Abstract
The toxicity of Xanthorrhoea johnsonii methanolic leaf extract was investigated using the Artemia franciscana nauplii bioassay. At 24 h, the extract produced an EC50 of 361.0 ± 41.8 μg/ml, indicating that it was substantially more toxic than the pesticide Mevinphos (1346.2 ± 80.1 μg/ml) and approximately 4 fold less toxic than potassium dichromate (87.1 ± 5.2 μg/ml). Whilst potassium dichromate LC50 values remained constant across the 72-h test period, these values decreased for the extract and Mevinphos to similar values (199.8 ± 60.5 and 114 ± 12.8 μg/ml, respectively), indicating their similar level of efficacy. Noteworthy was the apparent anesthetic effect of X. johnsonii leaf extract. Although the extract initially appeared to kill the A. franciscana nauplii, they were seen to temporarily recover by 48 h only to die by 72 h.
Keywords: Australian plants, grass tree, methanol extracts, toxic plants, Xanthorrhoea johnsonii
INTRODUCTION
Plants produce a wide variety of compounds, which in addition to giving them characteristic pigment, odor and flavor characteristics, may also have medicinal and therapeutic properties.[1] For thousands of years, traditional plant-derived medicines have been used in most parts of the world and their use in fighting disease is becoming the focus of intense study.[2,3] Much of the research into traditional medicinal plant use has focused on Asian,[4] African[5] and South American[6] plants. Relatively few studies have focused on the medicinal properties of Australian native plants.
Xanthorrhoea (grasstree) is a small genus of evergreen plants consisting of 28 species and 5 subspecies, all of which are endemic to Australia.[7] All species are long-lived with some plants being estimated at more than 550 years of age.[7–9] However, Xanthorrhoea species are also extremely slow growing. Indeed, the growth of Xanthorrhoea johnsonii has been estimated to be at a rate of 0.88 cm/year.[9,10] Some species are stemless whilst others develop a thick, sometimes branching, trunk topped by a dense crown of rigid leaves. In species with a trunk, this trunk may take up to 20 years to develop.[11]
Australian Aboriginal people had many traditional uses for Xanthorrhoea species including use as tools and weapons[12–14] and as a resin.[12,15] Xanthorrhoea species also provided a minor food source for Aboriginals. Tubers[13] and shoots[12] were eaten whilst nectar from the flowers was used to make a sweet drink.[13] The seeds could also be ground to make dough. Since European settlement, Xanthorrhoea species were extensively harvested for their resin[7] until they were declared protected species.
Being such a slow growing group of plants, it is likely that Xanthorrhoea species have developed protective mechanisms to deter animal foraging which could potentially threaten their survival. Indeed, the list of animals that use Xanthorrhoea species as a major food source is relatively small.[7] Whilst several species of insects, birds and mammals are known to use Xanthorrhoea for food, they almost exclusively feed on the flowers and seeds, leaving all but the youngest leaves largely untouched. Indeed, X. johnsonii has been reported to poison cattle that graze on its leaves.[16–19] Cattle poisoned by Xanthorrhoea become uncoordinated and lose condition, become dehydrated, and in severe cases die. It is possible that a chemical protectant/deterrent produced by Xanthorrhoea species discourages foraging by herbivores, allowing juvenile plants to reach maturity.
This study was undertaken to examine the toxicity of X. johnsonii leaves using the A. franciscana nauplii (brine shrimp larvae) lethality bioassay. This assay has been used to examine the toxicity of a wide variety of compounds.[20] It is an efficient, inexpensive and a relatively rapid way to detect toxic compounds, requiring only low amounts of sample (<20 mg). This test correlates well with cytotoxic activity of some human tumors and, therefore, has the potential to detect new antitumour agents.[21]
MATERIALS AND METHODS
Plant collection and extraction
X. johnsonii is one of the two species of Xanthorrhoea occurring naturally in Toohey Forest, Brisbane, Australia (along with Xanthorrhoea macronema) and is by far the dominant species. X. johnsonii leaves were collected from Toohey Forest and were identified with reference to a taxonomic key to Toohey Forest plants.[22] Samples were dried in a Sunbeam food dehydrator and then ground to a coarse powder. One gram of the powdered leaves was extracted extensively in 50 ml methanol (Ajax, AR grade) for 24 h at 4°C with gentle shaking. The extract was passed through filter paper (Whatman No. 54) under vacuum, followed by drying by rotary evaporation in an Eppendorf concentrator 5301. The resultant pellet was dissolved in 10 ml of 20% methanol. The extract was passed through 0.22 μm filter (Sarstedt) and stored at 4°C.
Reference toxins for biological screening
Potassium dichromate (K2Cr2O7) (AR grade, Chem-Supply, Australia) was prepared as a 1.6 mg/ml solution in distilled water and was serially diluted in synthetic seawater (described below) for use in the A. franciscana nauplii bioassay. Mevinphos (2-methoxycarbonyl-1-methylvinyl dimethyl phosphate) was obtained from Sigma-Aldrich as a mixture of cis (76.6%) and trans (23.0%) isomers and prepared as a 4 mg/ml stock in distilled water. The stock was serially diluted in artificial seawater for use in the bioassay.
A. franciscana nauplii toxicity screening
Toxicity was tested using the A. franciscana nauplii lethality assay developed by Meyer et al,[20] for the screening of active plant constituents, with the following modifications. A. franciscana cysts were obtained from North American Brine Shrimp, LLC, USA (harvested from the Great Salt Lake, Utah). Synthetic seawater was prepared using Reef Salt, AZOO Co., USA. Seawater solutions at 34 g/l distilled water were prepared prior to use. Two grams of A. franciscana cysts were incubated in 1 l synthetic seawater under artificial light (2000 Lux) at 25 °C with continuous aeration. Hatching commenced within 16–18 h of incubation. Newly hatched A. franciscana (nauplii) were used within 10 h of hatching. Nauplii were separated from the shells and remaining cysts and were concentrated to a suitable density by placing an artificial light at one end of their incubation vessel and the nauplii-rich water closest to the light was removed for biological assays. Four hundred microliters of seawater containing approximately 50 (mean 53, n = 168, SD 12) nauplii was added to wells of a 48-well plate and immediately used for bioassay. The plant extract was diluted to 2 mg/ml in seawater for toxicity testing, resulting in a 1 mg/ml concentration in the bioassay. Four hundred microliters of diluted plant extract and the reference toxins were transferred to the wells and incubated at 25 ± 1 °C under artificial light (1000 Lux). A negative control (400 μl seawater) was run in at least triplicate for each plate. All the treatments were performed in at least triplicate. The wells were checked at regular intervals and the number of dead counted. The nauplii were considered moribund if no movement of the appendages was observed within 10 s. After 72 h, all nauplii were sacrificed and counted to determine the total number per well. The LC50 with 95% confidence limits for each treatment was calculated using probit analysis.[23]
RESULTS AND DISCUSSION
One gram of powdered dried X. johnsonii leaves was extensively extracted with methanol and dried under vacuum, resulting in 39 mg of dried extracted material. Resuspension of the dried fraction in 10 ml of 10% methanol resulted in the crude test extract concentration of 3.9 mg/ml. The extract was diluted to 2000 μg/ml in artificial seawaterfor toxicity testing, resulting in a 1000 μg/ml concentration in the A. franciscana lethality bioassay. A. franciscana nauplii were also tested against methanol dilutions to determine the effect of residual methanol on toxicity. No increase in mortality above the control values was seen when testing methanol concentrations up to 5% in the assay, indicating that the residual methanol in the re-suspended extracts was not responsible for nauplii mortality (unpublished results). The results of A. franciscana bioassay screening of the X. johnsonii methanolic extract and the control toxins are shown in Figure 1.
Figure 1.
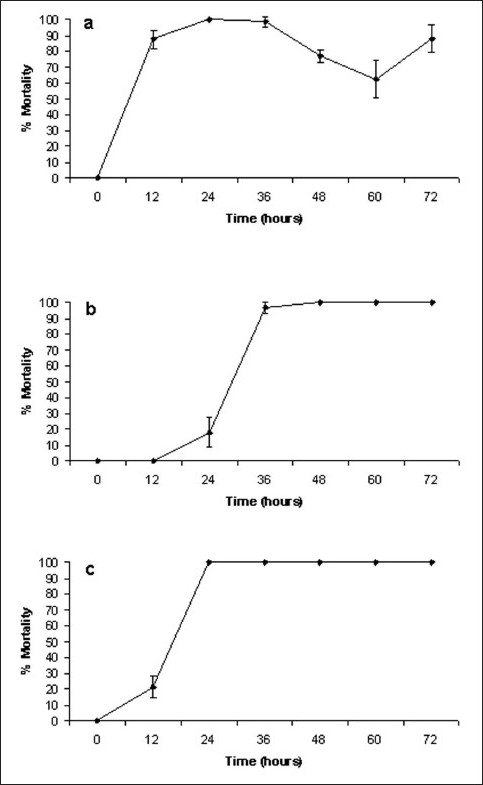
Brine shrimp lethality of (a) X. johnsonii extract (1000 µg/ml), (b) Mevinphos (2000 µg/ml), (c) potassium dichromate (800 µg/ml). All bioassays were performed in at least triplicate and are expressed as mean ± standard deviation
Two interesting features of X. johnsonii toxicity were noted. Firstly, X. johnsonii extract induced apparent morbundity much more rapidly than was observed for either of the positive control toxins. High levels of apparent morbundity (87.6 ± 5.8%) were seen with X. johnsonii extract after 12-h exposure, with 100% mortality seen by 24 h. In contrast, both Mevinphos and potassium dichromate took much longer to achieve high levels of mortality. Following 12 h exposure, both Mevinphos and potassium dichromate had only induced low levels of mortality (0 and 21.3 ± 6.9%, respectively). Indeed, 36 h was required by Mevinphos to achieve approximately 100% mortality (96.9 ± 3.3%).
Perhaps more interesting was the decrease in apparent morbundity seen between 36 and 60 h of exposure. Throughout the course of these studies and previous studies within this laboratory, lack of movement of A. franciscana appendages for 10 s was considered as an indication of apparent morbundity. Many other studies have been less rigorous in their definition of apparent morbundity when determining LC50 /EC50 values. Several studies have defined death (for LC50 determination) as the lack of controlled forward movement. As this could indicate intoxication from which the brine shrimp could recover, we have defined a total lack of movement, over a period of 10 s, as our endpoint. Surprisingly, many of the A. franciscana nauplii used in these studies recovered from their apparent “death” and were seen to swim normally by 48 h exposure. These studies have been repeated several times using a longer time period (10 min) for lack of movement of appendages as a definition of death and the same trend is evident. It appears that the X. johnsonii extract has an anesthetic affect similar to the effect previously reported for curare,[24–26] from which the A. franciscana nauplii are able to temporarily recover. However, at 60 h, it became evident that the nauplii were experiencing difficulty in swimming (“strobe-like” swimming). Following 60 h, the percentage apparent moribundity again began to increase. The nauplii did not recover from this moribund state a second time.
A concentration–response analysis was undertaken to determine the dependence of percentage apparent moribundity on X. johnsonii extract concentration. Due to the previously described decrease in moribundity with time, we report both on the percentage dead (as defined by lack of movement of appendages for 10 s) [Figure 2] and percentage affected (lack of controlled forward movement) [Figure 3]. The same trend previously described for 1000 μg/ml X. johnsonii extract was also apparent in these concentration-response studies. Apparent moribundity of A. franciscana exposed to 1000 μg/ml X. johnsonii extract again decreased from 100% moribund at 24 h to 76.7% (±3.6) by 48 h and increased again to 87.9% (±8.5) by 72 h. The trend was even more apparent when percentage affected was monitored [Figure 3]. The percentage of affected A. franciscana exposed to 1000 μg/ml X. johnsonii extract decreased from 100% affected at 24 h to 63.2% (±3.9) by 48 h and increased again to 93.4% (±6.6) by 72 h.
Figure 2.
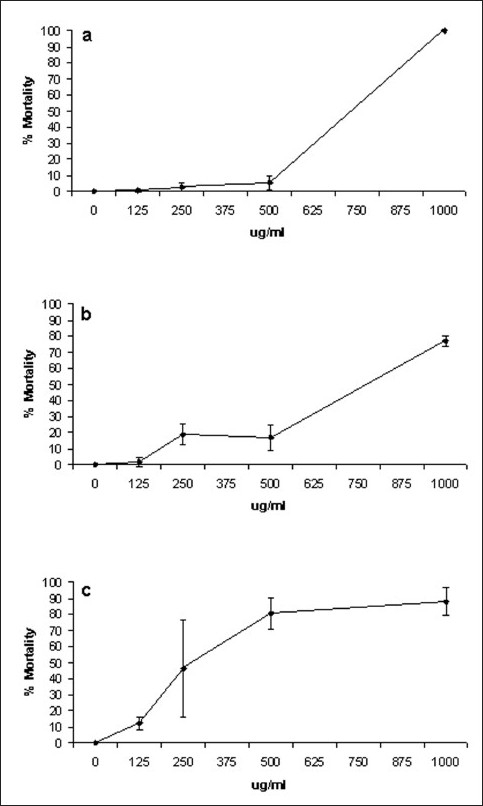
Dependence of X. johnsonii extract concentration on A. franciscana apparent moribundity following exposure for (a) 24 h, (b) 48 h, (c) 72 h. X. johnsonii extract was diluted freshly prior to use in deionized water; 400 µl of juice dilution was added to 400 µl of saline containing A. franciscana nauplii. Moribundity was defined as the lack of appendage movement for at least 10 s. All the bioassays were performed in at least triplicate
Figure 3.
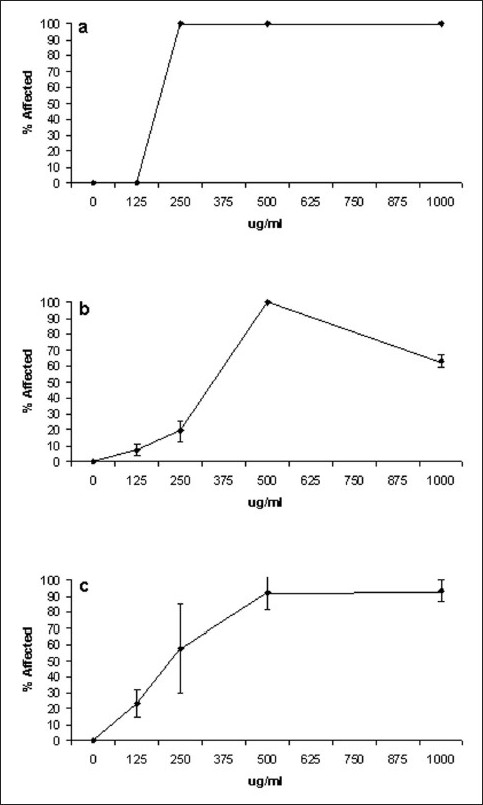
Dependence of X. johnsonii extract concentration on percentage of affected A. franciscana, following exposure for (a) 24 h, (b) 48 h, (c) 72 h. X. johnsonii extract was diluted freshly prior to use in deionized water; 400 µl of juice dilution was added to 400 µl of saline containing A. franciscana nauplii. Affected nauplii were defined as the lack of controlled forward movement. All bioassays were performed in at least triplicate
LC50 values for the X. johnsonii extract, potassium dichromate and mevinphos were calculated by probit analysis [Table 1]. Similarly, EC50 values for X. johnsonii extract were also calculated. Both the extract and the control toxins displayed significant correlation between the concentration/dilution and the percentage affected. The R2 coefficients for the lines of regression of each test sample were greater than 0.9 with the response linear and statistically significant at P < 0.05.
Table 1.
LC50 and EC50 determinations of X. johnsonii extract and the reference toxins Mevinphos and potassium dichromate; values indicate the mean of triplicate determinations ± standard deviation
| Treatment | LC50 value in µg/ml (95% confidence interval) at time (h) |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| X. johnsonii extract | 361.0 ± 41.8 | 402.1 ± 15.7 | 199.8 ± 60.5 |
| X. johnsonii extract* | 220.1 ± 8.6* | 250.0 ± 9.8* | 187.5 ± 19.8* |
| Mevinphos | 1346.2 ± 80.1 | 523.3 ± 39.0 | 114 ± 12.8 |
| Potassium dichromate | 87.1 ± 5.2 | 81.9 ± 3.8 | 79.6 ± 4.6 |
Indicates EC50 determinations (where effect is defined as lack of controlled forward movement)
CONCLUSIONS
The results reported here demonstrate the toxicity of methanolic extracts of X. johnsonii leaves, providing a possible explanation for the lack of use of the leaves as a food source for Australian native herbivores. Moreover, as the A. franciscana nauplii bioassay used in these studies correlates well with antitumor activity,[21] it is possible that X. johnsonii leaf extracts may also be effective as anti-tumor agents. Further studies are needed to determine whether this is the case and to identify the factors involved and their mechanism of action.
Perhaps more interesting was the apparent anesthetic effect of the methanol extract on the A. franciscana nauplii. This effect is similar to the effects previously described for tubocurarine, dimethyltubocurarine and alcuronium (collectively known as curare, a South American arrow poison) from Chondrodendron tomentosum.[24–26] Curare is a neuromuscular blocker that was commonly used as a muscle relaxant by anesthesiologists prior to the development of safer, more effective muscle relaxants. Curare functions by blocking neuronal nicotinic acetylcholine receptors (nAChR),[27] thus blocking neurotransmission. Determining whether the X. johnsonii leaf methanolic extract functions by a similar mechanism or not, was outside the scope of this study. Further studies are needed to isolate individual components from X. johnsonii leaf and examine their mechanism/s of action. It is possible that novel anesthetics/muscle relaxants may be discovered by such studies.
Acknowledgments
Financial support for this work was provided by the School of Biomolecular and Physical Sciences, Griffith University, Australia. The authors would like to thank Ella Merriman for assistance with collection, identification and processing of plant specimens.
Footnotes
Source of Support: School of Biomolecular and Physical Sciences, Griffith University, Australia
Conflict of Interest: None declared
REFERENCES
- 1.Cowan MM. Plant products as antibacterial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhavnani SM, Ballow CH. New agents for Gram-positive bacteria. Curr Opin Microbiol. 2000;3:528–34. doi: 10.1016/s1369-5274(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 3.Chiariandy CM, Seaforth CE, Phelps RH, Pollard GV, Khambay BP. Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J Ethnopharmacol. 1999;64:265–70. doi: 10.1016/s0378-8741(98)00130-5. [DOI] [PubMed] [Google Scholar]
- 4.Patwardhan B, Warude D, Pushpangadan P, Bhatt N. Ayurveda and traditional Chinese medicine: A comparative overview. Evid Based Complement Alternat Med. 2005;2:465–73. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hostettmann K, Marston A, Ndjoko K, Wolfender J. The potential of African plants as a source of drugs. Curr Org Chem. 2000;4:973–1010. [Google Scholar]
- 6.Alonso Paz E, Cerdeiras MP, Fernandez J, Ferreira F, Moyna P, Soubes M, et al. Screening of Uruguayan medicinal plants for antimicrobial activity. J Ethnopharmacol. 1995;45:67–70. doi: 10.1016/0378-8741(94)01192-3. [DOI] [PubMed] [Google Scholar]
- 7.Borsboom AC. Queensland: Environmental Protection Agency; 2005. Xanthorrhoea: A review of current knowledge with a focus on X.johnsonii and X. latifolia, two Queensland protected plants-in-trade. [Google Scholar]
- 8.Stanley TD, Ross EM. Vol. 3. Brisbane: Miscellaneous Publication QM88001, Queensland Department of Primary Industries; 1989. Flora of South-eastern Queensland; p. 45. [Google Scholar]
- 9.Bulow-Olsen A, Just J, Liddle MJ. Growth and flowering history of Xanthorrhoea johnsonii Lee (Liliaceae) in Toohey Forest Queensland. Bot J Linn Soc. 1982;84:195–207. [Google Scholar]
- 10.Lamont BB, Downes S. The longevity, flowering and fire history of the grasstrees Xanthorrhoea preissii and Kingia australis. J Appl Ecol. 1979;16:893–9. [Google Scholar]
- 11.Burnie G, Forrester S, Greig D, Guest S, Harmony M, et al. Botanica: The Illustrated A - Z of Over 10,000 Garden Plants for Australian Gardens and How to Cultivate Them. In: Page S, Olds M, editors. Australia: Random House; 2004. [Google Scholar]
- 12.Anderson ER. Information Series QI 92037. Queensland: Department of Primary Industries; 3. Plants of Central Queensland: Their Identification and uses; p. 119. [Google Scholar]
- 13.Gott B. Use of Victorian plants by Koories. In: Foremen DB, Walsh NG, editors. Flora of Victoria. Vol 1. Melbourne: Inkarta Press; 1993. pp. 195–211. [Google Scholar]
- 14.Bedford DJ. Australian grasstrees. Aus Nat Hist. 1992;24:42–50. [Google Scholar]
- 15.Staff IA, Waterhouse JT. Arborescent monocotyledons. In: Pate JS, McComb AJ, editors. The Biology of Australian Plants. Nedlands: University of Western Australia Press; 1981. pp. 238–57. [Google Scholar]
- 16.McKenzie R. Australian native poisonous plants, Australian Society for Growing Australian Plants (Australia) Available from: http://farrer.csu.edu.au/ASGAP?Apol7/sep97–4.html [last updated on 1997] [last accessed on 2010 Jan 12]
- 17.Everist SL. Botanical affinities of Australian poisonous plants. In: Keeler RF, Van Kampen KR, Lynn LJ, editors. Effects of Poisonous Plants on Livestock. London: Academic Press; 1978. pp. 93–100. [Google Scholar]
- 18.Hall WT. Xanthorrhoea hastile poisoning of cattle. Queens J Agric Sci. 1956;13:1–10. [Google Scholar]
- 19.Hurst E. Sydney: Snelling Printing Works; 1942. The poison plants of New South Wales. [Google Scholar]
- 20.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 21.McLaughlin JL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf J. 1998;32:513–24. [Google Scholar]
- 22.Coutts RH, Catterall CP. Nambour, Australia: Ecos Educational Publishers; 1908. Identifying the plants of Toohey Forest. [Google Scholar]
- 23.Finney DJ. 3rd ed. Nambour, Australia: Ecos Educational Publishers; 1971. Probit Analysis. [Google Scholar]
- 24.Bisset NG. Arrow and dart poisons. J Ethnopharmacol. 1989;25:1–41. doi: 10.1016/0378-8741(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 25.Bisset NG. Curare. In: Pelletier WS, editor. Alkaloids: Chemical and Biological Perspectives. Vol 8. Berlin: Springer; 1992. pp. 3–150. [Google Scholar]
- 26.Bisset NG. War and hunting poisons of the New World: Part 1: Notes on the early history of curare. J Ethnopharmacol. 1992;36:1–26. doi: 10.1016/0378-8741(92)90056-w. [DOI] [PubMed] [Google Scholar]
- 27.Strecker GJ, Jackson MB. Curare binding and the curare-induced subconductance state of the acetylcholine receptor channel. Biophys J. 1989;56:795–806. doi: 10.1016/S0006-3495(89)82726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


