Abstract
The objective of this work was to evaluate the physical properties and in vivo circulation of chitosan (CH)-coated liposomes of mitoxantrone (MTO). Changes in particle size and zeta potential confirmed the existence of a coating layer on the surface of liposomes. The in vitro release of adsorbed CH from the liposomes was significantly slower than CH solution, indicating the stable interaction between CH and liposomes. The physical stability of the CH-coated liposomes was evaluated by measuring the change in particle size before and after freeze-drying and rehydration. The smallest change was observed when saturated adsorption of CH occurred (0.3%). The sustained release in vitro of MTO from CH-coated liposomes confirmed the increased stability of liposomes. Systemic circulation of CH-coated MTO liposomes was examined. The 0.3% CH-coated liposomes showed the longest circulation time. It could be concluded that the prolonged retention time of the liposomes was closely related with CH coating and its stability effect.
Keywords: liposomes, chitosan coating, stability, in vitro release, prolonged retention time
Introduction
Liposomes are promising drug carriers because of their increased therapeutic efficacy and decreased toxicity compared with the drug prototype. Liposomes for anti-tumor drugs can passively accumulate in tumor tissues as a result of the enhanced permeability of tumor vasculature, thereby limiting drug distribution to normal tissues, which also helps decrease drug toxicity.1,2 However, the stability problem of liposomes in vitro and in vivo limits their application and development as liposomes tend to degrade or aggregate and fuse, which leads to the leakage of entrapped drug during storage3 and rapid clearance from the circulation system after intravenous injection.4 Many studies have found that, among all the factors affecting the stability of liposomes, surface characteristics of the carriers, such as hydrophobicity,4–6 charge,7,8 and fluidity,9 are of great importance. The mechanisms explaining their effect are associated with the inter-molecular interaction and the interaction with various plasma components.10 Therefore, the simple surface modification of the carriers using polymers with specific properties may be an easy approach to modulating their in vitro and in vivo stability.
Polymer coating is a promising way of modifying the surface characteristics of liposomes, which simply mixes a liposome suspension with a polymer solution without chemically linking the polymers to the lipid molecules.11 By coating the liposomes with polymers, the stability of liposomes can be improved during storage due to the long-range mutual repulsion between adjacent bilayers. It has been reported that a variety of polymers have been incorporated into the modification of liposomes, including natural components such as polysaccharides, or synthetic polymers such as polyethylene, polyvinyl alcohol, or polyacrylamide,11–13 among which CH, a positively charged polysaccharide, is one with a promising future in the pharmaceutical and medical fields. CH ((1→4)-2-amino-2deoxy-β-D-glucan), a deacetylated derivative of chitin, is an abundant and natural carbohydrate polymer. CH is regarded as nontoxic, biocompatible, biodegradable,14 and is widely used as a useful excipient in various drug delivery systems. To date, CH has been mainly applied to coat liposomes as a mucoadhesive polymer, and as a permeation enhancer for non-invasive drug delivery due to its ability to open epithelial tight junctions to allow for an increase in paracellular transport of macromolecular drugs. Another major application for CH is to form complex with vaccines or plasmid DNA in non-viral gene therapy.15 Studies on CH-coated liposomes as anti-tumor drug carriers for injection use are rare. In addition, most papers focused only on the in vitro properties of CH-coated liposomes, with few dealing with the systemic evaluation of them. In this paper, we tried to give an overall investigation on the in vitro and in vivo stability of CH-coated liposomes.
In this study, MTO, a synthetic anthraquinone derivative, was chosen as a model drug. MTO has been extensively used for the treatment of various cancers; however, the dose-related cardiotoxicity and myelosuppression of anthraquinone drugs limit their clinical use to a large extent. In recent years, preparations of MTO liposomes have been developed in an effort to decrease its adverse effects.16 Clinically, MTO is of special effectiveness in the treatment of acute myelogenous leukemia. Therefore, the CH-coated MTO-containing liposomes are expected to enhance storage stability and prolong systemic circulation.
The objectives of the present work were: first, to obtain and characterize liposomes coated with CH by measuring the particle size and the zeta potential and second, to give a thorough investigation of the effect of polymer coating on in vitro and in vivo behavior of the obtained CH-coated liposomes by evaluating the physical stability through freeze-drying followed by rehydration (FD-RH) and the systemic circulation of the liposomes. More specifically, for the first time, we evaluated the in vitro desorption of CH from the liposomes using CH solution as control, further clarifying the lasting protective effect of CH for liposomes after entering systemic circulation in vivo in rat.
Methods and materials
Soybean phospholipid was a kind gift from Evonik Degussa China Co., Ltd (Shanghai, China) (purity > 90%). Cholesterol was purchased from Huixing Biochemical Reagent Co., Ltd (Shanghai, China). CH (degree of deacetylation: 92%, viscosity average molecular weight: 70,000 Da) was provided by Nantong Shuanglin Biochemical Co., Ltd. (Jiangsu, China). Mitoxantrone dihydrochloride (99.1%) was obtained from Sichuan Shenghe Pharmaceutical Co., Ltd. Methanol was of HPLC grade. Other reagents were of analytical grade. Water was deionized before use.
Preparation of liposomes and CH-coated liposomes
Liposomes containing MTO were prepared by an ammonium sulfate gradient loading procedure.17 Unloaded liposomes composed of lipids and cholesterol (5:1 in molar ratio) were prepared by thin film hydration followed by sonication. Briefly, 200 mg of PC and 20 mg of cholesterol were dissolved in a small amount of a mixture solution of methanol and chloroform, and the solution was rotary evaporated at 37°C under reduced pressure to obtain a thin lipid film. The thin lipid film was dried in vacuum overnight to ensure complete removal of the solvent and hydration was carried out with 10 mL of aqueous ammonium sulfate (0.3 M) using a vortex mixer. The resultant multilamellar liposomal suspension was sonicated at 4°C using a micro-tip probe sonicator, JY 92-II ultrasonic processor (Syklon, Zhejiang, China) to obtain small unilamellar liposomes. After sonication, the unloaded liposomes were dialyzed against normal saline to remove the extraliposomal ammonium sulfate. The drug-loading liposomes were prepared by incubating the solution of MTO (4 mg/mL) with the suspension of the unloaded liposomes at 37°C for 20 min.
For preparation of CH-coated liposomes, an appropriate amount of CH was dissolved in 1% v/v of acetic acid solution and diluted with sodium acetate buffer solution (pH 6.0) to various concentrations (0.1%, 0.3%, 0.6% and 1.2%, w/v). Then, liposome suspension was added dropwise to CH solution of equal volume. The mixed solution was incubated at 10°C for 1 hour with continuous stirring. The final concentrations of the lipids and CH were half of the original solutions, and the final MTO concentration in the CH-coated liposomes was 0.5 mg/mL.
Characterization of CH-coated liposomes
Particle size and zeta potential of CH-coated liposomes were measured by a Zetasizer 3000 HS (Malvern Instruments, Malvern, UK). Samples for transmission electron microscopy (TEM) were prepared at room temperature by conventional negative staining using 0.3% phosphotungstic acid buffer (pH 6.0). Samples were viewed on a transmission electron microscope H 7650 (Hitachi High-Technologies, Tokyo, Japan).
Content and entrapment efficiency of drug
Concentration of MTO was determined by HPLC. The HPLC system (Shimadzu Corporation, Kyoto, Japan) consisted of a LC-10AT pump and a SPD-10A UV detector set at 658 nm. The analyte was determined at room temperature on a 150 mm × 4.6 mm Diamonsil® C18 column (Dikma Technologies, Beijing, China). The mobile phase consisted of methanol – ammonium acetate buffer solution (20 mM) (48:52 v/v). The ammonium acetate buffer solution was adjusted to pH 2.0 with sulfuric acid before mixed with methanol. The mobile phase was pumped through the system at a rate of 1 mL/min.
The total drug content of suspensions was determined by dissolving liposomes in appropriate amount of methanol containing 1% (v/v) 1 M HCl (acidified methanol) and measured by HPLC.
The entrapment efficiency of the liposomes was determined using a gel filtration method. Free drug was separated from encapsulated MTO over a Sephadex G-25 (1.4 cm × 35 cm) column. MTO-loaded liposomal suspension (0.5 mL) was added to the column. The encapsulated drug was eluted with 5% glucose solution and then the free drug was eluted with normal saline. One milliliter of the liposome elute was diluted with acidified methanol for HPLC and the eluted free drug was injected into the HPLC system directly. The total drug content of the suspensions was also determined. The encapsulation efficiency (EE) was calculated using Eq. (1):
| (1) |
where AUCE and AUCT are the area under the elution profile curve of the encapsulated and total drug in the feed, respectively.18
Coating efficiency of CH
Aliquots of CH-coated liposome suspensions (0.4 mL) were centrifuged at 12,000 rpm for 5 min in an ultimate filtration tube (molecular weight cut 10,000). The non-adhered CH at the bottom of the tube was determined by a colorimetric method described by Gao et al with a slight modification.19 A solution of Alizarin Red S was prepared in deionized water, at a concentration of 1.5 mg/mL. To prepare the standard curve, a stock solution of CH was prepared at a concentration of 1 mg/mL. Different volumes of the stock solution were transferred into volumetric flasks. Aliquots of the dye solution (0.8 mL) were added to each flask, after which the volume in each flask was filled to 10 mL with sodium acetate buffer solution at a pH of 5.0. The UV adsorption was detected at 580 nm with an 8453 UV-Visible Spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) within 2 hours.
In vitro desorption of CH from liposomes
The aim of the study was to investigate the desorption of CH from the surface of the liposomes. To protect the liposomes from deteriorating by the plasma components, CH should not be removed immediately on dilution after entering the systemic circulation. In this experiment, 0.3% CH solution was first diluted with sodium acetate buffer solution at pH 6.0 to half of the original concentration and used as control and CH (0.3%)-coated liposomes without MTO were first prepared according to the method described in “Preparation of liposomes and CH-coated liposomes.” Thereafter, 4 mL of the CH-coated liposomes or 4 mL of CH solution was respectively placed in a cellulose membrane tubing (molecular weight cut-off was 10,000, membrane pore size 10 nm). Then the tubing was tightened and soaked in 40 mL of phosphate buffer solution (pH 7.4) as dissolution medium. The experiments were carried out at 37°C for 8 hours in 3 replicates. At predetermined intervals, 0.5 mL of the dissolution medium was withdrawn and replaced with the same amount of pre-warmed dissolution medium. The samples of the replicates were pipetted directly into disposable cuvettes and analyzed for CH content as described in “Coating efficiency of CH.”
Statistical analysis was performed using Student’s t-test with P < 0.05 as the minimal level of significance on the raw data of the release rate of CH solution and adsorbed CH from liposomes at each time point.
Physical stability studies
The liposomal suspension was subjected to FD-RH with water to assess the physical stability of the liposomes. One mL of liposomal suspension was preparatively frozen at −70°C and applied to the LGJ-10 Freeze-Dry Machine, (Songyuan Huaxing Technology Development Co., Ltd., Beijing, China) for 24 hours. The freeze-dried cake was rehydrated with 1 mL of distilled water containing equal amount of 1% acetic acid (for uncoated liposomes, with water) by vortex-mixing till the cake was optically clear. The particle size of the dispersed liposomes was measured with a Zetasizer 3000 HS (Malvern Instruments, Malvern, UK).
In vitro drug release
In vitro release of MTO from liposomal formulations was analyzed by membrane dialysis. Pre-experiments were carried out on the in vitro release of MTO from liposomes with or without CH coating in phosphate buffer solution (pH 7.4), and less than 5% of MTO was released up to 48 hours from each preparation (data not shown). In this study, equal amount of fetal cattle serum (FCS) was added to the liposome to simulate the in vivo environment. Briefly, 4 mL of CH-coated liposomes mixed with 4 mL of FCS was placed in the cellulose membrane tubing (molecular weight cut-off was 8,000–10,000). For uncoated liposomes, sodium acetate buffer solution (pH 6.0) was added to the liposomes to adjust the concentration. Meanwhile, MTO aqueous solution was treated in the same way and used as control. Then the tubing was tightened and soaked in 200 mL of PBS 7.4 as dissolution medium. The experiments were carried out at 37°C for 48 hours in 3 replicates. At predetermined intervals, 5 mL of the dissolution medium was withdrawn and replaced with the same amount of pre-warmed dissolution medium. The samples of the replicates were pipetted directly into disposal cuvettes and analyzed by an 8453 UV-Visible Spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) at 658 nm.
In vivo studies
Animals
Wistar rats (200–240 g, male), obtained from the animal center of China Pharmaceutical University (Nanjing, China), were maintained under uniform experimental conditions (temperature 25 ± 2°C; humidity 60 ± 5%, 12 hours dark/light cycle) for 1 week prior to experiments. They were fed with standard laboratory chow. All rats were fasted overnight before the experiment while water was provided ad libitum. All animal treatments followed the recommendations of the Regulations for the Administration of Affairs Concerning Experimental Animals.
Drug administration and blood sampling
Four groups of 6 rats each were treated with the normal saline solution of MTO, uncoated liposomes and CH-coated liposomes (0.3% and 0.6%, w/v) at a single MTO dose of 13 mg/m2 via femoral vein injection, respectively. At each time point (5, 15, and 30 minutes and 1, 2, 4, 6, and 8 hours), blood samples (0.3 mL) were collected by oculi chorioidea vein into pre-labeled chilled micro-tubes containing heparin as an anticoagulant. Immediately after collection, each blood sample was gently inverted several times to ensure complete mixing with the anticoagulant. The blood samples were centrifuged for 10 minutes at 4000 × g to separate plasma, and the latter was transferred to cryotubes and stored at −20°C until analysis.
Sample preparation
Plasma containing MTO was extracted by previously reported protein precipitation with slight modification.20 One hundred μL of plasma was mixed with 200 μL of extraction solution (methanol containing 0.5 M hydrochloric acid: acetonitrile (90:10, v:v)). After vigorous vortexing, the samples were centrifuged for 10 minutes at 12,000 × g. The supernatants were removed and 20 μL was injected into the HPLC column for analysis.
Data analysis
Plasma concentrations versus time data were analyzed by Kinetica 4.4 (Thermo Electron Corporation, Waltham, MA, USA). Parameters including AUC, MRT, and t1/2 were determined.
A one-way ANOVA analysis was performed with P < 0.05 as the minimal level of significance.
Results and discussion
Morphological shape
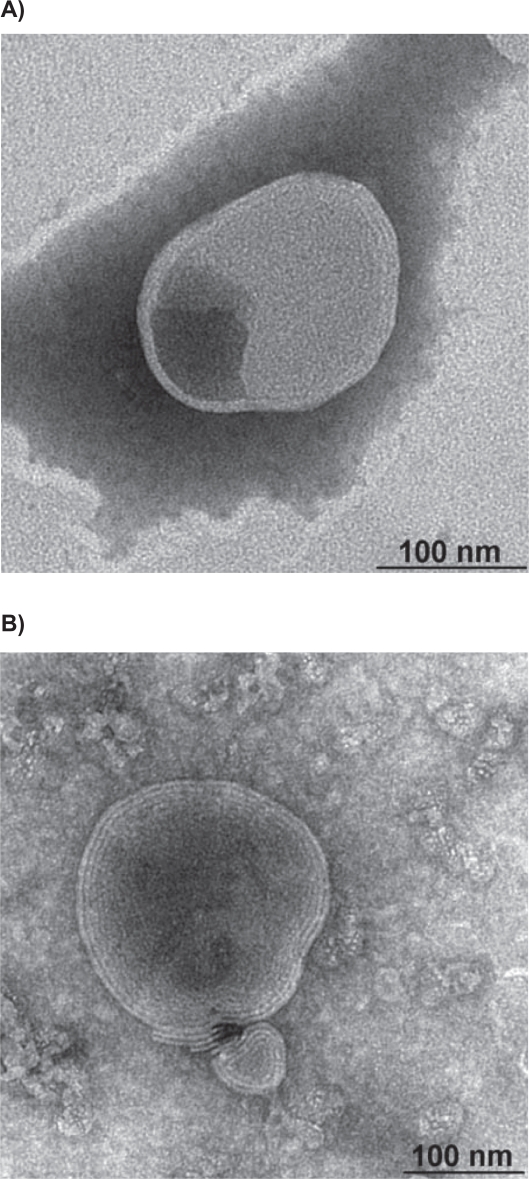
The morphological appearance of CH-coated liposomes and uncoated liposomes was visualized using TEM (Figure 1). It could be seen that both types of liposomes presented a spherical morphology, yet no significant morphological differences between liposomes with or without CH coating were observed. This is probably due to the strong adsorption between the polymer and the liposomal bilayer, resulting in a flat configuration and making it difficult to observe the polymer at the liposome surface. The same situation has been reported by others.21,22
Figure 1.
Transmission electron micrographs of A) uncoated liposomes and B) CH-coated liposomes. Bar is 100 nm.
Particle size and zeta potential
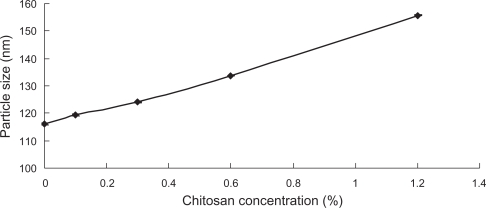
The liposomes were coated with CH at various concentrations in the solution. The particle size of the liposomes is shown in Figure 2. For measuring the particle size, liposomes were diluted by 20-fold with water. The particle size of CH-coated liposomes increased with the increased concentration of polymer solution, suggesting the formation of coating layer on the surface of the liposomes, and an increase in the thickness of the coating layer with the increase of the polymer concentration. However, the size did not change much before and after coating, only 1.34-fold as large as the original size even at the highest concentration of CH (1.2%, w/v). The small change in particle size of liposomes is preferable for circulation, since larger particles tend to be removed from the blood by the cells of the mononuclear phagocyte system.23 The presence of strong intra-molecular electrostatic interactions is an explanation for the thin layer formed around the liposomes.24 After adsorption, the strong attraction between segments of the polyelectrolyte chains and oppositely charged groups on the surface forces them to come into close proximity, forming a thin layer around the surface.25
Figure 2.
The influence of CH concentration on the size of CH-coated liposomes (n = 3).
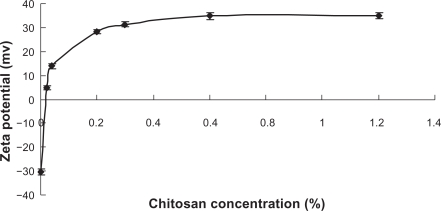
The uncoated liposome had a marked negative charge (−30.4 mv) which, after the incubation with CH at various concentrations (0.02%, 0.04%, 0.2%, 0.3%, 0.6%, and 1.2% w/v), was inverted to positive value. This inversion of the surface charge of the liposomes confirmed the presence of CH coating on the surface of the liposomes. Therefore, the main interaction between liposomes and CH was electrostatic attraction. The zeta potential reached a relatively constant value as the CH concentration increased to 0.3% (Figure 3), indicating the saturated adsorption of CH to liposomes.
Figure 3.
The influence of CH concentration on the zeta potential of CH-coated liposomes (n = 3).
Entrapment efficiency of MTO
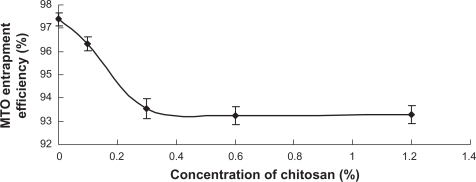
The entrapment efficiency of MTO was 97.4% for uncoated liposomes. The addition of CH decreased the entrapment efficiency slightly (Figure 4), probably due to the interaction of CH with the apolar head groups on the surface of phospholipid bilayers. When the concentration of CH increased to 0.3%, no more leakage of MTO was observed. Since saturated adsorption has achieved (see “Particle size and zeta potential”) and the stable coating layer formed, the excess CH could only surround the outside of the liposomes and were not able to interfere with the bilayer. A similar phenomenon has been reported previously.26
Figure 4.
The effect of CH concentration on the entrapment efficiency of MTO (n = 3).
Coating efficiency of CH
The maximum adsorption of CH to the liposomes was achieved at a polymer concentration of 0.3% (see “Particle size and zeta potential”). The coating efficiency at maximum adsorption was estimated by measuring the concentration of free CH after centrifugation. The coating efficiency at maximum adsorption is 82%, and when calculated on a weight basis, the weight ratio of CH to phospholipids is 1/8.13 at a polymer concentration of 0.3%.
In vitro desorption of CH from liposomes
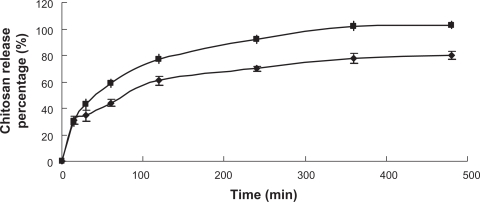
Figure 5 depicts the CH desorption profile from the liposomes using CH solution as control. Both adsorbed CH and CH solution released 30% of their total amount within the first 5 min. After that, the release rate of adsorbed CH from liposomes was significantly slower than that of CH solution (P < 0.05). Maximum release of CH was obtained both at 8 h with a release of 80% of adsorbed CH from liposomes in contrast to 100% of CH solution. CH combines with the phospholipid bilayers mainly through electrostatic interactions. In addition, CH’s hydrophobic side chains can also exert hydrophobic interaction with the phospholipid bilayers.27 Due to the effect of the two kinds of interactions, the release of adsorbed CH was mostly retarded over the period. Therefore, it is reasonable that CH can still protect the liposomes after entering the systemic circulation, and won’t be easily removed through dilution. The initial rapid release of adsorbed CH in the first 5 minutes was probably due to the free CH molecules existing in the preparation, as shown by 82% coating efficiency of CH (0.3%).
Figure 5.
The desorption of CH from the coated liposomes (♦) using CH solution as control (▪) (n = 3).
Physical stability of CH-coated liposomes
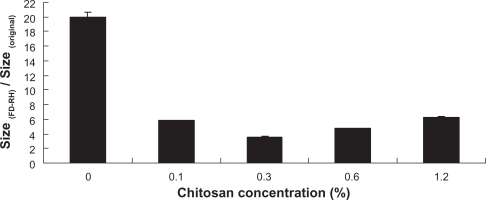
The physical stability of the liposomes was evaluated by measuring the change in particle size after perturbing the system by FD-RH. Figure 6 illustrates the ratios of particle size for CH-coated or uncoated liposomes to original values after FD-RH. The higher values of particle size ratio for uncoated liposomes suggest aggregation and/or fusion of liposomal particles during FD-RH. When liposomes are cooled to a temperature where ice crystals form in the aqueous phase, the liposome particles are forced closer together, leading to the fusion of the particles.25 The addition of CH improved the stability of liposomes against severe physical stress (−70°C). As the CH’s concentration increased from 0% to 0.3%, the values of particle size ratio decreased from 20 to 3.5. Chitosan contains hydroxyl groups along its backbone which can form hydrogen bonds between the polymer and water molecules. A similar protective mechanism for the aggregation and/or fusion of liposomes during freezing or dehydration as in the case of carbohydrates is expected for the polymer layer on the surface of liposomes.23 In addition, the protective effect of CH was partially attributed to the increased steric repulsion between the particles.28 Furthermore, after dehydration, CH formed the “wall material” surrounding the liposomal particles in the powder by forming a puseudohydration phase through their interaction with phospholipid head groups,25 further improving the stability of the particles.
Figure 6.
Relative particle size of liposomes coated with CH after FD-RH compared to that before FD-RH.
As the concentration of CH further increased from 0.3% to 1.2%, the protective effect against the physical stress with FD-RH slightly decreased. The values of particle size ratio increased from 3.5 (0.3% CH) to 6.25 (1.2% CH). Since the liposomal membrane was fully covered with CH molecules, the adsorbed polymer is sufficiently strong to prevent particle–particle interaction by steric stabilization, and the excess CH would not help to further increase the stability of liposomes. On the contrary, the stability of liposomes would be slightly impaired. Laye et al29 have proposed the so-called saturation concentration (Csat), which is the minimum concentration of polymer required to cover the oppositely charged particles, and an empirical model to calculate it via the change in zeta potential:25
where ζ(c) is the zeta potential of liposomes at a CH concentration of c, and ζ0 and ζsat are the initial and saturation liposomal charges, respectively. According to the equation, the calculated Csat was 0.28%, which well agreed with the result we obtained in the zeta potential measurement (0.3%). According to Laye’s explanation, addition of CH to liposomes below and above the saturation concentration can both lead to the destabilization of liposomal dispersions.29 At insufficient polymer concentrations, the anionic phospholipid molecules of liposomes may have been bound to the cationic CH molecules to form coacervates rather than the cationic CH molecules wrapping themselves around the surfaces of the liposome particles. At excess polymer concentrations, the exclusion of polymer molecules from a narrow region surrounding the particle surfaces generates an attractive force that is strong enough to overcome the inter-molecular repulsive forces and to bring the particles together, making the liposomes susceptible to depletion flocculation.25,29 The narrow region (ie, “depletion zone”) was formed due to the conformational entropy restrictions. The “zone” existed between the surface of chitosan-coated particles and the surface in contact with a solution of non-adsorbing polymer. The polymer concentration in the depletion zone is lower than that of the bulk solution, resulting in an attractive force between the particles, which is proportional to the osmotic pressure of the polymer in the bulk solution.30,31
In vitro drug release
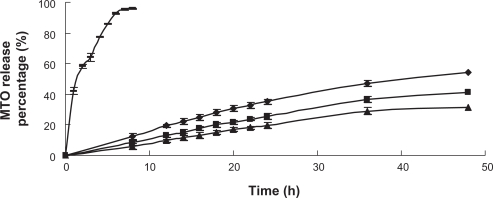
In the in vitro release study, an aqueous solution of MTO was released rapidly and almost completed within 7 h. However, the uncoated and CH-coated liposomes (CH concentration 0.3% and 0.6%) released 31.39%–54.10% of MTO within 48 h of dialysis as shown in Figure 7. The encapsulation of MTO into liposomes significantly slowed its release indicating the depot effect of liposomes, especially the CH-coated liposomal formulations. The sustained release behavior of MTO from polymer-coated liposomes was attributed to the retarded effect of CH coating and the stabilization of coated liposomes in the serum. Besides, CH at a concentration of 0.3% had a better protective effect over CH at a concentration of 0.6%, which was in excellent agreement with the result obtained in FD-RH stability studies (“Physical stability of CH-coated liposomes”).
Figure 7.
In vitro release of MTO from uncoated liposomes (♦), CH-coated liposomes (0.6%) (▪), and CH-coated liposomes (0.3%) (▴) in serum using MTO aqueous solution as control (–) (n = 3).
Pharmacokinetics of the CH-coated liposomes after intravenous injection in rats
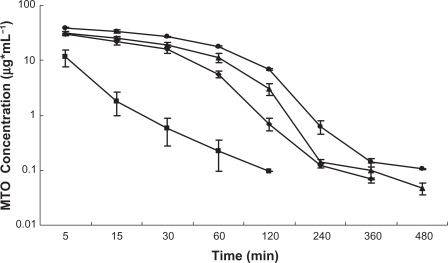
Figure 8 depicts the plasma level-time profiles of MTO saline solution, uncoated liposomes and CH-coated liposomes after intravenous injection in rats. CH at a concentration of 0.3% and 0.6% were used to study the polymer coating effect on the systemic circulation of the liposomes. A rapid decrease of the drug from the systemic circulation for MTO solution was observed within 15 minutes, followed by a relatively slower and steady decrease. After 2 hours, the plasma concentration decreased to around the detection limit (20 ng/mL). Encapsulation of MTO in the liposomes significantly increased the retention time and plasma concentration (P < 0.05). The area under the curve (AUC) for the uncoated liposomes was 7.35-fold of that for the MTO solution (calculated from the data shown in Table 1). Comparing the pharmacokinetic profiles of the uncoated liposomes and CH-coated liposomes, an even higher retention time and plasma concentration were observed for the latter, confirming the effectiveness of the steric coating layer of CH formed on the liposomal surface for prolonging the retention time of the carriers.
Figure 8.
Semi-log plasma concentration-time profiles in rats after intravenous administration of MTO solution (▪), uncoated liposomes (♦), CH-coated liposomes (0.6%) (▴), and CH-coated liposomes (0.3%) (•) (n = 6).
Table 1.
Mean pharmacokinetic parameters of MTO after intravenous administration of MTO solution, uncoated liposomes, CH (0.3%) and CH (0.6%)-coated liposomes
| Parameters | Solution | Uncoated liposomes | CH-coated liposomes (0.6%) | CH-coated liposomes (0.3%) |
|---|---|---|---|---|
| AUC (μg*mL−1* min) | 162.33 ± 12.10 | 1193.50 ± 143.27* | 1709.09 ± 203.53*,# | 2738.16 ± 17.86*,#,
|
| MRT (min) | 18.73 ± 0.52 | 35.67 ± 4.76* | 47.74 ± 0.96*,# | 62.10 ± 2.15*#,
|
| t1/2 (min) | 19.33 ± 1.23 | 35.07 ± 5.25* | 46.73 ± 2.84*,# | 50.22 ± 1.04*# |
Notes: Each value is the mean ± standard deviation, n = 6.
P < 0.05, compared with MTO solution;
P < 0.05, compared with uncoated liposomes;
 P < 0.05, compared with CH-coated liposomes (0.6%).
P < 0.05, compared with CH-coated liposomes (0.6%).
However, as the concentration of CH increased from 0.3% to 0.6%, the retention of the liposome did not increase accordingly, on the contrary, a decrease of both AUC and MRT were observed. The AUC and MRT value for CH-coated liposomes at a concentration of 0.3% was 1.60-fold and 1.30-fold of that for CH at a concentration of 0.6%, respectively (calculated from the data shown in Table 1). This is in agreement with the result in physical stability and in vitro release studies, the excess CH leading to the decreased protective effect for liposomes, which means the dominant mechanism of prolonged retention of MTO in CH-coated liposomes is to stabilize the lipid bilayer membranes after entering the systemic circulation.
Conclusion
CH formed a hydrophilic layer on the surface of liposomes by a simple method of mixing a liposomal suspension with a polymer solution followed by incubation, resulting in particle size increment and positive zeta potential. The effectiveness of CH coating was confirmed by a sustained release of adsorbed CH from the liposomes compared with the CH solution. The enhancing physical stability of the resultant CH-coated liposomes was observed by FD-RH. The presence of free excess CH would decrease the stability of the liposomes. By comparing the circulating profiles of various liposomes with different coating amounts of CH, it was concluded that both the steric property of the coating layer and stabilization of the lipid bilayer membranes can affect the systemic circulation of the liposomes.
Polymer coating is an easy and flexible way to alter the surface properties of the liposomes, and CH, one of the most promising coating materials, is expected to be fully examined.
Acknowledgments/disclosures
The authors report no conflicts of interest in this work. This study is financially supported by National Natural Science Foundation of China (Project No. 30873183).
References
- 1.Chang CC, Liu DZ, Lin SY, et al. Liposome encapsulation reduces cantharidin toxicity. Food Chem Tox. 2008;46(9):3116–3121. doi: 10.1016/j.fct.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 2.Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery – liposomes versus lipid nanoparticles. Int J Nanomed. 2007;2(4):595–607. [PMC free article] [PubMed] [Google Scholar]
- 3.Pajean M, Huc A, Herbage D. Stabilization of liposomes with collagen. Int J Pharm. 1991;77(1):31–40. [Google Scholar]
- 4.Senior JH. Fate and behavior of liposomes in vivo: a review of controlling factors. Crit Rev Ther Drug Carrier Syst. 1987;3(2):123–193. [PubMed] [Google Scholar]
- 5.Storm G, Belliot SO, Daemeh T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17(1):31–48. [Google Scholar]
- 6.Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers long-circulating. Adv Drug Deliv Rev. 1995;16(2–3):141–155. [Google Scholar]
- 7.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146(12):4234–4241. [PubMed] [Google Scholar]
- 8.Juliano RL, Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem Biophys Res Commun. 1975;63(3):651–658. doi: 10.1016/s0006-291x(75)80433-5. [DOI] [PubMed] [Google Scholar]
- 9.Patel HM. Serum opsonins and liposomes: their interaction and opsonophagocytosis. Crit Rev Ther Drug Carrier Syst. 1992;9(1):39–90. [PubMed] [Google Scholar]
- 10.Chonn A, Semple SC, Cullis PR. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J Biol Chem. 1992;267(26):18759–18765. [PubMed] [Google Scholar]
- 11.Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Polymer coating of liposomes with a modified polyvinyl alcohol and their systemic circulation and RES uptake in rats. J Control Rel. 2000;68(2):195–205. doi: 10.1016/s0168-3659(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 12.Mu X, Zhong Z. Preparation and properties of poly(vinyl alcohol)-stabilized liposomes. Int J Pharm. 2006;318(1–2):55–61. doi: 10.1016/j.ijpharm.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Zhu JB, Wen N, Xiong F. Stability and pharmacokinetic studies of O-palmitoyl amylopectin anchored dipyridamole liposomes. Int J Pharm. 2006;313(1–2):136–143. doi: 10.1016/j.ijpharm.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Ravi Kumar MNV. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. [Google Scholar]
- 15.Borchard G, Junginger HE. Modern drug delivery applications of chitosan. Adv Drug Deliv Rev. 2001;52(2):103. doi: 10.1016/s0169-409x(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 16.Ugwu S, Zhang A, Parmar M, Miller B, Sardone T, Peikov V, Ahmad I. Preparation, characterization, and stability of liposome-based formulations of mitoxantrone. Drug Dev Ind Pharm. 2005;31(2):223–229. doi: 10.1081/ddc-200047850. [DOI] [PubMed] [Google Scholar]
- 17.Duan Y, Yu B, Zhang Z. Preparation of long circulating mitoxantrone liposomes and its pharmacokinetics. Acta Pharm Sinica. 2002;37(6):465–468. [PubMed] [Google Scholar]
- 18.Rengel RG, Barisic K, Pavelic Z, Grubisic TZ, Cepelak I, Filipovic-Grcic J. High efficiency entrapment of superoxide dismutase into mucoadhesive chitosan-coated liposomes. Eur J Pharm Sci. 2002;15(5):441–448. doi: 10.1016/s0928-0987(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 19.Gao GZ, Jiao QC, Ding YL, Chen L. Study on quantitative assay of chitosan in compound sample with a spectrophotometric method. Food and Ferm Ind. 2003;29(5):49–52. [Google Scholar]
- 20.Johnson JL, Ahmad A, Khan S, et al. Improved liquid chromatographic method for mitoxantrone quantification in mouse plasma and tissues to study the pharmacokinetics of a liposome entrapped mitoxantrone formulation. J Chromatogr B. 2004;799(1):149–155. doi: 10.1016/j.jchromb.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Blakmeer J, Behmer MR, Cohen Stuart MA, Fleer GJ. Adsorption of weak polyelectrolytes on highly charged surfaces: polyfacrylic acid on olystyrene latex with strong cationic groups. Macromol. 1990;23(8):2301–2309. [Google Scholar]
- 22.Henriksen I, Smistad G, Karlsen Interactions between liposomes and chitosan. Int J Pharm. 1994;101(3):227–236. [Google Scholar]
- 23.Takeuchi H, Yamamoto H, Toyoda T, Toyobuku H, Hino T, Kawashima Y. Physical stability of size controlled small unilameller liposomes coated with a modified polyvinyl alcohol. Int J Pharm. 1998;164(1–2):103–111. [Google Scholar]
- 24.Schonhoff M. Layered polyelectrolyte complexes: physics of formation and molecular properties. J Phys Condens Matter. 2003;15(49):1781–1808. [Google Scholar]
- 25.Guzey D, McClements DJ. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv Colloid Interface Sci. 2006;(21):128–130. 227–248. doi: 10.1016/j.cis.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Ping Q, Jiang G, Huang L, Tong Y. Chitosan-coated liposomes: characterization and interaction with leuprolide. Int J Pharm. 2003;260(2):167–173. doi: 10.1016/s0378-5173(03)00254-0. [DOI] [PubMed] [Google Scholar]
- 27.Fang N, Chan V, Mao HQ, Leong KW. Interactions of phospholipid bilayer with chitosan: effect of molecular weight and pH. Biomacromol. 2001;2(4):1161–1168. doi: 10.1021/bm015548s. [DOI] [PubMed] [Google Scholar]
- 28.Kellaway IW, Najib NM. Hydrophilic polymers as stabilizers and flocculants of sulphadimidine suspensions. Int J Pharm. 1981;9(1):59–66. [Google Scholar]
- 29.Laye C, McClements DJ, Weiss J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J Food Sci. 2008;73(5):N7–N15. doi: 10.1111/j.1750-3841.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins P, Snowden M. Depletion flocculation in colloidal dispersions. Adv Colloid Interface Sci. 1996;68(1):57–96. [Google Scholar]
- 31.Kwaambwa HM. Stability and phase separation behavior of systems of particles in a medium with added polymer. Water SA. 2006;32(1):111–114. [Google Scholar]