Abstract
Plants are used worldwide for the treatment of diseases, and novel drugs continue to be developed through research from plants. There are more than 20,000 species of plants used in traditional medicines, and these are all potential reservoirs for new drugs. Cucurbita pepo has been used in traditional folk medicine to treat cold and alleviate ache. Previous pharmacological tests have shown that it possesses antiviral, anti-inflammatory, and analgesic effects. Also, Solanum nigrum has been used as a diuretic and an antipyretic agent and it has also been used to cure inflammation, edema, mastitis and hepatic cancer. In this investigation, cytotoxicity of specific concentrations of hydro-alcoholic extracts of C. pepo and S. nigrum was studied on normal [Chinese hamster ovarian cells (CHO) and rat fibroblast] and cancer (HepG2 and CT26) cell lines. The cytotoxic effects and IC50 of the extracts on the selected cell lines were studied followed by colonogenic assay method. The results showed that IC50 of S. nigrum extract was significantly lower than that of the C. pepo extract on all four cell lines (P < 0.05). On the other hand, IC50 of S. nigrum extract was significantly higher than the extract of Taxus baccata and Cisplatin, herbal and chemical control positive anticancer compounds, respectively, on all four cell lines (P < 0.05). As a result, it is concluded that the extract of S. nigrum has almost similar cytotoxicity to the extract of T. baccata on cancer cells.
Keywords: Cell lines, colonogenic assay, Cucurbita pepo, cytotoxicity, Solanum nigrum
INTRODUCTION
Plants are used worldwide for the treatment of diseases, and novel drugs continue to be developed through research from plants. There are more than 20,000 species of plants used in traditional medicines, and these are all potential reservoirs for new drugs.[1] With the advance in modern medicine and drug research, chemical synthesis has replaced plants as the primary source of medicinal agents in industrialized countries. In developing countries, the majority of the world’s population cannot afford pharmaceutical drugs and use their own plant-based indigenous medicines. Traditional medicinal plants have received considerable attention because their bioactive components may lead to new drug discoveries.[2–10]
Solanum nigrum Linn. (Solanaceae), commonly known as “Black nightshade,” has been extensively used in traditional medicine in different parts of world to cure liver disorders, chronic skin ailments (Psoriasis and ringworm), inflammatory conditions, painful periods, fevers, diarrhea, eye diseases, hydrophobia, seizure, etc.[11–14] Also, the fruits are believed to have anti-tumor properties, although the mechanism for the activity remains to be elucidated.
Cucurbita pepo (Cucurbitaceae), commonly known as “pumpkin,” distributed in different regions of world, has been employed in folk medicine to treat colds, alleviate aches, benign prostatic hypertrophy (BPH), etc. Previous pharmacological tests have shown that it possesses antibacterial, antiviral, anti-inflammatory and analgesic effects.[15,16] Taxol is an intense anti-tumor compound extracted from the plants belonged to Taxus species, such as Taxus baccata. However, difficulty in obtaining this compound from yew trees has limited its clinical use.[17]
In this investigation, cytotoxic effects and IC50 of specific concentrations of hydro-alcoholic extracts of fruits of S. nigrum and leaves of C.pepo were compared with hydro-alcoholic extract of bark of T. baccata and Cisplatin, well-known herbal and chemical anticancer compounds, respectively, on normal [Chinese hamster ovarian cells (CHO) and rat fibroblast] and cancer (HepG2 and CT26) cell lines.
MATERIALS AND METHODS
Plant material
Leaves of C. pepo and bark of T. baccata were collected from the northern regions of Iran (Neka city in Mazandaran province) in August 2007. Also, fruits of S. nigrum were collected from downtown of Isfahan, Isfahan province. Botanical identification was confirmed by morphologic characteristics at Department of Pharmacognosy, Sari Faculty of Pharmacy.
Extraction and isolation
A measured quantity of 50 g of dried and powdered parts of each plant was chopped and soaked in 500 ml of ethanol (50% v/v) for 48 h and then percolated (10 drops/min).[18,19] The hydro-alcoholic extracts (1:10) were separately concentrated over a rotary vacuum evaporator and specific concentrations of the hydro-alcoholic extracts (5, 25, 50, 100, 150 µg/ml) were prepared using phosphate buffer (pH = 7.4).
Cell lines
Normal CHO, normal rat fibroblast, HepG2 (human hepatocarcinoma) and CT26 (human colon carcinoma) cell lines were purchased from Pasture Institute of Iran in Tehran.
The completed media were sterilized by 0.22 µm microbiological filters and kept at 4°C before use.
Colonogenic assay
In colonogenic assay, 50 µl of DMEM/F12 including 500–700 cells was added to three wells of six well/plates for each concentration of the extracts and Cisplatin. Then, they were incubated for 48 h. After incubation, the cell lines were exposed to 50 µl of 0 (phosphate buffer), 5, 25, 50, 100, 150 µg/ml of hydro-alcoholic extracts of C. pepo, S. nigrum and T. baccata, and 50 µl of 0, 2.5, 5, 10, 25 µg/ml of Cisplatin for 2 h, and then washed using sterile normal saline 0.09%. Then, 4 ml fresh culture media was added to the wells and incubated for 7 days. After this period, the contents of wells were excluded; the cells were fixed with formalin 9%, and dyed with trypan blue 4% (w/v) for 20 min. Then, trypan blue was excluded, and the six well/plates were washed using sterile normal saline 0.09%. At the end, the dyed colonies were counted using a light microscope.[19–21]
Statistical analysis
Prism ver.3 Software was used to perform statistical tests. One way analysis of variance (ANOVA) followed by Tukey test were used to determine the differences among the groups (P < 0.05).
RESULTS
The results showed that the extract of S. nigrum possesses lower IC50 compared to that of the C. pepo extract on all four normal and cancer cell lines (P < 0.05). But IC50 of the S. nigrum extract was significantly higher than that of the extract of T. baccata and Cisplatin on all four normal and cancer cell lines (P < 0.05) [Table 1]. The lower IC50 represents the higher potency of a compound to inhibit the growth of cells and cause toxicity and death of cells.
Table 1.
The evaluated IC50 of C. pepo, S. nigrum and T. baccata extracts, and Cisplatin on the selected normal and cancer cell lines
| Compound | Cell lines |
|||
|---|---|---|---|---|
| Cancer cell line |
Normal cell line |
|||
| HepG2 (1C50a ± SD)* | CT26 (1C50a ± SD)* | CHO (1C50a ± SD)* | Fibroblast (1C50a ± SD)* | |
| C. pepo (leaves) | 132.6 ± 4.3 | 167.2 ± 6.3 | 239.2 ± 10.3 | 241.4 ± 9.6 |
| S. nigrum (fruits) | 56.4 ± 9.3 | 77.6 ± 1.2 | 98.2 ± 2.1 | 102.3 ± 6.1 |
| T. baccata (bark) | 16.6 ± 0.8 | 18.7 ± 0.3 | 20.4 ± 1.2 | 19.1 ± 1.1 |
| Cisplatin | 0.07 ±0.87 | 1.2 ± 0.03 | 5.5 ± 0.21 | 1.6 ± 0.21 |
µg/ml
P < 0.05
Comparison of the evaluated IC50 of the S. nigrum and C. pepo extracts with that of the T. baccata extract and Cisplatin on normal and cancer cell lines are shown in Figure 1. However, the lowest and highest IC50 values were related to Cisplatin and hydro-alcoholic extract of C. pepo in all cell lines.
Figure 1.
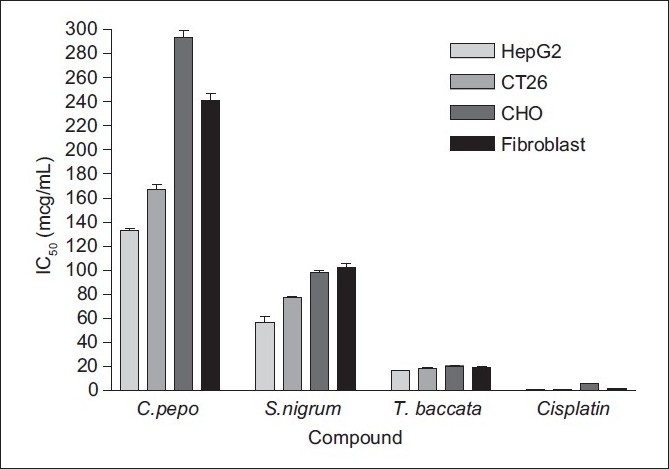
The evaluated IC50 of C. pepo, S. nigrum and T. baccata extracts, and Cisplatin on the selected normal and cancer cell lines
IC50 of the compounds on the four cell lines increased in the following rank order: Cisplatin < T. baccata < S. nigrum < C. pepo. The IC50 of Cisplatin on the four cell lines decreased in the following rank order of cells: CHO > fibroblast > CT26 > HepG2. Also, the IC50 of hydro-alcoholic extract of T. baccata on the four cell lines decreased in the following rank order of cells: CHO > fibroblast > CT26 > HepG2.
On the other hand, the IC50 of hydro-alcoholic extract of S. nigrum on the four cell lines increased in the following rank order of cells: fibroblast < CHO < CT26 < HepG2. As a result, the highest and lowest cytotoxicity of the S. nigrum extract was related to HepG2 (IC50 = 56.4 ± 9.3 µg/ml) and CHO (IC50 = 102.3 ± 6.1 µg/ml) cell lines.
The IC50 of hydro-alcoholic extract of C. pepo on the four cell lines increased in the following rank order of cells: CHO < fibroblast < CT26 < HepG2. Also, the highest and lowest cytotoxicity of the C. pepo extract was related to HepG2 (IC50 = 132.6 ± 4.3 µg/ml) and fibroblast (IC50 = 293.2 ± 10.3 µg/ml) cell lines.
DISCUSSION
For many years, plants have been used for treating different types of diseases in human beings. As per the World Health Organization (WHO) calculation, about 80% of the world’s population presently uses medicinal herbal drugs for their primary health care.[22,23] Cancer is one of the main health problems of the world′s population. Current chemotherapeutic drugs are not useful in all cases and have severe side effects on human health. As a result, a search for other alternatives, preferably natural products, seems necessary and beneficial. This leaves an open door for new and better compounds. In continuation of our search for substances of plant origin with pharmacological effects, we have isolated and screened the plants S. nigrum and C. pepo for their cytotoxic activities.
According to the results, IC50 of the S. nigrum and C. pepo extracts, and T. baccata extract and Cisplatin, as herbal and chemical control positive compounds, respectively, on normal cell lines were higher than that on cancer cell lines. This difference can result from dysfunction of cellular organisms following cancer incidence which causes higher rate of proliferation and increased cellular intake. Also, defensive disorders and effusion insufficiency to escape toxic substances from cells can lead to a lower necessity of the amounts of cytotoxic compounds to inhibit the growth of cancer cells, in comparison with normal cells.[19–21]
A study carried out by Patel et al., evaluated that methanolic extract of S. nigrum fruits has potential activity on HeLa cell and lesser effect on Vero cell. So, the drug has considerable anticancer activity on cervical cancer.[24] Wang et al. showed antitumor effects of methanolic extract of S. nigrum fruits on U266 cells.[25] During Wang et al.’s search for new anti-tumor agents, the ethanolic extract of fruits of C. pepo was observed to exhibit a significant dose-dependent inhibitory effect against HeLa cell growth.[26]
In our study, MTT[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay shows considerable activity of S. nigrum on HepG2 cell and little beat effect on CT26 cell. Also, the viability percents of the cells exposed to the extract of S. nigrum and similarity of IC50 of this extract with the T. baccata extract, a common natural anticancer product, allows us to conclude that extracts of different parts of S. nigrum are good candidates for further studies of activity-monitored fractionation to identify their active components. Our results further support the idea that these medicinal plants can be promising sources of potential anticancer agents to treat or control a variety of cancers such as liver and colon cancers. In addition, the results of this study can form the basis on which the researchers can conduct cytotoxic studies on different cancer cells and find the mechanisms of cytotoxicity of the plants.
Acknowledgments
This study was supported by a grant from the Vice-chancellor of Research of Mazandaran University of Medical Sciences, Sari, Iran.
Footnotes
Source of Support: Research of Mazandaran University of Medical Sciences, Sari, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Hamamouchi M. Medicinal plants in Morocco: Traditional use, marketing, and strategies for conservation and increasing value. Esperance Med. 2002;9:454–8. [Google Scholar]
- 2.Huang CH, Kingston DG, Magri NF, Samaranayake G, Boettner FE. New taxanes from Taxus brevifolia. J Nat Prod. 1986;49:665–9. doi: 10.1021/np50046a018. [DOI] [PubMed] [Google Scholar]
- 3.Van Uden W, Homan B, Woerdenbag HJ, Pras N, Malingre TM, Wichers HJ, et al. Isolation, purification, and cytotoxicity of 5-methoxypodophyllotoxin, a lignan from a root culture of Linum flavum. J Nat Prod. 1992;55:102–10. doi: 10.1021/np50079a015. [DOI] [PubMed] [Google Scholar]
- 4.Prasain JK, Stefanowicz P, Kiyota T, Habeichi F, Konishi Y. Taxines from the needles of Taxus wallichiana. Phytochem. 2001;58:1167–70. doi: 10.1016/s0031-9422(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 5.Dehkordi AJ, Emami SA, Saeidi M, Sadeghi H. Cytotoxicologic Studies of the Extracts of Iranian Juniperus Sabina and Platycladus orientalis on Cancer Cells. J Res Med Sci. 2004;5:205–9. [Google Scholar]
- 6.Kim JS. US6984405; 2006. Compositions for inducing secretion of insulin-like growth factor-1. [Google Scholar]
- 7.Limem-Ben Amor I, Boubaker J, Ben Sgaier M, Skandrani I, Bhouri W, Neffati A, et al. Phytochemistry and biological activities of Phlomis species. J Ethnopharmacol. 2009;125:183–202. doi: 10.1016/j.jep.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahimzadeh MA, Mahmoudi M, Karami M, Saeedi Saravi SS, Ahmadi AH, Salimi E. Separation of active and toxic portions in Sambucus ebulus. Pakistan J Biol Sci. 2007;10:4171–3. doi: 10.3923/pjbs.2007.4171.4173. [DOI] [PubMed] [Google Scholar]
- 9.Saeedi Saravi SS, Shokrzadeh M. The chemistry, pharmacology and clinical properties of Sambucus ebulus: A review. J Med Plant Res. 2010;4:95–103. [Google Scholar]
- 10.Saeedi Saravi SS, Shokrzadeh M. Histopathological and Biochemical Disorders Following Administration of Sambucus ebulus Extract on Mice and Rats and Preventive Effects of Vitamins C and E on Renal and Hepatic Disorders. Phcog Mag. 2008;5:131–5. [Google Scholar]
- 11.Kritikar KR, Basu BD. 2nd ed. vol 2. Allahabad: Lalit Mohan Basu Publications; 1935. Indian Medicinal Plants; pp. 457–8. [Google Scholar]
- 12.Jainu M, Shyamala Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: Possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006;104:156–63. doi: 10.1016/j.jep.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 13.Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl4- induced oxidative damage in rats. Chem Biol Interact. 2008;171:283–93. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Wannang NN, Anuka JJ, Kwanashie HO, Gyang SS, Auta A. Anti-seizure activity of the aqueous leaf extract of Solanum nigrum linn (solanaceae) in experimental animals. African Health Sci. 2008;8:74–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DA, Pan HU, Deng XM, Xiang H, Gao HY, Cai H, et al. Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua. J Asian Nat Product Res. 2007;9:525–9. doi: 10.1080/10286020600782538. [DOI] [PubMed] [Google Scholar]
- 16.Hegi G. Berlin: Parey Verlag; 1979. Illustrierte Flora von Mitteleuropa. Band 6, Teil 2; pp. A29–36. [Google Scholar]
- 17.Bonfill M, Expüsito O, Onrubia M, Jané A, Cusidü RM, Palazün J. Effect of external factors on the production of taxol and other taxanes in cell cultures of Taxus baccata. J Biotechnol. 2007;131:S45. [Google Scholar]
- 18.Prasad SB, A Giri. Antitumor effect of Cisplatin against murine ascites Dalton’s lymphoma. Indian J Exp Biol. 1994;32:57–62. [PubMed] [Google Scholar]
- 19.Shokrzadeh M, Azadbakht M, Ahangar N, Naderi H, Saeedi Saravi SS. Comparison of Cytotoxic Effects of Juniperus sabina and Zataria multiflora Extracts With Taxus baccata Extract and Cisplatin on Normal and Cancer Cell Lines. Phcog Mag. 2010;6:102–5. doi: 10.4103/0973-1296.62894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shokrzadeh M, Saeedi Saravi SS, Mirzayi M. Cytotoxic Effects of Ethyl Acetate Extract of Sambucus ebulus Compared With Etoposide on Normal and Cancer Cell Lines. Phcog Mag. 2009;5:316–9. [Google Scholar]
- 21.Shokrzadeh M, Azadbakht M, Ahangar N, Naderi H, Saeedi Saravi SS. Cytotoxic effects of hydroalcoholic extract of Juniperus sabina compared with hydroalcoholic extract of Taxus baccata and Cisplatin on normal and cancer cell lines. Planta Med. 2009;75:986–7. [Google Scholar]
- 22.Geneva: World Health Organization, Technical Report Series, 916; 2003. WHO. Diet, Nutrition and the Prevention of Chronic Diseases. [PubMed] [Google Scholar]
- 23.Etkin NL, Hausa A. Herbal Pharmacopoeia: Biomedical Evaluation of Commonly used Plant Medicines. J Ethnopharmacol. 1981;4:75–98. doi: 10.1016/0378-8741(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 24.Patel S, Gheewala N, Suthar A, Shah A. In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int J Pharm Pharmaceut Sci. 2009;1:38–46. [Google Scholar]
- 25.Wang W, Lu DP. An in vitro study of cytotoxic and antineoplastic effect of Solanum nigrum L extract on U266. Beijing Da Xue Xue Bao. 2005;37:240–4. [PubMed] [Google Scholar]
- 26.Wang DC, Pan HY, Deng XM, Xiang H, Gao HY, Cai H, et al. Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua. J Asian Nat Pro Res. 2007;9:525–9. doi: 10.1080/10286020600782538. [DOI] [PubMed] [Google Scholar]


