Abstract
The degree to which a water sample can potentially support the growth of human pathogens was evaluated. For this purpose, a pathogen growth potential (PGP) bioassay was developed based on the principles of conventional assimilable organic carbon (AOC) determination, but using pure cultures of selected pathogenic bacteria (Escherichia coli O157, Vibrio cholerae, or Pseudomonas aeruginosa) as the inoculum. We evaluated 19 water samples collected after different treatment steps from two drinking water production plants and a wastewater treatment plant and from ozone-treated river water. Each pathogen was batch grown to stationary phase in sterile water samples, and the concentration of cells produced was measured using flow cytometry. In addition, the fraction of AOC consumed by each pathogen was estimated. Pathogen growth did not correlate with dissolved organic carbon (DOC) concentration and correlated only weakly with the concentration of AOC. Furthermore, the three pathogens never grew to the same final concentration in any water sample, and the relative ratio of the cultures to each other was unique in each sample. These results suggest that the extent of pathogen growth is affected not only by the concentration but also by the composition of AOC. Through this bioassay, PGP can be included as a parameter in water treatment system design, control, and operation. Additionally, a multilevel concept that integrates the results from the bioassay into the bigger framework of pathogen growth in water is discussed. The proposed approach provides a first step for including pathogen growth into microbial risk assessment.
Pathogenic bacteria can survive and also grow in low-nutrient aquatic environments, such as surface waters or man-made water treatment systems (2, 17, 30). Studies on pathogen survival and/or die-off (including disinfection) in water are common, but little is known about the fundamental factors governing their growth in the environment (34, 35). Understanding the growth of pathogenic bacteria in aquatic ecosystems is essential for a holistic approach to microbial risk assessment as well as for improving drinking water treatment design and operation.
A key factor governing growth of all organisms is nutrient availability. All human pathogens are heterotrophs, utilizing organic compounds as their carbon and energy source. Natural organic matter in water comprises a broad spectrum of many different compounds; it is usually determined as a bulk parameter, such as dissolved organic carbon (DOC). Only a fraction (0.1 to 44%) of this DOC pool is readily available for bacterial growth (18, 33). This bioavailable fraction is quantified using bioassays, such as the biodegradable dissolved organic carbon (BDOC) assay (27) or the assimilable organic carbon (AOC) assay (31). Typically, AOC represents small molecules readily available for growth, whereas BDOC can also include larger molecular compounds, which require predegradation before they can be taken up by microbial cells. Results from both of these assays are commonly used as indicators for bacterial growth potential and have previously been associated with regrowth and biofilm formation in drinking water distribution systems (7, 20, 32).
Previous studies have pointed toward an apparent correlation between the concentration of AOC and the presence of enteric bacteria. For example, during two large surveys of drinking water treatment systems across North America, the occurrence (presence/absence) of coliform bacteria was found to be elevated above an AOC concentration of 100 μg liter−1 (4, 21). Other studies also found that AOC concentrations were directly correlated to growth of pathogenic bacteria (30, 34, 35). However, AOC is a bulk parameter, which includes many different substrates (e.g., amino acids, sugars, and fatty acids) readily available for heterotrophic growth. Hence, its composition can differ distinctly, and it is assumed that every aquatic environment carries a complex and unique “fingerprint” of utilizable organic carbon compounds (22). Moreover, the spectrum of growth-supporting substrates (carbon compounds) of individual bacterial strains is specific—a fact also used for the classification of bacteria for taxonomic purposes. This principle has been integrated into conventional AOC assays, where the specific substrate spectrum of different pure cultures can be used to quantify different types of compounds present in water (26, 33). The term “pathogenic bacteria” is a collective term for many different bacterial species that can all cause disease in humans but their individual substrate spectra are unique for each species. Thus, we have hypothesized that the total concentration of AOC alone is not a sufficient parameter for describing the growth potential of pathogenic bacteria; the quality of the available carbon compounds has to be considered as well.
There is no existing method that is capable of fractionating organic carbon in a way that allows for the quantification of individual compounds that support growth of specific pathogens. In this study, we have developed a pathogen growth potential (PGP) assay by combining the conventional AOC assay (31) with flow cytometric quantification of bacterial growth (11) and using pathogens as inocula. The PGP assay yields two main results, namely, (i) the extent of pathogen growth, and (ii) the relative fraction of AOC consumed by a pathogen. With this approach, we investigated the growth potential of three model pathogens from three different genera, namely, Escherichia coli O157, Vibrio cholerae O1, and Pseudomonas aeruginosa, in a broad range of water samples, differing considerably in their origin and quality.
MATERIALS AND METHODS
Bacterial strains used and precultivation.
The verotoxin-negative Escherichia coli O157 (NENT 2540-04; National Centre for Enteropathogenic Bacteria [NENT], Lucerne, Switzerland), Vibrio cholerae O1 Ogawa biotype El Tor (NENT 720-95), and Pseudomonas aeruginosa (BCCM/LMG 14073; University of Ghent, Belgium) were kept at −80°C before use. The cryocultures were streaked onto tryptic soy agar plates (Bio-Rad, Reinach, Switzerland) and incubated for 24 h at 37°C. One colony was transferred with a loop into Luria-Bertani (LB) broth (10 g liter−1 tryptone [Applichem, Darmstadt, Germany], diluted 10 times 5 g liter−1 yeast extract [Biolife, Milan, Italy], 10 g liter−1 NaCl [Fluka Chemie AG, Buchs, Switzerland]), and the culture was incubated overnight at 37°C. Cells from this overnight culture (initial concentration of 5 × 103 cells ml−1) were transferred into LB medium diluted 10,000 times, incubated for 4 days at 30°C until the cells reached stationary phase and subsequently used as inoculum. Fresh inocula were prepared for each experiment.
Preparation of carbon-free materials.
Carbon-free bottles (Schott, Mainz, Germany) and vials (Supelco, Bellefonte, PA) were prepared by the method of Hammes and Egli (11). In short, all glassware was first washed with a common detergent and thereafter rinsed three times with deionized water. Then the glassware was submerged overnight in 0.2 N HCl and subsequently rinsed with deionized water again and air dried. Finally, the bottles and vials were baked in a Muffel furnace at 500°C for at least 6 h. Teflon-coated screw caps for the glassware were washed and treated identically with acid (0.2 N HCl). Caps were thereafter soaked in a 10% sodium persulfate solution (60°C, 1 h), rinsed three times with deionized water, and finally air dried.
Water samples.
A list of the 19 water samples used in the present study is given in Table 1. Two different full-scale drinking water treatment plants (plants A and B), one wastewater treatment plant, and river water (Glatt River, Dübendorf, Switzerland) were included in this study. The river water was treated with ozone (5 mg liter−1) for different time periods, resulting in ozone exposures (ct values) of 0, 1, 5, and 10 mg min liter−1 and subsequently quenched with nitrite before use.
TABLE 1.
Parameters of the 19 different water samples analyzed in this studya
| Source and sample or treatmentb | DOCc (mg liter−1) | AOCd (μg liter−1) | pH | Salinity (μS) |
|---|---|---|---|---|
| DWTP A | ||||
| RW | 1.21 | 56 ± 4 | 7.79 | 296 |
| 1. Oz | 1.17 | 129 ± 4 | 7.8 | 290 |
| RSF | 0.97 | 66 ± 4 | 8.05 | 301 |
| 2. Oz | 0.94 | 65 ± 6 | 8.06 | 299 |
| GAC + SSF | 0.73 | 37 ± 2 | 7.9 | 297 |
| DWTP B | ||||
| RW | 20.9 | 395 ± 46 | 7.82 | 514 |
| 1. Oz | 21.4 | 520 ± 20 | 7.6 | 293 |
| RSF | 9.40 | 67 ± 15 | 6.78 | 344 |
| 2. Oz | 7.25 | 177 ± 2 | 6.57 | 337 |
| GAC | 6.45 | 66 ± 5 | 6.51 | 341 |
| Cl | 7.04 | 75 ± 22 | 6.71 | 341 |
| WWTP | ||||
| RW | 33.6 | 5,497 ± 730 | 7.16 | 649 |
| Bio | 3.56 | 349 ± 23 | 7.17 | 657 |
| Oz | 3.38 | 753 ± 38 | 7.14 | 623 |
| SSF | 3.15 | 424 ± 18 | 7.86 | 633 |
| Ozonated river water | ||||
| RW | 3.70 | 331 ± 61 | 8.22 | 415 |
| ct 1 | 3.70 | 439 ± 32 | 8.13 | 468 |
| ct 5 | 3.66 | 515 ± 15 | 8.16 | 438 |
| ct 10 | 3.71 | 525 ± 39 | 8.14 | 434 |
The water samples were always taken immediately after the treatment step or process.
The samples or steps in the treatment are as follows: for drinking water treatment plant A (DWTP A), untreated (raw) water (RW); the first ozonation (1. Oz), rapid sand filtration (RSF), the second ozonation (2. Oz), granular activated carbon filtration and slow sand filtration (GAC + SSF); for drinking water treatment plant B (DWTP B), untreated (raw) water (RW), the first ozonation (1. Oz), rapid sand filtration (RSF), the second ozonation (2. Oz), granular activated carbon filtration (GAC), and chlorination (Cl); for the wastewater treatment plant (WWTP), biological wastewater treatment (Bio), ozonation (Oz), slow sand filtration (SSF); for ozonated river water, untreated (raw) water (RW) and oxidation (degree of oxidation) (mg·min/liter) (ct 1 to 10).
DOC, dissolved organic carbon. The standard deviation for DOC measurements was always <5%.
AOC, assimilable organic carbon. The means ± standard deviations are shown.
Sampling and sterilization.
Water samples (500 ml) were collected in a carbon-free flask (Schott, Mainz, Germany) and closed with a carbon-free Teflon cap. To quench the remaining oxidant in waters treated with ozone or chlorine, nitrite (double the molar concentration of the oxidant) was added to the flask before sampling. The samples were then prepared by the method of Vital et al. (35), i.e., pasteurized (60°C for 30 min), filtered (0.2-μm Millex syringe filter [Millipore, Billerica, MA]), and aliquoted (15 ml) into 30-ml carbon-free glass vials (Supelco, Bellefonte, PA) in triplicate. Pasteurization was specifically included in the procedure, since it was shown that 0.2-μm filtration still allows the passage of a considerable fraction of bacteria present in freshwater (38). After pasteurization, samples were filtered in order to remove particles, which can interfere with the flow cytometric analysis. Syringe filters were prewashed with at least 200 ml of carbon-free water before use in order to eliminate residual organic carbon.
Pathogen growth potential (PGP) assay.
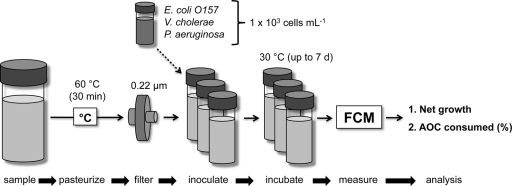
Each pathogen was precultivated as described above and separately inoculated into three vials, containing 15 ml of the sterile sample (starting concentration of 1 × 103 bacteria ml−1). Three extra vials served as a sterile control. Furthermore, AOC-free bottled mineral water (Evian, France) was treated in the same way and inoculated with the individual pathogens serving as a negative control to assess for possible AOC contamination. The inoculated water samples were then incubated at 30°C in the dark until stationary phase was reached (up to 7 days). A schematic presentation of the PGP assay is shown in Fig. 1. All vials were put into a sonication bath (Scherrer AG, Wil, Switzerland) (35 kHz, 10 min) before analysis in order to dislodge possible surface-attached bacteria into the liquid.
FIG. 1.
Schematic overview of the developed assay investigating the growth potential of pathogenic bacteria in different water samples. Abbreviations: FCM, flow cytometric measurement.
Flow cytometric measurements.
Flow cytometry was used to determine the final cell concentration and average biovolume after growth. A 10-μl aliquot of SYBR green stain (Molecular Probes, Basel, Switzerland), diluted 100 times in dimethyl sulfoxide (Fluka Chemie AG, Buchs, Switzerland), was added to 1 ml of a bacterial suspension and incubated for 15 min at room temperature in the dark before analysis. For outer membrane permeabilization, EDTA (pH 8) was added (5 mM final concentration) to the sample together with the stain (1). If a sample contained more than 106 cells ml−1, a dilution prior to the staining procedure was done. All samples were measured on a CyFlow Space flow cytometer (Partec, Münster, Germany) equipped with a 200-mW solid-state laser emitting a fixed wavelength of 488 nm and equipped with volumetric counting hardware. The trigger was set on the green fluorescence (520-nm) channel, and signals for total cell counting were collected on the combined 520-nm/630-nm (red fluorescence) dot plot. For cellular biovolume estimations, additional signals were collected on the combined 520-nm/side scatter (SSC) dot plot. An experimentally derived correlation factor was then used to determine the cellular biovolume (15). The quantification limit of the instrument was below 1,000 cells ml−1 with an average standard deviation of less than 5% (14).
AOC determination.
AOC was determined as described previously (11, 34). This bioassay is similar to the PGP assay presented here; a water sample is filtered, inoculated with bacteria from a natural bacterial community, and the final cell concentrations are measured after 3 days using flow cytometry (11). In this study, pasteurization of the water samples was included as well (as described above). The bacterial community used for AOC determination consisted of bacteria derived from river water (Glatt River, Dübendorf, Switzerland) and unchlorinated tap water (Dübendorf, Switzerland). The same community was used for all experiments.
DOC determination.
The total organic carbon (TOC) was measured based on the combustion catalytic oxidation/nondispersive infrared analysis (NDIR) method (TOC-V CPH; Shimadzu, Duisburg, Germany). Before analysis, samples were filtered (prewashed 0.2-μm Millex syringe filter [Millipore, Billerica, MA]), and the TOC was then referred to as DOC.
Data analysis and presentation. (i) “Growth potential”: net growth of pathogens.
The growth potential in a water sample was measured as the final cell concentration in stationary phase. A minimum increase of 1 × 103 cells ml−1 was required for a result to be judged positive. The presented results are expressed as net growth where the inoculum cell concentration was subtracted from the final cell concentrations of individual water samples.
(ii) “AOC quality”: fraction of consumed AOC by the individual pathogens.
AOC assays normally estimate the amount of AOC from the concentrations of cells produced. However, cells of the pathogenic bacteria used in this study are considerably larger than those of a bacterial community (15, 35). Hence, relating only the final cell concentration of the different pathogens to that of the bacterial community used for AOC determination (see below) would underestimate the growth of the pathogens (thus also AOC consumption) relative to the bacterial community. Multiplying the average cell volume (μm3 cell−1) derived from flow cytometric analysis with the final cell concentration yielded the estimated total biovolume (μm3) for individual cultures (15). We then calculated the percentage of AOC available for the pathogens by dividing the estimated total biovolume of each pathogen with the estimated total biovolume of the bacterial community used for AOC determination. Where the obtained value exceeded 100% (4 out of 59 samples), we display this point as 100% in the figures.
Statistics.
Correlations were derived from linear regression analysis and are expressed as R2 values. A correlation was regarded as significant when the P value was below 0.01. All correlations shown in Table 2 are also displayed as figures (Fig. S2 to S4) in the supplemental material.
TABLE 2.
Correlation (R2) between the growth potential of the individual pathogens and the DOC and AOC concentration for the 19 water samples analyzed in this studya
| Parameter or pathogen |
R2 value for: |
||
|---|---|---|---|
| P. aeruginosa | V. cholerae | E. coli O157 | |
| DOC | 0.03 | 0.12 | 0.24 |
| AOC | 0.51* | 0.88* | 0.55* |
| E. coli O157 | 0.47* | 0.42* | |
| V. cholerae | 0.29 | ||
| P. aeruginosa | |||
Due to the very high concentration of assimilable organic carbon (AOC) and dissolved organic carbon (DOC), the results for raw wastewater were not included in the calculation of correlations. The correlations shown here are also shown in Fig. S2 to S4 in the supplemental material. Significant correlations (P < 0.01) are indicated by an asterisk.
RESULTS
We modified an existing AOC assay (11) to assess the growth potential of selected pathogenic bacteria in any water sample (Fig. 1). In this bioassay, a water sample is tested with respect to (i) the net growth of a pathogenic bacterium, measured as an increase in cell concentration, representing the “growth potential,” and (ii) the fraction of AOC consumed by a pathogen, representing the “AOC quality.” With respect to the latter, “AOC quality” is not a compositional analysis of individual AOC compounds but an expression of the accessibility of AOC for a specific organism.
General overview of all data.
We analyzed 19 water samples from different locations. The DOC and AOC concentrations of the different samples varied considerably, ranging between 0.73 and 33.6 mg liter−1 of DOC and 37 to 5,497 μg liter−1 of AOC, respectively (Table 1), with AOC accounting for 0.7% to up to 22% of the DOC. Overall, no significant correlation (R2 = 0.08; P = 0.29; see Fig. S1 in the supplemental material) between AOC and DOC was observed, which reaffirms that mere DOC analysis is insufficient evidence of the general growth potential of a water sample. Looking at all results in general, the growth potential of E. coli O157 ranged from 0 to 2.87 × 106 cells ml−1 and the growth potential for V. cholerae ranged from 0 to 1.35 × 107 cells ml−1, whereas P. aeruginosa grew in all samples tested and its growth potential varied from 4 × 103 to 2.3 × 106 cells ml−1 (Fig. 2 to 5). There was no significant correlation (P value always higher than 0.01) between DOC concentration and net growth of the pure cultures (Table 2; see Fig. S2 in the supplemental material), whereas the concentration of AOC, reflecting the fraction of nutrients readily available for heterotrophic growth, could explain the growth of the different pathogens better (Table 2; see Fig. S3 in the supplemental material). The correlations (R2) between the growth potential and AOC ranged from 0.51 for P. aeruginosa up to 0.88 for V. cholerae (Table 2; see Fig. S3 in the supplemental material). From this, one can conclude that in general, elevated concentrations of AOC also support enhanced pathogen growth.
FIG. 2.
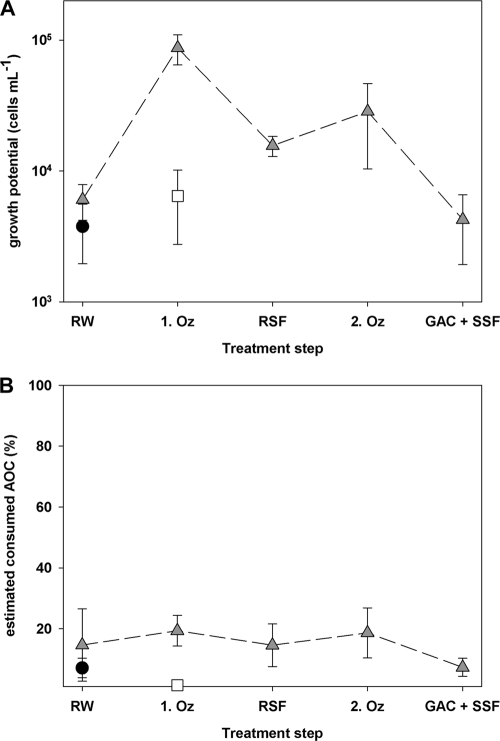
Growth of the three pathogens, E. coli O157 (white squares), V. cholerae (black circles), and P. aeruginosa (gray triangles), in water samples taken after the individual treatment steps from drinking water treatment plant A. The growth potential, i.e., net grown cells (A), as well as the fraction of estimated consumed assimilable organic carbon (AOC) for each pathogen (B) are shown. The samples or treatment steps are shown in order from left to right as follows: RW, untreated (raw) water; 1. Oz, the first ozonation; RSF, rapid sand filtration; 2. Oz, the second ozonation; GAC + SSF, granular activated carbon filtration and slow sand filtration. The error bars indicate the standard deviations for three replicate samples.
However, it was only partly possible to predict the growth potential of the individual pathogens using the concentration of AOC. The data scattering along the regression lines was considerable, with notable outliers (see Fig. S3 in the supplemental material). Furthermore, correlations (R2) between the pure cultures with each other were poor, ranging from only 0.29 (V. cholerae versus P. aeruginosa) to 0.47 (E. coli O157 versus P. aeruginosa) (Table 2; see Fig. S4 in the supplemental material), and the pathogen displaying the highest growth potential was different for different samples. The three pathogens never grew to the same concentration in any water sample, and the relative ratio of the cultures to each other was unique in each sample (Fig. 2 to 5). Additionally, we also investigated the “quality” of AOC in the different water samples by estimating the fraction of AOC consumed by a pathogen. For E. coli O157 and V. cholerae, the values obtained ranged from 1% up to 100%, whereas P. aeruginosa consumed between 7% and 71% (see Fig. S5 in the supplemental material). This further illustrates the differing accessibility of AOC for pathogen growth. All these results imply that the pathogens responded dissimilarly in the different waters tested and that not only the concentration but also the “quality” of AOC are key factors controlling their growth.
PGP in water samples from drinking water treatment plant A.
The treatment train of drinking water treatment plant A is composed of two sequential ozonation-biofiltration steps (Fig. 2). Overall, the PGP in water samples from this plant was very low, as were AOC concentrations. Growth of V. cholerae was detected only in the raw water samples, and no other water sample allowed proliferation of this pathogen, whereas E. coli O157 grew only in the water sample collected after the first ozonation treatment (Fig. 2A). In contrast, P. aeruginosa multiplied in all samples. The two ozonation steps resulted in increased growth of P. aeruginosa, whereas the growth potential of this pathogen was markedly decreased after slow sand filtration and very low (net growth of only 4 × 103 cells ml−1) in the finished water. We observed a similar pattern for the AOC concentration (Table 1). The estimated fraction of AOC consumed by P. aeruginosa was, however, always below 20%, and hence, only a small fraction of the AOC was always available for the pathogen (Fig. 2B).
PGP in water samples from drinking water treatment plant B.
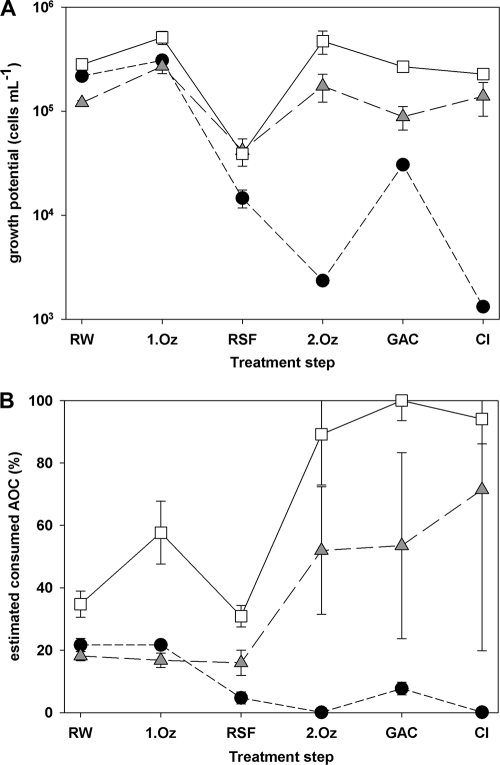
The treatment train of the second drinking water treatment plant is similar to that of plant A and includes two sequential ozonation-biofiltration steps as well (Fig. 3). However, it includes no slow sand filtration step, and the finished water is chlorinated at the end of the treatment as a final disinfection step. The water quality was, however, distinctly different in comparison to plant A: DOC and AOC concentrations were considerably higher, as was the growth for all three pathogens (Table 1 and Fig. 3A). The growth potential of E. coli O157 and of P. aeruginosa displayed the same trend, i.e., more growth in the samples after ozonation, followed by a decline in the growth potential after biofiltration. Interestingly, although the total AOC concentration decreased considerably through the treatment process, the growth potential of E. coli O157 and of P. aeruginosa in samples of the finished water (2.27 × 105 cells ml−1 and 1.39 × 105 cells ml−1, respectively) were similar to those observed in the raw water (2.81 × 105 cells ml−1 and 1.21 × 105 cells ml−1, respectively). This observation is also illustrated in Fig. 3B, where the accessed fraction of AOC for the pathogens increased during the water treatment steps and displayed very high values, i.e., 94% for E. coli O157 and 71% for P. aeruginosa in the finished water. V. cholerae displayed a distinctly different pattern. After a slight increase in growth after the first ozonation step, its growth potential sharply decreased after rapid sand filtration and second ozonation. Finally, only a little growth potential of V. cholerae was detected in the finished water (Fig. 3A).
FIG. 3.
Growth of the three pathogens, E. coli O157 (white squares), V. cholerae (black circles), and P. aeruginosa (gray triangles), in samples taken after the individual treatment steps from drinking water treatment plant B. The growth potential, i.e., net grown cells (A), as well as the fraction of estimated consumed assimilable organic carbon (AOC) for each pathogen (B) are shown. The samples or treatment steps are shown in order from left to right as follows: RW, untreated (raw) water; 1.Oz, the first ozonation; RSF, rapid sand filtration; 2.Oz, the second ozonation; GAC, granular activated carbon filtration; Cl, chlorination. The error bars indicate the standard deviations for three replicate samples.
PGP in water samples from a wastewater treatment plant.
In the wastewater treatment plant, the effluent of the conventional biological treatment is subjected to additional treatment consisting of a combined ozonation-biofiltration step before it is finally fed into a river. For all three pathogens, the raw wastewater, which contained a high AOC concentration (5.5 mg liter−1), promoted the highest growth of all waters tested in this study (Fig. 4 A). In samples after biological treatment, the growth potential was reduced by 93% for E. coli O157 and by 98% for V. cholerae and P. aeruginosa. The following ozonation-biofiltration step resulted in a similar pattern as observed in the two drinking water treatment plants, i.e., ozonation generated AOC and led to an increased growth potential of E. coli O157 and P. aeruginosa, which was followed by reduced growth after biofiltration (Fig. 4A). In this case, the growth potential of V. cholerae displayed a pattern similar to that of the two other pathogens during treatment. The estimated AOC consumption differed distinctly between the individual pure cultures; the data suggest that V. cholerae consumed the most AOC, followed by E. coli O157 and P. aeruginosa (Fig. 4B).
FIG. 4.
Growth of the three pathogens, E. coli O157 (white squares), V. cholerae (black circles), and P. aeruginosa (gray triangles), in samples taken after the individual treatment steps from a wastewater water treatment plant. The growth potential, i.e., net grown cells (A), as well as the fraction of estimated consumed assimilable organic carbon (AOC) for each pathogen (B) are shown. The samples or treatment steps are shown in order from left to right as follows: RW, untreated (raw) water; Bio, biological treatment; Oz, ozonation; SSF, slow sand filtration. The error bars indicate the standard deviations for three replicate samples.
PGP in ozonated river water.
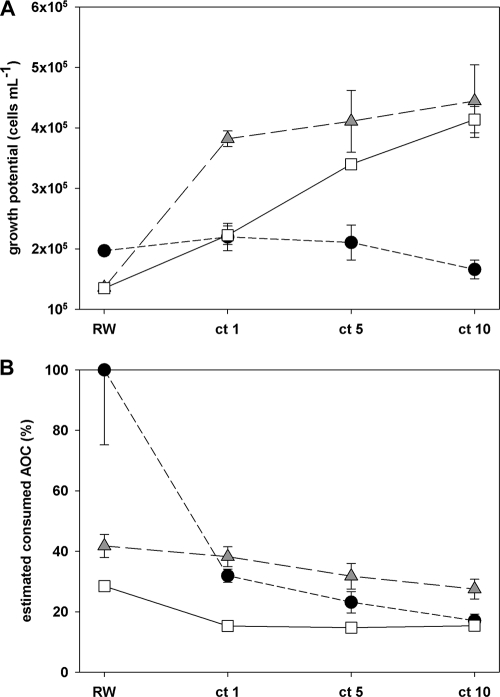
Ozonation is a key step in the water treatment plants studied above. Since it is a common method to treat water of different types, we investigated the effect of ozonation on the growth of the three pathogens in detail. As observed before (Fig. 2A, 3A, and 4A), the AOC concentration increased with increasing ozone exposure, and the growth potential of E. coli O157 and of P. aeruginosa was also positively affected by ozonation; both pathogens produced around three times more cells in the most oxidized water (ct = 10) compared to untreated river water (Fig. 5 A). However, the estimated AOC consumption of the two pathogens showed a decrease with increasing oxidation, suggesting that the nutrient availability for the pathogens was considerably altered during the treatment (Fig. 5B). The data presented in Fig. 3 and 4 indicate that V. cholerae followed a pattern different from those of the other pathogens when it was grown in oxidized water. Also in this experiment, the growth potential of this pathogen was hardly affected by the oxidation treatment (Fig. 5A).
FIG. 5.
Growth of the three pathogens, E. coli O157 (white squares), V. cholerae (black circles), and P. aeruginosa (gray triangles), in river water exposed to ozone. The growth potential, i.e., net grown cells (A), as well as the fraction of estimated consumed assimilable organic carbon (AOC) for each pathogen (B) are shown. RW, untreated (raw) river water; ct 1 to ct 10, degree of oxidation (mg·min/liter). Error bars indicate the standard deviations for three replicate samples.
DISCUSSION
Despite the holistic approaches of modern day risk assessment, growth of pathogenic bacteria is only vaguely considered (23). This risk component is usually restricted to tables grouping pathogens into those that may grow in water and those that do not (39). The neglect of pathogen growth in risk assessment can partly be explained by the limited knowledge of the factors governing their growth (35). It has, however, been shown previously that pathogenic bacteria, including enteric pathogens, can grow at low nutrient concentrations in waters used for drinking or personal hygiene (3, 17, 29, 30, 34, 35). This warrants further investigation on this topic, and we have, therefore, developed a bioassay specifically assessing the pathogen growth potential (PGP) in water.
Nearly all treatment processes affect the carbon pool of water with respect to both concentration and composition. Despite exciting recent advances in DOC fractionation (8), an accurate and quantitative method for the determination of all individual carbon compounds in natural waters and in particular of those available for certain bacterial strains, such as pathogens, is not available. Thus, an analysis of the PGP in water as presented here provides researchers and water utilities with an additional decision making tool for optimum design and operation of water treatment systems in order to minimize the risk of pathogen growth. For example, ozonation is used in (drinking) water treatment for the oxidation of micropollutants and for disinfection (7, 36, 37). It is commonly known that oxidation processes generate AOC and BDOC, thus increasing the overall bacterial growth potential of a water (12, 13, 36) (Table 1). This increase in AOC also enhanced the growth potential of E. coli O157 and P. aeruginosa after ozonation (Fig. 2, 3, 4, and 5), albeit to a different extent in different waters. Ozonation is, however, an effective disinfection step against pathogenic bacteria (16, 37), and our work should not be understood as a suggestion to omit this process to improve treatment efficiency and lower the risk of pathogen growth. Rather, the presented PGP assay can be used to characterize and optimize the ozonation or subsequent processes in terms of the trade-off between maximum disinfection and minimum formation of AOC available for pathogens. Moreover, to reduce the risk of pathogen growth, our results suggest that a biofiltration step should always directly follow this oxidation step, as done in the treatment trains analyzed here and also described previously (5, 28). All biofiltration steps analyzed in this study (biological wastewater treatment, rapid sand filtration, granular activated carbon filtration, and slow sand filtration) reduced AOC concentrations and the growth potential of E. coli O157 and P. aeruginosa. The PGP assay can, therefore, be used to evaluate, optimize, and compare different biofilter systems. Interestingly, V. cholerae often responded uniquely in samples collected after oxidation steps. It appears that during oxidation the quality of carbonaceous compounds in the water is altered in such a way that the potential risk for growth of this pathogen is in fact reduced, although the concentration of AOC increases (Fig. 2, 3, and 5). This illustrates the necessity of testing the growth potential for different pathogens separately. Another good example demonstrating the advantage of individual testing is drinking water treatment plant B (Fig. 3). Although a low concentration of AOC (75 μg liter−1) was recorded in the finished water, the growth potential of E. coli O157 and of P. aeruginosa was disproportionally high. The supporting data presented in Fig. 3B suggest that the quality of AOC was favoring the growth of these two pathogens. Interestingly, a previous study demonstrated that E. coli can indeed grow in biofilms of this particular drinking water treatment system, contributing up to 0.1% of the total bacterial population (17).
In this study, we used the three pathogenic bacteria tested previously for their growth properties at low nutrient concentration (34, 35). However, the PGP assay is not restricted to these organisms but is essentially applicable to any bacterium of interest. For example, Rice et al. (25) used a similar approach to analyze coliform growth in water samples. However, for some heterotrophic bacterial species, there are factors other than the availability of AOC that affect their growth potential. For example, Legionella pneumophila requires cysteine and iron for proliferation (6); such specific physiological properties should thus be considered when designing a PGP assay and interpreting the results.
Several other factors also determine the growth of pathogenic bacteria in water, along with nutrient availability. Integration of the developed PGP assay into a bigger framework of pathogen growth in water is outlined in Fig. 6. In this flow chart or scheme, we identified three main levels to be considered, namely: (i) the presence of pathogens, (ii) an environment supporting their growth, and (iii) competition with the indigenous microbial flora for AOC. First, the starting concentration of the pathogen(s) present (or contaminating) has to be known, since growth cannot occur in their absence. The second level pertains to whether the physicochemical environment allows growth. This information is obtained directly from the results of the PGP assay. For example, in finished water samples from drinking water treatment plant A (Fig. 2), no growth of E. coli O157 and V. cholerae was detected, and hence, their growth potential can be considered zero. Moreover, the PGP assay does not consider the possible presence of disinfection residuals and in situ temperatures of the water. It is well documented that pathogens require a certain “minimum temperature” for growth that is normally in the range between 10 and 20°C, depending on the bacterial species (24). Therefore, if the PGP assay yields a positive result but the actual water temperature is below the “minimum temperature” or disinfection residuals are present at concentrations inhibiting bacterial proliferation, the risk of growth is zero as well. If all three parameters (PGP assay, temperature, and disinfection residuals) allow for growth, a risk of pathogen multiplication in the system is automatically given. In other words, if all requirements for level two are fulfilled, then there is a risk of growth (Fig. 6). At the third level, the extent of pathogen growth in a given water sample is of interest. The results of the PGP assay can serve as a first indication; the higher the growth potential, the more growth can be expected. Since pathogens, however, normally grow together with the indigenous bacterial flora, and not in pure culture, the extent of their growth is strongly influenced by competition for AOC (level three in Fig. 6). Competition is a complex interplay controlled by several factors, such as the concentrations of competing species, their stoichiometric and kinetic properties, and several physicochemical parameters as well as the nutrient supply dynamics (9, 10). Unfortunately, the current state of knowledge on the mechanisms governing competition of bacteria in natural waters is very limited. However, new methods measuring bacteria at low cell concentrations at a high throughput are rapidly providing new information that are shifting existing paradigms regarding pathogen growth in water (19, 34, 35). Such data combined with mathematical modeling could be used in the future to estimate the actual degree of pathogen growth in a given water sample and to investigate potential risk scenarios of interest in silico.
FIG. 6.

Schematic overview of the three different levels that are important in assessing the risk of pathogen growth in water. For explanation, see the discussion of Fig. 6 in the Discussion section. PGP, pathogen growth potential.
Conclusions.
A novel bioassay was developed to investigate the potential of any water sample to support growth of pathogenic microorganisms. The data from this study showed unique growth behavior of the different pathogens in each water sample, relative to each sample and to each other. Furthermore, AOC concentrations could explain their net growth only to some extent, indicating that the quality/composition of AOC is critical for pathogen growth. The developed pathogen growth potential (PGP) assay can be used as a screening tool complementary to conventional AOC assays in order to compare different water samples or treatment steps. The results of the PGP assay can also be incorporated into a multilevel concept assessing the risk of pathogen growth in water.
Supplementary Material
Acknowledgments
Research on this project was financially supported by the 6th Framework European integrated project TECHNEAU (018320).
We specifically acknowledge M. Ramseier for assistance with the ozonation experiments.
Footnotes
Published ahead of print on 6 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Berney, M., F. Hammes, F. Bosshard, H. U. Weilenmann, and T. Egli. 2007. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73:3283-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camper, A. K., M. W. LeChevallier, S. C. Broadaway, and G. A. McFeters. 1985. Growth and persistence of pathogens on granular activated carbon filters. Appl. Environ. Microbiol. 50:1378-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camper, A. K., G. A. McFeters, W. G. Characklis, and W. L. Jones. 1991. Growth kinetics of coliform bacteria under conditions relevant to drinking water distribution systems. Appl. Environ. Microbiol. 57:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camper, A. K., P. W. Butterfield, B. D. Ellis, W. L. Jones, P. M. Huck, W. B. Anderson, C. Volk, N. J. Welch, and M. W. LeChevallier. 2000. Investigation of the biological stability of water in treatment plants and distribution systems. American Water Works Association Research Foundation, Denver, CO.
- 5.Chen, C., X. Zhang, W. He, W. Lu, and H. Han. 2007. Comparison of seven kinds of drinking water treatment processes to enhance organic material removal: a pilot test. Sci. Total Environ. 382:93-102. [DOI] [PubMed] [Google Scholar]
- 6.Devos, L., N. Boon, and W. Verstraete. 2005. Legionella pneumophila in the environment: occurrence of a fastidious bacterium in oligotrophic conditions. Rev. Environ. Sci. Biotechnol. 4:61-74. [Google Scholar]
- 7.Escobar, I. C., A. A. Randall, and J. S. Taylor. 2001. Bacterial growth in distribution systems: effect of assimilable organic carbon and biodegradable dissolved organic carbon (BDOC). Environ. Sci. Technol. 35:3442-3447. [DOI] [PubMed] [Google Scholar]
- 8.Frazier, S. W., L. A. Kaplan, and P. G. Hatcher. 2005. Molecular characterization of biodegradable dissolved organic matter using bioreactors and [12C/13C] tetramethylammonium hydroxide thermochemolysis GC-MS. Environ. Sci. Technol. 39:1479-1491. [DOI] [PubMed] [Google Scholar]
- 9.Fredrickson, A. G., and G. Stephanopoulos. 1981. Microbial competition. Science 213:972-979. [DOI] [PubMed] [Google Scholar]
- 10.Gottschal, J. C. 1993. Growth kinetics and competition-some contemporary comments. Antonie Van Leeuwenhoek 63:299-313. [DOI] [PubMed] [Google Scholar]
- 11.Hammes, F. A., and T. Egli. 2005. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 39:3289-3294. [DOI] [PubMed] [Google Scholar]
- 12.Hammes, F., E. Salhi, O. Koster, H. P. Kaiser, T. Egli, and U. von Gunten. 2006. Mechanistic and kinetic evaluation of organic disinfection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res. 40:2275-2286. [DOI] [PubMed] [Google Scholar]
- 13.Hammes, F., S. Meylan, E. Salhi, O. Koster, T. Egli, and U. von Gunten. 2007. Formation of assimilable organic carbon (AOC) and specific natural organic matter (NOM) fractions during ozonation of phytoplankton. Water Res. 41:1447-1454. [DOI] [PubMed] [Google Scholar]
- 14.Hammes, F., M. Berney, Y. Wang, M. Vital, O. Koster, and T. Egli. 2008. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 42:269-277. [DOI] [PubMed] [Google Scholar]
- 15.Hammes, F., F. Goldschmidt, Y. Wang, M. Vital, and T. Egli. 2010. Sensitive measurement and interpretation of bacterial adenosine tri-phosphate (ATP) in freshwater environments. Water Res. 44:3915-3923. [DOI] [PubMed] [Google Scholar]
- 16.Hunt, N. K., and B. J. Marinas. 1997. Kinetics of Escherichia coli inactivation with ozone. Water Res. 31:1355-1362. [Google Scholar]
- 17.Juhna, T., D. Birzniece, S. Larsson, D. Zulenkovs, A. Sharipo, N. F. Azevedo, F. Menard-Szczebara, S. Castagnet, C. Feliers, and C. W. Keevil. 2007. Detection of Escherichia coli in biofilms from pipe samples and coupons in drinking water distribution networks. Appl. Environ. Microbiol. 73:7456-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan, L. A., T. L. Bott, and D. J. Reasoner. 1993. Evaluation and simplification of the assimilable organic carbon nutrient bioassay for bacterial growth in drinking water. Appl. Environ. Microbiol. 59:1532-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirschner, A. K., J. Schlesinger, A. H. Farnleitner, R. Hornek, B. Suss, B. Golda, A. Herzig, and B. Reitner. 2008. Rapid growth of planktonic Vibrio cholerae non-O1/non-O139 strains in a large alkaline lake in Austria: dependence on temperature and dissolved organic carbon quality. Appl. Environ. Microbiol. 74:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeChevallier, M. W., W. Schulz, and R. G. Lee. 1991. Bacterial nutrients in drinking water. Appl. Environ. Microbiol. 57:857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeChevallier, M. W., N. J. Welch, and D. B. Smith. 1996. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 62:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Münster, U. 1993. Concentrations and fluxes of organic carbon substrates in the aquatic environment. Antonie Van Leeuwenhoek 63:243-274. [DOI] [PubMed] [Google Scholar]
- 23.Organisation for Economic Co-operation and Development-World Health Organization. 2003. Assessing microbial safety of drinking water: improving approaches and methods. OECD Publishing, Paris, France.
- 24.Raghubeer, E. V., and J. R. Matches. 1990. Temperature range for growth of Escherichia coli serotype O157:H7 and selected coliforms in E. coli medium. J. Clin. Microbiol. 28:803-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice, E. W., P. V. Scarpino, G. S. Logsdon, D. J. Reasoner, P. J. Mason, and J. C. Blannon. 1990. Bioassay procedure for predicting coliform bacterial growth in drinking water. Environ. Technol. 7:821-828. [Google Scholar]
- 26.Sack, E. L., P. W. van der Wielen, and D. van der Kooij. 2010. Utilization of oligo- and polysaccharides at microgram-per-litre levels in freshwater by Flavobacterium johnsoniae. J. Appl. Microbiol. 108:1430-1440. [DOI] [PubMed] [Google Scholar]
- 27.Servais, P., G. Billen, and M. C. Hascoet. 1987. Determination of the biodegradable fraction of dissolved organic matter in waters. Water Res. 21:445-450. [Google Scholar]
- 28.Siddiqui, S. S., L. A. Gary, and B. D. Murphy. 1997. Ozone enhanced removal of natural organic matter from drinking water sources. Water Res. 31:3098-3106. [Google Scholar]
- 29.Szewzyk, U., R. Szewzyk, W. Manz, and K.-H. Schleifer. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81-127. [DOI] [PubMed] [Google Scholar]
- 30.Torvinen, E., S. Suomalainen, M. J. Lehtola, I. T. Miettinen, O. Zacheus, L. Paulin, M. L. Katila, and P. J. Martikainen. 2004. Mycobacteria in water and loose deposits of drinking water distribution systems in Finland. Appl. Environ. Microbiol. 70:1973-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Kooij, D., A. Visser, and W. A. M. Hijnen. 1982. Determination of easily assimilable organic carbon in drinking water. J. Am. Water Works Assoc. 74:540-545. [Google Scholar]
- 32.Van der Kooij, D. 1992. Assimilable organic carbon as indicator of bacterial regrowth. J. Am. Water Works Assoc. 84:57-65. [Google Scholar]
- 33.Van der Kooij, D. 2002. Assimilable organic carbon (AOC) in treated water: determination and significance, p. 312-327. In G. Bitton (ed.), Encyclopedia of environmental microbiology. John Wiley & Sons, Hoboken, NJ.
- 34.Vital, M., H. P. Fuchslin, F. Hammes, and T. Egli. 2007. Growth of Vibrio cholerae O1 Ogawa Eltor in freshwater. Microbiology 153:1993-2001. [DOI] [PubMed] [Google Scholar]
- 35.Vital, M., F. Hammes, and T. Egli. 2008. Escherichia coli O157 can grow in natural freshwater at low carbon concentrations. Environ. Microbiol. 10:2387-2396. [DOI] [PubMed] [Google Scholar]
- 36.Von Gunten, U. 2003. Ozonation of drinking water: part I. Oxidation kinetics and product formation. Water Res. 37:1443-1467. [DOI] [PubMed] [Google Scholar]
- 37.Von Gunten, U. 2003. Ozonation of drinking water: part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 37:1469-1487. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Y., F. Hammes, N. Boon, and T. Egli. 2007. Micro-filterable bacteria are omnipresent in natural freshwater. Environ. Sci. Technol. 41:7080-7086. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. 2008. Guidelines for drinking-water quality, 3rd ed. Volume 1. Recommendations. World Health Organization, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.