of Rub1 conjugation is unclear (39). Defects in the neddylation pathway display severe defects in cell growth and development, ranging from cell cycle arrest in S. pombe to arrest in embryonic development for C. elegans (30). A current hypothesis for the reason for neddylation is for facilitating the attachment of ubiquitin E2 to the E3 ligase to allow for efficient proteosome targeting of proteins (31). We showed that rub1Δ mutants exhibit severe survival defects when grown in CSF (Fig. 2B). Perhaps this is due to the mutant's inability to achieve optimal protein degradation as it is upregulating genes and turning over proteins in response to the potentially hostile CSF environment. Also, the rub1Δ mutant showed a partial temperature sensitive (ts) growth defect at 37°C. Generally, ts mutants survive poorly in the rabbit, yet the rub1Δ mutant also showed attenuated virulence in C. elegans at a permissive temperature of 25°C. Thus, RUB1 may play several subtle roles in cryptococcal disease.
PIK1 is a 1-phosphatidylinositol-4-kinase (PI4K) and functions in the inositol metabolism and phosphatidylinositol signaling pathways. The kinase catalyzes the first step in the synthesis of phosphatidylinositol phosphates (PIPs), the conversion from phosphatidylinositol (PI) to phosphatidylinositol-4-phospate (PI4P). Besides being structural components of plasma membranes, PIPs regulate a wide variety of cellular functions, including cell wall integrity, actin cytoskeleton organization, and vesicle-mediated membrane trafficking. Through cleavage by phospholipase C, PIPs are precursors to inositol phosphates (IPs), which are intracellular signaling molecules with multiple functions, such as Ca2+ release (7, 49). Because of their multifaceted nature, it is unclear which aspect is responsible for the CSF survival defect of this mutant. Recently, 1-phosphatidylinositol-3-kinase (PI3K) was found to be required for the formation of autophagic vesicles under nutrient starvation and linked to the virulence of C. neoformans (23). Nutrient starvation, however, is not likely responsible for the severe CSF phenotype because the pik1Δ mutant survived like the WT in minimal media such as PBS and CSF itself contains readily available carbon sources (55). Additionally, pik1Δ strains survived like the WT when placed under osmotic stress (Fig. 4A); thus, cell integrity appears to not play a crucial role in this CSF survival phenotype. Improper vesicle trafficking, however, appears to be a more likely cause of the CSF phenotype of this mutant. As C. neoformans acclimates to the hostile environment of the CSF, it is probable that rapid remodeling of the cell surface proteins and uptake of extracellular molecules through rapid endosomal transport will be necessary, and the inability of the pik1Δ mutant to efficiently adapt may result in reduced CSF survival.
In summary, we have confirmed that CSF is a hostile microenvironment, requiring unique transcriptional and biochemical responses for C. neoformans to survive. We focused on 3 genes among the 13 CSF survival-defective mutants, ENA1, RUB1, and PIK1, whose functions were found to be necessary for survival in CSF and intracellular compartments and for full virulence. Single mutations in multiple, disparate pathways can result in CSF survival defects. Perhaps disruptions of several critical cellular processes will act synergistically with CSF to impede fungal survival. Taking our data together, we posit that in vitro CSF survival is a virulence marker for cryptococcal meningitis. The ease of in vitro screening for CSF survival will likely reveal additional genetic mechanisms responsible for adapting to this microenvironment. The compartments of the body (fluids and tissues) put unique demands on fungal pathogens, and how they are able to respond to these host environments determines how effectively they produce disease. Identification of mutants with reduced CSF survival may allow us to pick a novel target(s) for future antifungal drug development.
Supplementary Material
Acknowledgments
We thank Aimee Zaas for helpful discussions regarding the manuscript and Hana Sychrova for the BW31 strain.
J.R.P. was supported by PHS grants AI73896 and AI28388. A.L. was supported by an A. B. Duke Memorial Scholarship and a Trinity College Deans' Summer Research Fellowship.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 9 August 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ackermann, K., A. Waxmann, C. V. Glover, and W. Pyerin. 2001. Genes targeted by protein kinase CK2: a genome-wide expression array analysis in yeast. Mol. Cell. Biochem. 227:59-66. [PubMed] [Google Scholar]
- 2.Ahmed, K., D. A. Gerber, and C. Cochet. 2002. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 12:226-230. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1):16-23. [DOI] [PubMed] [Google Scholar]
- 4.Bairamian, D., C. E. Johanson, J. T. Parmelee, and M. H. Epstein. 1991. Potassium co-transport with sodium and chloride in the choroid plexus. J. Neurochem. 56:1623-1629. [DOI] [PubMed] [Google Scholar]
- 5.Bañuelos, M. A., and A. Rodríguez-Navarro. 1998. P-type ATPases mediate sodium and potassium effluxes in Schwanniomyces occidentalis. J. Biol. Chem. 273:1640-1646. [DOI] [PubMed] [Google Scholar]
- 6.Benito, B., B. Garciadeblás, and A. Rodríguez-Navarro. 2002. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology (Reading, Engl.) 148:933-941. [DOI] [PubMed] [Google Scholar]
- 7.Bennett, M., S. M. N. Onnebo, C. Azevedo, and A. Saiardi. 2006. Inositol pyrophosphates: metabolism and signaling. Cell. Mol. Life Sci. 63:552-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankenship, J. R., and J. Heitman. 2005. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect. Immun. 73:5767-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, P. D., S. L. Davies, T. Speake, and I. D. Millar. 2004. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129:957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chayakulkeeree, M., and J. R. Perfect. 2006. Cryptococcosis. Infect. Dis. Clin. North Am. 20:507-544. [DOI] [PubMed] [Google Scholar]
- 11.Cox, G. M., T. S. Harrison, H. C. McDade, C. P. Taborda, G. Heinrich, A. Casadevall, and J. R. Perfect. 2003. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 71:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, G. M., H. C. McDade, S. C. Chen, S. C. Tucker, M. Gottfredsson, L. C. Wright, T. C. Sorrell, S. D. Leidich, A. Casadevall, M. A. Ghannoum, and J. R. Perfect. 2001. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 39:166-175. [DOI] [PubMed] [Google Scholar]
- 13.Cox, G. M., J. Mukherjee, G. T. Cole, A. Casadevall, and J. R. Perfect. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson, T., L. Liu, A. Gueyikian, X. Zhu, J. Gibbons, and P. R. Williamson. 2001. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol. Microbiol. 42:1121-1131. [DOI] [PubMed] [Google Scholar]
- 15.Fan, W., A. Idnurm, J. Breger, E. Mylonakis, and J. Heitman. 2007. EcaI, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 75:3394-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser, J. A., S. S. Giles, E. C. Wenink, S. G. Geunes-Boyer, J. R. Wright, S. Diezmann, A. Allen, J. E. Stajich, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360-1364. [DOI] [PubMed] [Google Scholar]
- 18.Fraser, J. A., R. L. Subaran, C. B. Nichols, and J. Heitman. 2003. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginisty, H., F. Amalric, and P. Bouvet. 1998. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 17:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladfelter, A. S., L. Kozubowski, T. R. Zyla, and D. J. Lew. 2005. Interplay between septin organization, cell cycle and cell shape in yeast. J. Cell Sci. 118:1617-1628. [DOI] [PubMed] [Google Scholar]
- 21.Haro, R., B. Garciadeblas, and A. Rodríguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 22.Harvey, S. L., A. Charlet, W. Haas, S. P. Gygi, and D. R. Kellogg. 2005. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell 122:407-420. [DOI] [PubMed] [Google Scholar]
- 23.Hu, G. W., M. Hacham, S. R. Waterman, J. Panepinto, S. Shin, X. G. Liu, J. Gibbons, T. Valyi-Nagy, K. Obara, H. A. Jaffe, Y. Ohsumi, and P. R. Williamson. 2008. PI3K signaling of autophagy is required for starvation tolerance and virulence of Cryptococcus neoformans. J. Clin. Invest. 118:1186-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husted, R. F., and D. J. Reed. 1977. Regulation of cerebrospinal fluid bicarbonate by the cat choroid plexus. J. Physiol. (Lond.) 267:411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Icehour, C. R., D. L. Toffaletti, and J. R. Perfect. 2006. Abstr. 106th Gen. Meet. Am. Soc. Microbiol., abstr. F-020.
- 26.Idnurm, A., Y. S. Bahn, K. Nielsen, X. Lin, J. A. Fraser, and J. Heitman. 2005. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3:753-764. [DOI] [PubMed] [Google Scholar]
- 27.Idnurm, A., J. L. Reedy, J. C. Nussbaum, and J. Heitman. 2004. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryot. Cell 3:420-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idnurm, A., F. J. Walton, A. Floyd, J. L. Reedy, and J. Heitman. 2009. Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot. Cell 8:315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johanson, C. E., J. A. Duncan, P. M. Klinge, T. Brinker, E. G. Stopa, and G. D. Silverberg. 2008. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, D., and E. P. M. Candido. 2000. The NED-8 conjugating system in Caenorhabditis elegans is required for embryogenesis and terminal differentiation of the hypodermis. Dev. Biol. 226:152-165. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami, T., T. Chiba, T. Suzuki, K. Iwai, K. Yamanaka, N. Minato, H. Suzuki, N. Shimbara, Y. Hidaka, F. Osaka, M. Omata, and K. Tanaka. 2001. NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J. 20:4003-4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kidd, S. E., F. Hagen, R. L. Tscharke, M. Huynh, K. H. Bartlett, M. Fyfe, L. Macdougall, T. Boekhout, K. J. Kwon-Chung, and W. Meyer. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. U. S. A. 101:17258-17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, W., Z. Xue, and T. Melese. 1991. The NSR1 gene encodes a protein that specifically binds nuclear localization sequences and has two RNA recognition motifs. J. Cell Biol. 113:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, J.-J., and V. A. Zakian. 1994. Isolation and characterization of two Saccharomyces cerevisiae genes that encode proteins that bind to (TG1-3)n single strand telomeric DNA in vitro. Nucleic Acids Res. 22:4906-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, O. W., C. D. Chun, E. D. Chow, C. Chen, H. D. Madhani, and S. M. Noble. 2008. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135:174-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mylonakis, E., A. Idnurm, R. Moreno, J. El Khoury, J. B. Rottman, F. M. Ausubel, J. Heitman, and S. B. Calderwood. 2004. Cryptococcus neoformans Kin1 protein kinase homologue, identified through a Caenorhabditis elegans screen, promotes virulence in mammals. Mol. Microbiol. 54:407-419. [DOI] [PubMed] [Google Scholar]
- 38.Park, B. J., K. A. Wannemuehler, B. J. Marston, N. Govender, P. G. Pappas, and T. M. Chiller. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525-530. [DOI] [PubMed] [Google Scholar]
- 39.Parry, G., and M. Estelle. 2004. Regulation of cullin-based ubiquitin ligases by the Nedd8/RUB ubiquitin-like proteins. Semin. Cell Dev. Biol. 15:221-229. [DOI] [PubMed] [Google Scholar]
- 40.Perfect, J. R. 2005. Cryptococcus neoformans: a sugar-coated killer with designer genes. FEMS Immunol. Med. Microbiol. 45:395-404. [DOI] [PubMed] [Google Scholar]
- 41.Petzold, E. W., U. Himmelreich, E. Mylonakis, T. Rude, D. Toffaletti, G. M. Cox, J. L. Miller, and J. R. Perfect. 2006. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 74:5877-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price, M. S., C. B. Nichols, and J. A. Alspaugh. 2008. Cryptococcus neoformans Rho-GDP dissociation inhibitor mediates intracellular survival and virulence. Infect. Immun. 76:5729-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rude, T. H., D. L. Toffaletti, G. M. Cox, and J. R. Perfect. 2002. Relationship of the glyoxylate pathway to the pathogenesis of Cryptococcus neoformans. Infect. Immun. 70:5684-5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz, A., and J. Ariño. 2007. Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot. Cell 6:2175-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 46.Sherman, F. 2002. Getting started with yeast. Methods Enzymol. 350:3-41. [DOI] [PubMed] [Google Scholar]
- 47.Sridhar, S., M. Ahluwalia, E. Brummer, and D. A. Stevens. 2000. Characterization of an anticryptococcal protein isolated from human serum. Infect. Immun. 68:3787-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephen, C., S. Lester, W. Black, M. Fyfe, and S. Raverty. 2002. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 43:792-794. [PMC free article] [PubMed] [Google Scholar]
- 49.Strahl, T., and J. Thorner. 2007. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta 1771:353-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenney, K. A., and C. V. Glover. 1999. Transcriptional regulation of the S. cerevisiae ENA1 gene by casein kinase II. Mol. Cell. Biochem. 191:161-167. [PubMed] [Google Scholar]
- 51.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Roermund, C. W. T., M. de Jong, L. Ijlst, J. van Marle, T. B. Dansen, R. J. A. Wanders, and H. R. Waterham. 2004. The peroxisomal lumen in Saccharomyces cerevisiae is alkaline. J. Cell Sci. 117:4231-4237. [DOI] [PubMed] [Google Scholar]
- 53.Verheggen, C., J. Mouaikel, M. Thiry, J. M. Blanchard, D. Tollervey, R. Bordonne, D. L. Lafontaine, and E. Bertrand. 2001. Box C/D small nucleolar RNA trafficking involves small nucleolar RNP proteins, nucleolar factors and a novel nuclear domain. EMBO J. 20:5480-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verleur, N. E. H. Hettema, C. W. T. van Roermund, H. F. Tabak, and R. J. A. Wanders. 1997. Transport of activated fatty acids by the peroxisomal ATP-binding-cassette transporter Pxa2 in a semi-intact yeast cell system. Eur. J. Biochem. 249:657-661. [DOI] [PubMed] [Google Scholar]
- 55.Wishart, D. S., M. J. Lewis, J. A. Morrissey, M. D. Flegel, K. Jeroncic, Y. Xiong, D. Cheng, R. Eisner, B. Gautam, D. Tzur, S. Sawhney, F. Bamforth, R. Greiner, and L. Li. 2008. The human cerebrospinal fluid metabolome. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871:164-173. [DOI] [PubMed] [Google Scholar]
- 56.Wormley, F. L., G. Heinrich, J. L. Miller, J. R. Perfect, and G. M. Cox. 2005. Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect. Immun. 73:5022-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan, X., and D. M. Desiderio. 2005. Proteomics analysis of human cerebrospinal fluid. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 815:179-189. [DOI] [PubMed] [Google Scholar]
- 58.Yuan, X., and D. M. Desiderio. 2005. Proteomics analysis of prefractionated human lumbar cerebrospinal fluid. Proteomics 5:541-550. [DOI] [PubMed] [Google Scholar]
- 59.Zhu, X., and P. Williamson. 2003. A CLC-type chloride channel gene is required for laccase activity and virulence in Cryptococcus neoformans. Mol. Microbiol. 50:1271-1281. [DOI] [PubMed] [Google Scholar]
- 60.Zougman, A., B. Pilch, A. Podtelejnikov, M. Kiehntopf, C. Schnabel, C. Kumar, and M. Mann. 2008. Integrated analysis of the cerebrospinal fluid peptidome and proteome. J. Proteome Res. 7:386-399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.