Abstract
Bacillus subtilis and Bacillus licheniformis are widely used for the large-scale industrial production of proteins. These strains can efficiently secrete proteins into the culture medium using the general secretion (Sec) pathway. A characteristic feature of all secreted proteins is their N-terminal signal peptides, which are recognized by the secretion machinery. Here, we have studied the production of an industrially important secreted protease, namely, subtilisin BPN′ from Bacillus amyloliquefaciens. One hundred seventy-three signal peptides originating from B. subtilis and 220 signal peptides from the B. licheniformis type strain were fused to this secretion target and expressed in B. subtilis, and the resulting library was analyzed by high-throughput screening for extracellular proteolytic activity. We have identified a number of signal peptides originating from both organisms which produced significantly increased yield of the secreted protease. Interestingly, we observed that levels of extracellular protease were improved not only in B. subtilis, which was used as the screening host, but also in two different B. licheniformis strains. To date, it is impossible to predict which signal peptide will result in better secretion and thus an improved yield of a given extracellular target protein. Our data show that screening a library consisting of homologous and heterologous signal peptides fused to a target protein can identify more-effective signal peptides, resulting in improved protein export not only in the original screening host but also in different production strains.
Gram-positive bacteria of the genus Bacillus are industrially well-established microorganisms for the production of extracellular proteins. Due to the availability of relatively cheap large-scale production systems combined with the ability of bacteria to secrete up to 20 to 25 g/liter of a target protein into the growth medium, about 60% of commercially available enzymes are presently produced in Bacillus species (14, 28).
The closely related species Bacillus subtilis and Bacillus licheniformis are widely used as production hosts on an industrial scale, and, in contrast to the well-known production species Escherichia coli, they are free of endotoxin and have GRAS (generally regarded as safe) status. The complete genome sequences of strains B. subtilis 168 (1, 18) and B. licheniformis DSM13 (isogenic to ATCC 14580) (26, 32) are available, greatly facilitating the construction of improved production strains.
The Sec pathway constitutes the main secretion pathway in B. subtilis and B. licheniformis. Proteins secreted via the Sec pathway are initially synthesized with an N-terminal hydrophobic signal peptide (SP) consisting of a positively charged N domain followed by a longer, hydrophobic H domain and a C domain consisting of three amino acids which form the signal peptidase recognition site (35). Targeting of a secreted protein to the membrane, the translocation process itself, and subsequent processing by a signal peptidase represent the major bottlenecks for efficient translocation and thus production of heterologous proteins (20).
SPs play a crucial role in the efficient translocation of secretory proteins by the Sec machinery. They interact with the SecA protein, the signal recognition particle (SRP), and the signal peptidase (16, 30). The interaction between the SP and the mature protein is known to influence protein export as well (9, 16, 17). Therefore, the choice of an efficient signal peptide for any given target protein is of utmost importance, and several approaches to identify efficient SPs for different target proteins were taken (2, 4, 6, 15, 21, 38).
Among the huge number of enzymes produced on a large scale by Bacillus species, proteases are important for diverse industrial applications (25), with subtilisins being used as additives in household detergents (22, 28). We have chosen as a model for secretion optimization the subtilisin “Bacillus protease novo type” (BPN′) from Bacillus amyloliquefaciens ATCC 23844, a well-known enzyme belonging to the alkaline serine proteases (5).
We present a novel approach to improve the extracellular production of this protease using different Bacillus host strains. A total number of 393 SPs were fused to the target protein, with 173 SPs originating from B. subtilis (termed homologous SPs) and 220 SPs from B. licheniformis DSM13 (termed heterologous SPs). The fusion constructs were cloned and expressed in B. subtilis, and the resulting library was screened for extracellular protease activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cloning.
The bacterial strains and plasmids used in this study are listed in Table 1. B. subtilis TEB1030 was used as an expression host and for cloning and plasmid preparation. B. licheniformis H402 and MW3 were used as expression hosts. All DNA manipulations followed standard procedures (27).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| B. subtilis TEB1030 | trpC2 his nprE aprE bpf ispI lipA lipB | 13 |
| B. licheniformis DSM13 | Wild type (isogenic to ATCC 14580) | DSMZ, Germany |
| B. licheniformis MW3 | B. licheniformis DSM13 (hsdR1 hsdR2) | 37 |
| B. licheniformis H402 | Henkel AG & Co. KGaA, Germany | |
| B. amyloliquefaciens ATCC 23844 | Wild type | ATCC |
| Plasmids | ||
| pBSMul5 | Bacillus vector PHpaII; repB Kmr | This study |
| pBSMul5-BPN | pBSMul5 containing 1,059-bp EcoRI-BamHI fragment of the BPN′ coding sequence from B. amyloliquefaciens ATCC 23844 without signal sequence | This study |
Media, growth conditions, and preparation of culture supernatants.
Bacterial strains were grown in LB medium (5 g yeast extract liter−1, 10 g tryptone liter−1, 10 g NaCl liter−1, pH 7) at 37°C supplemented with 50 μg/ml kanamycin. Protease production was monitored on agar plates containing 1% (vol/vol) skim milk. For screening, the cultures were grown for 20 h at 37°C in deep-well microtiter plates (96 wells, 1 ml LB medium per well; Greiner Bio-One, Frickenhausen, Germany) using a microplate shaker (600 rpm; TiMix 5; Edmund Bühler GmbH, Hechingen, Germany). Culture supernatants were prepared by centrifugation (20 min, 3,200 × g, 4°C) and used immediately to determine proteolytic activity. Each strain was cultured 3-fold in parallel in 3 different wells. Cell-free culture supernatants were prepared by microfiltration (membrane filter, cellulose acetate/nitrate, pore size, 0.22 μm) and used for Western blot analysis. A comparison of proteolytic activities in culture supernatants prepared with and without microfiltration did not reveal any differences, thus indicating that the proteolytic activities could be assigned to cell-free, i.e., secreted, proteases.
High-cell-density fermentation of B. subtilis.
Fed-batch fermentations were performed for 48 h with a Labfors fermentor (volume: 7.5 liter; Infors, Germany) using a high-cell-density cultivation process developed for B. subtilis (24). The dissolved oxygen level was set to 30 to 40%, and glucose was fed from a stock solution (500 g glucose/liter). The pH was adjusted by addition of 10% NH3 (wt/vol) or 4 N phosphoric acid.
Transformation of DNA.
B. subtilis was transformed by protoplast transformation essentially as described by Chang and Cohen (7). B. licheniformis was transformed by electroporation (3, 31).
PCR conditions and amplification of B. licheniformis DSM13 signal peptides.
Amplification of DNA was performed under standard PCR conditions in a 50-μl reaction volume with 1 pmol of each primer, 1 U of Phusion high-fidelity polymerase (New England Biolabs), and 100 ng of template DNA. Amplified DNA was purified after gel electrophoresis using the Qiaex II gel extraction kit (Qiagen, Germany).
Signal peptide-encoding DNA sequences for B. licheniformis DSM13 were predicted from the genome sequence based on the presence of a signal peptidase type I (SPase I) recognition site (34). Two hundred twenty SP-encoding fragments were amplified and used for screening in combination with 173 SPs previously amplified from B. subtilis (4).
Cloning strategy and construction of the controls.
SP libraries were cloned into pBSMul5 as described by Brockmeier et al. (4). The fusion of subtilisin BPN′ with the wild-type SP (wtSP) originating from B. amyloliquefaciens was used as the benchmark construct, which gave an extracellular protease activity in B. subtilis of 0.83 ± 0.1 U/ml. Each tested microtiter plate contained as an internal standard a clone expressing the benchmark construct. The BPN′-encoding gene lacking the DNA encoding the SP, the ribosomal binding site, and the spacer sequence served as a control; it produced an extracellular protease activity of <0.1 U/ml.
Proteolytic activity assay.
Protease activity was determined with the substrate succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (AAPF; Bachem AG, Weil am Rhein, Germany) dissolved in dimethyl sulfoxide and finally diluted in 0.1 M Tris-HCl buffer, pH 8.6, containing 1% Brij35 to a final concentration of 1.1 mM. Assays were performed with the same buffer in microtiter plates (96 wells, 250-μl reaction volume per well; Greiner Bio-One, Frickenhausen, Germany) at 30°C for 5 min. The amount of p-nitroanilide released was measured at 405 nm using a molar absorption coefficient of 8,480/M × cm (11). One unit is defined as the activity releasing 1 μmol of p-nitroanilide per minute. Clones showing high proteolytic activity in the culture supernatants were isolated and independently recultivated in microtiter plates (3-fold replicative determination). The amount of protease protein and proteolytic activity were determined by SDS-PAGE and Western blotting in culture supernatants of 35 clones with high extracellular proteolytic activity.
Protein analysis and immunodetection of BPN′.
SDS-polyacrylamide gel electrophoresis was performed using a 5% (wt/vol) stacking gel and a 15% separating gel (19). For Western blot analysis, 10 μl of culture supernatant was loaded on a gel and the separated proteins were subsequently blotted onto a polyvinylidene difluoride membrane (Sequiblot membrane; Bio-Rad, Munich, Germany) (12). BPN′ protein was detected with a rabbit polyclonal antiserum and horseradish peroxidase-labeled goat anti-rabbit secondary antibodies (Bio-Rad, Munich, Germany) using the ECL Western blot kit (Thermo Scientific).
RESULTS
Design and construction of a signal peptide library consisting of Sec type signal peptides.
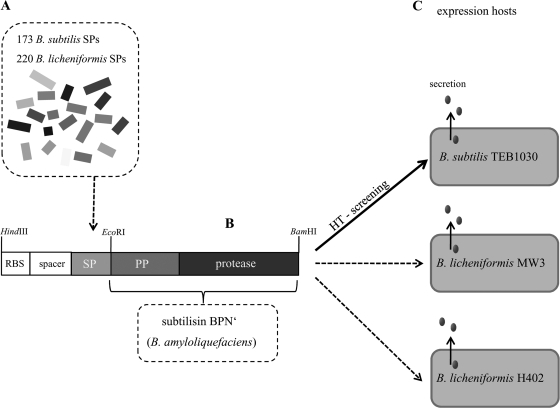
For optimization of heterologous protein export in different Bacillus expression strains, a signal peptide library consisting of 220 B. licheniformis SPs and 173 B. subtilis SPs was constructed. Subtilisin protease BPN′ from B. amyloliquefaciens ATCC 23844 (NCBI accession no. K02496.1) served as the secretion target, and B. subtilis was used as the expression host strain. DNA fragments encoding SPs were cloned upstream of BPN′ into plasmid pBSMul5, and the resulting library was screened for extracellular protease activity in B. subtilis as summarized in Fig. 1.
FIG. 1.
Strategy used for secretion optimization in different Bacillus hosts. Three hundred ninety-three SPs originating from B. subtilis and B. licheniformis (A) were cloned in front of the gene encoding subtilisin BPN′ from B. amyloliquefaciens with its propeptide (PP) (B), which was used as a heterologous secretion target protein. Each SP was amplified with an artificial ribosome binding site (RBS), followed by a spacer region and ATG as the standardized start codon. (C) B. subtilis was used for screening of the signal peptide library, and two B. licheniformis strains were used to assess protease secretion levels for the best-performing SP-BPN′ fusions identified previously. HT, high throughput.
Homologous and heterologous signal peptides significantly improve BPN′ export in B. subtilis.
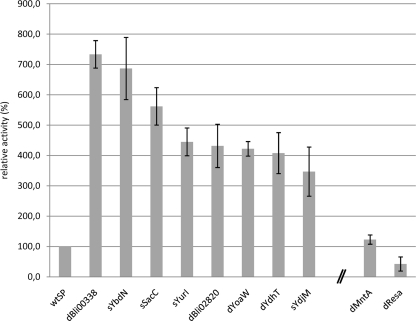
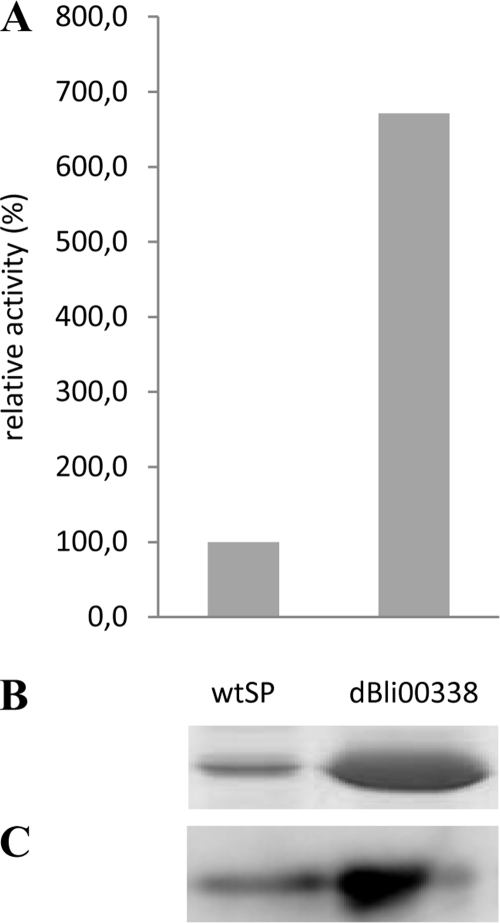
B. subtilis was transformed with the SP library as described above, and about 1,800 clones (4-fold oversampling) were tested for extracellular protease production on agar plates containing skim milk. About 900 clones formed a clear halo around the colony; they were cultivated in duplicate or triplicate in different microtiter plates, and proteolytic activities in the culture supernatants were determined, allowing identification of eight SPs which efficiently mediated secretion of BPN′ by B. subtilis (Fig. 2). SPs dBli00338 and sYbdN increased the extracellular level of BPN′ by about 7-fold compared to the B. amyloliquefaciens wild-type SP (Fig. 2). Characteristics of the best-performing SPs are shown in Table 2. Strikingly, we found that not only SPs from the homologous host B. subtilis but also SPs isolated from the heterologous host B. licheniformis efficiently mediated BPN′ secretion. In fact, B. licheniformis SP dBli00338 was among the most efficient SPs we have identified. B. subtilis clones expressing constructs with the benchmark SP, wtSP, and the best-performing SP, dBli00338, were also cultivated in a laboratory scale fermentor. BPN′ fused to SP dBli00338 was also efficiently secreted under the conditions of high-cell-density fed-batch cultivation. After 48 h of cultivation, proteolytic activities were 8.9 units/ml for wtSP (at a cell density corresponding to an optical density at 600 nm [OD600] of 68.3) and 59.7 units/ml for dBli00338 (OD600 = 65.3), corresponding to a 6- to 7-fold increase of proteolytic activity in the culture supernatant (Fig. 3 A), with a concomitant increase in extracellular protease protein (Fig. 3B and C).
FIG. 2.
SPs which produced the most efficient secretion of BPN′, identified by screening in B. subtilis TEB1030. SPs originating from B. licheniformis DSM13 are labeled with the prefix “d,” and SPs from B. subtilis 168 are labeled with “s.” The extracellular enzyme activity obtained with the wild-type SP (wtSP) of BPN′ was defined as the benchmark (100%, corresponding to 0.83 units per ml). Error bars indicate standard deviations between proteolytic activities detected for each construct in at least three independent cultivations.
TABLE 2.
Characteristics of the SPs identified for BPN′ in the SP screening
| SP | Amino acid sequence | Length (aae) | D scorea | Net charge of N domainb | Hydrophobicity (%)c |
|---|---|---|---|---|---|
| dBli00338 | MLINKSKKFFVFSFIFVMMLSLSFVNGEVAKA | 32 | 0.897 | 3 | 53 |
| sYbdN | MVKKWLIQFAVMLSVLSTFTYSASA | 25 | 0.930 | 2 | 48 |
| sSacC | MKKRLIQVMIMFTLLLTMAFSADA | 24 | 0.927 | 3 | 58 |
| sYurI | MTKKAWFLPLVCVLLISGWLAPAASASA | 28 | 0.915 | 2 | 68 |
| dBli02820 | MRMKRLRMRKHLLIAVCTLALLLSSPIVSDA | 31 | 0.936 | 6 | 52 |
| dYoaW | MKKIVCLMVFSIMTAFGIHIQPAEA | 25 | 0.864 | 2 | 56 |
| dYdhT | MKKNIVCSIFALLLAFAVSQPSYA | 24 | 0.870 | 2 | 58 |
| sYdjM | MLKKVILAAFILVGSTLGAFSFSSDASA | 28 | 0.915 | 2 | 57 |
| dMntAd | MKWKQTLAIAAALILILAAGCSSKSSS | 27 | 0.783 | 2 | 59 |
| dResAd | MKKKRFYIRTGILLVLLAALGYTLYSAVFQNTESV | 35 | 0.617 | 5 | 51 |
| wtSPd | MRGKKVWISLLFALALIFTMAFGSTSSAQA | 30 | 0.930 | 3 | 57 |
D score calculated by SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP/).
Calculated with amino acids D and E defined as −1, R and K defined as +1, and any other amino acid as 0.
Percentage of hydrophobic amino acids in each signal sequence, calculated with amino acids G, A, V, L, I, M, F, W, and P defined as hydrophobic and any other amino acid defined as hydrophilic.
Control construct.
aa, amino acids.
FIG. 3.
High-cell-density cultivation of B. subtilis TEB1030 carrying BPN′ fused to SPs wtSP and dBli00338. Cells were grown in fed-batch mode in a fermentor (culture volume: 3 liters), and culture supernatants were analyzed for extracellular protease activity (A) and amount of extracellular protease protein (B and C) by SDS-PAGE and subsequent staining with Coomassie brilliant blue (B) and Western blotting using antibodies against subtilisin BPN′ (C). The protein bands correspond to a molecular mass of 28 kDa, as deduced from positions of molecular weight standards (New England Biolabs [NEB]; broad range, 2 to 212 kDa, not shown) and thus correspond to the theoretical molecular mass of 27.6 kDa of mature BPN′.
Different SPs and expression hosts affect extracellular BPN′ levels.
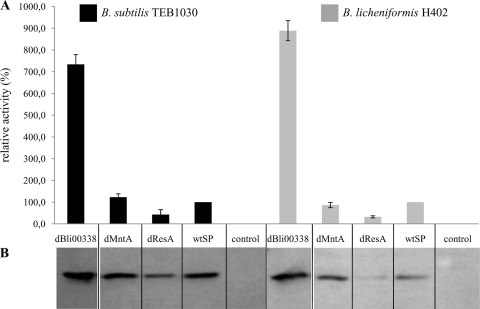
Next, we studied whether the best-performing constructs identified in B. subtilis would also mediate secretion in two distinct B. licheniformis strains of biotechnological relevance. As shown in Fig. 4, we chose three SP-BPN′ fusions with clearly different extracellular protein levels of BPN′. We transformed these into B. licheniformis H402 and determined extracellular proteolytic activity and amount of BPN′ protein (Fig. 4).
FIG. 4.
Extracellular activities (A) and amount of protein (B) determined by Western blotting of subtilisin BPN′ in culture supernatants of screening host B. subtilis TEB1030 and expression host B. licheniformis H402. The protein bands correspond to a molecular mass of 28 kDa, as deduced from positions of molecular weight standards (NEB; broad range, 2 to 212 kDa, not shown) corresponding to the theoretical molecular mass of 27.6 kDa of mature BPN′. A B. subtilis strain with the vector encoding BPN′ without SP served as a control. Error bars indicate the standard deviations of proteolytic activities detected for each construct in at least three independent cultivations.
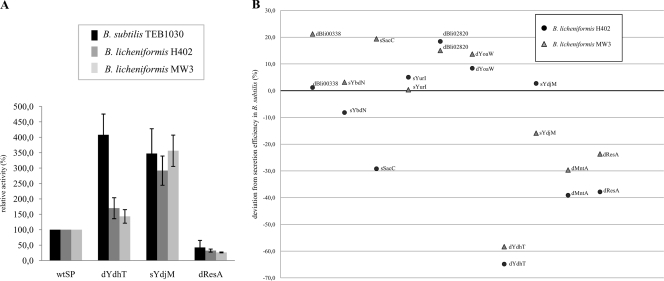
SP dBli00338 produced a 9-fold increase of BPN′ secretion in B. licheniformis H402 compared to the wild-type SP of BPN′ based on extracellular proteolytic activity. For all constructs tested, levels of protein export of BPN′ were similar in both B. subtilis TEB1030 and B. licheniformis H402. These results prompted us to comparatively analyze the previously identified SPs in more detail. We additionally chose as an expression host B. licheniformis MW3, which is a variant of strain DSM13 and can easily be transformed. The eight best-performing SP-BPN′ fusions previously identified in B. subtilis were analyzed, together with two fusions which produced a significant decrease in extracellular BPN′. Extracellular protease activities were compared to those detected in the screening host B. subtilis TEB1030 (Fig. 5 A). The fusion sYdjM::BPN′ produced an approximately 3.5-fold increase of protease secretion in each of the three expression hosts, while dYdhT::BPN′ produced decreased export relative to the benchmark, wtSP::BPN′, in both B. licheniformis hosts. The deviations of relative BPN′ export levels in the two B. licheniformis hosts with respect to B. subtilis levels were compared for 10 different SP-BPN′ fusions (Fig. 5B). Interestingly, the relative protein export levels of the analyzed SP-BPN′ fusions for both B. licheniformis strains were comparable to those observed in B. subtilis, with deviations of less than 20%. BPN′ fused to SP dYdhT from B. licheniformis represents a clear exception, showing in both B. licheniformis strains an extracellular protease level 2-fold lower than that for B. subtilis TEB1030 (Fig. 5B).
FIG. 5.
Extracellular enzyme activities of SP-BPN′ fusions identified by screening in B. subtilis and additionally expressed in B. licheniformis H402 and B. licheniformis MW3. (A) Protease activities in culture supernatants of SP-BPN′ constructs expressed in three different Bacillus strains were compared to the benchmark construct. (B) Secretion efficiencies in B. licheniformis H402 and MW3 shown as percent increases or decreases compared to that in B. subtilis. The boldface line represents the secretion efficiency in B. subtilis, which is defined as 0% deviation.
DISCUSSION
Bacteria belonging to the genus Bacillus represent the most important strains for the industrial production of secreted proteins (28). Hence, extensive research into the optimization of protein secretion was performed in Bacillus, and several patents which address strategies to relieve bottlenecks for protein secretion were filed (14, 20, 23). SPs from extracellular proteins known to be secreted at high levels were identified and modified, e.g., SPs from NprE and AprE (14, 23). Additionally, homologous and heterologous SPs were tested in different hosts (8, 36); however, only very few examples of heterologous SPs producing significant increases in secretion efficiency have been described (10).
Screening projects published to date have exclusively been performed with SPs obtained from homologous hosts (4, 21, 38, 39). Here, we have used subtilisin BPN′ from B. amyloliquefaciens as a model protein and have studied the influence of homologous and heterologous SPs on export efficiency of BPN′ by using three different Bacillus strains.
A library consisting of about 400 different SPs fused to subtilisin BPN′ was constructed and tested by high-throughput activity screening of B. subtilis culture supernatants. Eight SPs were identified, with dBli00338 and sYbdN producing a 7-fold increase of BPN′ activity in the culture supernatant compared to wild-type SP. The results obtained by SP screening in microtiter plates were confirmed for wtSP and SP dBli00338 by high-cell-density cultivation of B. subtilis in a 3-liter laboratory scale fermentor. Interestingly, our screen did not identify SPs from enzymes like AprE and NprE, which are known to be secreted at high levels. Instead, we predominantly identified SPs originating from so far uncharacterized so-called “Y” proteins. An analysis of the extracellular proteomes of B. subtilis (29) and B. licheniformis (34) for the presence of proteins carrying the SPs shown in Fig. 2 revealed a significant amount of secretion only for protein YbdN. All other proteins were present in only very small amounts or were not identified at all. It should be noted that, apart from SPs, additional effects like promoter strengths, gene regulation mechanisms, transcriptional and translational efficiencies, and protein folding also affect the amount of secreted proteins. We have also analyzed these secreted proteins with respect to their size, function, and pI, and we were unable to detect any significant similarities between them or between them and subtilisin BPN′. These results suggest that the general approach of choosing an SP from an efficiently secreted protein to direct secretion of another target protein is clearly not the best strategy.
A detailed analysis of the eight best-performing SPs did not reveal any significant similarities with respect to charge of the N region, hydrophobicity, signal peptidase recognition site, or D score, as calculated with the SignalP prediction tool. Thus, high-throughput SP screening as described here is the method of choice to quickly identify the most efficient SP for any protein to be secreted.
Another important aspect of this work relates to the fact that industrially relevant production strains are often not amenable to molecular manipulations, including DNA transformation (22, 37). Hence, the use of closely related but better-accessible strains may be required to accomplish high-throughput screening procedures. Therefore, we have determined the levels of extracellular proteases of different SP-BPN′ fusions, which were originally identified in B. subtilis, in the industrially relevant B. licheniformis strain H402 and in B. licheniformis type strain DSM13 (MW3). Interestingly, the majority of the SP-BPN′ fusions revealed comparable levels of extracellular protease in all three Bacillus expression strains. These results suggest that high-throughput prescreening in B. subtilis and subsequent transfer of the best-performing constructs into B. licheniformis for further testing should be performed. SPs interact with components of the Sec machinery, but it is also known that the interaction of SPs and the mature part of the secreted proteins influences the secretion efficiency (9, 16, 17). Although B. subtilis and B. licheniformis are closely related, significant differences in components of their Sec machineries and especially their signal peptidases have been described (32, 33). Most of the constructs with different SPs showed similar relative protease export levels in different Bacillus hosts. However, the dYdhT::BPN′ fusion revealed a significantly decreased level of extracellular protease upon transfer into the B. licheniformis hosts, indicating that the efficiency of SPs can still not be predicted a priori.
In summary, we have demonstrated that both homologous and heterologous SPs, when fused to a given target protein, can produce significantly increased amounts of extracellular protein, which can easily be recovered from the culture supernatant. Furthermore, we have demonstrated that a genetically accessible host strain like B. subtilis can be used for a preliminary high-throughput screening of an SP library and that the best-performing SP fusions can subsequently be transferred into a production strain to improve protein export.
Acknowledgments
We thank Ulf Brockmeier for providing the Bacillus subtilis signal peptide library.
Footnotes
Published ahead of print on 13 August 2010.
REFERENCES
- 1.Barbe, V., S. Cruveiller, F. Kunst, P. Lenoble, G. Meurice, A. Sekowska, D. Vallenet, T. Wang, I. Moszer, C. Medigue, and A. Danchin. 2009. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155:1758-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchert, T. V., and V. Nagarajan. 1991. Effect of signal sequence alterations on export of levansucrase in Bacillus subtilis. J. Bacteriol. 173:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigidi, P., E. De Rossi, G. Riccardi, and D. Matteuzzi. 1991. A highly efficient electroporation system for transformation of Bacillus licheniformis. Biotechnol. Techniques 5:5-8. [Google Scholar]
- 4.Brockmeier, U., M. Caspers, R. Freudl, A. Jockwer, T. Noll, and T. Eggert. 2006. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J. Mol. Biol. 362:393-402. [DOI] [PubMed] [Google Scholar]
- 5.Brode, P. F., III, C. R. Erwin, D. S. Rauch, B. L. Barnett, J. M. Armpriester, E. S. Wang, and D. N. Rubingh. 1996. Subtilisin BPN′ variants: increased hydrolytic activity on surface-bound substrates via decreased surface activity. Biochemistry 35:3162-3169. [DOI] [PubMed] [Google Scholar]
- 6.Caspers, M., U. Brockmeier, C. Degering, T. Eggert, and R. Freudl. 2010. Improvement of Sec-dependent secretion of a heterologous model protein in Bacillus subtilis by saturation mutagenesis of the N-domain of the AmyE signal peptide. Appl. Microbiol. Biotechnol. 86:1877-1885. [DOI] [PubMed] [Google Scholar]
- 7.Chang, S., and S. N. Cohen. 1979. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol. Gen. Genet. 168:111-115. [DOI] [PubMed] [Google Scholar]
- 8.Chen, M., and V. Nagarajan. 1993. The roles of signal peptide and mature protein in RNase (barnase) export from Bacillus subtilis. Mol. Gen. Genet. 239:409-415. [DOI] [PubMed] [Google Scholar]
- 9.Choo, K. H., and S. Ranganathan. 2008. Flanking signal and mature peptide residues influence signal peptide cleavage. BMC Bioinformatics 9(Suppl. 12):S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier, D. N. 1994. Escherichia coli signal peptides direct inefficient secretion of an outer membrane protein (OmpA) and periplasmic proteins (maltose-binding protein, ribose-binding protein, and alkaline phosphatase) in Bacillus subtilis. J. Bacteriol. 176:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DelMar, E. G., C. Largman, J. W. Brodrick, and M. C. Geokas. 1979. A sensitive new substrate for chymotrypsin. Anal. Biochem. 99:316-320. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, S. D. 1986. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal. Biochem. 157:144-153. [DOI] [PubMed] [Google Scholar]
- 13.Eggert, T., U. Brockmeier, M. J. Droge, W. J. Quax, and K. E. Jaeger. 2003. Extracellular lipases from Bacillus subtilis: regulation of gene expression and enzyme activity by amino acid supply and external pH. FEMS Microbiol. Lett. 225:319-324. [DOI] [PubMed] [Google Scholar]
- 14.Fu, L. L., Z. R. Xu, W. Li, J. B. Shuai, P. Lu, and C. X. Hu. 2007. Protein secretion pathways in Bacillus subtilis: implication for optimization of heterologous protein secretion. Biotechnol. Adv. 25:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Fu, L. L., Z. R. Xu, J. B. Shuai, C. X. Hu, W. Dai, and W. F. Li. 2008. High-level secretion of a chimeric thermostable lichenase from Bacillus subtilis by screening of site-mutated signal peptides with structural alterations. Curr. Microbiol. 56:287-292. [DOI] [PubMed] [Google Scholar]
- 16.Harwood, C. R., and R. Cranenburgh. 2008. Bacillus protein secretion: an unfolding story. Trends Microbiol. 16:73-79. [DOI] [PubMed] [Google Scholar]
- 17.Kaderbhai, M. A., H. M. Davey, and N. N. Kaderbhai. 2004. A directed evolution strategy for optimized export of recombinant proteins reveals critical determinants for preprotein discharge. Protein Sci. 13:2458-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Li, W., X. Zhou, and P. Lu. 2004. Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Res. Microbiol. 155:605-610. [DOI] [PubMed] [Google Scholar]
- 21.Mathiesen, G., A. Sveen, M. B. Brurberg, L. Fredriksen, L. Axelsson, and V. G. Eijsink. 2009. Genome-wide analysis of signal peptide functionality in Lactobacillus plantarum WCFS1. BMC Genomics 10:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurer, K. H. 2004. Detergent proteases. Curr. Opin. Biotechnol. 15:330-334. [DOI] [PubMed] [Google Scholar]
- 23.Nijland, R., and O. P. Kuipers. 2008. Optimization of protein secretion by Bacillus subtilis. Recent Pat. Biotechnol. 2:79-87. [DOI] [PubMed] [Google Scholar]
- 24.Park, Y. S., K. Kai, S. Iijima, and T. Kobayashi. 1992. Enhanced beta-galactosidase production by high cell-density culture of recombinant Bacillus subtilis with glucose concentration control. Biotechnol. Bioeng. 40:686-696. [DOI] [PubMed] [Google Scholar]
- 25.Rao, M. B., A. M. Tanksale, M. S. Ghatge, and V. V. Deshpande. 1998. Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 62:597-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey, M. W., P. Ramaiya, B. A. Nelson, S. D. Brody-Karpin, E. J. Zaretsky, M. Tang, A. Lopez de Leon, H. Xiang, V. Gusti, I. G. Clausen, P. B. Olsen, M. D. Rasmussen, J. T. Andersen, P. L. Jorgensen, T. S. Larsen, A. Sorokin, A. Bolotin, A. Lapidus, N. Galleron, S. D. Ehrlich, and R. M. Berka. 2004. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol. 5:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 28.Schallmey, M., A. Singh, and O. P. Ward. 2004. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 50:1-17. [DOI] [PubMed] [Google Scholar]
- 29.Tjalsma, H., H. Antelmann, J. D. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J. Y. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68:207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vehmaanperä, J. 1989. Transformation of Bacillus amyloliquefaciens by electroporation. FEMS Microbiol. Lett. 52:165-169. [DOI] [PubMed] [Google Scholar]
- 32.Veith, B., C. Herzberg, S. Steckel, J. Feesche, K. H. Maurer, P. Ehrenreich, S. Baumer, A. Henne, H. Liesegang, R. Merkl, A. Ehrenreich, and G. Gottschalk. 2004. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J. Mol. Microbiol. Biotechnol. 7:204-211. [DOI] [PubMed] [Google Scholar]
- 33.Voigt, B., H. Antelmann, D. Albrecht, A. Ehrenreich, K. H. Maurer, S. Evers, G. Gottschalk, J. M. van Dijl, T. Schweder, and M. Hecker. 2009. Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 16:53-68. [DOI] [PubMed] [Google Scholar]
- 34.Voigt, B., T. Schweder, M. J. Sibbald, D. Albrecht, A. Ehrenreich, J. Bernhardt, J. Feesche, K. H. Maurer, G. Gottschalk, J. M. van Dijl, and M. Hecker. 2006. The extracellular proteome of Bacillus licheniformis grown in different media and under different nutrient starvation conditions. Proteomics 6:268-281. [DOI] [PubMed] [Google Scholar]
- 35.von Heijne, G. 1990. The signal peptide. J. Membr. Biol. 115:195-201. [DOI] [PubMed] [Google Scholar]
- 36.Wang, L. F., A. A. Kortt, and D. J. Stewart. 1993. Use of a Gram-negative signal peptide for protein secretion by Gram-positive hosts: basic protease of Dichelobacter nodosus is produced and secreted by Bacillus subtilis. Gene 131:97-102. [DOI] [PubMed] [Google Scholar]
- 37.Waschkau, B., J. Waldeck, S. Wieland, R. Eichstadt, and F. Meinhardt. 2008. Generation of readily transformable Bacillus licheniformis mutants. Appl. Microbiol. Biotechnol. 78:181-188. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe, K., Y. Tsuchida, N. Okibe, H. Teramoto, N. Suzuki, M. Inui, and H. Yukawa. 2009. Scanning the Corynebacterium glutamicum R genome for high-efficiency secretion signal sequences. Microbiology 155:741-750. [DOI] [PubMed] [Google Scholar]
- 39.Zhu, F. M., S. Y. Ji, W. W. Zhang, W. Li, B. Y. Cao, and M. M. Yang. 2008. Development and application of a novel signal peptide probe vector with PGA as reporter in Bacillus subtilis WB700: twenty-four tat pathway signal peptides from Bacillus subtilis were monitored. Mol. Biotechnol. 39:225-230. [DOI] [PubMed] [Google Scholar]