Abstract
Head blight, caused by Gibberella zeae, is a significant disease among cereal crops, including wheat, barley, and rice, due to contamination of grain with mycotoxins. G. zeae is spread by ascospores forcibly discharged from sexual fruiting bodies forming on crop residues. In this study, we characterized a novel gene, ROA, which is required for normal sexual development. Deletion of ROA (Δroa) resulted in an abnormal size and shape of asci and ascospores but did not affect vegetative growth. The Δroa mutation triggered round ascospores and insufficient cell division after spore delimitation. The asci of the Δroa strain discharged fewer ascospores from the perithecia but achieved a greater dispersal distance than those of the wild-type strain. Turgor pressure within the asci was calculated through the analysis of osmolytes in the epiplasmic fluid. Deletion of the ROA gene appeared to increase turgor pressure in the mutant asci. The higher turgor pressure of the Δroa mutant asci and the mutant spore shape contributed to the longer distance dispersal. When the Δroa mutant was outcrossed with a Δmat1-2 mutant, a strain that contains a green fluorescence protein (GFP) marker in place of the MAT1-2 gene, unusual phenotypic segregation occurred. The ratio of GFP to non-GFP segregation was 1:1; however, all eight spores had the same shape. Taken together, the results of this study suggest that ROA plays multiple roles in maintaining the proper morphology and discharge of ascospores in G. zeae.
Gibberella zeae (anamorph: Fusarium graminearum) causes Fusarium head blight in wheat, barley, and rice, as well as ear rot and stalk rot in maize (20, 23). The infected grains are frequently contaminated by mycotoxins, such as trichothecenes and zearalenone, which are harmful to humans and animals (6). The fungus overwinters in crop debris in the form of storage hyphae and develops ephemeral fruiting bodies (perithecia) at warmer temperatures. Ascospores formed within the perithecia are forcibly discharged into the air and are believed to serve as the primary inoculum of the disease (7, 27, 37, 39–42). Therefore, sexual development and ascospore discharge are important factors in fungal survival and disease initiation.
In fungi of the phylum Ascomycota, the sexual cycle is initiated when two genetically distinct nuclei combine to form a binucleate cell (31). As a homothallic fungus, G. zeae possesses the two mating type genes MAT1-1 and MAT1-2 in the haploid genome and therefore does not require a mating partner for sexual development (22, 46). Perithecium initials give rise to small, coiled initials that develop into perithecia filled with asci, tubular sacs of ascospores, which are the products of meiosis. Mature asci extend through the ostiole of perithecia and discharge their ascospores (40).
Unique features of cell differentiation are involved in ascus and ascospore morphogenesis. Ascospore delimitation within the ascus and the development of a cell wall between the ascus and ascospore membranes are unique features of the process (31). Most studies of morphogenesis have described these changes in detail; however, much of these data have been limited to microscopic observations. Several genes involved in ascospore morphogenesis have been identified in Neurospora crassa (30), but the detailed mechanisms and genes involved in ascus and ascospore morphogenesis remain to be elucidated. The Round spore (R) mutant of N. crassa was shown to have round ascospores (24), and the gene responsible for this phenotype, rsp, was subsequently cloned (28). However, in G. zeae, no genes have been identified that are involved in ascus and ascospore morphogenesis.
Although recent research has shed light on the physiological basis of ascospore discharge, the genetic basis remains largely unknown (38). The main force responsible for the observed shooting is turgor pressure within the extended asci. In G. zeae, a buildup of K+ and Cl− ions drives the influx of water and causes turgor pressure that stretches the asci (41). Asci can accumulate polyols as well as ions. In a previous study, it was shown that the polyols are comprised mainly of mannitol and glucose; however, the concentration of these polyols is too low to make a significant contribution to turgor pressure (42). When the turgor pressure exceeds the threshold of the asci, apical pores rupture and ascospores are forcibly discharged (38). Trail et al. (41) estimated that the acceleration of ascospores in G. zeae is 8,500,000 m s−2 using an iterative model to predict initial velocity. Recently, Yafetto et al. (44) used high-speed video photography to examine several large-spore fungi, including Ascobolus immerses, and to predict acceleration during dispersal. The asci of A. immerses are more than 12-fold larger in diameter than the asci of G. zeae (38). The size difference between these fungi greatly affects the behavior of their projectiles and results in an initial speed for G. zeae that is too great for application of the video photography method (for further discussion, see the supplemental material).
To date, only one gene from G. zeae, the calcium ion channel gene cch1, has been shown to be involved in ascospore discharge (12). Deletion of this gene was shown to arrest ascospore discharge without affecting spore and ascus morphology. Since the genomic sequence of G. zeae is now available, the functional analysis of genes involved in sexual development has been accelerated. Random insertional mutagenesis is one strategy that has been used to identify novel genes associated with sexual development (13, 34). Previously, we produced a collection of more than 20,000 mutants from G. zeae by using the restriction enzyme-mediated integration (REMI) transformation procedure (13). In this study, the G. zeae mutant Z43R9901, which was isolated from a screening of REMI transformants, showed an unusual phenotype during sexual development. Further analysis demonstrated that the novel gene ROA is involved in ascospore morphogenesis and discharge in G. zeae. The results of this study increase our understanding of sexual development in the fungus.
MATERIALS AND METHODS
Strains and culture conditions.
The wild-type strain Z03643 of G. zeae (3) and mutants derived from this strain were used for this study (see Table S1 in the supplemental material). The mutant strain Z43R9901 was generated by REMI mutagenesis (13). All strains were stored as mycelia and conidia in a 20% glycerol solution at −80°C. Standard laboratory methods and culture media for the Fusarium species were used (23). For conidial production, the strains were inoculated in carboxymethyl cellulose medium (4).
Nucleic acid manipulations, primers, and PCR conditions.
Fungal genomic DNA was extracted as previously described (23). Total RNA was isolated from mycelia that were ground in liquid nitrogen using an Easy-Spin total RNA extraction kit (Intron Biotech, Seongnam, Republic of Korea). Standard procedures were used for restriction endonuclease digestion, agarose gel electrophoresis, and Southern and Northern analysis (33). The PCR primers (see Table S2 in the supplemental material) for this study were synthesized by the Bionics oligonucleotide synthesis facility (Seoul, Republic of Korea). General PCR was performed, following the manufacturer's instructions (Takara Bio Inc., Otsu, Japan). Plasmid DNA was purified from Escherichia coli grown in 3 ml of lysogeny broth using a plasmid purification kit (Intron Biotech, Seongnam, Republic of Korea). DNA sequence analysis of the rescued plasmids was initiated at a location close to the HindIII site on the REMI vector pUCH1 (43) and performed with specific primers for pUCH1-P1 and pUCH1-P5.
Targeted gene deletion and complementation.
Fusion PCR products for targeted gene deletion were constructed using a slightly modified double-joint PCR (DJ-PCR) procedure (45). The 5′ and 3′ regions of the target gene were amplified by PCR with the primer pairs ROA-5F/ROA-5R and ROA-3F/ROA-3R, respectively. The Geneticin resistance gene cassette (gen), which carries the Aspergillus nidulans trpC promoter and terminator, was amplified from pII99 (26). The three amplicons (5′ region, 3′ region, and gen) were mixed in a 1:1:3 molar ratio and fused by a second round of PCR. The fused constructs were amplified with the new nested primers ROA-NF and ROA-NR. To complement the Δroa mutant, the entire ROA gene, including the promoter and terminator, was amplified from the wild-type strain using the ROA-NF/ROA-NR primer pair. The amplicon was cloned into the pGEM-T-Hyg vector, which contained the hygromycin B resistance gene, and the plasmid was transformed into the Δroa strain to generate a Δroa::ROA strain using the polyethylene glycol (PEG)-mediated fungal transformation method as previously described (18). Transformants were first selected from regeneration medium amended with 50 μg/ml of Geneticin (Sigma-Aldrich, St. Louis, MO) or hygromycin (Sigma-Aldrich), and each transformant was transferred to fresh complete medium amended with 100 μg/ml of the antibiotic. The numbers of independent transformants are listed in Table S1 in the supplemental material.
Self-fertilization.
One hundred microliters of conidial suspension (105 spores/ml) was spread across a petri dish (9-cm diameter) containing carrot agar and incubated at 25°C for 5 days (12). The cultures were mock fertilized with 1 ml of 2.5% Tween 60 solution to induce sexual development (23) and were continuously incubated under near-UV light (wavelength, 365 nm; HKiv Co., Ltd., Xiamen, China) at 25°C.
Ascospore discharge was observed in small acrylic chambers (1 by 2.5 by 5 cm) that were constructed to minimize free convection (1). A semicircular agar block (11 mm in diameter) that was covered with mature perithecia was placed on a coverslip in the chamber for 24 h (41). This placement allowed the ascospores to be discharged horizontally down the length of the chamber onto the coverslip. Images were captured on a DE/Axio Imager A1 microscope (Carl Zeiss, Oberkochen, Germany) with a charge-coupled-device (CCD) camera, and the shooting distance of the ascospores was measured at intervals of 0.5 mm using the AxioVision release 4.7 software program (Carl Zeiss).
Outcrosses.
Outcrosses were performed to characterize the inheritance of the trait of the Δroa mutant under the conditions described above. A MAT1-2 deleted mutant (Δmat2) tagged with cytoplasmic green fluorescent protein (GFP) (22), a Δroa Δmat2 double mutant, and a MAT1-1-1-deleted mutant (Δmat1) tagged with hH1-GFP (14) were fertilized individually with 1 ml of a conidial suspension (106 spores/ml in 2.5% Tween 60 solution) of the Δroa mutant or the wild-type strain. Following perithecium formation, progeny were collected and phenotypically characterized.
Epiplasmic fluid analysis.
Five days after induction (DAI) of sexual development, cultures were inverted so that the ascospores could discharge onto petri dish lids. On 14 DAI, distilled water was added to the petri dish lids and the ascospores were collected. Ascospores were collected by centrifugation for 10 min at 4,000 × g, resuspended in distilled water, and counted with a hemocytometer. Spore washes were frozen at −20°C and then dried in a Speed Vac instrument (Savant Instrument, Inc., Farmingdale, NY) as previously described (42). The identification and quantification of simple sugar alcohols in the epiplasmic fluid were accomplished by high-performance anion-exchange chromatography with pulsed amperometric detection (Dionex Corp., Sunnyvale, CA) using an anion-exchange column (CarboPac MA1, 4 by 250 mm; Dionex Corp.). The mobile phase was a 0.48 M NaOH solution at a flow rate of 0.4 ml/min. Confirmation of the sugar alcohols was performed by gas chromatography-mass spectrometry (GC-MS). Sugar alcohols in the epiplasmic fluid were derivatized by the acetylation method as previously described (17). Briefly, each extract was dissolved in dry pyridine (1.2 ml) followed by acetic anhydride (3 ml), and the mixture was stirred at 100°C. After 1 h, the mixture was cooled at room temperature, and 20 ml of cold water containing ice was added. The aqueous layer was extracted twice with 5 ml of diethyl ether. The combined extracts were then dried over anhydrous MgSO4 and evaporated until completely dry. The residue was dissolved in 500 μl of ethyl acetate, and 1 μl of the solution was subsequently injected into a capillary Shimadzu QP-5050 GC-MS (Shimadzu, Kyoto, Japan). The analytical conditions were as follows: a DB-5 fused silica column was used (30 m by 0.25 mm [inside diameter], 0.25-μm film; J&W Scientific, Folsom, CA); the column temperature was 80°C for 4 min and then was increased to 220°C at a rate of 10°C/min; the injector temperature was 250°C; the interface temperature was 250°C; the ionizing voltage was 70 eV; and the ionizing current was 300 μA. The K+ and Na+ contents were determined on an inductively coupled plasma-atomic emission spectrometer (ICP-AES) at an emission-line wavelength of 766.490 nm using the Jobin-Yvon Model 170 ICP emission spectrometer Ultrace (Jobin-Yvon Ultima, Longjumeau, France). The Cl− content was quantified by ion chromatography (IC) using a Metrohm model 761 Compact IC (Metrohm, Herrisau, Switzerland) with a suppressor module and equipped with an ICSep AN2 analytical column (4.6 by 250 mm) and an ICSep AN2 guard column (4.6 by 50 mm). The ions were detected using a suppressed conductivity detector that had a full scale of 250 μS/cm, which was optimized with respect to the maximum signal-to-noise ratio for the anions being analyzed.
Mathematical model for predicting initial velocity and turgor pressure.
Previously, we used a computer program which predicted initial velocity based on successive approximations, working back from the mean distance traveled (41). Determining the launch speed of the spores emerging from the ascus is an essential step in predicting the turgor pressure. We used two methods for this prediction here, modifying the iterative program to use Stokes' law and a “full-model” approach. The program was the same for both except in the way it approximated drag, varying drag coefficients for the “full model” and using the standard 24/Re drag coefficient reflected in Stokes' law. The effective size was calculated using predictions of drag at two extremes (spore flying lengthwise and spore flying crosswise with respect to the trajectory). The results were averaged, and then the sphere diameter that gives an equivalent result was used (41). The diameter for the wild type and revertant was 11.03 μm; for the mutant, it was 11.40 μm; density was taken as 1,200 kg m−3. The iterative interval was 20 ms, which produced about 1,000 steps, and the launch pitch angle was 2 degrees. Launch speeds were determined from calculated trajectories that produced the observed horizontal ranges, as done previously (41).
The following formulas were used to approximate the drag (D) of spore shapes, cylinders or long ellipsoids, with their long axes normal and parallel to flow, respectively: D = (4πμvl)/[ln(l/a) + 0.193] and D = (24πμvl)/[ln(l/a) − 0.807], where l and a are length and radius, respectively, of the cylinder or ellipsoid. An earlier publication with these formulas contained an error in the second equation (41).
Staining of asci and ascospores.
Nuclei and chromosomes of G. zeae were stained with acriflavin following the procedure described by Raju (29). Discharged ascospores and perithecia were hydrolyzed in 4 M HCl at 30°C for 15 to 20 min. The samples were washed twice with distilled water and stained in acriflavin solution (100 μg of acriflavin and 5 mg of K2S2O in 1 ml of 0.1 M HCl) for 20 min. The stained samples were washed three times (2 min each) in an HCl–70% ethanol mixture (2:98 [vol/vol]) and then twice in distilled water.
Specimen fixation and preparation for light microscopy.
The perithecia were collected from the carrot agar surface by gently scraping with a scalpel. The collected perithecia were fixed, dehydrated, and embedded in Spurr's resin (36) as previously described (19). The resin blocks were sectioned at 1 μm with a glass knife on an MT-X ultramicrotome (RMC, Tucson, AZ) and stained with 1% toluidine blue for light microscopy (40).
GFP tagging.
To check the cellular localization of ROA, the ROA open reading frame (ORF), which included its own terminator region, was amplified from the wild-type genomic DNA with ROA-AUG(−) and ROA-3R primers. The 5′ flanking region of ROA, which included its own promoter, was amplified with the ROA-promoter and ROA-GFP 5R primers. The GFP was amplified from pIGPAPA (15) with the EGFP-M and EGFP-p1 primers and fused to the N terminus and 5′ flanking region of ROA by DJ-PCR. The fused construct was then amplified with the nested primers ROA-promoter N and ROA-3N. The fusion construct was cloned into the pGEM-T easy vector (Promega Corp., Madison, WI) and subcloned into pUCH1 (43). The clone was transformed into Δroa or the wild-type strain, and the phenotypes of the transformants were compared with those of the wild-type strain.
Microscopy.
The perithecia were dissected on glass slides in a drop of 20% glycerol, and the asci were flattened under the cover glass. Both GFP-tagged and acriflavin-stained nuclei were examined with the 488-nm excitation and 515/530-nm emission wavelength filters. Differential interference contrast (DIC) and fluorescence images were captured on a DE/Axio Imager A1 microscope (Carl Zeiss) with a CCD camera. The sizes of the asci and ascospores were measured using the AxioVision release 4.7 software program (Carl Zeiss).
RESULTS
Phenotype of REMI mutant Z43R9901.
Following self-fertilization, the wild-type Z03643 strain produced eight normal, spindle-shaped ascospores containing two to four cells each, while the asci produced by the mutant Z43R9901 contained eight ascospores that were round or oval and contained one or two cells each. The numbers of perithecia and ascospores per perithecium that were produced by the mutant were similar to those of the wild-type strain. In addition, we did not observe any significant difference between the wild-type strain and the REMI mutant in mycelial growth, conidiation, spore germination, or virulence (see Table S3 in the supplemental material).
Molecular characterization of the ROA gene.
The genomic DNA from strain Z43R9901 was digested with HindIII or BglII for Southern analysis. The digested DNA was hybridized with the entire REMI vector pUCH1. The hybridization pattern confirmed a single integration site of the vector in the Z43R9901 genome. Genomic DNA of Z43R9901 digested with BglII was self-ligated and transformed in E. coli for sequencing. The BLAST results from the sequence flanking the inserted vector showed that the vector integrated at a site 407 bp upstream of an ORF annotated as FGSG_08667.3. We designated this putative ORF round ascospore (ROA) after the phenotype of its REMI mutant. ROA encodes a 770-amino-acid protein and has three putative introns (see Fig. S1 in the supplemental material). ROA is conserved in filamentous fungi (see Fig. S2), and the predicted protein sequence of ROA has high identity (>60%) to the hypothetical proteins in Podospora anserina, Magnaporthe grisea, N. crassa, Chaetomium globosum, and other Fusarium species. The protein sequence contained the conserved domain ketopantoate reductase, which was located near the N terminus (1 to 315 amino acid residues) and is known to be involved in the coenzyme A biosynthesis pathway (8). Examination of expression of FGSG_08667 using Affymetrix GeneChip data in culture during sexual development and wheat colonization (9–11) indicated that it is consistently highly expressed across a range of conditions.
Targeted deletion of the ROA gene and complementation.
The ROA ORF from the wild-type genome was replaced with the gen cassette to generate the ROA deletion mutant (Δroa). When probed with the 5′ flanking region, the genomic DNA digested with EcoRV from four independent transformants had a single 12-kb hybridized DNA fragment instead of the 2.2-kb fragment found in the wild-type strain (Fig. 1A), which confirmed that the 2.5-kb ROA locus in G. zeae had been replaced with the gen cassette. The Δroa mutant was complemented by introducing ROA into the Δroa strain, and the presence of the inserted ROA gene was confirmed by Southern blot analysis (Fig. 1B). The shapes of asci and ascospores in the Δroa::ROA strain were restored to those of the wild-type strain.
Fig. 1.
Targeted deletion and complementation of ROA. For each panel, the left and right sides show the strategy and Southern analysis, respectively. (A) Targeted deletion of ROA from the genome of the wild-type strain. E, EcoRV; gen, Geneticin resistance gene; lane 1, wild-type strain; lane 2, Δroa mutant. The 5′ flanking region of ROA (black bar) was used as a probe. (B) Complementation of ROA in the Δroa strain. H, HindIII; hygB, hygromycin resistance gene; lane 1, wild-type strain; lane 2, Δroa; lanes 3, Δroa::ROA. The partial ROA ORF (black bar) and hygB ORF (gray bar) were used as probes for the left and right blots, respectively. The sizes of the standards (kb) are indicated on the left of each blot.
Sexual development of the Δroa mutant.
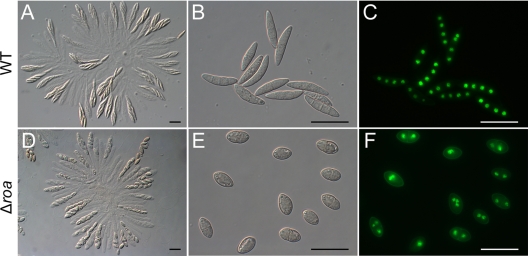
Similarly to the REMI mutant, the Δroa mutants showed no difference in mycelial growth, conidiation, and germination from the wild-type strain. Both Δroa and wild-type perithecia contained rosettes of asci of similar sizes, with each ascus containing eight ascospores (Fig. 2). The average ascus length in the Δroa mutant was 61 μm, which was shorter than that of the wild-type asci (66 μm) (P < 0.01). The shape of Δroa ascospores was round or oval, while the wild-type ascospores were slender and spindle shaped. The wild-type ascospores usually contained four cells; however, the mutant ascospores had one or two cells (Fig. 2). The nuclei in the ascospores were counted by acriflavin staining, showing that approximately 40% of the Δroa ascospores had two cells, with each one containing one nucleus. In addition, 26% of the ascospores were composed of one cell with two nuclei. Some of the Δroa ascospores had three nuclei in one or two cells. In contrast, most of the wild-type ascospores (∼85%) had four cells, with one nucleus per cell (Fig. 2). A few of the wild-type ascospores had only two cells, but ascospores with one cell were not found in the wild-type strain. The average length and width of the Δroa ascospores were 12 μm and 7.0 μm, respectively, while those of the wild-type ascospores were 23 μm and 4.5 μm, respectively. Therefore, ascospores from the Δroa strain were shorter and wider than wild-type ascospores (P < 0.01). The ascus shape is partially determined by the ascospores, and the shorter ascus may be due to the wider, shorter ascospores. We estimated a cylindrical shape for the volume calculation of the wild-type ascospores and an ellipsoid shape for the Δroa ascospores. A cylindrical shape overestimates volume, since the ascospores taper at their end, and the ascospore volume was divided by 1.2 to adjust the overestimation (41). Based on these measurement criteria, the average volumes of the wild-type and Δroa ascospores were by chance the same and were estimated to be 2.94 × 10−16 m3.
Fig. 2.
Morphology of asci rosettes and ascospores. (A and D) Microscopic observation of 8-DAI asci rosettes of the wild-type (WT) (A) or Δroa (D) strain. (B and E) Discharged ascospores of the wild-type (B) or Δroa (E) strain. (C and F) Nuclei of the discharged ascospores were stained with acriflavin. Scale bar = 20 μm.
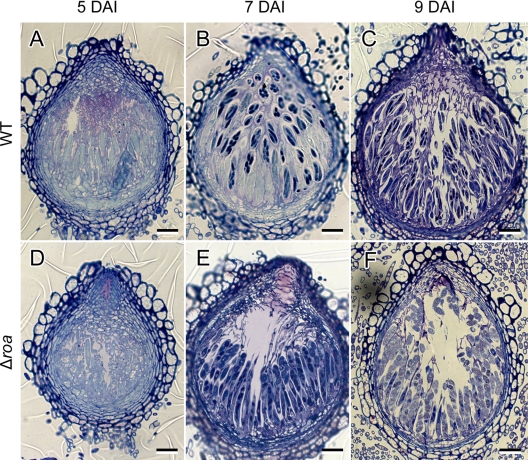
Approximately 300 perithecia from the wild-type and Δroa strains were fixed and sectioned for microscopic observation. The number and shape of perithecia from Δroa strain were similar to those of the wild-type strain (Fig. 3) despite having a reduced number of discharged ascospores. In contrast to the tightly packed asci in wild-type perithecia, the arrangement of Δroa asci was loose and sparse. Intact ascus rosettes were rarely observed in the old perithecia from Δroa at the 9-DAI time point, while perithecia from the wild-type strain at the same time point exhibited intact asci (Fig. 3). When mature perithecia from the Δroa strain were dissected with needles under a microscope, the ascospores were scattered from the perithecia without forming the typical rosettes of asci. However, wild-type perithecia at the 14-DAI time point still contained intact asci that were ready for the release of ascospores.
Fig. 3.
Light microscopy of developing asci and ascospores. Perithecia of wild-type (A, B, and C) or Δroa (D, E, and F) strains were stained with toluidine blue. Perithecia were collected from carrot agar 5, 7, and 9 days after sexual induction (DAI). Scale bar = 20 μm.
Ascospore discharge.
The wild-type strain discharged ascospores for more than 9 days after 5 DAI (maturation point), while the Δroa strain discharged ascospores for only 3 days (from 6 to 8 DAI). Approximately 67% of the ascospores from the Δroa strain were discharged at 6 DAI. We collected ascospores from the lid of the culture plate until 14 DAI and estimated the total number of discharged ascospores per perithecium. The wild-type strain discharged approximately 3,800 ascospores per perithecium on average, while the Δroa strain discharged 630 ascospores per perithecium (P < 0.01).
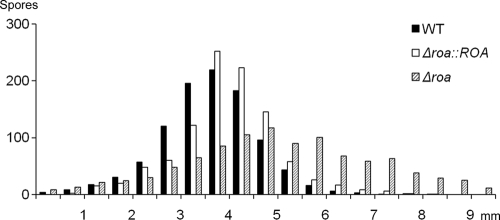
The shooting distance of more than 1,000 ascospores from each strain was measured in still air. The mean distances of discharged ascospores were 3.6 mm, 5.0 mm, and 4.0 mm in the wild type, Δroa, and Δroa::ROA strains, respectively (Fig. 4 and 5). The Δroa strain discharged ascospores farther than the wild-type strain and showed a broader range of shooting distance. The discharge pattern of the ascospores from the Δroa::ROA strain was similar to that of the wild type.
Fig. 4.
Forcible ascospore discharge of the wild-type (WT), Δroa, and Δroa::ROA strains. Photographs were taken 48 h after the assay was initiated. A semicircular agar block (arrowhead) covered with perithecia was placed on a coverslip in the chamber. This placement allowed for ascospores (arrow) to be discharged horizontally down the length of the chamber onto the coverslip.
Fig. 5.
Number of ascospores discharged at indicated distances in still air. Wild-type (black bar), Δroa (dashed bar), and complement Δroa::ROA strains (white bar) are shown. One thousand spores from each strain were assessed.
The launch speed of the ascospores was estimated from the discharge distance. In addition, the turgor pressure required for discharge was estimated from the launch speed (Table 1). The discharge distance was set to a range where 95% of the ascospores dispersed from the mean distance. Ascospores from the Δroa strain were launched at a higher speed than wild-type ascospores, suggesting that they possessed a higher turgor pressure in the asci. The estimated launch speed and turgor pressure of the Δroa::ROA strain were similar to those of the wild type.
Table 1.
Estimation of launch speed and turgor pressure in G. zeae
| Parameter | Value in model for genotyped |
|||||
|---|---|---|---|---|---|---|
| Full |
Stokes' law |
|||||
| WT | Δroa | Δroa::ROA | WT | Δroa | Δroa::ROA | |
| Measured distance (mean, range) (mm) | 3.6 (1.4–5.5) | 5 (1.1–8.7) | 4 (1.7–6.2) | |||
| Estimated mean launch speed (m s−1)a | 12.0 (3.9–20.6) | 16.9 (2.7–36.0) | 12.5 (4.9–24.2) | 8.4 (3.2–12.9) | 11.0 (2.4–19.0) | 8.8 (3.9–14.5) |
| Estimated pressure (MPa)b | 0.57 | 1.13 | 0.62 | 0.27 | 0.69 | 0.31 |
| Measured turgor pressure (MPa)c | 0.41 | 1.6 | 0.40 | |||
Launch speed was estimated from measured distance.
Estimated pressure was the pressure that is required for discharging ascospores calculated from the launch speed.
Measured pressure was the osmotic pressure calculated using the concentration of osmolytes in the epiplasmic fluid.
WT, wild type.
Epiplasmic fluid analysis.
Based on estimates of epiplasmic fluid volume, the Δroa strain contained a higher concentration of ions (K+, Na+, and Cl−) and sugar alcohols in the fluid per ascus than the wild-type strain (Fig. 6). The asci from the Δroa strain contained approximately four times more ions than the wild-type asci (Fig. 6A). Glycerol, arabitol, mannitol, and glucose were found in the epiplasmic fluids from both the Δroa and wild-type strains (see Fig. S3 in the supplemental material). Among the polyols, glycerol was the major component of the epiplasmic fluid of both strains (Fig. 6B), and both the wild-type and Δroa strains had similar patterns of polyol production (Fig. 7). The concentration of polyols in the mutant asci was also markedly higher than that in the wild-type asci. The total concentration of the identified sugar alcohols per ascus was approximately 20 times higher in the Δroa strain than in the wild-type strain.
Fig. 6.
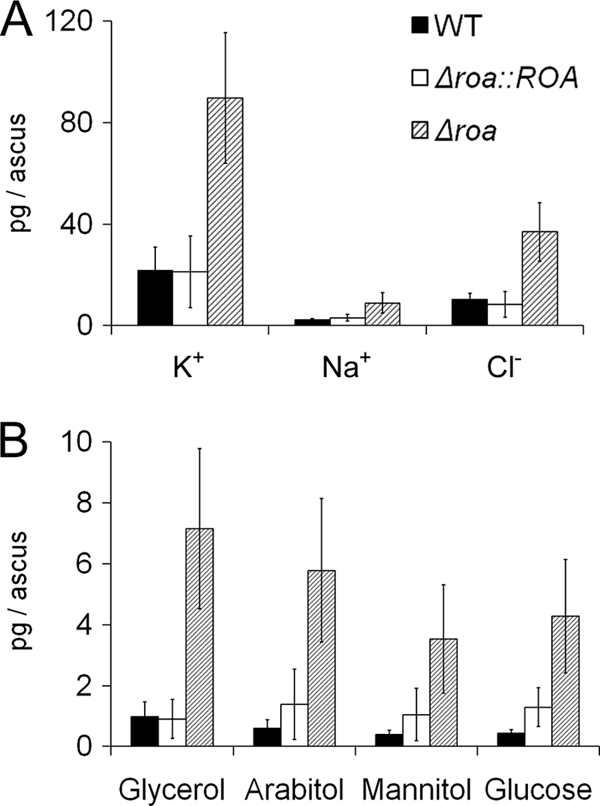
Mass of epiplasmic fluid components per ascus. Wild-type (black bar), Δroa (dashed bar), and complement Δroa::ROA (white bar) strains are shown.
Fig. 7.
High-performance liquid chromatography (HPLC) chromatogram of polyol standards (A), polyols in the epiplasmic fluid of the wild-type strain (B), or polyols in the epiplasmic fluid of the Δroa strain (C). Peaks 1 to 5 are glycerol, erythritol, arabitol, mannitol, and glucose, respectively.
The volume of epiplasmic fluid was calculated as the volume of extended asci minus the measured volume of spores (41). Since the ascospore volumes were equal in the wild-type, Δroa, and Δroa::ROA strains, the volume of the epiplasmic fluid was set as the same (5.77 × 10−15 m3). The volume of epiplasmic fluid in the unextended mature ascus was calculated previously (2.1 × 10−15 m3) (41). The concentration of osmolytes in the extended asci was calculated from the mass of the osmolytes and the volume of epiplasmic fluid. The turgor pressure in the extended asci was estimated from the concentration of osmolytes (Table 1). The Δroa strain showed a significantly higher predicted turgor pressure than the wild-type strain (P < 0.01). In contrast, the turgor pressures of the Δroa::ROA and wild-type strains were similar.
Effects of ROA in outcrossing.
The outcrossing between the female Δmat1 strain tagged with hH1-GFP and the male Δroa strain showed that the GFP and non-GFP ascospores within an individual ascus had a 1:1 segregation ratio (Fig. 8). However, the shapes of eight ascospores in each individual ascus were identical. Approximately 10% of asci contained eight round ascospores (5 to 14 μm in length), similar to case with the Δroa strain, while ∼60% had eight spores with wild-type shapes (20 to 30 μm in length). In addition, ∼30% of ascospores had a shape that resembled an intermediate form (15 to 19 μm in length). The progeny of the cross between the female Δmat2 and male Δroa strains and the female Δroa Δmat2 and male wild-type strains showed the same phenotype (data not shown). When we randomly isolated ascospores from the three outcrossing sets, cultured them, and induced sexual development, they produced their respective wild-type or round ascospores regardless of the initial shape. When ascospores that discharged from the outcrossing between the female Δmat2 (genotype mat1-2 ROA) strain and the male Δroa (genotype MAT1-2 roa) strain were randomly isolated, the segregation ratio of the genotypes was 25:7:10:24 (mat1-2 ROA/mat1-2 roa/MAT1-2 ROA/MAT1-2 roa, respectively). The genetic distance of the two linked genes (MAT1-2 and ROA) was estimated to be 19 cM based on chromosome maps of G. zeae (21), and therefore, the segregation ratio was consistent with the expected number (χ2 = 2.8).
Fig. 8.
Outcrosses between Δmat1 female and Δroa male. In the heterothallic Δmat1 strain, the histone H1 gene was fused with GFP. DIC, differential interference contrast image; GFP, GFP fluorescence image; DIC + GFP, DIC images merged with GFP fluorescence image. Scale bar = 20 μm.
GFP localization.
We tried complementing the Δroa strain by introducing a plasmid that contained the ROA gene fused to GFP. We obtained 60 transformants from three independent transformation trials, and GFP expression was detected in 24 transformants, of which only one strain fully restored the wild-type phenotype. However, this complemented strain carried multiple copies of the construct, and other strains that carried a single copy of the construct did not restore the wild-type phenotype. We amplified the construct from each transformant for sequencing and found each construct carried several point mutations resulting in nonsynonymous substitution. Regardless of the patterns of point mutation and integrated copy number, GFP in the 24 transformants was localized in the cytoplasm in all stages of the life cycle, including ascospores, conidia, and mycelia, but was not detected in young asci before spore delimitation. We could not determine GFP expression in perithecia of the transformants, because perithecia produced by the wild-type strain had strong autofluorescence. We also introduced the construct into the wild-type strains and selected 30 transformants. Southern blot analyses showed that five independent mutants carried a single integration of the construct in their genome and that two of them carried an integration at the 5′ flanking region of ROA (see Fig. S4 in the supplemental material). In both strains, GFP was also localized in the cytoplasm in all fungal stages except for young asci (Fig. 9).
Fig. 9.
Localization of ROA in mycelia (A and B) or ascospores (C and D). Young asci before spore delimitation and ascospores within asci are indicated by arrowheads and arrows, respectively. DIC, DIC image; GFP, GFP fluorescence image. Scale bar = 20 μm.
DISCUSSION
In this study, we identified a novel gene, ROA, from the screening of a REMI mutant collection. The Δroa mutant showed unique characteristics of ascospore morphology and discharge without any defects in vegetative growth, conidiation, and conidia germination. Despite their abnormal shape, germination of Δroa ascospores occurred normally. These results suggest that ROA has a specific role in ascospore morphology and discharge in G. zeae. The most striking phenotypes of the Δroa mutant are the reduction in the number of ascospores discharged from the mature perithecia and the increased distance that these spores are fired.
We hypothesized that the mutant had a defect in the shooting of ascospores, since the total number of ascospores per perithecium was the same in the Δroa and wild-type strains. The turgor pressure in asci is known to be the predominant driving force behind the discharge of ascospores from asci (32, 38, 42). In addition, components of the ascus epiplasmic fluid have been identified and quantified in G. zeae (39). Therefore, we analyzed the osmolyte components of the ascus fluid. Assuming the volumes of the epiplasmic fluid are equal in asci of the mutant and the wild type, the measured turgor pressure of the Δroa asci is four times higher than that of the wild-type strain.
The difference between the estimated and measured pressures (Table 1) for both the mutant and the wild type is relatively small, and the full model did a better job of predicting the measured pressure for the mutant. If our assumption that the epiplasmic fluid volume is equal in the mutant and wild-type is incorrect, and there is greater fluid in the mutant, then the measured turgor pressure would be lower and may align more closely with the estimated pressure (Table 1). Wild-type spores appear to nest against each other in the ascus, whereas the shape of the mutants prevents this (Fig. 2A and D), which may increase the fluid in the discharging mutant ascus. For further discussion of the model, see the supplemental material.
The change in spore shape may also contribute to the distance fired. Recent work (32) weighed the evolutionary pressures on spore shape from friction at the ascus pore to reduction of drag and suggests the latter as the driving force for spores without appendages or multiple cells. Our data from a previous publication (41) were used to develop this model, although G. zeae spores have multiple cells. Additionally, one of the assumptions of the model is that spores exit the ascus singly and without accompanying ascus fluid, an assumption, which was based on our previous results (42). The figure in question, however, shows accompanying fluid clinging to 7 of 8 discharged spores. However, for the Δroa mutants, the aspect ratio (length divided by width) plotted against Reynold's number (Re) fits within 1% of that predicted by the model for minimum drag, whereas the wild-type spores fall outside this region. The study also predicts that the rounder shape of the mutant would minimize friction during exit of the ascus pore, resulting in a longer shooting distance.
The asci of G. zeae were shown previously to accumulate predominantly mannitol and glucose (42). In this study, we identified several other sugar alcohols in the epiplasmic fluid of G. zeae asci, including glycerol and arabitol (Fig. 6). The polyol profile of epiplasmic fluid is consistent with several reports of other fungi (16). Polyol profiles may vary according to conditions under which the fungi are grown. Magnaporthe oryzae was shown to use glycerol to drive turgor pressure in the appressoria (5). However, the concentration of the glycerol (4.6 mM) in G. zeae asci is too low to reach the required turgor pressure (0.57 MPa) for the release of ascospores. In contrast, M. grisea accumulates a large amount of glycerol (3.3 M) in the appressorium that creates a sufficient force (5.8 MPa) for penetration (5). Therefore, G. zeae uses ions (predominantly K+ and Cl−) instead of polyols to increase the turgor pressure in asci, as previously reported (41). An analysis of ion components has not been published for the M. oryzae system.
Although the morphologies of the ascopores from the Δroa and wild-type strains were quite different, acriflavin staining showed that the Δroa strain maintained normal meiosis and a third division of the nuclei. Three nuclear divisions (2 meiotic and 1 mitotic) that generated eight nuclei occurred normally in the young Δroa asci before spore delimitation; however, further cell division in these ascospores did not accompany these divisions as expected. Ascospores are formed from the compartmentalization of the ascus cytoplasm during ascospore delimitation (2), and the shape of the young ascus may govern the shape of ascospores produced within it (30). Therefore, insufficient cell division after spore delimitation in the Δroa strain may be a consequence of abnormal ascus physiology.
Unusual phenotype segregation occurred in the outcross between the Δmat1 mutant tagged with hH1-GFP and the Δroa mutant (Fig. 8). All eight spores from one ascus had the same shape; however, the GFP/non-GFP segregation ratio was 1:1. Genotypes of F1 progeny ascospores had an expected segregation ratio and confirmed that the outcross underwent normal sexual recombination. This result showed that the initial shape of F1 ascospores from the outcross was not determined by the spore genotype. This unusual phenomenon may be caused by a diffusible factor, such as a protein or RNA that gets captured by asci at various concentrations, since the asci produced by the outcross have haploinsufficiency in ROA. This phenomenon also can result independently of haploinsufficiency. Genetic studies of N. crassa showed a phenomenon similar to the unusual phenotypic segregation observed in this study, but this phenomenon in N. crassa is not related to haploinsufficiency. Crosses of the N. crassa wild-type strain with the Round spore mutant (R) strain produced 100% round-spore asci, suggesting that R is ascus dominant (24). Shiu et al. (35) hypothesized that this phenomenon was a result of meiotic silencing by unpaired DNA (MSUD) and involved a molecular mechanism that was similar to RNA silencing: when DNA from the parental cells is unpaired in early stages of meiosis, both the unpaired DNA and its homologue were silenced. When SAD-1, an important gene involved in MSUD, is self-silenced, the ascus dominance of R becomes suppressed (semidominance). A cross of sad-1UV with R produced 35% round-spore asci in Neurospora (35). In this regard, MSUD in G. zeae may be partially functioning, since this semidominance is similar to the outcross results of Δmat1 and Δroa in G. zeae in our study and major genes of MSUD are conserved in G. zeae (25).
In conclusion, the novel gene ROA is required for proper sexual development in G. zeae. Interestingly, the round-spore mutant has a possible selective advantage in that it shoots farther, which would help in distribution, but it does not shoot as many spores. The round-spore mutant has not been previously identified in nature, which may reflect the balance of propagule number versus firing distance. Deletion of the ROA gene suggests an increase in turgor pressure in the ascus through the accumulation of ions. In addition, this mutation triggered the formation of round ascospores and caused insufficient cell divisions after spore delimitation. In this study, we have shown a close relationship between ascospore morphology, turgor pressure, and ascospore discharge and have identified additional polyol components in asci. Future work will explore the mechanisms by which ROA affects turgor pressure. These studies will also help explain how the physiology and structure of asci affects turgor pressure and the morphogenesis of ascospores.
Supplementary Material
ACKNOWLEDGMENTS
We thank Namboori B. Raju for valuable advice on cytology, Hansol Choi for technical assistance, and Wonsang Woo for estimating the launch speed of the ascospores.
This work was supported by a grant (CG 1140) from the Crop Functional Genomics Center of the 21st Century Frontier Research Program funded by the Korean Ministry of Education, Science, and Technology and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2010-0001826). K. Min was supported by graduate fellowships from the Korean Ministry of Education, Science, and Technology through the Brain Korea 21 project. The contributions of F. Trail were supported in part by the Michigan Agricultural Experiment Station.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 27 August 2010.
REFERENCES
- 1.Aylor D. E., Anagnostakis S. L. 1991. Active discharge distance of ascospores of Venturia inaequalis. Phytopathology 81:548–551 [Google Scholar]
- 2.Beckett A. 1981. Ascospore formation, p. 107–129 InTurian G., Hohl H. (ed.), The fungal spore: morphogenetic controls. Academic Press, London, United Kingdom [Google Scholar]
- 3.Bowden R. L., Fuentes-Bueno I., Leslie J. F., Lee J., Lee Y.-W. 2008. Methods for detecting chromosome rearrangements in Gibberella zeae. Cereal Res. Commun. 36(Suppl. 6):603–608 [Google Scholar]
- 4.Cappellini R. A., Peterson J. L. 1965. Macroconidium formation in submerged cultures by a non-sporulating strain of Gibberella zeae. Mycologia 57:962–966 [Google Scholar]
- 5.De Jong J. C., McCormack B. J., Smirnoff N., Talbot N. J. 1997. Glycerol generates turgor in rice blast. Nature 389:244–245 [Google Scholar]
- 6.Desjardins A. E. 2006. Fusarium mycotoxins: chemistry, genetics, and biology. APS Press, St. Paul, MN [Google Scholar]
- 7.Fernando W. G. D., Paulitz T. C., Seaman W. L., Dutilleul P., Miller J. D. 1997. Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87:414–421 [DOI] [PubMed] [Google Scholar]
- 8.Genschel U. 2004. Coenzyme A biosynthesis: reconstruction of the pathway in archaea and an evolutionary scenario based on comparative genomics. Mol. Biol. Evol. 21:1242–1251 [DOI] [PubMed] [Google Scholar]
- 9.Guenther J. C., Hallen-Adams H. E., Bücking H., Shachar-Hill Y., Trail F. 2009. Triacylglyceride metabolism by Fusarium graminearum during colonization and sexual development on wheat. Mol. Plant Microbe Interact. 22:1492–1503 [DOI] [PubMed] [Google Scholar]
- 10.Güldener U., Seong K.-Y., Boddu J., Cho S., Trail F., Xu J.-R., Adam G., Mewes H.-W., Muehlbauer G. J., Kistler H. C. 2006. Development of a Fusarium graminearum Affymetrix GeneChip for profiling fungal gene expression in vitro and in planta. Fungal Genet. Biol. 43:316–325 [DOI] [PubMed] [Google Scholar]
- 11.Hallen H. E., Huebner M., Shiu S.-H., Güldener U., Trail F. 2007. Gene expression shifts during perithecium development in Gibberella zeae (anamorph Fusarium graminearum), with particular emphasis on ion transport proteins. Fungal Genet. Biol. 44:1146–1156 [DOI] [PubMed] [Google Scholar]
- 12.Hallen H. E., Trail F. 2008. The L-type calcium ion channel cch1 affects ascospore discharge and mycelial growth in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot. Cell 7:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y.-K., Lee T., Han K.-H., Yun S.-H., Lee Y.-W. 2004. Functional analysis of the homoserine O-acetyltransferase gene and its identification as a selectable marker in Gibberella zeae. Curr. Genet. 46:205–212 [DOI] [PubMed] [Google Scholar]
- 14.Hong S.-Y., So J., Lee J., Min K., Son H., Park C., Yun S.-H., Lee Y.-W. 2010. Functional analyses of two syntaxin-like SNARE genes, GzSYN1 and GzSYN2, in the ascomycete Gibberella zeae. Fungal Genet. Biol. 47:364–372 [DOI] [PubMed] [Google Scholar]
- 15.Horwitz B. A., Sharon A., Lu S.-W., Ritter V., Sandrock T. M., Yoder O. C., Turgeon B. G. 1999. A G protein alpha subunit from Cochliobolus heterostrophus involved in mating and appressorium formation. Fungal Genet. Biol. 26:19–32 [DOI] [PubMed] [Google Scholar]
- 16.Jennings D. H., Burke R. M. 1990. Compatible solutes—the mycological dimension and their role as physiological buffering agents. New Phytol. 116:277–283 [Google Scholar]
- 17.Jerković I., Mastelić J. 2004. GC-MS characterization of acetylated β-D-glucopyranosides: transglucosylation of volatile alcohols using almond β-glucosidase. Croat. Chem. Acta 77:529–535 [Google Scholar]
- 18.Kim J.-E., Jin J., Kim H., Kim J.-C., Yun S.-H., Lee Y.-W. 2006. GIP2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl. Environ. Microbiol. 72:1645–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K. W., Hyun J.-W. 2007. Nonhost-associated proliferation of intrahyphal hyphae of citrus scab fungus Elsinoe fawcettii: refining the perception of cell-within-a-cell organization. Micron 38:565–571 [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Chang I.-Y., Kim H., Yun S.-H., Leslie J. F., Lee Y.-W. 2009. Genetic diversity and fitness of Fusarium graminearum populations from rice in Korea. Appl. Environ. Microbiol. 75:3289–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Jurgenson J. E., Leslie J. F., Bowden R. L. 2008. Alignment of genetic and physical maps of Gibberella zeae. Appl. Environ. Microbiol. 74:2349–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J., Lee T., Lee Y.-W., Yun S.-H., Turgeon B. G. 2003. Shifting fungal reproductive mode by manipulation of mating type genes: obligatory heterothallism of Gibberella zeae. Mol. Microbiol. 50:145–152 [DOI] [PubMed] [Google Scholar]
- 23.Leslie J. F., Summerell B. A. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames, IA [Google Scholar]
- 24.Mitchell M. B. 1966. A round-spore character in Neurospora crassa. Neurospora Newslett. 10:6 [Google Scholar]
- 25.Nakayashiki H. 2005. RNA silencing in fungi: mechanisms and applications. FEBS Lett. 579:5950–5957 [DOI] [PubMed] [Google Scholar]
- 26.Namiki F., Matsunaga M., Okuda M., Inoue I., Nishi K., Fujita Y., Tsuge T. 2001. Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis. Mol. Plant Microbe Interact. 14:580–584 [DOI] [PubMed] [Google Scholar]
- 27.Parry D. W., Jenkinson P., McLeod L. 1995. Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol. 44:207–238 [Google Scholar]
- 28.Pratt R. J., Lee D. W., Aramayo R. 2004. DNA methylation affects meiotic trans-sensing, not meiotic silencing, in Neurospora. Genetics 168:1925–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raju N. B. 1986. A simple fluorescent staining method for meiotic chromosomes of Neurospora. Mycologia 78:901–906 [Google Scholar]
- 30.Raju N. B. 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96:241–262 [Google Scholar]
- 31.Read N. D., Beckett A. 1996. Ascus and ascospore morphogenesis. Mycol. Res. 100:1281–1314 [Google Scholar]
- 32.Roper M., Pepper R. E., Brenner M. P., Pringle A. 2008. Explosively launched spores of ascomycete fungi have drag-minimizing shapes. Proc. Natl. Acad. Sci. U. S. A. 105:20583–20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 34.Seong K., Hou Z., Tracy M., Kistler H. C., Xu J.-R. 2005. Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum. Phytopathology 95:744–750 [DOI] [PubMed] [Google Scholar]
- 35.Shiu P. K. T., Raju N. B., Zickler D., Metzenberg R. L. 2001. Meiotic silencing by unpaired DNA. Cell 107:905–916 [DOI] [PubMed] [Google Scholar]
- 36.Spurr A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31–43 [DOI] [PubMed] [Google Scholar]
- 37.Sutton J. C. 1982. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant Pathol. 4:195–209 [Google Scholar]
- 38.Trail F. 2007. Fungal cannons: explosive spore discharge in the Ascomycota. FEMS Microbiol. Lett. 276:12–18 [DOI] [PubMed] [Google Scholar]
- 39.Trail F. 2009. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 149:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trail F., Common R. 2000. Perithecial development by Gibberella zeae: a light microscopy study. Mycologia 92:130–138 [Google Scholar]
- 41.Trail F., Gaffoor I., Vogel S. 2005. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fuarium graminearum). Fungal Genet. Biol. 42:528–533 [DOI] [PubMed] [Google Scholar]
- 42.Trail F., Xu H., Loranger R., Gadoury D. 2002. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia 94:181–189 [PubMed] [Google Scholar]
- 43.Turgeon B. G., Garber R. C., Yoder O. C. 1987. Development of a fungal transformation system based on selection of sequences with promoter activity. Mol. Cell. Biol. 7:3297–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yafetto L., Carroll L., Cui Y., Davis D. J., Fischer M. W. F., Henterly A. C., Kessler J. D., Kilroy H. A., Shidler J. B., Stolze-Rybczynski J. L., Sugawara Z., Money N. P. 2008. The fastest flights in nature: high-speed spore discharge mechanisms among fungi. PLoS One 3:e3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu J.-H., Hamari Z., Han K.-H., Seo J.-A., Reyes-Dominguez Y., Scazzocchio C. 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41:973–981 [DOI] [PubMed] [Google Scholar]
- 46.Yun S.-H., Arie T., Kaneko I., Yoder O. C., Turgeon B. G. 2000. Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet. Biol. 31:7–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.