Abstract
Candida glabrata is a haploid opportunistic fungal pathogen that is phylogenetically related to Saccharomyces cerevisiae. Even though C. glabrata has no known sexual cycle, it contains, like S. cerevisiae, three mating type-like loci (MTL) called MTL1, MTL2, and MTL3, as well as most of the genes required for mating, meiosis, and sporulation. MTL1 is localized at an internal position on chromosome B and is thought to be the locus corresponding to the MAT locus in S. cerevisiae. MTL2 and MTL3 are localized close to two telomeres on different chromosomes (29.4 kb from Chr E-L and 10.5 kb from Chr B-L, respectively). By using URA3 reporter gene insertions at the three MTL loci, we found that in contrast to the case for S. cerevisiae, only MTL3 is subject to transcriptional silencing while MTL2 is transcriptionally active, and this is in agreement with previously reported data. We found that the silencing of MTL3 is nucleated primarily at the left telomere of chromosome B and spreads over 12 kb to MTL3, rather than nucleating at flanking, closely positioned cis-acting silencers, like those flanking HMR and HML of S. cerevisiae. Interestingly, the silencing of MTL3 absolutely requires the yKu70, yKu80, and Rif1 proteins, in sharp contrast to the silencing of the HM loci of S. cerevisiae. In addition, we found that several cell type-specific genes are expressed in C. glabrata regardless of the presence, or even absence, of mating type information at any of the MTL loci.
Candida glabrata is a haploid yeast found as a normal part of the mammalian microflora, but in recent years it also has emerged as a common opportunistic pathogen of humans, and now it accounts for about 12% of all Candida infections worldwide, second only to Candida albicans (29, 36, 37, 39, 50). C. glabrata and Saccharomyces cerevisiae are closely related phylogenetically, and both genomes conserve a high degree of synteny, whereas C. albicans is more distantly related (6, 14).
Sexual reproduction has been documented in many fungal species; however, several human fungal pathogens either do not reproduce sexually or very rarely do so (33). As the genomes of more fungal species are sequenced it has become clear, however, that the vast majority of the species have highly conserved genes involved in sexual reproduction, and yet some species have not been observed to mate (5, 6, 16). In some cases, a cryptic sexual cycle and even same-sex mating have been discovered recently, as is the case for C. albicans and Aspergillus fumigatus (1, 34). C. glabrata has no known sexual cycle even though it contains the vast majority of the genes required for mating (6, 14), and some mating type identity is maintained (32).
In many fungal species, mating and cell type identity are determined by the information encoded by a locus called MAT (mating type) in the budding yeast S. cerevisiae or MTL (mating type like) in other fungi. The information contained in the MAT locus of S. cerevisiae can be either type a or type α and consists of three transcriptional regulators that control the sexual cycle. Mating type a cells (MATa cells) contain the gene a1, and MATα cells contain the genes α1 and α2. Haploid MATa cells can mate only with cells carrying the MATα information (and vice versa) to form diploid a/α cells that are unable to mate (21, 26). In addition to the MAT locus, the S. cerevisiae chromosome III contains two silent loci, HMR and HML, located near each telomere (∼22.7 and ∼13.0 kb, respectively). The HMR and HML loci usually contain identical copies of MATa and MATα, respectively, that are normally maintained efficiently repressed through a chromatin-based mechanism called silencing (21, 44). The silencing of these loci (HM) is essential for maintaining cell type identity and the occurrence of a sexual cycle. The HM loci are each flanked by two small regulatory sites (∼150 bp) called silencers, and each one contains binding sites for the proteins Rap1, the origin of recognition complex (ORC), and Abf1 in different combinations. These silencer-binding proteins recruit the four Sir proteins (Sir1 to -4) to nucleate silencing. Once the Sir proteins are at the silencer, Sir2, an NAD+-dependent histone deacetylase (25, 46), removes acetyl groups from the N-terminal tails of histones H3 and H4 of the adjacent nucleosomes.
The Rif1 and Rif2 proteins of Saccharomyces cerevisiae compete with Sir3 and Sir4 for Rap1 binding, and S. cerevisiae rif1Δ mutants display increased silencing at subtelomeric regions and a modest decrease in silencing at the HM loci (20, 55). These phenotypes presumably are due to the titration of the available Sir proteins that are recruited efficiently by Rap1 to the longer telomeres that rif1Δ mutants display.
The Sir2-4 complex is absolutely required for the establishment and maintenance of silent chromatin, whereas Sir1 is not essential and contributes only modestly to establish the silent chromatin at the HM loci (17, 38, 44). It is thought that the yeast Ku complex (yKu70/80) is not required for silencing at the HM loci, although it is essential for silencing at the subtelomeric regions (4, 28, 43, 49). Recently, however, it was found that Sir1 has a small, redundant role with yKu70 and yKu80 for silencing at HM loci and that the yKu complex binds these loci in a process dependent on Sir4 (35, 53).
C. glabrata, like S. cerevisiae, contains three mating type-like loci called MTL1, MTL2, and MTL3, which correspond to MAT, HMR, and HML, respectively (47). Initially it was thought that only MTL1 was transcriptionally active while MTL2 and MTL3 were maintained in a silent state, like HMR and HML. Recently, it was reported that unlike the case for S. cerevisiae, the MTL2 locus is transcriptionally active and some cell type identity is maintained through the cell type-specific regulation of the expression of the α1 and α2 genes and the cell type-specific processing of the a1 transcript, which contains two introns (32). In this regard it should be noted that C. glabrata does not have an SIR1 orthologue (15), which in S. cerevisiae contributes to establishing the silent chromatin at the HM loci (38).
To better understand how C. glabrata achieves the mating type-specific expression of the α genes and to determine unambiguously from which of the MTL loci the transcription of the a1 gene comes in our C. glabrata strain, we constructed a collection of single-deletion mutants of each MTL locus, as well as all of the combinations of double mutants and the triple-deletion mutant, which contains no mating type information. By using these mutants and URA3 reporter gene insertions throughout the three MTL loci, we found four major differences between C. glabrata and S. cerevisiae with respect to the regulation of the expression of mating type-specific genes. First, we found, in agreement with Muller et al. (32), that MTL2, unlike HMR in S. cerevisiae, is transcriptionally active. Second, C. glabrata does not seem to maintain cell type identity like S. cerevisiae. Third, MTL3 is subject to subtelomeric silencing rather than silencing mediated by discrete, cis-acting silencer elements. Finally, we found that unlike silencing at HML of S. cerevisiae, MTL3 silencing absolutely requires Rif1, yKu70, and yKu80 proteins. Taken together, our results highlight several critical differences between the regulation of the expression of the genes located at the MTL loci, as well as some genes involved in the maintenance of cell type identity and sexual reproduction, compared to that of S. cerevisiae.
MATERIALS AND METHODS
Strains, plasmids, and primers.
All strains, plasmids, and primers used in the present study are listed in Tables S1, S2, and S3, respectively, in the supplemental material.
Media.
Yeast cells were grown in standard yeast media as described previously (45), and 2% agar was added for plates. Synthetic complete (SC) medium contained 1.7 g/liter yeast nitrogen base [without (NH4)2SO4 and amino acids], 5 g/liter (NH4)2SO4, 0.6% Casamino Acids, and 2% glucose. Yeast extract-peptone-dextrose (YPD) medium contained 10 g/liter yeast extract, 20 g/liter peptone, 2% glucose, and 25 mg/liter uracil. When required, YPD plates were supplemented with 420 μg/ml hygromycin (Invitrogen, Carlsbad, CA). To score 5-fluoroorotic acid resistance (5-FOA; Toronto Research Chemicals, North York, Canada), 0.9 g/liter 5-FOA and 25 mg/liter uracil were added to the SC plates (5-FOA plates).
Bacteria were grown in Luria-Bertani (LB) medium as described previously (2) with 1.5% agar added for plates. LB medium contained 5 g/liter yeast extract, 10 g/liter tryptone, and 5 g/liter NaCl, and when needed, it was supplemented with either 50 μg/ml carbenicillin or 50 μg/ml carbenicillin plus 30 μg/ml kanamycin. All plasmid constructs were introduced into strain DH10B by electroporation.
Cloning and sequencing of the three MTL loci of C. glabrata.
To clone the three mating type loci of our wild-type strain, BG14 (ura3Δ::Tn903 G418r) (11), we amplified by PCR three different fragments encompassing the three regions where the MTL loci are located, using primers specific for each MTL locus containing added BamHI sites. These fragments were cloned into the cloning vector pAP502 (12) or pCR2.1 TOPO (Invitrogen, Carlsbad, CA) and sequenced.
Sequence analysis revealed that our wild-type strain (BG14) contains a information at MTL1 and MTL2 and α information at MTL3. The 2.997-kb PCR fragment containing the MTL1 locus was amplified with primers 160 and 161 (see Table S3 in the supplemental material) and was cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA) to generate pIC122. pIC122 then was digested with NcoI, blunted with Klenow fragment of DNA polymerase I, and then digested with MscI to delete most of the kanamycin resistance gene to generate pSD10. A 2.734-kb PCR fragment containing the MTL2 locus was amplified with primers 2434 and 2435 (see Table S3) and was cloned into pAP502 (12) to generate pAP699. A 3.319-kb PCR fragment containing the MTL3 locus was amplified with primers 2438 and 2439 (see Table S3) and was cloned into pAP502 to generate pAP695.
Construction of URA3 insertions throughout the three MTL loci.
To assess whether the MTL loci are transcriptionally silenced, we inserted a URA3 reporter gene at different positions throughout the MTL1, MTL2, and MTL3 loci using a modified Tn7 transposon carrying the URA3 gene (9). For this, we mutagenized in vitro each of the plasmids containing each MTL locus (pSD10, pAP699, and pAP695) with Tn7 UKR as described previously (9). We obtained hundreds of independent insertions for each plasmid transformed in Escherichia coli. Insertions first were mapped by colony PCR to identify insertions within each MTL locus using primers that anneal with the ends of the Tn7 (oriented outwards) transposon and primers annealing at different positions within the cloned MTL fragment. In this way, we picked for further study a total of 5 independent URA3 insertions throughout MTL1 (pSD plasmids), 7 along MTL2, and 10 in the MTL3 locus (pRZ plasmids). All of these insertions (a total of 22) were sequenced, and the results are described in Table S4 in the supplemental material.
All of the 22 insertions were introduced into the C. glabrata genome by homologous recombination at their respective loci by the one-step gene replacement method as described previously (10, 12). The homologous recombination of each insertion was verified by PCR using locus-specific genomic primers that hybridized outside the region cloned in the plasmids carrying the insertion and primers annealing to the ends of Tn7. Because the transposition of Tn7 occurs in vitro and subsequently selects transposition reactions in E. coli, only one Tn7 (URA3) insertion is obtained in C. glabrata after homologous recombination has occurred following transformation with linearized plasmids. Once it has recombined in C. glabrata, Tn7 is stable and does not transpose, since it does not contain the transposase genes. To show that C. glabrata mtl::URA3 strains contain only one copy of the URA3 gene at the indicated locus, we used three different insertions at each MTL locus and replaced the relevant mtl::URA3 insertion with the hygromicin cassette we used to generate mtlΔ mutants. We used the knockout plasmids described in supplemental Table S2 (pSD7, pRZ15, and pRZ17) to transform the different URA3 insertions and selected resistance to hygromicin. Correct transformants (where the hygromicin cassette was integrated by homologous recombination at each MTL) were screened for the Ura− phenotype. All of the correct Hygr transformants were uracil auxotrophs and resistant to 5-FOA, indicating that mtl1,2,3::URA3 insertions contain only one insertion at the indicated MTL locus (data not shown). We also performed Southern blotting with three URA3 insertions at each MTL locus using an internal URA3 fragment as the probe. Genomic DNA from each strain was digested with SspI, and 80 μg of DNA was run in a 0.8% agarose gel and transferred to a Hybond membrane. The membrane was hybridized overnight with peroxidase-labeled probe for 12 h and washed under stringent conditions. The Southern blot for the nine insertions showed that there is only one band of the expected size corresponding to only one URA3 insertion in each strain (data not shown).
Reporter URA3 gene expression assays (5-FOA sensitivity assays).
To assess the degree of silencing of the URA3 gene inserted at different positions throughout the MTL loci, we carried out 5-FOA growth assays as described previously (42). Briefly, strains containing the different URA3 insertions along the MTL loci were grown in YPD to stationary phase for around 36 h. The cultures were adjusted to an optical density at 600 nm (OD600) of 1 with sterile water, and 10-fold serial dilutions were made in 96-well plates. Five μl of each dilution was spotted onto three different plates, YPD, SC without uracil (SC-ura), and SC plus 5-FOA, after which the plates were incubated at 30°C for 48 h and photographed.
Generation of strains with deletion of silencing genes.
To generate derivatives with the deletion of silencing genes in strains with three representative URA3 insertions along the MTL3 silent locus, we followed the one-step gene replacement procedure using disruption plasmids for each gene to be deleted (SIR2, SIR3, SIR4, HDF1, HDF2, and RIF1) (42) (see Table S1 in the supplemental material). Disruption plasmids contain a hygromycin resistance cassette flanked by 5′- and 3′-flanking regions of each gene (all of the plasmids used for allele replacements are listed in Table S2 in the supplemental material). Each disruption plasmid was digested with enzymes that cut at both ends, within the 5′ and 3′ cloned flanking regions, generating ends homologous to each specific gene to be deleted in the C. glabrata genome (of each insertion strain to be deleted). The released fragment was used to transform C. glabrata selected on plates supplemented with 420 μg/ml hygromycin. Homologous recombination at each locus was verified by PCR with a primer that anneals in the sequences external to the cloned 5′ and 3′ fragments and a primer annealing within the hygromycin cassette. We also verified the absence of each gene deleted by the inability to PCR amplify a small internal fragment from each deleted gene (<300 bp). Each of these derivatives was used for 5-FOA plate growth assays as described above.
Construction of strains with deletions of two MTL loci.
To construct strains that carry only one type of mating information, we set out to construct strains containing deletions of two MTL loci (leaving only one MTL locus). The first deletion was generated by allele replacement as described above. To construct double or triple mutants, the hygromycin cassette used to generate the first deletion was excised by expressing ScFLP1 product from plasmid pMZ18 (see Table S2 in the supplemental material). ScFLP1 encodes a site-specific recombinase that recognizes two direct repeats, the Flp1 recognition target (FRT) sites that flank the hygromycin cassette of the disruption plasmids. The Flp1 recombinase then induces recombination between FRT sites and the hygromycin marker is excised from the chromosome, resulting in an unmarked deletion of the corresponding MTL locus and leaving one copy of the FRT site. Unmarked deletion strains are identified as Hygs colonies on YPD hygromycin plates, and the deletion is confirmed by PCR using primers annealing in the chromosome outside the 5′ and 3′ regions used for the disruption.
The resulting Hygs strains then are used as recipients for double or triple deletions using the same hygromycin resistance cassette and selecting for Hygr colonies.
RT-PCR.
RNA was extracted from stationary-phase cells (36 h in YPD) using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions and treated with DNase I (Sigma-Aldrich, St. Louis, MO). The synthesis of cDNA and PCR was carried out using the AccessQuick reverse transcription-PCR (RT-PCR) system (Promega, Madison, WI). The RT primers used for each gene are listed in Table S3 in the supplemental material. The cDNA synthesis reaction was carried out at 45°C for 45 min for all of the genes. The PCR was carried out at 57°C for a1, STE3 (CAGL0M08184g), STE12 (CAGL0H02154g), STE20 (CAGL0K02673g), and ACT1 (CAGL0K12694g); 53°C for MF(ALPHA) (CAGL0H03135g) and HOG1 (CAGL0M11748g); 55°C for MFA1 (CAGL0C01919g); 50°C for α1 (CAGL0B1243g), α2 (CAGL0B1265g), and STE2 (CAGL0K12430g); and 58°C for BAR1 (CAGL0J02288g) and HO (CAGL0G05423g). A reaction mixture without reverse transcriptase was included as a negative control in all of the RNA samples and with every pair of primers. No bands were obtained, indicating that the RNA preparations had no DNA contamination.
RESULTS
The MTL3 locus is the only silent mating type-like locus in C. glabrata.
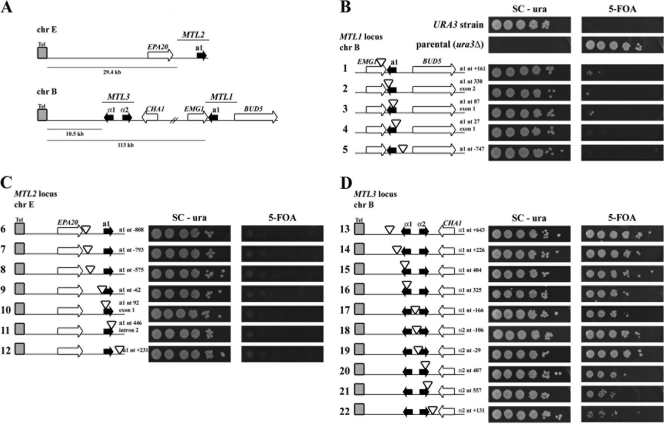
C. glabrata possesses three MTL loci arranged in a configuration similar to that of the mating type loci of Saccharomyces cerevisiae (47). MTL1 is the only one localized at an internal position within chromosome B (approximately 113 kb from the left telomere of this chromosome), while MTL2 and MTL3 loci are located close to two telomeres, at approximately 29.4 and 10.5 kb from the left telomeres of chromosomes E (Chr E-L) and B (Chr B-L), respectively (Fig. 1A). We have shown previously that genes in subtelomeric regions of several chromosomes in C. glabrata are subject to chromatin-based transcriptional silencing in a process that depends on Sir2, Sir3, Sir4, Rap1, the Ku70/80 complex, and Rif1 (10, 12). To assess whether MTL2 and MTL3 are subject to silencing, we constructed strains containing URA3 reporter gene insertions at different positions throughout the three MTL loci; the precise insertion site for each one is described in Table S4 in the supplemental material. Strains containing URA3 insertions were plated on medium containing the drug 5-fluoroorotic acid (5-FOA) and on plates lacking uracil (SC-ura plates) to assay for cells expressing URA3. The amount of growth on medium containing 5-FOA reflects the extent of the transcriptional silencing of URA3.
Fig. 1.
MTL3 is the only MTL locus subject to silencing in Candida glabrata. (A) Schematic representation of the MTL1, MTL2, and MTL3 loci of C. glabrata. MTL1 and MTL3 are located on chromosome B. MTL3 is placed 10.5 kb away from the left telomere of chromosome B (ChrB-L), and MTL1 is at an internal position on this chromosome (113 kb away from ChrB-L). MTL2 is located 29.4 kb from the left telomere of chromosome E (ChrE-L). (B, C, and D) Plate growth assay to determine the expression of the URA3 reporter gene (shown as empty triangles) inserted across the three MTL loci in C. glabrata. To the left of each panel is shown a schematic representation of each MTL locus and the relative positions of the reporter gene insertions with respect to the a1, α1, or α2 gene (represented by black arrows that indicate the direction of transcription). The exact position within the a1, α1, or α2 gene of each insertion is indicated. Strains of C. glabrata containing 5 URA3 insertions throughout the MTL1 locus (B), 7 in the MTL2 locus (C), or 10 in the MTL3 locus (D) were grown to stationary phase in YPD, and 10-fold serial dilutions in sterile water were made. Equal numbers of cells of each dilution were spotted onto SC-ura and SC plates containing 5-FOA. Plates were incubated at 30°C for 48 h and photographed.
As shown in Fig. 1B, we isolated five URA3 reporter insertions throughout the MTL1 locus. As expected, all of the insertions at MTL1 are transcriptionally active as measured by the lack of growth on 5-FOA plates and robust growth on SC-ura plates. The parental strain (BG14 ura3Δ::Tn903), used as a control, is not able to grow in the absence of uracil, but it grows well on plates containing 5-FOA. Unlike what has been reported for S. cerevisiae (19, 40, 48), seven different URA3 reporter insertions along the MTL2 locus (homologous to HMR) show that the chromatin in this locus is transcriptionally active, as evidenced by their ability to grow on SC-ura plates and the failure to do so on 5-FOA plates (Fig. 1C). MTL2 has been reported to be transcriptionally active in C. glabrata as assayed by the quantitative PCR of the a1 gene in several strains (32).
At the MTL3 locus, however, the first eight URA3 reporter gene insertions throughout the locus are subject to transcriptional silencing, as shown by the robust growth on 5-FOA plates (Fig. 1D). A significant proportion of the cells from cultures containing these reporter insertions at MTL3 also are able to grow on media lacking uracil, indicating the expression of URA3 in these cells. The leakiness of the silencing at MTL3 contrasts sharply with the silencing observed at the homologous HML locus of S. cerevisiae, where no expression of reporter genes introduced at this site is detected (3, 40). The last two insertions in MTL3, located at the C-terminal end or downstream from the α2 gene, show a decreased level of silencing (Fig. 1D).
Native genes contained at MTL3 are not expressed, while genes at MTL1 and MTL2 are expressed.
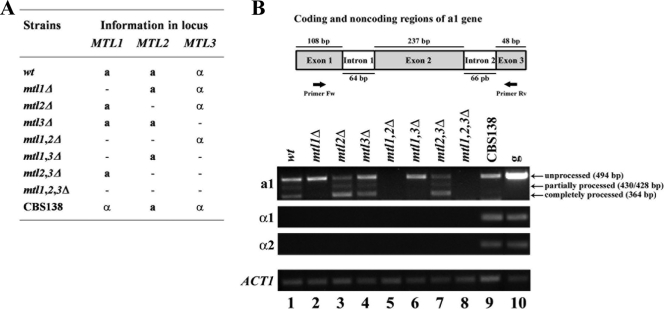
The complete processing of the a1 gene is achieved only when transcribed from MTL1. To determine whether the native a1, α1, and α2 genes from the three MTL loci are expressed, we constructed a series of null mutants in each MTL locus, all of the combinations of double mutants and the triple knockout (Fig. 2A), and performed RT-PCR experiments on all of them. As shown in Fig. 2B, α1 and α2 genes are not expressed under the conditions tested in any of the strains derived from the BG14 background (MTL1a, MTL2a, and MTL3α), where α information comes from MTL3 only (but see below). However, a transcript is clearly detectable from the reference, sequenced strain ATCC2001 (CBS138), where the α genes are located at both MTL1 and MTL3 (http://www.genolevures.org/cagl.html). In our strain background, the a1 gene, which contains two introns, is expressed from both loci, MTL1a and MTL2a, but it is processed (both partially and completely) only when the transcript is expressed from MTL1. Figure 2B, lanes 2 and 6, shows that for strains mtl1Δ and (mtl1,mtl3)Δ, where a1 is transcribed only from MTL2a, only the unprocessed form of the a1 transcript is detected, while in strains mtl2Δ and (mtl2,mtl3)Δ, where a1 is transcribed only from MTL1, the partially and totally processed forms can be seen (Fig. 2B, lanes 3 and 7). In the CBS138 strain, only the unprocessed form of the a1 transcript is detected, since in this strain type a information is present only at the MTL2 locus (Fig. 2B, lane 9). These results are in agreement with previously reported data for C. glabrata (32) and indicate that in contrast to S. cerevisiae, C. glabrata does not maintain repression at MTL2, which is the orthologue of HMR.
Fig. 2.
a1 transcript, which contains two introns, is processed only when transcription comes from MTL1 but not when it is initiated at MTL2. (A) Description of the mating type information present at each MTL locus in the collection of strains containing single, double, and triple deletion mutations in the MTL loci. (B) Locus-specific processing of the a1 transcript. Top panel, schematic representation of the a1 gene showing the two introns and the positions of the primers used for the PCR (black arrows). Bottom panel, the indicated strains containing deletions in the MTL loci were grown to stationary phase and RNA was extracted and used for RT-PCR (see Materials and Methods). a1 transcript was detected using primers indicated for RT-PCR in the top panel. The unprocessed transcript is 494 bp long, the partially processed transcripts are 430 and 428 bp long, and the completely processed transcript is 364 bp long. Note that the complete processing of the a1 gene can be detected only when transcription comes from the MTL1 locus [lanes 3 and 7; mtl2Δ and (mtl2, mtl3)Δ] but not when transcription is initiated at MTL2 [lanes 2 and 6; mtl1Δ and (mtl1, mtl3)Δ]. Strains containing deletions of both MTL1 and MTL2 [lanes 5 and 8; (mtl1, mtl2)Δ and (mtl1, mtl2, mtl3)Δ] were used as negative controls, since there is no a1 gene. Lane 9, RNA from strain CBS138 (reference strain ATCC2001 containing α information in MTL1) was used as a positive control for the transcription of the α1 or α2 gene. Lane 10, genomic DNA from the wild-type strain (g) was used as a positive control for PCR. ACT1 RT-PCR was used as an internal control. Negative control experiments where no reverse transcriptase was added did not amplify any bands (data not shown).
C. glabrata cells containing different mating type information at the MTL loci express both a-specific and α-specific genes.
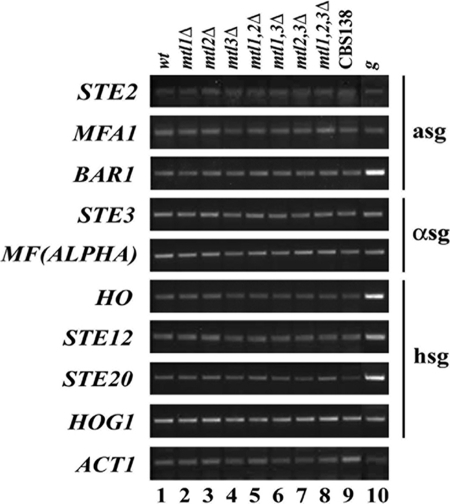
Because of the imperfect silencing at MTL3, it is possible that some cells containing a information at MTL1 and α at MTL3 express both types of mating information (RT-PCR using increased number of cycles shows detectable α1 and α2 transcripts from MTL3; data not shown). It also is possible that a very small fraction of the a1 transcript expressed from MTL2 is processed correctly, such that in cells containing α information at MTL1 there would be the expression of both types of mating information in a given cell. To maintain cell type identity in S. cerevisiae, MATa or MATα cells use a regulatory circuit controlled by the mating type genes a1, α1, and α2 in which a cells express specific genes present only in MATa cells (a-specific genes, or asg) and MATα cells express a set of genes present only in these cells (α-specific genes, or αsg). In diploid cells (after mating; MATa/α cells), a heterodimer repressor is made consisting of the a1/α2 proteins that represses the transcription of several genes expressed only in haploid cells (haploid-specific genes, or hsg). We asked whether some of the known asg, αsg, or hsg in S. cerevisiae are regulated in the same way in C. glabrata. For this, we used the strains containing all of the deletion combinations of the MTL loci, so that there are strains that contain only one type of information. We performed RT-PCR for three orthologues of the S. cerevisiae asg (STE2, MFA1, and BAR1), two of the αsg [STE3, MF(ALPHA)], and four of the hsg (STE12, STE20, HOG1, and HO). Figure 3 shows that all of the genes we tested are expressed at relatively moderate levels in all of the MTL mutant strains, including the strain that contains no mating type information [mtl(1,2,3)Δ] (Fig. 3, lane 8) and also the parental strain and CBS138 that contain a and α information, respectively, at MTL1. This suggests that C. glabrata does not keep a cell type identity, as has been described for S. cerevisiae.
Fig. 3.
Analysis of the expression of several cell type-specific genes by RT-PCR. Genes analyzed are STE2, MFA1, and BAR1 as examples of a-specific genes (asg); STE3 and MF(ALPHA) are α-specific genes (αsg), and HO, STE12, STE20, and HOG1 are haploid-specific genes (hsg). RNA was extracted from stationary-phase cultures of the collection of single, double, and triple mutants in the MTL loci as indicated. C. glabrata strain CBS138 (ATCC2001) was used as a positive control for the expression of α-specific genes. ACT1 RT-PCR was used as an internal control and genomic DNA from the wild-type strain (g) as a positive control for the PCR. Negative-control experiments where no reverse transcriptase was added did not amplify any bands (data not shown).
Silencing at the MTL3 locus requires Sir2, Sir3, Sir4, yKu70, yKu80, and Rif1.
The yKu70 and yKu80 proteins are absolutely required for silencing at the subtelomeric regions in S. cerevisiae (telomere position effect, or TPE) (44), while at the silent HM loci these proteins play only a minor role, performing an overlapping function with Sir1 at establishing the silent chromatin (35, 53).
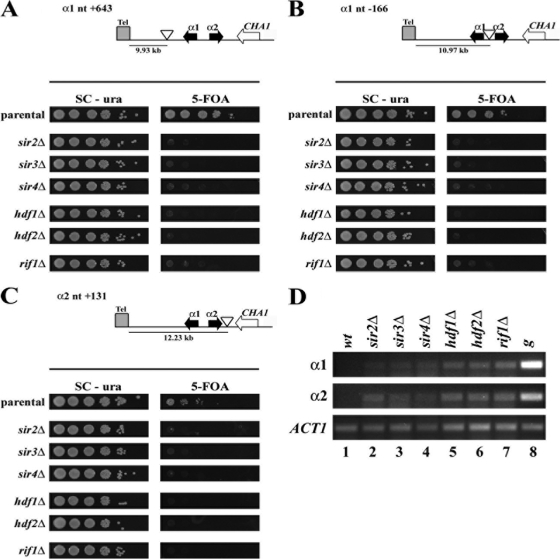
Silencing at the subtelomeric regions of C. glabrata requires the Sir proteins (Sir2-4), Rap1, yKu70, and yKu80 and Rif1 (10, 12). To determine whether the silencing proteins also are required for silencing at MTL3, we made deletion/insertion mutations in each of the SIR2, SIR3, SIR4, HDF1, HDF2, and RIF1 genes in three different C. glabrata strains containing the URA3 reporter insertions at representative locations within MTL3 (Fig. 4). As expected, the silencing of the reporter URA3 at the three positions tested in MTL3 absolutely requires the Sir2 to -4 proteins, as measured by the lack of growth on 5-FOA plates (Fig. 4A to C). Surprisingly, silencing at this locus also requires yKu70, yKu80, and Rif1 proteins (Fig. 4A to C). We determined by RT-PCR that the expression of the native genes α1 and α2 in each of the mutants in silencing proteins is strongly derepressed (Fig. 4D).
Fig. 4.
Silencing at MTL3 depends on the silencing proteins Sir2, Sir3, Sir4, yKu70, yKu80, and Rif1. The top parts of A, B, and C show three representative URA3 insertions across the MTL3 locus (indicated by empty triangles) that were selected to be introduced independently in each one of the deletion/insertion mutations in SIR2, SIR3, SIR4, HDF1 (yKu70), HDF2 (yKu80), and RIF1. Stationary-phase cultures of strains of C. glabrata containing the shown URA3 insertions and the indicated deletion alleles, sir2Δ, sir3Δ, sir4Δ, hdf1Δ, hdf2Δ, and rif1Δ, were diluted in sterile water. Equal numbers of cells of each dilution were spotted onto SC-ura and SC plates containing 5-FOA. Plates were incubated at 30°C for 48 h and photographed. (A) Strains of C. glabrata containing the indicated silencing mutations and the URA3 insertion closest to the ChrB-L telomere (9.93 kb from the telomere repeats). (B) Strains containing the indicated silencing mutations and the URA3 insertion between α1 and α2 of MTL3 (10.97 kb from the telomere). (C) Strains containing the indicated silencing mutations and the URA3 insertion farthest away from the MTL3 telomere (12.23 kb). (D) Analysis of the expression of α1 and α2 genes from MTL3 by RT-PCR. Strains containing deletion alleles in SIR2, SIR3, SIR4, HDF1 (yKu70), HDF2 (yKu80), and RIF1 were grown to stationary phase, and RNA was extracted to perform RT-PCR. ACT1 RT-PCR was used as an internal control. Control experiments where no reverse transcriptase was added did not amplify any bands (not shown). Genomic DNA from the wild-type strain (g) was used as a positive control for the PCR (lane 8).
In S. cerevisiae the silencers flanking the silent mating loci contain binding sites for Rap1, ORC, and Abf1. These silencers are not conserved in MTL2 and MTL3 of C. glabrata (although there are two conserved putative Abf1 binding sites flanking MTL3 as well as one ORC putative binding site). The absence of conserved silencers at MTL3 in C. glabrata could explain the leaky silencing observed that depends on the nucleation and spreading of the silent chromatin from the telomere.
Silencing at MTL3 is nucleated predominantly at the telomere and spreads through the MTL3 locus.
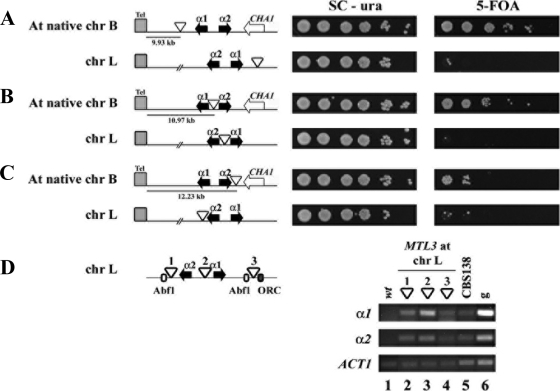
The dependence of silencing at the MTL3 locus on yKu70, yKu80, and Rif1 is reminiscent of the subtelomeric silencing we have observed at other telomeres in C. glabrata (10, 12, 42). To determine whether silencing at MTL3 is nucleated at the left telomere of chromosome B (which is only 10.5 kb away) and then propagates into the MTL3 locus, we introduced this locus carrying each of the three representative URA3 insertions into a region away from any telomere. We chose a large, internal, intergenic region 224 kb away from the left telomere of chromosome L that is not subject to silencing to integrate the entire MTL3 locus with the reporter insertions. These constructs consist of a 3.313-kb fragment (1.262 kb downstream from α1 and 0.531 kb downstream from α2) containing the MTL3 locus as well as the flanking sequences containing the conserved putative Abf1 and ORC binding sites and each of the three URA3 reporter insertions. As shown in Fig. 5 A to C, moving MTL3 away from the left telomere of chromosome B, where it normally resides, into an internal location of chromosome L results in an almost-complete loss of silencing as measured by the lack of growth on 5-FOA plates (compare growth on 5-FOA of strains with URA3 insertions at chromosome L with that of their counterpart strains with URA3 insertions integrated at the subtelomeric region of chromosome B). The fact that there is no silencing of the MTL3::URA3 reporter system when moved to a nonsubtelomeric region suggests that the flanking regions of MTL3 do not contain a cis-acting silencer and that the silencing observed at this locus is nucleated mainly at the telomere and propagates into MTL3 from there. In agreement with the expression of the reporter URA3 at this location, we could detect the expression of the α1 and α2 genes at this internal position (Fig. 5D).
Fig. 5.
Silencing at MTL3 requires the telomere context. The entire MTL3 region containing the three representative URA3 insertions at MTL3 and the flanking sequences that contain Abf1 and ORC putative binding sites (indicated by white and black small rectangles, respectively) were integrated at an internal, large intergenic region in chromosome L, and silencing at these positions was assayed by the plate growth assay as described for Fig. 1. (A) Silencing of the URA3 insertion closest to the telomere, at its native location 9.93 kb from the left telomere of chromosome B (top row) and when integrated at an internal position on chromosome L (bottom row). (B) Silencing of the URA3 insertion between α1 and α2 genes, at its native location 10.97 kb from the left telomere of chromosome B (top row) and when integrated at an internal position on chromosome L (bottom row). (C) Silencing of the URA3 insertion farthest from the telomere at its native site 12.23 kb from the left telomere of chromosome B (top row) and when integrated at an internal position on chromosome L (bottom row). (D) Expression measured by RT-PCR of α1 and α2 genes from MTL3 when inserted at an internal location on chromosome L.
DISCUSSION
The occurrence of a sexual cycle in human pathogenic fungi is uncommon, and it has been suggested that mating sorts out specific combinations of genes required for virulence or survival in the host (33). However, in several cases of human fungal pathogens, a cryptic sexual cycle has been found. One example is the obligate diploid Candida albicans, where the vast majority of strains are heterozygous at the mating type-like locus (MTL) containing both types of information (a/α). In this human pathogen, mating was shown to occur if two requirements are fulfilled: first, the information present at the MTL locus needs to be homozygous (cell expressing either a/a or α/α information), and second, cells must switch to the mating-competent type or opaque cell type. Under these conditions, a parasexual cycle can be detected where opaque cells expressing only one type of information can mate with another opaque cell expressing only the opposite type of information (23, 24, 30, 31). Furthermore, it was shown recently that C. albicans also can undergo same-sex mating between homozygous strains in a way similar to what has been reported for some other medically relevant fungi (1).
Candida glabrata has never been shown to mate, and all isolates analyzed to date are haploids. Using a population genetics-based analysis it was determined that the population structure is predominantly clonal, with some evidence for recombination (13).
The genes that control mating and cell type identity in fungi are in the MAT locus in S. cerevisiae and MTL in other fungi. C. glabrata, like S. cerevisiae, contains three mating type loci, MTL1, MTL2, and MTL3, and only MTL1 was thought to be transcriptionally active, corresponding to the functional homologue of the MAT locus, so that the information present at MTL1 is thought to determine the mating type of the cell (32, 47). In a recent report, Muller et al. (32) found that α1 and α2 are expressed in a mating type-specific way while a1 is transcribed in both mating types, and mating type identity was proposed to be maintained in C. glabrata through the mating type-specific processing of the a1 gene (32). In this work, we looked at the transcriptional activity of the three MTL loci and determined that MTL1 and MTL2 are expressed and only MTL3 is silenced; in fact, MTL3α (an orthologue of HMRα) is imperfectly silenced in C. glabrata, most likely through silencing nucleated at the telomere that propagates more than 11 kb to MTL3. Surprisingly, the silencing of MTL3α absolutely depends on the silencing proteins yKu70, yKu80, and Rif1p. In S. cerevisiae, these proteins do not have a predominant role in the silencing of the mating type silent cassettes, and only in the absence of Sir1 could a small effect of the Ku proteins in silencing the HM loci be detected (35, 53).
C. glabrata MTL1a and MTL2a loci are transcriptionally active.
By introducing multiple insertions of the reporter URA3 gene at various positions along MTL1 and MTL2 loci, we found, in agreement with Muller et al. (32), that both MTL1a and MTL2a (the functional homologue of HMRa) are transcriptionally active (Fig. 1B and C). This is in sharp contrast to S. cerevisiae, where both HMRa and HMLα are very efficiently silenced (3, 22, 27).
Locus-specific processing of the a1 transcript.
By using a collection of single, double, and triple MTL deletion strains to unambiguously determine whether the transcription of the native a1 gene comes from MTL1 or MTL2 (since both contain this gene), we determined that this gene is transcribed from both loci, but there is a locus-specific processing of the a1 transcript. When the transcription of a1 comes from MTL2, as in strains mtl1Δ and (mtl1, mtl3)Δ (Fig. 2B, lanes 2 and 6), we cannot detect the processing of the transcript, whereas when transcription comes from MTL1, as in strains mtl2Δ and (mtl2, mtl3)Δ (Fig. 2B, lanes 3 and 7), we can detect both the fully processed transcripts and the intermediates of the partially processed transcript. These results are consistent with previously reported data and are thought to demonstrate the mechanism by which C. glabrata maintains some cell type identity (32). The sequences of both a1 genes at MTL1 and MTL2 loci in our background are identical for up to 500 bp on the 5′-flanking sequence, except for two mismatches at positions −375 and −360 from the start codon. At the 3′ region, the identity extends for 370 bp downstream from the stop codon; further upstream or downstream the sequences are completely different. It is possible that the two differences in the 5′ region or the sequences upstream from −500 bp or downstream from +370 lead to differential splicing. Another possibility is that there are as-yet-unidentified mechanisms, possibly chromatin based or others, that detect where the a1 transcript originates and only process it when it comes from MTL1, but the identification of this regulation needs further studies.
C. glabrata cells express a-specific, α-specific, and haploid-specific genes regardless of the mating type information present at MTL loci.
Surprisingly, we found that unlike S. cerevisiae, C. glabrata expresses at least some of the genes that are cell type specific in S. cerevisiae. For example, three S. cerevisiae a-specific genes (STE2, MFA1, and BAR1) as well as two of the α-specific genes [STE3 and MF(ALPHA)] are expressed at moderate levels in C. glabrata independently of the presence or absence of mating type information at any of the MTL loci (Fig. 3). The transcription of four of the S. cerevisiae haploid-specific genes (STE12, STE20, HOG1, and HO) (18, 26) also was detected in all of the strains tested, including the triple mutant strain (mtl1, mtl2, mtl3)Δ (Fig. 3, lane 8). This suggests that C. glabrata does not regulate the expression of cell type-specific genes in the same way that S. cerevisiae regulates these genes, and this probably leads to a lack of cell type identity of C. glabrata. This is also different from C. albicans, where the expression of a-specific genes is detected only when a cells are induced with alpha pheromone (51), while in C. glabrata expression is detected in the absence of pheromone and in all mtl mutant strains. In this regard, both MFA1 and BAR1 promoter regions contain relatively conserved putative Mcm1 and α2 recognition sequences while STE2 is less conserved, and the spacing differs from that of S. cerevisiae (52). For the αsg, the promoter region of STE3, but not MF(ALPHA), contains putative Mcm1 and α1 recognition sequences.
Genes negatively regulated by the a1/α2 heterodimer are a subset of the haploid-specific genes in S. cerevisiae, and of the four hsg we assayed, only the HO promoter region contains an a1/α2 heterodimer recognition sequence. The result, however, is the same for all of the genes we tested, since all were expressed at moderate levels in all of the strains, regardless of the information at the MTL loci and of the presence or absence of conserved binding sites for the different transcription factors. It is not known if the a1, α1, and α2 proteins are functional in C. glabrata, or whether they control a different set of genes. It is possible that C. glabrata has undergone a rewiring of the transcriptional regulators of the mating pathway, and that they do not regulate sexual reproduction or cell type identity genes. It also should be noted that both α1 and α2 are not closely conserved, sharing only 27 and 38% identity, respectively, across the entire lengths of the proteins.
MTL3 locus is subject to subtelomeric silencing that depends on yKu70, yKu80, and Rif1.
Unlike MTL2, MTL3 is subject to transcriptional silencing as shown by growth on 5-FOA plates of all the strains with the insertions we isolated across the entire locus (Fig. 1D). The degree of silencing decreases as the distance between the URA3 insertions and the telomere increases (the farthest insertion is located 12.233 kb from the telomere [insertion no. 22; Fig. 1D, last row]). Relieving subtelomeric silencing by making deletions of each of the genes coding for silencing proteins results in the expression not only of the reporter URA3 gene but also of the native α1 and α2 genes at MTL3 (Fig. 4D).
It should be noted that in S. cerevisiae, the functional orthologous locus HMLα is very efficiently silenced in a process depending on Sir1 to -4, Rap1, ORC, and Abf1, but not on the yKu proteins (4, 28, 43, 49). However, a small effect of yKu70 and yKu80 on both of the HM silent loci that is redundant with Sir1 recently was discovered in S. cerevisiae (35, 53). Rif1 also was shown to contribute to silencing but only at a weakened hmrΔA silencer (20, 55).
In contrast, in C. glabrata the silencing of MTL3 absolutely requires Sir2 to -4, Rif1, yKu70, and yKu80 (Fig. 4). In this regard, silencing at MTL3 is reminiscent of the silencing we have found at telomeres Chr C-L and Chr I-R, in which silencing appears to be nucleated at the telomere and propagates over long distances to some EPA genes (like EPA7, EPA4, and EPA5). A consequence of this type of silencing is that MTL3 is not as efficiently silenced as the HM loci of S. cerevisiae, resulting in a sizable proportion of the population in each culture expressing, at least transitorily, the reporter gene (Fig. 1). Sir1 is required for the establishment of silencing at the HM loci in S. cerevisiae, and C. glabrata does not contain the SIR1 gene (15). This could explain in part the lack of silencing at MTL2 and the imperfect silencing at MTL3. In contrast, silencing at the HM loci in S. cerevisiae is nucleated at discrete silencers flanking each locus that depend on Rap1, ORC, and Abf1 but not on the yKu proteins. At MTL3 in C. glabrata, however, the sequences of the silencers are not conserved, although there are two putative Abf1 binding sites flanking α1 and α2 genes and an ORC consensus site downstream from α1. These conserved, putative binding sites, however, are not sufficient to mediate the silencing of the α1 and α2 genes independently of the telomere, since when moving three representative URA3 reporter insertions in the MTL3 locus (along with its flanking regions and putative binding sites for Abf1 and ORC) to an internal, intergenic region in chromosome L (200 kb away from the telomere), both the URA3 reporter and α1 and α2 genes are expressed (Fig. 5A, B, C and D).
The leaky silencing of MTL3 could have implications in C. glabrata, since strains containing a1 at MTL1 could form the a1/α2 heterodimer in at least some cells in any given population. This could lead to the repression of several genes if, in C. glabrata, this heterodimer functions in the same way as it does in S. cerevisiae, where it is a repressor of many haploid-specific genes. We are investigating this possibility.
Taken together, our results highlight some important differences between C. glabrata and S. cerevisiae in recruiting silencing machinery to their respective mating loci. There are differences in the expression of cell type-specific genes as well that could explain in part the apparent absence of cell type identity and sexual reproduction in C. glabrata.
It has been proposed that many human fungal pathogens rarely undergo sexual reproduction, which possibly gives them an advantage to proliferate in the host, but under stressful conditions, a sexual or parasexual cycle can be achieved, allowing for adaptation to hostile environments (33). Genomic data from many fungal species shows that the vast majority of them have conserved almost all the genes needed to reproduce sexually (5, 6), even in those species where no sexual cycle has been found. Although it is not known why they have kept all of these genes, it is possible that some of them have been rewired in the asexual pathogenic species and now play an additional role important to their survival as commensals or pathogens in their hosts. For example, it has been reported that in C. albicans, C. glabrata, and C. neoformans some components of the pheromone response pathway play a role in the virulence of these pathogens, and this implies that the components of the pheromone response pathway, rather than the actual mating, are important for pathogenesis (5, 7, 8, 41, 54). Further studies on the regulation of cell type-specific genes and pheromone response pathway genes will help define whether C. glabrata can undergo sexual reproduction and whether some of these genes have been rewired to control other processes important for its survival in the mammalian host.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Cormack for providing strains and helpful discussions and A. D. Johnson for kindly providing space and reagents. We are indebted to O.E. Arroyo-Helguera and E. E. Uresti-Rivera for excellent technical assistance.
This work was supported by CONACyT fellowships to C.Y.R.-Z. (164574) and to G.E.S.-D. (209281), as well as CONACyT grant no. CB-2005-48304 to I.C.N. and UC-MEXUS CONACYT grant no. CN-06-53 to I.C.N. and A.D.J.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 30 July 2010.
REFERENCES
- 1.Alby K., Schaefer D., Bennett R. J. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (ed.). 2001. Current protocols in molecular biology. Wiley & Sons, Inc., New York, NY [Google Scholar]
- 3.Bi X., Braunstein M., Shei G. J., Broach J. R. 1999. The yeast HML I silencer defines a heterochromatin domain boundary by directional establishment of silencing. Proc. Natl. Acad. Sci. U. S. A. 96:11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulton S. J., Jackson S. P. 1998. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17:1819–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler G. 2010. Fungal sex and pathogenesis. Clin. Microbiol. Rev. 23:140–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler G., Rasmussen M. D., Lin M. F., Santos M. A. S., Sakthikumar S., Munro C. A., Rheinbay E., Grabherr M., Forche A., Reedy J. L., Agrafioti I., Arnaud M. B., Bates S., Brown A. J. P., Brunke S., Costanzo M. C., Fitzpatrick D. A., de Groot P. W. J., Harris D., Hoyer L. L., Hube B., Klis F. M., Kodira C., Lennard N., Logue M. E., Martin R., Neiman A. M., Nikolaou E., Quail M. A., Quinn J., Santos M. C., Schmitzberger F. F., Sherlock G., Shah P., Silverstein K. A. T., Skrzypek M. S., Soll D., Staggs R., Stansfield I., Stumpf M. P. H., Sudbery P. E., Srikantha T., Zeng Q. D., Berman J., Berriman M., Heitman J., Gow N. A. R., Lorenz M. C., Birren B. W., Kellis M., Cuomo C. A. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcagno A. M., Bignell E., Rogers T. R., Canedo M., Muhlschlegel F. A., Haynes K. 2004. Candida glabrata Ste20 is involved in maintaining cell wall integrity and adaptation to hypertonic stress, and is required for wild-type levels of virulence. Yeast 21:557–568 [DOI] [PubMed] [Google Scholar]
- 8.Calcagno A. M., Bignell E., Warn P., Jones M. D., Denning D. W., Muhlschlegel F. A., Rogers T. R., Haynes K. 2003. Candida glabrata STE12 is required for wild-type levels of virulence and nitrogen starvation induced filamentation. Mol. Microbiol. 50:1309–1318 [DOI] [PubMed] [Google Scholar]
- 9.Castano I., Kaur R., Pan S., Cregg R., De Las Penas A., Guo N., Biery M. C., Craig N. L., Cormack B. P. 2003. Tn7-based genome-wide random insertional mutagenesis of Candida glabrata. Genome Res. 13:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaño I., Pan S. J., Zupancic M., Hennequin C., Dujon B., Cormack B. P. 2005. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 55:1246–1258 [DOI] [PubMed] [Google Scholar]
- 11.Cormack B. P., Falkow S. 1999. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Las Peñas A., Pan S. J., Castano I., Alder J., Cregg R., Cormack B. P. 2003. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 17:2245–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodgson A. R., Pujol C., Pfaller M. A., Denning D. W., Soll D. R. 2005. Evidence for recombination in Candida glabrata. Fungal Genet. Biol. 42:233–243 [DOI] [PubMed] [Google Scholar]
- 14.Dujon B., Sherman D., Fischer G., Durrens P., Casaregola S., Lafontaine I., De Montigny J., Marck C., Neuveglise C., Talla E., Goffard N., Frangeul L., Aigle M., Anthouard V., Babour A., Barbe V., Barnay S., Blanchin S., Beckerich J. M., Beyne E., Bleykasten C., Boisrame A., Boyer J., Cattolico L., Confanioleri F., De Daruvar A., Despons L., Fabre E., Fairhead C., Ferry-Dumazet H., Groppi A., Hantraye F., Hennequin C., Jauniaux N., Joyet P., Kachouri R., Kerrest A., Koszul R., Lemaire M., Lesur I., Ma L., Muller H., Nicaud J. M., Nikolski M., Oztas S., Ozier-Kalogeropoulos O., Pellenz S., Potier S., Richard G. F., Straub M. L., Suleau A., Swennen D., Tekaia F., Wesolowski-Louvel M., Westhof E., Wirth B., Zeniou-Meyer M., Zivanovic I., Bolotin-Fukuhara M., Thierry A., Bouchier C., Caudron B., Scarpelli C., Gaillardin C., Weissenbach J., Wincker P., Souciet J. L. 2004. Genome evolution in yeasts. Nature 430:35–44 [DOI] [PubMed] [Google Scholar]
- 15.Fabre E., Muller H., Therizols P., Lafontaine I., Dujon B., Fairhead C. 2005. Comparative genomics in hemiascomycete yeasts: evolution of sex, silencing, and subtelomeres. Mol. Biol. Evol. 22:856–873 [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick D. A., Logue M. E., Stajich J. E., Butler G. 2006. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol. Biol. 6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox C. A., McConnell K. H. 2005. Toward biochemical understanding of a transcriptionally silenced chromosomal domain in Saccharomyces cerevisiae. J. Biol. Chem. 280:8629–8632 [DOI] [PubMed] [Google Scholar]
- 18.Galgoczy D. J., Cassidy-Stone A., Llinas M., O'Rourke S. M., Herskowitz I., DeRisi J. L., Johnson A. D. 2004. Genomic dissection of the cell type-specification circuit in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 101:18069–18074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63:751–762 [DOI] [PubMed] [Google Scholar]
- 20.Hardy C. F., Sussel L., Shore D. 1992. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 6:801–814 [DOI] [PubMed] [Google Scholar]
- 21.Herskowitz J., Rine J., Strathern J. 1992. Mating-type determination and mating-type interconversion in Saccharomyces cerevisiae, vol. 2 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 22.Huang Y. 2002. Transcriptional silencing in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Nucleic Acids Res. 30:1465–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hull C. M., Johnson A. D. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271–1275 [DOI] [PubMed] [Google Scholar]
- 24.Hull C. M., Raisner R. M., Johnson A. D. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307–310 [DOI] [PubMed] [Google Scholar]
- 25.Imai S., Armstrong C. M., Kaeberlein M., Guarente L. 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800 [DOI] [PubMed] [Google Scholar]
- 26.Johnson A. D. 1995. Molecular mechanisms of cell-type determination in budding yeast. Curr. Opin. Genet. Dev. 5:552–558 [DOI] [PubMed] [Google Scholar]
- 27.Kimmerly W., Buchman A., Kornberg R., Rine J. 1988. Roles of two DNA-binding factors in replication, segregation and transcriptional repression mediated by a yeast silencer. EMBO J. 7:2241–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laroche T., Martin S. G., Gotta M., Gorham H. C., Pryde F. E., Louis E. J., Gasser S. M. 1998. Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr. Biol. 8:653–656 [DOI] [PubMed] [Google Scholar]
- 29.Leroy O., Gangneux J. P., Montravers P., Mira J. P., Gouin F., Sollet J. P., Carlet J., Reynes J., Rosenheim M., Regnier B., Lortholary O. 2009. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit. Care Med. 37:1612–1618 [DOI] [PubMed] [Google Scholar]
- 30.Magee B. B., Magee P. T. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310–313 [DOI] [PubMed] [Google Scholar]
- 31.Miller M. G., Johnson A. D. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 32.Muller H., Hennequin C., Gallaud J., Dujon B., Fairhead C. 2008. The asexual yeast Candida glabrata maintains distinct a and alpha haploid mating types. Eukaryot. Cell 7:848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen K., Heitman J. 2007. Sex and virulence of human pathogenic fungi. Adv. Genet. 57:143–173 [DOI] [PubMed] [Google Scholar]
- 34.O'Gorman C. M., Fuller H. T., Dyer P. S. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474 [DOI] [PubMed] [Google Scholar]
- 35.Patterson E. E., Fox C. A. 2008. The Ku complex in silencing the cryptic mating-type loci of Saccharomyces cerevisiae. Genetics 180:771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller M. A., Diekema D. J. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaller M. A., Messer S. A., Boyken L., Tendolkar S., Hollis R. J., Diekema D. J. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS antifungal surveillance program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillus L., Rine J. 1989. Epigenetic inheritance of transcriptional states in Saccharomyces cerevisiae. Cell 59:637–647 [DOI] [PubMed] [Google Scholar]
- 39.Richardson M., Lass-Florl C. 2008. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 14:5–24 [DOI] [PubMed] [Google Scholar]
- 40.Rivier D. H., Ekena J. L., Rine J. 1999. HMR-I is an origin of replication and a silencer in Saccharomyces cerevisiae. Genetics 151:521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Román E., Arana D. M., Nombela C., Alonso-Monge R., Pla J. 2007. MAP kinase pathways as regulators of fungal virulence. Trends Microbiol. 15:181–190 [DOI] [PubMed] [Google Scholar]
- 42.Rosas-Hernández L. L., Juarez-Reyes A., Arroyo-Helguera O. E., De Las Penas A., Pan S. J., Cormack B. P., Castano I. 2008. yku70/yku80 and Rif1 regulate silencing differentially at telomeres in Candida glabrata. Eukaryot. Cell 7:2168–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy R., Meier B., McAinsh A. D., Feldmann H. M., Jackson S. P. 2004. Separation-of-function mutants of yeast Ku80 reveal a Yku80p-Sir4p interaction involved in telomeric silencing. J. Biol. Chem. 279:86–94 [DOI] [PubMed] [Google Scholar]
- 44.Rusche L. N., Kirchmaier A. L., Rine J. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72:481–516 [DOI] [PubMed] [Google Scholar]
- 45.Sherman F., Fink G. R., Hicks J. B. 1986. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 46.Smith J. S., Brachmann C. B., Celic I., Kenna M. A., Muhammad S., Starai V. J., Avalos J. L., Escalante-Semerena J. C., Grubmeyer C., Wolberger C., Boeke J. D. 2000. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. U. S. A. 97:6658–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikantha T., Lachke S. A., Soll D. R. 2003. Three mating type-like loci in Candida glabrata. Eukaryot. Cell 2:328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sussel L., Vannier D., Shore D. 1993. Epigenetic switching of transcriptional states: cis- and trans-acting factors affecting establishment of silencing at the HMR locus in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:3919–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tham W. H., Zakian V. A. 2002. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene 21:512–521 [DOI] [PubMed] [Google Scholar]
- 50.Trick W. E., Fridkin S. K., Edwards J. R., Hajjeh R. A., Gaynes R. P. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627–630 [DOI] [PubMed] [Google Scholar]
- 51.Tsong A. E., Miller M. G., Raisner R. M., Johnson A. D. 2003. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell 115:389–399 [DOI] [PubMed] [Google Scholar]
- 52.Tsong A. E., Tuch B. B., Li H., Johnson A. D. 2006. Evolution of alternative transcriptional circuits with identical logic. Nature 443:415–420 [DOI] [PubMed] [Google Scholar]
- 53.Vandre C. L., Kamakaka R. T., Rivier D. H. 2008. The DNA end-binding protein Ku regulates silencing at the internal HML and HMR loci in Saccharomyces cerevisiae. Genetics 180:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P., Nichols C. B., Lengeler M. B., Cardenas M. E., Cox G. M., Perfect J. R., Heitman J. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1:257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wotton D., Shore D. 1997. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 11:748–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.