Abstract
The ability of Candida albicans to reversibly switch morphologies is important for biofilm formation and dispersion. In this pathogen, Nrg1p functions as a key negative regulator of the yeast-to-hypha morphogenetic transition. We have previously described a genetically engineered C. albicans tet-NRG1 strain in which NRG1 expression levels can be manipulated by the presence or absence of doxycycline (DOX). Here, we have used this strain to ascertain the role of Nrg1p in regulating the different stages of the C. albicans biofilm developmental cycle. In an in vitro model of biofilm formation, the C. albicans tet-NRG1 strain was able to form mature biofilms only when DOX was present in the medium, but not in the absence of DOX, when high levels of NRG1 expression blocked the yeast-to-hypha transition. However, in a biofilm cell retention assay in which biofilms were developed with mixtures of C. albicans tet-NRG1 and SC5314 strains, tet-NRG1 yeast cells were still incorporated into the mixed biofilms, in which an intricate network of hyphae of the wild-type strain provided for biofilm structural integrity and adhesive interactions. Also, utilizing an in vitro biofilm model under conditions of flow, we demonstrated that C. albicans Nrg1p exerts an exquisite control of the dispersal process, as overexpression of NRG1 leads to increases in dispersion of yeast cells from the biofilms. Our results demonstrate that manipulation of NRG1 gene expression has a profound influence on biofilm formation and biofilm dispersal, thus identifying Nrg1p as a key regulator of the C. albicans biofilm life cycle.
Candidiasis represents one of the most important nosocomial infections in hospitals worldwide, affecting an increasing number of immunosuppressed as well as other at-risk patients, and Candida albicans remains the most common causative agent of candidiasis (1, 2, 21). These infections are frequently associated with the formation of biofilms on the surfaces of medical devices. For example, the presence of central venous catheters is a major risk factor for candidiasis, and yeasts (mainly C. albicans) are the third most frequent cause of catheter-associated bloodstream infections (1, 2, 7). Because biofilms constitute a reservoir for infections and a protective environment, as cells within biofilms display increased antifungal drug resistance and protection from host immune defenses, the ability of C. albicans to form biofilms is considered one of its main virulence factors.
In C. albicans, biofilm development progresses through multiple developmental stages, with two major processes, adhesion and filamentation, playing vital roles in biofilm formation (4, 13, 31). The first stage involves attachment of C. albicans cells to a substrate, closely followed by cellular proliferation, hyphal development, and synthesis of exopolymeric material, leading to the formation of an architecturally complex, three-dimensional biofilm (4, 23, 26). The confluent hyphal layers, comprising the bulk of the biofilm, form yeast cells that are continually released from the biofilms (34). This completes the biofilm developmental life cycle, as dispersed cells will eventually colonize new distal sites and the entire process can start all over again. Several molecular determinants are known to play important roles at different stages of biofilm development. Early events in biofilm formation are known to be orchestrated in part by interactions between cell wall proteins Als3 and Hwp1, which are required for complementary adhesive interactions (17, 19, 20, 36). Transcriptional factors such as Efg1p, Tec1p, and Bcr1p also play vital roles in early stages of biofilm formation. For example, Δefg1 and Δtec1 mutants are unable to undergo morphogenesis, leading to a biofilm formation defect (18, 25), while the Δbcr1 mutant is deficient in manifestations of the cell-cell adhesion important for biofilm maturation (17, 18). More recently, we showed that C. albicans biofilm dispersion can be controlled by manipulating levels of expression of two key morphogenetic genes, UME6 (involved in hyphal elongation) and PES1 (the “pescadillo” homolog involved in the reverse morphological transition, from hyphae to yeast, and lateral yeast formation) (34).
While several genes that control individual stages of biofilm formation and dispersion have been described, a common genetic determinant that might contribute throughout the biofilm life cycle has not yet been identified. Here we report on the transcriptional regulator NRG1, which influences multiple different steps during the C. albicans biofilm developmental cycle. This gene encodes Nrg1p, a DNA-binding protein with a zinc finger domain that functions as a negative regulator of filamentation (3, 15). Despite the functional significance of NRG1, its role during C. albicans biofilm growth has not yet been investigated. With the help of a genetically engineered strain of C. albicans in which NRG1 is under the control of a tetracycline-regulatable promoter (30), we show that levels of expression of NRG1 exert an exquisite control over the processes of biofilm formation and dispersion in C. albicans.
MATERIALS AND METHODS
C. albicans strains and strain construction.
The strains used in this study were C. albicans strains SC5314 (wild type), the C. albicans SSY50-B tetracycline-regulatable tet-NRG1 strain, which has been previously described by our group (30), and a novel green fluorescent protein (GFP)-tagged tet-NRG1 strain which constitutively expresses GFP under the control of the C. albicans ACT1 promoter, constructed as follows: a 2.4-kb fragment containing the sequence for a C. albicans codon-modified GFP was cut out of pMG1646 (8) (kind gift from Judith Berman) by using EcoRI/HindIII. This fragment was then ligated into EcoRI/HindIII-digested CIpSATSA (6) to create CIpSATSA-GFP. This plasmid was then linearized with StuI and transformed into SSY50-B (30) by using a modified electroporation transformation method (11). Digestion at the StuI site produces the RP10 homologous ends, which facilitate integration of the plasmid into the C. albicans genome at this locus. Nourseothricin-resistant transformants were selected on yeast extract-peptose-dextrose (YPD) agar plates containing 200 μg ml−1 nourseothricin (Werner Bioagents, Jena, Germany) as described previously (27) and screened for GFP activity by fluorescence microscopy. To confirm the integration of the GFP construct into the Candida genome at the RP10 locus, DNA was extracted from transformants demonstrating GFP activity by using a commercially available kit (Masterpure; Epicentre Technologies, Madison, WI), digested with HindIII, transferred to a nylon membrane (Nytran; Schleicher & Schuell, Keene, NH), and subjected to Southern blot analysis using an established method (5) with RP10 as the probe. Stock cultures were stored in 15% glycerol at −80°C.
Strains were routinely grown under non-filament-inducing conditions (media at 30°C) in the presence or absence of 20 μg/ml of doxycycline (DOX). Media used for culturing both planktonic and biofilm cells were YPD (0.5% yeast extract, 1% Bacto peptone, 1% glucose), and RPMI 1640 (Sigma, St. Louis, MO) with morpholinepropanesulfonic acid (MOPS) buffer.
C. albicans biofilm development assays.
C. albicans biofilms were formed in vitro under either static conditions or under conditions of flow. For static conditions, biofilms were formed in 96-well polystyrene microtiter plates as previously described by our group (22, 24). Briefly, cells were grown in YPD overnight at 37°C and resuspended in RPMI buffered with MOPS, with or without DOX (20 μg/ml) depending on the specific experimental design, at a final concentration of 106 cells/ml based on hemocytometer counts. An inoculum (100 μl) was added to each well of a 96-well flat-bottom plate. After 24 h of incubation at 37°C, the wells were washed with phosphate-buffered saline (PBS) three times to remove any nonadherent cells. The extent of biofilm formation was estimated using a semiquantitative colorimetric 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay as reported previously by our group (22, 26). For some experiments relating to mixed biofilms, biofilms were also developed under static conditions on six-well polystyrene plates. The general procedure for biofilm development was similar to that for 96-well plates, with the exception of the volume of the medium containing cells (3 ml) used for the adhesion step. For easy handling of samples for scanning electron microscopy (SEM), biofilms were developed on Thermanox coverslips (Nunc, Thermo Fisher Scientific, Rochester, NY) in 24-well microtiter plates.
C. albicans biofilms were also cultured under conditions of flow using a simple flow biofilm model described recently by our group (33). This model involves a controlled flow of fresh medium via Tygon tubing (Cole-Parmer, Vernon Hills, IL) into a 15-ml polypropylene conical tube (BD, Franklin, NJ) holding a silicon elastomer (SE) strip. Medium flow is controlled by connecting the tubing to a peristaltic pump (Masterflex L/S Easy-Load II; Cole-Parmer). The whole apparatus was placed inside a 37°C incubator to facilitate biofilm development at a controlled temperature. Briefly, SE strips (1 by 9 cm; Cardiovascular Instrument Corp., Wakefield, MA) were sterilized by autoclaving and pretreated for 24 h with bovine serum. C. albicans was grown overnight at 30°C, washed, and diluted to an optical density at 600 nm (OD600) of 0.5 in (1:1-diluted) YPD medium. The SE strips were incubated with the diluted C. albicans suspension at 37°C for 90 min at 100 rpm agitation for the initial adhesion of cells. Next, the strip was inserted into the conical tube and the peristaltic pump was turned on. The medium flow rate was maintained at 1 ml/min. The medium used for biofilm development was YPD (diluted 1:1 with distilled H2O), with or without DOX (20 μg/ml). At various time points during biofilm development, cells released from the biofilm in the flowthrough liquid were collected from the bottom of the conical tube. The dispersed cells were enumerated by hemocytometer and by colony counts on solid medium (YPD-agar plates). For some experiments the dispersed cells were visualized and enumerated under a Leica DMR epifluorescence microscope (Leica Microsystems, Wetzlar, Germany) with an attached cooled charge-coupled-device SPOT RT camera (Diagnostic Instruments Inc., Sterling Heights, MI). The images were processed and analyzed using the Photoshop 7.0 software (Adobe, Mountain View, CA).
Scanning electron microscopy.
Biofilms grown on Thermanox coverslips were placed in fixative (4% [vol/vol] formaldehyde, 1% [vol/vol] glutaraldehyde in PBS) overnight. The samples were rinsed in 0.1 M phosphate buffer (two times for 3 min each) and then placed in 1% Zetterquist's osmium for 30 min. The samples were subsequently dehydrated in a series of ethanol washes (70% for 10 min, 95% for 10 min, and 100% for 20 min), treated (two times for 5 min each) with hexamethyldisilizane (Polysciences Inc., Warrington, PA), and finally air dried in a desiccator. The samples were rinsed in 0.1 M phosphate buffer and then coated with gold/palladium (40%/60%) and observed in a scanning electron microscope (Leo 435 VP) in high-vacuum mode at 15 kV. The images were processed for display using Photoshop software (Adobe, Mountain View, CA.).
Confocal scanning laser microscopy.
Biofilms grown in six-well plates for 24 h were stained with 25 μg/ml concanavalin A (ConA)-Alexa Fluor 594 conjugate (C-11253; Molecular Probes, Eugene, OR) for 1 h in the dark at 37°C. Confocal scanning laser microscopy (CSLM) was performed with a Zeiss LSM 510 upright confocal microscope using a Zeiss Achroplan 40×, 0.8-W objective. Concanavalin A conjugate staining was observed using a HeNe1 laser with an excitation wavelength of 543 nm. For GFP visualization, an argon laser was used with 458-, 488-, and 514-nm excitation wavelengths. Images were assembled into side views using the Zeiss LSM Image Browser v4.2 software.
RNA extraction and real-time PCR.
RNA was extracted from 24-h biofilms grown under static conditions in six-well microtiter plates under static conditions using the MasterPure yeast RNA purification kit (Epicentre Biotechnologies, Madison, WI). The integrity of the RNA was tested via gel electrophoresis. A total of 1 μg of RNA was treated with amplification-grade DNase I (Invitrogen, Carlsbad, CA) and used for cDNA synthesis with the cMaster RT kit (Eppendorf AG, Hamburg, Germany) as per the manufacturer's instructions. The following primer sets were used in conjunction with SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) and Twin.tec real-time 96-well PCR plates (Eppendorf AG, Hamburg, Germany) in an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA): NRG1 (CAC CTC ACT TGC AAC CCC & GCC CTG GAG ATG GTC TGA) (32), ACT1 (ATG TGT AAA GCC GGT TTT GCC G & CCA TAT CGT CCC AGT TGG AAA C) (32), HWP1 (TCA GCC TGA TGA CAA TCC TC & GCT GGA GTT GTT GGC TTT TC), and ALS3 (CAA CTT GGG TTA TTG AAA CAA AAA CA & AGA AAC AGA AAC CCA AGA ACA ACC T) (16). Parameters for primer design were set according to the recommendations of Applied Biosystems. Briefly, the primer sizes were between 20 and 25 bases in length, and the Tm of each primer was 58°C. The amplicons were between 90 and 110 bp in size. Each reaction mixture was set up in triplicate in a 25.0-μl volume with 25 ng of cDNA for 40 cycles (thermal cycling conditions were initial steps of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min). Relative gene expression was quantified using the threshold cycle (CT) method with the 7300 System sequence detection software with the RQ study application from Applied Biosystems (35). The target genes were normalized to the housekeeping gene ACT1. The change was calculated for each sample by using the equation, 2−ΔΔCT, and results from the different replicates were averaged after the 2−ΔΔCT calculations.
RESULTS AND DISCUSSION
Control of C. albicans biofilm formation by NRG1 gene expression levels.
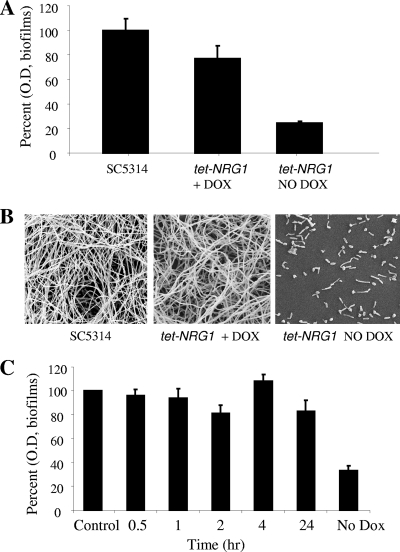
C. albicans Nrg1p is a negative regulator of filamentation (3, 15). Because of the well-established link between filamentation and biofilm formation in C. albicans, we postulated that altering the levels of Nrg1p in the cell may affect biofilm development in C. albicans. For this, we utilized a genetically engineered C. albicans tet-NRG1 strain (SSY50-B), in which NRG1 is placed under the control of a tetracycline-regulatable promoter so that morphology can be manipulated in vitro by adding or omitting DOX in the growth medium (30). Cells from this strain were used to seed the wells of microtiter plates using RPMI medium and incubated at 37°C, in the presence or absence of DOX. C. albicans SC5314 biofilms were also formed in parallel and served as controls. After 24 h, the extent of biofilm formation was estimated by measuring the metabolic activity of cells within the biofilms using an XTT colorimetric assay. At least five replicate wells were prepared for biofilm formation for each condition. Results indicated that robust biofilm formation in the C. albicans tet-NRG1 strain only occurs in the presence of antibiotic, when levels of Nrg1p are low and filamentation is allowed to progress. As shown in Fig. 1, in the absence of DOX (which translates to NRG1 overexpression), the strain developed very poor biofilms, about 77% less robust than the control biofilms formed by strain SC5314 (Fig. 1A). Instead, addition of the antibiotic yielded much more robust biofilms as estimated by colorimetric readings (Fig. 1A). Although the difference between control and tet-NRG1 biofilms in the presence of DOX was significant (P ≤ 0.05, by analysis of variance [ANOVA]), results from SEM revealed that this difference perhaps may not be sufficient for a biological difference.
Fig. 1.
Biofilm formation in the C. albicans tet-NRG1 strain occurs only in the presence of DOX, and not in the absence of the antibiotic when NRG1 is overexpressed. (A) Biofilm formation by the C. albicans wild-type strain (SC5314) in the absence of DOX and by the tet-NRG1 strain in the presence (20 μg/ml) or absence of DOX. Biofilms were formed in 96-well mictrotiter plates under biofilm-inducing conditions with suspensions of 1.0 × 106 cells/ml in RPMI and incubated at 37°C for 24 h. The extent of biofilm formation was estimated using the XTT colorimetric reduction assay. Results are expressed as percent OD readings compared to wild-type SC5314 biofilms. (B) The extent of biofilm formation by SC5314 and tet-NRG1 was further visualized by scanning electron microscopy. (C) C. albicans strain tet-NRG1 is capable of forming biofilms when DOX is added to medium after initial attachment of yeast cells. Yeast cells of the tet-NRG1 strain were allowed to adhere to the surface of wells in microtiter plates for 30 min and 1, 2, 4, and 24 h before the wells were washed with PBS and fresh medium containing DOX was added to the wells. After addition of the antibiotic-containing medium, plates were then incubated for an additional 24 h at 37°C. Biofilms were processed using the XTT reduction assay. Results are expressed as the percent OD readings compared to those of control biofilms obtained with the C. albicans tet-NRG1 strain grown in the continuous presence of DOX from the zero time point.
As visualized by SEM, a severe defect in biofilm formation was observed for the C. albicans tet-NRG1 strain in the absence of DOX, when NRG1 was overexpressed (Fig. 1B). Only a monolayer of yeast and pseudohyphal cells was found attached to the substrate, without the presence of hyphae. These “biofilms” were very similar to those formed by the Δefg1 mutant (25). On the other hand, in the presence of the antibiotic, when NRG1 expression is low, biofilms formed by this tet-NRG1 strain appeared indistinguishable from those formed by the wild-type strain SC5314, with abundant hyphae and structural complexity.
During these experiments we observed that the absence of DOX led to the attachment of mostly yeast cells, which seemed to indicate that the very initial adhesion phase may not be influenced by Nrg1p levels. Hence, we asked whether addition of DOX externally after initially seeding the plates would rescue the biofilm formation defect and trigger subsequent biofilm formation by these attached cells. To answer this question we seeded the wells of microtiter plates with cells of the C. albicans tet-NRG1 strain in the absence of DOX and allowed the cells to adhere for various periods of time (30 min and 1, 2, 4, and 24 h). At the specified time points, wells were washed to remove nonadherent cells and replenished with fresh medium containing the antibiotic. Then, plates were incubated for an additional 24 h and the extent of biofilm formation was estimated using the XTT colorimetric assay. We found that downregulation of levels of expression of NRG1 by the addition of DOX in the growth medium triggered biofilm formation from the yeast cells and pseudohyphae that were originally attached to the wells (Fig. 1C). Even the wells containing cells for 24 h could regain their biofilm formation ability to levels comparable to those observed for cells incubated continuously in the presence of DOX, once the antibiotic was added to the medium (Fig. 1C). These observations have important clinical ramifications, as they demonstrate that C. albicans cells may initially attach to a surface, maintain a state of “dormancy” for extended periods of time, and then proliferate and be able to form biofilms upon the receipt of the right environmental cues. Of note, even in the absence of an extensive three-dimensional structure, attached cells already demonstrate increased levels of resistance to antifungal agents, particularly azole derivatives (9, 14), and this property may be further accentuated by the change in physiological status (nongrowing) associated with this dormant state (10, 12).
C. albicans tet-NRG1 yeast cells can get incorporated into a mixed biofilm with wild-type cells.
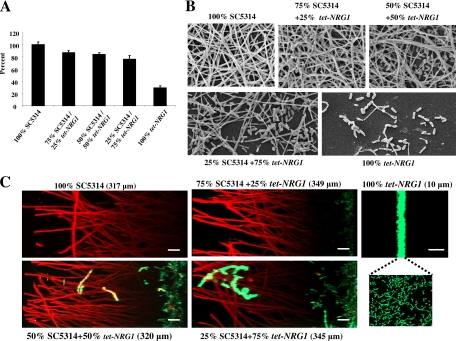
Overexpression of Nrg1p resulted in cells that displayed filamentation defects and, as a consequence, were defective in biofilm formation. In the next series of experiments we examined whether yeast cells of the tet-NRG1 strain could incorporate into a mixed biofilm with filamentous cells of the wild-type strain. Briefly, cells of C. albicans wild-type strain SC5314 and the tet-NRG1 strain were mixed at various ratios (100:0, 75:25, 50:50, 25:75, and 0:100) and used to seed wells of microtiter plates in the absence of DOX. Plates were incubated for 24 h at 37°C to promote biofilm formation. At least three replicates were performed under each condition of biofilm development. After 24 h, the biofilms were washed, and metabolic activity of cells within the biofilms was measured in an XTT assay. As expected, the C. albicans tet-NRG1 strain on its own was unable to develop a robust biofilm (Fig. 2A). A modest yet statistically significant (P ≤ 0.05, ANOVA) reduction was obtained in the XTT values of all mixed biofilms compared to the control biofilms formed by SC5314 cells (Fig. 2A). Also in these biofilms, increasing proportions of the tet-NRG1 strain (from 0% to 25% to 50%) corresponded to an increase in the population of yeast cells interspersed between abundant hyphae, as observed by SEM. In fact, the biofilm containing 75% tet-NRG1 cells was comprised of copious yeast and pseudohyphae at a proportion that appeared much higher than in the biofilm containing 100% tet-NRG1 cells (Fig. 2B). Overall, these results suggest that cells from the C. albicans tet-NRG1 strain, which alone are defective in biofilm formation, can effectively be incorporated and retained as a part of a mixed biofilm along with SC5314, as hyphal elements of this wild-type strain provide for structural integrity and adhesive interactions required for biofilm development.
Fig. 2.
C. albicans tet-NRG1 cells are incorporated into mixed biofilms. A biofilm retention assay was performed in which mixed biofilms were formed for 24 h with varied proportions of C. albicans SC5314 wild-type and tet-NRG1 strains. (A) Biofilms were formed in wells of microtiter plates, washed with PBS, and quantified in an XTT colorimetric reduction assay. Results are expressed as the percentage of wild-type SC5314 biofilms. (B) SEM observations of single and mixed biofilms formed by C. albicans strains SC5314 and tet-NRG1. (C) CSLM of single and mixed C. albicans strain SC5314 and GFP-labeled tet-NRG1 strain biofilms. Biofilms were stained with ConA for CSLM visualization, and side views were constructed to show both GFP expression (green) and ConA staining (red). Bars, 10 μm.
We note here that plating (in YPD-agar plates) of cells recovered from the biofilms formed in the wells of microtiter plates confirmed the presence of both tet-NRG1 and SC5314 strains in the mixed biofilms, since in the absence of DOX at 37°C SC5314 colonies are wrinkled whereas colonies formed by the C. albicans tet-NRG1 strain are smooth (results not shown).
To gain an even better understanding of the morphological and architectural characteristics associated with the mixed biofilms, we generated a GFP-labeled C. albicans tet-NRG1 strain and visualized the resulting mixed biofilms by using nondestructive CSLM. Initial experiments indicated that the integration of this reporter gene did not alter the biofilm formation defect of the tet-NRG1 strain in the absence of DOX. Single-strain and mixed biofilms (together with the wild-type SC5314 strain) were developed in six-well polystyrene plates, in RPMI medium without DOX. After 24 h of incubation, the biofilms were stained with ConA and examined by CSLM. As seen in Fig. 2C, the GFP-negative wild-type SC5314 strain developed robust biofilms approximately 317 μm deep. As expected, biofilms formed by the GFP-expressing tet-NRG1 strain were very poor, forming only a 10-μm-thick monolayer of mostly yeast cells and few pseudohyphae. A mixture of 75% SC5314 and 25% GFP-tet-NRG1 yielded a biofilm as deep (349 μm) as the one formed by the wild type only. In this mixed biofilm it was observed that GFP-tet-NRG1 cells grew mostly as yeast cells confined to the bottom layer of the biofilm, but the depth of this layer (about 20 μm) grew to twice that of the biofilms formed in the absence of strain SC5314. As the ratio of GFP-tet-NRG1 increased, so did the thickness of the bottom layer of the biofilm that it occupied. At 50% or 75% of the original proportion of the mixed-species biofilm, the green fluorescent layer measured 37 μm and 45 μm, respectively. Interestingly, a higher degree of filamentation was now observed in these basal layers, and some green fluorescent filaments were also observed extending into the middle layers of the biofilm. The overall thicknesses of the two resulting mixed biofilms (containing 50% or 75% GFP-tet-NRG1) were similar to that observed for biofilms formed by the SC5314 strain alone (Fig. 2C). Overall, it would seem that retention of yeast cells in the mixed biofilms is mostly through a mechanism of physical entrapment, where yeast cells are mostly confined to the layer closer to the substrate in which biofilms are formed, rather than through direct adhesive interactions between yeast cells of the tet-NRG1 strain and filaments of the wild-type SC5314. This picture is similar to the Δtec1 mutant that stays entrapped as a basal layer in a mixed biofilm setting along with the wild-type reference strain (18). While the tet-NRG1 strain is able to filament to a small extent in the mixed biofilm, its ability still is in stark contrast with the nonadhesive Δbcr1 mutant, which is able to filament and fully incorporate within the three-dimensional scaffold provided by filaments of the wild-type strain (18).
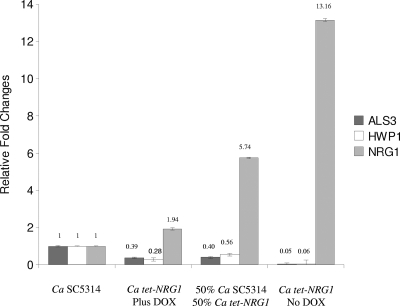
As a validation for our experimental system to control gene expression, for these series of experiments we also wanted to make sure that the observed morphological features and extent of biofilm formation in single and mixed biofilms correlated with levels of expression for NRG1 and were indeed a consequence of our ability to manipulate levels of NRG1 gene expression in the regulatable tet-NRG1 strain. Thus, we extracted RNA from biofilms formed by the C. albicans SC5314 and tet-NRG1 strains in both the presence and absence of DOX, as well as from mixed (50/50) biofilms in the absence of DOX. As seen in Fig. 3, measured levels of gene expression for NRG1 showed excellent correlation with the presence or absence of the antibiotic in the growing medium. Moreover, as expected since expression of these adhesins is known to be dependent on Nrg1p levels (15), control of NRG1 gene expression levels also resulted in accompanying changes in levels of gene expression for HWP1 and ALS3 (Fig. 3). These observations corroborate the finding that the observed results are in direct correlation with NRG1 expression levels. Furthermore, the results indicated that Nrg1p control of biofilm formation is likely through the regulation of key target genes, including ALS3 and HWP1, encoding complementary filament-specific adhesins that play a critical role for biofilm formation in C. albicans (20). Importantly, within the biofilm context, expression of these hypha-specific adhesins is also controlled by Bcr1p and Efg1p (17, 25). Thus, similar to filamentation, biofilm formation in C. albicans is orchestrated by a complex regulatory circuitry with a seemingly high level of redundancy. From an evolutionary point of view, this high level of complexity gives further credence to the importance of the ability to form biofilms in different aspects of C. albicans biology and pathogenesis.
Fig. 3.
Results of quantitive real-time PCR for gene expression levels of NRG1, ALS3, and HWP1 in biofilms formed by C. albicans SC5314 and tet-NRG1 strains, as well as from mixed biofilms (mixed biofilms were grown in the absence of DOX). Values are expressed as relative fold changes compared to those in wild-type strain SC5314, based on ΔΔCT values of the individual amplicons.
Control of C. albicans biofilm dispersion by Nrg1p.
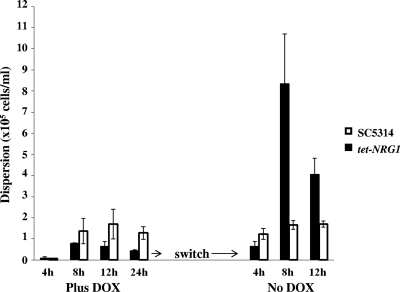
We next posited that, besides its role during biofilm formation, as a negative regulator of filamentation Nrg1p may also function during biofilm dispersion, as most cells dispersed from biofilms are in the yeast morphology (34). To test this hypothesis, we developed biofilms of strains SC5314 and tet-NRG1 on SE strips under conditions of flow using a model that, contrary to the static models, allows for the study of biofilm dispersion (33). As documented above, C. albicans strain tet-NRG1 is fully capable of developing a robust biofilm in the presence of DOX. Likely, DOX does not have any effect on biofilm formation properties of SC5314. At various time points during the course of biofilm formation (4, 8, 12, and 24 h) aliquots of flowthrough medium were collected and the number of cells released from the biofilms was enumerated. As shown in Fig. 4, the number of cells dispersed from biofilms formed by the tet-NRG1 strain in the presence of DOX was between 9,000 cells/ml (at 4 h) and 40,000 cells/ml (at 24 h). At all time points tested except for the first, the number of cells dispersed from biofilms formed by the tet-NRG1 strain was at least 1.5- to 3-fold lower than for cells released from SC5314 biofilms. After 24 h, the medium was switched to omit DOX. After just 4 h of this switch, there was a 1.5-fold increase in cell dispersion from tet-NRG1 biofilms. As time progressed, the plus-DOX-to-no-DOX switch triggered the hyphae in the biofilm to revert back to yeast cells that could easily disengage from the biofilms and disperse into the flowing medium. Dispersion in the absence of DOX was at levels that were at least 10 to 18 times higher than those observed for biofilms grown under the continuous presence of DOX. As expected, the control SC5314 biofilms showed no difference in the extent of biofilm dispersion, despite the switch to antibiotic-free medium. This adds to our previous observations on UME6 and PES1 control of biofilm dispersion (34) and also points to the complex regulatory mechanisms orchestrating this second part of the C. albicans biofilm developmental cycle.
Fig. 4.
Regulation of C. albicans biofilm dispersion by Nrg1p. Biofilms of the C. albicans SC5314 and tet-NRG1 strains were developed under conditions of flow for 24 h in medium with DOX, and the number of dispersed cells was counted at different times during biofilm development. After this period, DOX was withdrawn from the medium and the number of dispersed cells was counted at various time points after antibiotic removal.
Since mixed growth with SC5314 alleviated the biofilm-forming defects of the tet-NRG1 strain, we next questioned whether the wild-type strain's presence in a mixed biofilm would have an impact on the extent of biofilm dispersion. For this experiment nonfluorescent SC5314 cells and GFP-labeled tet-NRG1 cells were mixed at a 50/50 ratio and allowed to adhere to SE strips. Mixed biofilms were developed under conditions of flow during 24 h, in YPD medium containing DOX. Aliquots of cells dispersed from biofilms were collected at 5, 8, and 12 h, and the proportion of fluorescent versus nonfluorescent cells in the mixture was determined. At 5 h, dispersed aliquots contained similar numbers of green fluorescent tet-NRG1 cells and nonfluorescent SC5314 cells (Table 1). As time progressed, there was a decrease in the release of tet-NRG1 cells compared to SC5314 (approximately a 30/70 ratio) (Table 1). A completely opposite result was obtained when the antibiotic was removed from the growth medium after the initial 24 h of incubation. Only 5 h after the switch to DOX-free medium, the mixed-species biofilm released a large number of fluorescent cells. By 12 h, the dispersed population contained greater than 80% green fluorescent tet-NRG1 cells and only about 20% SC5314 cells (Table 1). This indicated that overexpression of NRG1 can still lead to increased biofilm dispersion despite its presence in a mixed biofilm with the wild-type strain.
Table 1.
Biofilm dispersion in mixed C. albicans SC5314 and GFP-labeled tet-NRG1 biofilmsa
| Time point (h) | % dispersed cells |
|||
|---|---|---|---|---|
| With DOX |
After switch to no DOX |
|||
| Fluorescent (GFP-tet-NRG1) | Nonfluorescent (SC5314) | Fluorescent (GFP-tet-NRG1) | Nonfluorescent (SC5314) | |
| 5 | 45.75 | 54.25 | 70.80 | 29.20 |
| 8 | 29.80 | 70.20 | 77.50 | 22.50 |
| 12 | 27 | 73 | 80.20 | 19.80 |
The numbers of dispersed cells from each strain were quantified using fluorescence microscopy at different time points during formation of mixed (50/50) biofilms under conditions of flow in the presence of DOX and after a subsequent switch to medium without the antibiotic.
In conclusion, we found that the C. albicans transcriptional repressor Nrg1p plays an important role in biofilm formation and dispersion. This adds to its critical functions in filamentation and virulence (28–30). Together, these observations suggest that regulation of NRG1 expression could potentially lead to control of biofilm-related disseminated diseases and, additionally, point to Nrg1p as an attractive target for the development of novel antifungal agents.
ACKNOWLEDGMENTS
Work in the laboratory is supported by grant numbers RO1AI063256 (to S.P.S.) and R21AI080930 (to J.L.L.-R.) from the National Institute of Allergy and Infectious Diseases. P.U. is supported by a postdoctoral fellowship, 10POST4280033, from the American Heart Association.
We thank the Research Center for Minority Institutions (RCMI) Advance Imaging Center, supported by grant number 5G12 RR01 3646-10, for use of the confocal microscope and Colleen Witt for assistance with confocal microscopy.
The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCR, the NIAID, the NIH, or AHA.
Footnotes
Published ahead of print on 13 August 2010.
REFERENCES
- 1.Banerjee S. N., Emori T. G., Culver D. H., Gaynes R. P., Jarvis W. R., Horan T., Edwards J. R., Tolson J., Henderson T., Martone W. J. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am. J. Med. 91:86S–89S [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sague C., Jarvis W. R. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. National Nosocomial Infections Surveillance System. J. Infect. Dis. 167:1247–1251 [DOI] [PubMed] [Google Scholar]
- 3.Braun B. R., Kadosh D., Johnson A. D. 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 20:4753–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra J., Kuhn D. M., Mukherjee P. K., Hoyer L. L., McCormick T., Ghannoum M. A. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church G. M., Gilbert W. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. U. S. A. 81:1991–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleary I. A., Mulabagal P., Reinhard S. M., Yadev N. P., Murdoch C., Thornhill M. H., Lazzell A. L., Monteagudo C., Thomas D. P., Saville S. P. 23July2010. Pseudohyphal regulation by the transcription factor Rfg1p in Candida albicans. Eukaryot. Cell doi:10.1128/EC.00088-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump J. A., Collignon P. J. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1–8 [DOI] [PubMed] [Google Scholar]
- 8.Gerami-Nejad M., Berman J., Gale C. A. 2001. Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast 18:859–864 [DOI] [PubMed] [Google Scholar]
- 9.Hawser S. P., Douglas L. J. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khot P. D., Suci P. A., Miller R. L., Nelson R. D., Tyler B. J. 2006. A small subpopulation of blastospores in Candida albicans biofilms exhibit resistance to amphotericin B associated with differential regulation of ergosterol and beta-1,6-glucan pathway genes. Antimicrob. Agents Chemother. 50:3708–3716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler G. A., White T. C., Agabian N. 1997. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J. Bacteriol. 179:2331–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaFleur M. D., Kumamoto C. A., Lewis K. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Ribot J. L. 2005. Candida albicans biofilms: more than filamentation. Curr. Biol. 15:R453–R455 [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P. K., Chandra J., Kuhn D. M., Ghannoum M. A. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murad A. M., Leng P., Straffon M., Wishart J., Macaskill S., MacCallum D., Schnell N., Talibi D., Marechal D., Tekaia F., d'Enfert C., Gaillardin C., Odds F. C., Brown A. J. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 20:4742–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nailis H., Coenye T., Van Nieuwerburgh F., Deforce D., Nelis H. J. 2006. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol. Biol. 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobile C. J., Andes D. R., Nett J. E., Smith F. J., Yue F., Phan Q. T., Edwards J. E., Filler S. G., Mitchell A. P. 2006. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobile C. J., Mitchell A. P. 2005. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 15:1150–1155 [DOI] [PubMed] [Google Scholar]
- 19.Nobile C. J., Nett J. E., Andes D. R., Mitchell A. P. 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 5:1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobile C. J., Schneider H. A., Nett J. E., Sheppard D. C., Filler S. G., Andes D. R., Mitchell A. P. 2008. Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 18:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89–S94 [DOI] [PubMed] [Google Scholar]
- 22.Pierce C. G., Uppuluri P., Tristan A. R., Wormley F. L., Jr., Mowat E., Ramage G., Lopez-Ribot J. L. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3:1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramage G., Saville S. P., Thomas D. P., Lopez-Ribot J. L. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramage G., Vande Walle K., Wickes B. L., Lopez-Ribot J. L. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramage G., VandeWalle K., Lopez-Ribot J. L., Wickes B. L. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214:95–100 [DOI] [PubMed] [Google Scholar]
- 26.Ramage G., Vandewalle K., Wickes B. L., Lopez-Ribot J. L. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163–170 [PubMed] [Google Scholar]
- 27.Reuss O., Vik A., Kolter R., Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 28.Saville S. P., Lazzell A. L., Bryant A. P., Fretzen A., Monreal A., Solberg E. O., Monteagudo C., Lopez-Ribot J. L., Milne G. T. 2006. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob. Agents Chemother. 50:3312–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saville S. P., Lazzell A. L., Chaturvedi A. K., Monteagudo C., Lopez-Ribot J. L. 2009. Efficacy of a genetically engineered Candida albicans tet-NRG1 strain as an experimental live attenuated vaccine against hematogenously disseminated candidiasis. Clin. Vaccine Immunol. 16:430–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saville S. P., Lazzell A. L., Monteagudo C., Lopez-Ribot J. L. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soll D. R. 2008. Candida biofilms: is adhesion sexy? Curr. Biol. 18:R717–R20 [DOI] [PubMed] [Google Scholar]
- 32.Toyoda M., Cho T., Kaminishi H., Sudoh M., Chibana H. 2004. Transcriptional profiling of the early stages of germination in Candida albicans by real-time RT-PCR. FEMS Yeast Res. 5:287–296 [DOI] [PubMed] [Google Scholar]
- 33.Uppuluri P., Chaturvedi A. K., Lopez-Ribot J. L. 2009. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia 168:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uppuluri P., Chaturvedi A. K., Srinivasan A., Banerjee M., Ramasubramaniam A. K., Kohler J. R., Kadosh D., Lopez-Ribot J. L. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 6:e1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao R., Daniels K. J., Lockhart S. R., Yeater K. M., Hoyer L. L., Soll D. R. 2005. Unique aspects of gene expression during Candida albicans mating and possible G1 dependency. Eukaryot. Cell 4:1175–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X., Daniels K. J., Oh S. H., Green C. B., Yeater K. M., Soll D. R., Hoyer L. L. 2006. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 152:2287–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]