Abstract
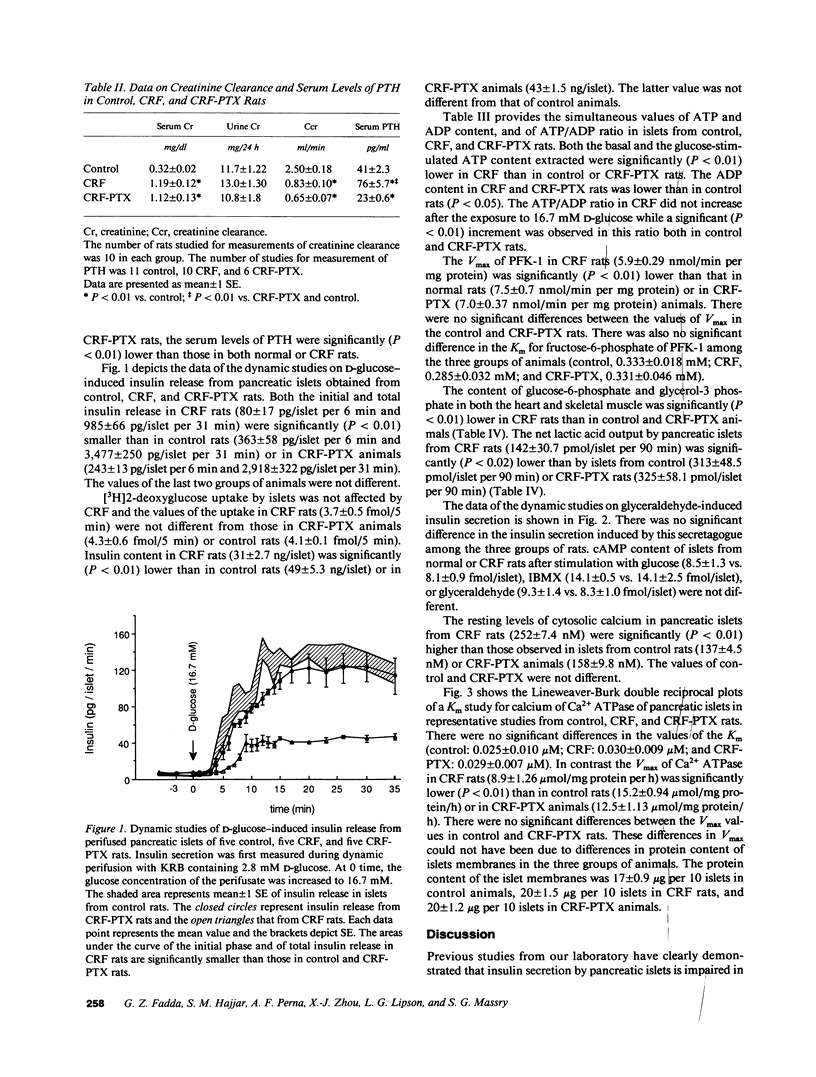
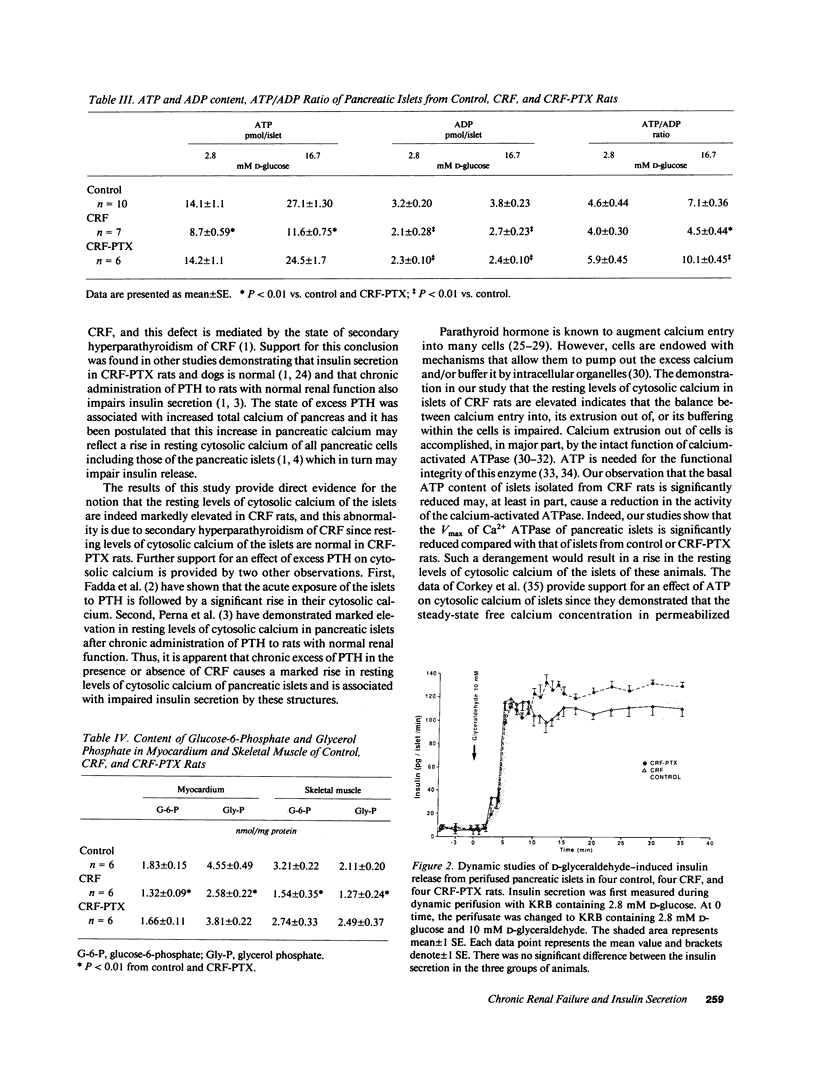
It has been suggested that a sustained rise in resting levels of cytosolic calcium [Ca2+]i of pancreatic islets is responsible for impaired insulin secretion in chronic renal failure (CRF). Evidence for such an event is lacking and the mechanisms through which it may affect insulin secretion are not known. Studies were conducted in normal, CRF, and normocalcemic, parathyroidectomized (PTX) CRF rats to answer these questions. Resting levels of [Ca2+]i of islets from CRF rats were higher (P less than 0.01) than in control of CRF-PTX rats. [3H]2-deoxyglucose uptake and cAMP production by islets were not different in the three groups. Insulin content of, and glucose-induced insulin secretion by islets from CRF rats was lower (P less than 0.01) than in control and CRF-PTX rats. In contrast, glyceraldehyde-induced insulin release by CRF islets was normal. Basal ATP content, both glucose-stimulated ATP content and ATP/ADP ratio, net lactic acid output, Vmax of phosphofructokinase-1, and Ca2+ ATPase of islets from CRF rats were lower (P less than 0.02-less than 0.01) than in normal or CRF-PTX animals. Data show that: (a) Glucose but not glyceraldehyde-induced insulin secretion is impaired in CRF; (b) the impairment in glucose-induced insulin release in CRF is due to a defect in the metabolism of glucose; (c) this latter defect is due to reduced ATP content induced partly by high [Ca2+]i of islets; and (d) the high [Ca2+]i in islets of CRF rats is due to augmented PTH-induced calcium entry into cells and decreased calcium extrusion from the islets secondary to reduced activity of the Ca2+ ATPase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akmal M., Massry S. G., Goldstein D. A., Fanti P., Weisz A., DeFronzo R. A. Role of parathyroid hormone in the glucose intolerance of chronic renal failure. J Clin Invest. 1985 Mar;75(3):1037–1044. doi: 10.1172/JCI111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczynski R., Massry S. G., Kohan R., Magott M., Saglikes Y., Brautbar N. Effect of parathyroid hormone on myocardial energy metabolism in the rat. Kidney Int. 1985 May;27(5):718–725. doi: 10.1038/ki.1985.71. [DOI] [PubMed] [Google Scholar]
- Baczynski R., Massry S. G., Magott M., el-Belbessi S., Kohan R., Brautbar N. Effect of parathyroid hormone on energy metabolism of skeletal muscle. Kidney Int. 1985 Nov;28(5):722–727. doi: 10.1038/ki.1985.190. [DOI] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J., Lu T. C., McCabe E. R. Separation and automated analysis of phosphorylated metabolic intermediates. Anal Biochem. 1974 Jun;59(2):533–546. doi: 10.1016/0003-2697(74)90307-8. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Calcium transport and buffering in neurons. Trends Neurosci. 1988 Oct;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Bogin E., Massry S. G., Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest. 1981 Apr;67(4):1215–1227. doi: 10.1172/JCI110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin E., Massry S. G., Levi J., Djaldeti M., Bristol G., Smith J. Effect of parathyroid hormone on osmotic fragility of human erythrocytes. J Clin Invest. 1982 Apr;69(4):1017–1025. doi: 10.1172/JCI110505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brautbar N., Tabernero-Romo J., Coats J. C., Massry S. G. Impaired myocardial lipid metabolism in phosphate depletion. Kidney Int. 1984 Jul;26(1):18–23. doi: 10.1038/ki.1984.128. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chausmer A. B., Sherman B. S., Wallach S. The effect of parathyroid hormone on hepatic cell transport of calcium. Endocrinology. 1972 Mar;90(3):663–672. doi: 10.1210/endo-90-3-663. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Corkey B. E., Deeney J. T., Glennon M. C., Matschinsky F. M., Prentki M. Regulation of steady-state free Ca2+ levels by the ATP/ADP ratio and orthophosphate in permeabilized RINm5F insulinoma cells. J Biol Chem. 1988 Mar 25;263(9):4247–4253. [PubMed] [Google Scholar]
- Draznin B., Sussman K. E., Eckel R. H., Kao M., Yost T., Sherman N. A. Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J Clin Invest. 1988 Dec;82(6):1848–1852. doi: 10.1172/JCI113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda G. Z., Akmal M., Lipson L. G., Massry S. G. Direct effect of parathyroid hormone on insulin secretion from pancreatic islets. Am J Physiol. 1990 Jun;258(6 Pt 1):E975–E984. doi: 10.1152/ajpendo.1990.258.6.E975. [DOI] [PubMed] [Google Scholar]
- Fadda G. Z., Akmal M., Premdas F. H., Lipson L. G., Massry S. G. Insulin release from pancreatic islets: effects of CRF and excess PTH. Kidney Int. 1988 Jun;33(6):1066–1072. doi: 10.1038/ki.1988.112. [DOI] [PubMed] [Google Scholar]
- Fadda G. Z., Akmal M., Soliman A. R., Lipson L. G., Massry S. G. Correction of glucose intolerance and the impaired insulin release of chronic renal failure by verapamil. Kidney Int. 1989 Nov;36(5):773–779. doi: 10.1038/ki.1989.262. [DOI] [PubMed] [Google Scholar]
- Fraser C. L., Sarnacki P., Budayr A. Evidence that parathyroid hormone-mediated calcium transport in rat brain synaptosomes is independent of cyclic adenosine monophosphate. J Clin Invest. 1988 Apr;81(4):982–988. doi: 10.1172/JCI113452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Levin S. R., Kasson B. G., Driessen J. F. Adenosine triphosphatases of rat pancreatic islets: comparison with those of rat kidney. J Clin Invest. 1978 Sep;62(3):692–701. doi: 10.1172/JCI109177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Hasenson M., Persson J., Pousette A. Estimation of biomass in growing cell lines by adenosine triphosphate assay. Methods Enzymol. 1986;133:27–42. doi: 10.1016/0076-6879(86)33053-2. [DOI] [PubMed] [Google Scholar]
- Lundin A., Richardsson A., Thore A. Continous monitoring of ATP-converting reactions by purified firefly luciferase. Anal Biochem. 1976 Oct;75(2):611–620. doi: 10.1016/0003-2697(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Carpinelli A. R., Herchuelz A., Sener A. The stimulus-secretion coupling of amino acid-induced insulin release: metabolism and cationic effects of leucine. Diabetes. 1980 Jun;29(6):431–437. doi: 10.2337/diab.29.6.431. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. The role of cyclic AMP in insulin release. Experientia. 1984 Oct 15;40(10):1068–1074. doi: 10.1007/BF01971453. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Premdas F. H., Klenck R. E., Eddlestone G., Oldham S. B., Lipson L. G. The dynamic insulin secretory response of isolated pancreatic islets of the diabetic mouse. Evidence for a gene dosage effect on insulin secretion. Diabetes. 1984 Nov;33(11):1120–1123. doi: 10.2337/diab.33.11.1120. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Premdas F. H., Lipson L. G. Insulin release in aging: dynamic response of isolated islets of Langerhans of the rat to D-glucose and D-glyceraldehyde. Endocrinology. 1985 Feb;116(2):821–826. doi: 10.1210/endo-116-2-821. [DOI] [PubMed] [Google Scholar]
- Perna A. F., Fadda G. Z., Zhou X. J., Massry S. G. Mechanisms of impaired insulin secretion after chronic excess of parathyroid hormone. Am J Physiol. 1990 Aug;259(2 Pt 2):F210–F216. doi: 10.1152/ajprenal.1990.259.2.F210. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDaniel M. L., Landt M., Bry C. G., Lacy P. E., McDonald J. M. Ca2+-activated ATPase and ATP-dependent calmodulin-stimulated Ca2+ transport in islet cell plasma membrane. Nature. 1980 Dec 4;288(5790):492–495. doi: 10.1038/288492a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. Measurement of lactic acid in nanomolar amounts. Reliability of such a method as an index of glycolysis in pancreatic islets. Biochem Med. 1976 Feb;15(1):34–41. doi: 10.1016/0006-2944(76)90072-7. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Leitner J. W., Draznin B. Cytosolic free-calcium concentrations in normal pancreatic islet cells. Effect of secretagogues and somatostatin. Diabetes. 1987 May;36(5):571–577. doi: 10.2337/diab.36.5.571. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]



