Abstract
The essential oil of the fruit of Illicium griffithii Hook f. et Thoms. was extracted using Clevenger’s apparatus. Forty-one compounds were characterized by gas chromatography-mass spectroscopy (GC-MS). 4-Methyl-6-(2-propenyl)-1,3-benzodioxole was characterized as the major constituent, followed by linalool amongst the volatile constituents. The essential oil was found to be effective against Aspergillus niger, Penicillium spp. and Saccharomyces cerevisiae and possessed considerable activity against Staphylococcus aureus and was inactive against Klebsiella pnemoniae, Pseudomonas aureginosae, Proteus vulgaris and Escherichia coli.
Keywords: Antifungal activity, essential oil, gas chromatography-mass spectroscopy, Illicium griffithii, linalool
INTRODUCTION
Illicium griffithii Hook. f. et Thoms. belongs to Illiciaceae family. It is a large shrub o13f 3–4.5 m height, found at an altitude of 1400–1700 m in northeastern states of India, Khasi hills and Bhutan. Its fruit is composed of compressed, beaked, incurved carpels, each containing one seed arranged in a single whorl. The fruit has a slightly aromatic, bitter and astringent taste. It is used as a stimulant and carminative.[1] Linalool, limonene, α-pinene, 1,8-cineole, β-methoxyphenyl acetone, terpinen-4-ol, (E)-anethole, safrole, germacrene B, cadinol, myristicin, α-selinene, δ-selinene, α-santalene, β-phellandandrene,[2–4] elemicin, (E)-caryophyllene and eugenol derivatives[5] were reported previously as the chemical constituents of the essential oil of I. griffithii, among which linalool was the major constituent.[2] p-Menth-1(7),4(8)-diene-3-O-β-D-glucoside was also reported to be present in the fruit.[6] The present study reports 41 volatile constituents from the essential oil of the fruit of I. griffithii and its antimicrobial activity.
MATERIALS AND METHODS
Plant material
The dried fruit of I. griffithii was procured from Arunachal Pradesh, India, and was identified by National Botanical Research Institute, Lucknow. A voucher specimen (no. I/145ICMR88) was deposited in the museum of this institute.
Instrument
Shimadzu GC 2010 was the instrument used for gas chromatography-mass spectroscopy (GC-MS) analysis. The constituents were identified by comparison of the mass fragments with the spectrum library NIST/EPA/NIH.
Test organisms
Organisms such as Klebsiella pnemoniae (ATCC 700603), Pseudomonas aureginosae (ATCC 27853), Escherichia coli (ATCC 25922), Proteus vulgaris (ATCC 9484) and Staphylococcus aureus (ATCC 25923) were used for the study. The organisms were procured from Christian Medical College, Vellore, and were maintained by serial sub-culturing every month on nutrient agar slants and incubating at 37°C for 18–24 hours. The cultures were stored under refrigerated condition. The antifungal activity of the oil was tested against Aspergillus niger, Penicillium spp. (both were isolated from soil) and Saccharomyces cerevisiae (isolated from dough).[7–9] Isolation and identification of the fungus was done by the microbiologist of this institute.
Extraction of essential oil
Exactly 200 g of the dried fruit of I. griffithii was coarsely powdered and transferred in to a 2-1 round bottom flask. Sufficient amount of water was added and fixed with Clevenger’s apparatus. This was boiled for 4 h and the steam-distilled essential oil (yield 0.85%) was collected, then dried over anhydrous sodium sulfate, transferred into an airtight sample tube and stored at 8°C.
Gas chromatography-mass spectroscopy analysis of the essential oil
One microliter of the essential oil of the fruit was injected into GC. The injector temperature was maintained at 250°C. The detector used was flame ionization detector which was maintained at 280°C. The pressure of the carrier gas, nitrogen, was kept at 10 psi. The oven temperature was set at 60–280°C with a gradual increment of 10°C/min. The injected oil was eluted in the DB-5 MS column of 30 m length and 0.25 mm inner diameter and the eluted constituents were detected by flame ionization detector and the GC chromatogram was recorded [Figure 1].
Figure 1.
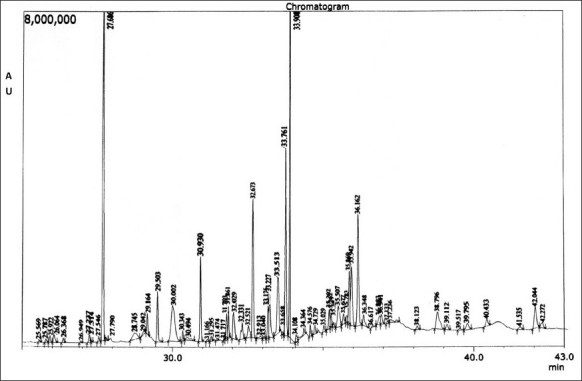
GC-MS chromatogram of essential oil of I. griffithii fruit
Antibacterial activity
Antibacterial activity was determined by the well diffusion method.[10] Petri plates containing 25 ml of nutrient agar medium were seeded with a 24-h culture of the bacterial strains. The inoculum’s size was adjusted so as to deliver a final inoculum of approximately 108 colony-forming units (CFU/ml). Wells (6 mm diameter) were made on solidified inoculated nutrient agar plates by using sterile plunger. Ten and 20 µl of the oil were transferred aseptically to the subsequent wells and labeled. Standard disc of ampicillin 30 µg (positive control) was placed on the inoculated plate to evaluate the potency of the oil. The plates were left undisturbed for 15 min at room temperature to aid in seeping of the oil and then the plates were incubated at 37°C for 24 h. The zone of inhibition was measured in millimeters.
Antifungal activity
The antifungal activity was tested by agar diffusion method.[11–13] Petri plates containing 25 ml of Sabouraud dextrose agar (SDA) medium were seeded with a 7-day old culture of fungus organisms. Wells (6 mm diameter) were made on solidified inoculated SDA plates by using sterile plunger. Ten and 20 µl of the oil were transferred aseptically to the subsequent wells and labeled. Standard disc of 30 µg of amphotericin B was loaded as a reference antifungal drug. The plates were incubated at 25 ± 2°C for 5 days. The zone of inhibition was measured in millimeters.
RESULTS
The volatile oil constituents along with their retention time and percentage obtained from the GC-MS analyzer are given in Table 1. The spectrum obtained is shown in the Figure 1. Forty-one constituents were identified by the detector.
Table 1.
GC-MS data of essential oil of I. griffithii fruit
| Compounds | Retention time (min.) | Area % |
|---|---|---|
| α-Terpinene | 25.569 | 0.07 |
| β-Terpinene | 25.787 | 0.14 |
| δ-Terpinene | 25.922 | 0.23 |
| Eucalyptol | 26.064 | 0.62 |
| α-Terpinolene | 26.949 | 0.07 |
| Linalool oxide | 27.237 | 0.28 |
| Linalool | 27.686 | 12.05 |
| δ-Candinene | 28.745 | 0.81 |
| α-Candinol | 29.042 | 3.86 |
| 4-Terpineol | 29.164 | 0.38 |
| Nerol | 30.343 | 0.31 |
| β-Eudesmol | 30.494 | 0.31 |
| Safrol | 30.930 | 2.47 |
| Phellandral | 31.166 | 0.07 |
| α-Cubene | 31.295 | 0.15 |
| Thymol | 31.474 | 0.07 |
| α-Ylangen | 31.717 | 0.04 |
| Copene | 31.793 | 0.76 |
| Nerol acetate | 31.861 | 0.86 |
| β-Bourbonen | 32.028 | 1.23 |
| α-Gurjunene | 32.331 | 0.83 |
| Calarene | 32.521 | 0.70 |
| Caryophyllene | 32.673 | 4.43 |
| Valencene | 32.913 | 0.24 |
| Cadinene | 33.040 | 0.08 |
| α-Caryophyllene | 33.175 | 1.18 |
| Isoledene | 33.227 | 1.73 |
| δ-Murolene | 33.513 | 3.18 |
| 4-Methyl-6-(2-propenyl)-1,3-benzodioxole | 33.908 | 22.64 |
| 2-Methyl eicosane | 34.108 | 0.35 |
| α-Calacorene | 34.364 | 0.42 |
| Elemol | 34.729 | 0.33 |
| Ledol | 35.029 | 0.25 |
| Spathulenol | 35.262 | 0.43 |
| (–)-Globulol | 35.347 | 0.14 |
| Di-epi-α-cedrene | 35.677 | 0.40 |
| Fonenol | 35.783 | 0.31 |
| Epizonaren | 35.869 | 1.86 |
| Eugenol | 36.941 | 0.44 |
| Rimuene | 39.795 | 0.51 |
| Suberosin | 42.272 | 0.34 |
The essential oil obtained from I. griffithii was tested for its antibacterial activity against five strains, viz., K. pnemoniae (ATCC 700603), Ps. aureginosae (ATCC 27853), E. coli (ATCC 25922), Pr. vulgaris (ATCC 9484) and St. aureus (ATCC 25923) and for the antifungal activity against A. niger, Penicillium spp. and Sa. cerevisiae. Tables 2 and 3 show the results of the observed antibacterial and antifungal activities, respectively.
Table 2.
GC-MS data of essential oil of I. griffithii fruit
| Organisms | Inhibition zone (mm) |
||
|---|---|---|---|
| Ampicillin (30 µg) | Essential oil (10 µl) | Essential oil (20 µl) | |
| K. pneumoniae | 14 | — | — |
| Ps. aeruginosae | 13 | — | — |
| E. coli | 13 | — | — |
| Pr. vulgaris | 14 | — | — |
| St. aureus | 14 | 14 | 14 |
Table 3.
Antifungal activity of essential oil of I. griffithii fruit
| Organisms | Inhibition zone (mm) |
||
|---|---|---|---|
| Ampicillin (30 µg) | Essential oil (10 µl) | Essential oil (20 µl) | |
| A. niger | 12 | 13 | 13 |
| Penicillium spp. | 10 | 9 | 9 |
| Sa. cerevisiae | 11 | 10 | 10 |
DISCUSSION
Of the 41 constituents identified, the percentage content of 4-methyl-6-(2-propenyl)-1,3-benzodioxole and linalool were observed to be 22.64 and 12.05%, respectively, whereas the content of caryophyllene, safrol, β-bourbonen, isoledene, δ-murolene, α-candinol, epizonaren were found to be <5%. Other constituents were <1%.
Ten and 20 µl of the oil showed antibacterial activity only against St. aureus and the inhibition zone was 14 mm and the positive control showed the inhibition zone ranging from 13 to 14 mm against K.pneumoniae, Ps. aureginosae, E. coli, Pr. vulgaris and St. aureus. There was no considerable change in inhibition found by increasing the concentration of the oil and thus the bacterial strains like K. pneumoniae, Ps. aureginosae, E. coli, Pr. vulgaris were susceptible to the oil.
Ten and 20 µl oil showed appreciable antifungal activity against A. niger, Penicillium spp. and Sa. cerevisiae. The inhibition zone of the oil for both the concentrations varied from 9 to 13 mm while for the positive control it was in the range of 10–12 mm. The results of the antifungal activity are shown in Table 3.
CONCLUSION
The essential oil isolated from I. griffithii was found to contain 41 volatile constituents, of which 4-methyl-6-(2-propenyl)-1,3-benzodioxole and linalool were the major compounds. The oil has significant activity against spoilage fungus A. niger, Penicillium spp. and Sa. cerevisiae and was active against the bacterial strain St. aureus. Therefore, it can be concluded that the oil can effectively be used as an antifungal agent and a food preservative, after detailed research.
Acknowledgments
Authors are thankful to ICMR, New Delhi for financial assistance and Director General, CCRAS, New Delhi for facilities.
Footnotes
Source of Support: ICMR, New Delhi
Conflict of Interest: None declared
REFERENCES
- 1.Kirtikar KR, Basu BD. 2 nd. Vol. 1. Delhi: Jayyed Press; 1975. Indian Medicinal Plants; p. 60. [Google Scholar]
- 2.Dung NX, Chinh ND, Leclercq PA. Volatile oil constituent of the fruit of Illicium griffithii Hook. f.et. Thoms. from Vietnam. 1995;7:451–2. [Google Scholar]
- 3.Dutta SC, Saha BN, Pathak MG, Kanjilal PB, Mathur RK. Essential oil of Illicium griffithii Hook.fand Thoms. J Essent Oil Res. 1997;9:227–8. [Google Scholar]
- 4.Agarwal SK, Siddiqui MS, Jain SP, Sushilkumar S. Chemotaxonomical study of Indian Illicium griffithii and Illicium verum fruits. J Med Aromat Plants Sci. 1999;21:945–6. [Google Scholar]
- 5.Howes MJ, Kite GC, Simmonds MS. Distinguishing Chinese star anise from Japanese star anise using thermal desorption - gas chromatography - mass spectrometry. J Agric Food Chem. 2009;57:5783–9. doi: 10.1021/jf9009153. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal SK, Sammal SS, Haneef M, Kumar S. A new monoterpenoid-glucoside from Indian Illicium griffithii. Indian J Chem B. 2002;41:675–6. [Google Scholar]
- 7.Villanova, PA, USA: Approved Standard NCCLS Publication M2-A5; 1993. NCCLS. Performance standards for antimicrobial disc suspectibility tests. [Google Scholar]
- 8.Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. 9 th. Baltimore: Williams and Wilkins; 1994. Bergey’s manual of determinative bacteriology; pp. 518–37. [Google Scholar]
- 9.Suhail M, Akhund S, Jatt T, Mangrio AM, Abro H. Isolation and identification of Pencillium spp., from the river Indus bed at Kotri. Pak J Bot. 2006;38:1289–92. [Google Scholar]
- 10.Nahvi I, Emtiazi G, Alkabi L. Isolation of a flocculating Saccharomyces cerevisiae and investigation of its performance in the fermentation of beet molasses to ethanol. Biomass and Bioenergy. 2005;90:333–40. [Google Scholar]; Tepe B, Daferera D, Sokmen A, Sokmen M, Sokmen M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem. 2002;23:481–6. [Google Scholar]
- 11.Gurgel LA, Sidrim JJC, Martins DT, Cechinel Filho V, Rao VS. In vitro antifungal activity of dragon’s blood from Croton urucurana against dermatophytes. J Ethnopharmacol. 2005;97:409–12. doi: 10.1016/j.jep.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Fontenelle1 RO, Morais SM, Brito EH, Kerntopf MR, Brilhante RS, Cordeiro RA, et al. Chemical composition, toxicological aspects and antifungal activity of essential oil from Lippia sidoides Cham. J Antimicrob Chemother. 2007;59:934–40. doi: 10.1093/jac/dkm066. [DOI] [PubMed] [Google Scholar]


