Abstract
Respiratory inhibitors are among the fungicides most widely used for disease control on crops. Most are strobilurins and carboxamides, inhibiting the cytochrome b of mitochondrial complex III and the succinate dehydrogenase of mitochondrial complex II, respectively. A few years after the approval of these inhibitors for use on grapevines, field isolates of Botrytis cinerea, the causal agent of gray mold, resistant to one or both of these classes of fungicide were recovered in France and Germany. However, little was known about the mechanisms underlying this resistance in field populations of this fungus. Such knowledge could facilitate resistance risk assessment. The aim of this study was to investigate the mechanisms of resistance occurring in B. cinerea populations. Highly specific resistance to strobilurins was correlated with a single mutation of the cytb target gene. Changes in its intronic structure may also have occurred due to an evolutionary process controlling selection for resistance. Specific resistance to carboxamides was identified for six phenotypes, with various patterns of resistance levels and cross-resistance. Several mutations specific to B. cinerea were identified within the sdhB and sdhD genes encoding the iron-sulfur protein and an anchor protein of the succinate dehydrogenase complex. Another as-yet-uncharacterized mechanism of resistance was also recorded. In addition to target site resistance mechanisms, multidrug resistance, linked to the overexpression of membrane transporters, was identified in strains with low to moderate resistance to several respiratory inhibitors. This diversity of resistance mechanisms makes resistance management difficult and must be taken into account when developing strategies for Botrytis control.
Chemical control methods are widely used to combat fungal plant pathogens in agriculture. Many synthetic fungicides have been used intensively since the 1950s, resulting in the selection of resistant mutants in natural populations of phytopathogenic fungi (17). The evolution of fungicide resistance has become a major problem worldwide, particularly in cases in which high-resistance factors have been reported and the frequencies of mutant phenotypes in the population are high. This phenomenon may greatly decrease the efficacy of the active ingredients concerned, increasing the cost of chemical control and potentially resulting in damage to the environment if repeated treatment is required (11).
Several resistance mechanisms have been identified, but changes in the target site proteins, which remain functional but are less susceptible to fungicides, are the most common resistance mechanism in plant pathogenic fungi. Target site changes may result from point or insertion/deletion mutations in the corresponding gene. However, other mechanisms have been reported at lower frequencies and include (i) target overproduction, (ii) activation of an alternative pathway compensating for inhibition of the major pathway, (iii) greater fungicide detoxification or lower levels of profungicide activation, and (iv) decreases in fungicide influx or increases in fungicide efflux, resulting in lower fungicide content within the cell (11). The first three mechanisms often lead to specific resistance, i.e., resistance concerning only one molecule or a class of fungicides. The last mechanism generally involves the constitutive overexpression of membrane drug efflux proteins, i.e., either ATP-binding cassette (ABC) or major facilitator superfamily (MFS) transporters. Due to their low substrate specificity, both types of transporters can mediate multidrug resistance (MDR) to various unrelated classes of fungicides, also considered multiple resistance even if driven by alteration of a single gene. MDR is an important phenomenon in human pathogenic microbes and cancer cells and is beginning to emerge in plant pathogenic fungi, even though resistance levels against individual fungicides are generally much lower than those achieved by target site mutations (16, 42).
Many of the fungicides used in agriculture inhibit respiration, and many are effective against a wide range of plant-pathogenic fungi. The oldest such fungicides are still in use and include dithiocarbamates (e.g., mancozeb, thiram) or trihalomethylthio derivatives (e.g., captafol, captan, folpet, dichlofluanid, tolylfluanid). These multisite inhibitors block several thiol-containing enzymes involved in respiration (50). A second group of these fungicides includes uncouplers of oxidative phosphorylation; the protonophore may be either a phenol group (i.e., dinocap) or a secondary amine group (i.e., fluazinam) (32, 50). A third group comprises inhibitors of mitochondrial complex III (the cytochrome bc1 complex), which bind to cytochrome b at either an outer quinol-oxidizing pocket (Qo site) or an inner quinone-reducing pocket (Qi site). Most Qo inhibitors (QoIs) are synthetic analogs of natural strobilurins (e.g., azoxystrobin, kresoxim-methyl) introduced in the mid-1990s to control a wide spectrum of fungal diseases (8). The only QiIs used in agriculture are sulfonamides (e.g., cyazofamid, amisulbrom), which are active only against oomycetes. The antibiotic antimycin A is also a Qi inhibitor (24). Inhibitors of succinate dehydrogenase (mitochondrial complex II), known as SDHIs, are the last major group of respiratory inhibitors used as agricultural fungicides (72). Chemically, many of these fungicides are carboxamides with a common “cis-crotonanilide” structure, as shown in Fig. SA1 in the supplemental material. They are derived from an α,β-unsaturated carboxylic acid, the double bond of which is incorporated into a benzene ring or conjugated to an electron-releasing atom, such as O, N, or S, giving rise to several subgroups. In addition to these “cis-crotonanilides,” several N-methylpyrazole carboxamides with a single double bond between the significant methyl and carboxanilide groups have been designed (see Fig. SA1 in the supplemental material). The first generation of carboxamide fungicides, including carboxin, was discovered in the mid-1960s, and these molecules were effective only against basidiomycetes (72). New carboxamides with a much wider spectrum of activity have recently been discovered (6). Many harbor a lipophilic substituent in the phenyl ring of the anilide (e.g., boscalid, bixafen). Such an extension of the spectrum of activity was described in 1967 for the experimental 2′-phenyl analog of carboxin (F427) (see Fig. SA1 in the supplemental material). All these carboxamides interfere with ubiquinone binding and affect three subunits of succinate dehydrogenase (33, 35, 36, 59, 72, 75).
Botrytis cinerea Pers. ex Fr., the anamorph of Botryotinia fuckeliana (de Barry) Whetzel, is a ubiquitous fungus causing gray mold on many crops, including vegetables, ornamental plants, and fruits, such as grapevine in particular (13). The losses for commercial wine production are especially high, being estimated up to 2 billion U.S. dollars per year worldwide (69), in addition to qualitative defaults observed in grapevine musts. Gray mold has recently been found to be caused by a complex of two related fungal species living in sympatry: Botrytis group II (B. cinerea sensu stricto, the most abundant species in the complex) and Botrytis group I (B. pseudocinerea), which represents less than 10% in French vineyards (25). It is not possible to surely distinguish between these two species on the basis of morphological criteria (25). Instead, these species can be separated on the basis of polymorphism in many genes (3, 4, 25) or microsatellite private alleles (26). In addition, Botrytis group I is naturally more tolerant to fenhexamid and more susceptible to fenpropidin than Botrytis group II (4, 47).
Despite the availability of a large number of botryticides, the chemical control of gray mold has been hindered by the emergence of resistant strains. This phenomenon is well documented for the use of benzimidazoles (e.g., carbendazim), dicarboximides (e.g., iprodione, procymidone), and to a lesser extent anilinopyrimidines (e.g., cyprodinil, pyrimethanil) and the hydroxyanilide fenhexamid in vineyards (47). Some shifts in susceptibility to multisite fungicides and fluazinam have also been recorded in ornamental plants and vegetables (44). Moreover, field strains of B. cinerea resistant to inhibitors of mitochondrial complexes II and/or III have recently been detected (7, 38, 40, 54, 65). Finally, strains displaying MDR have been isolated from French and German vineyards (42, 44).
In this study, our goal was to assess the susceptibility of B. cinerea strains from French and German vineyards to “risky” single-site respiratory inhibitors, including strobilurins and carboxamides, which are representative of the more recently introduced modes of action. We also aimed to identify the various possible mechanisms underlying resistance to these two classes of respiratory inhibitors and to investigate possible consequences for resistance management.
MATERIALS AND METHODS
Fungal strains.
Fifty B. cinerea field strains were isolated from infected berries collected from French and German vineyards (Table 1). They were plated on MYA medium (20 g malt, 5 g yeast extract, 12.5 g agar in 1 liter of deionized water). After one round of culture, highly diluted spore suspensions were obtained and poured onto GA medium (10 g glucose and 12.5 g agar in 1 liter of deionized water), and after 24 h, pieces of medium bearing single germinated conidia were cut off under the microscope in sterile conditions and isolated in a new petri dish. The single-spore isolates were maintained on the MYA medium, and large numbers of conidia were produced after 1 week of culture under white light at 19°C.
TABLE 1.
Origin and fungicide resistance phenotype of the tested strains
| Strain no. | Origina | Year | Location | Country | Botrytis groupb | Specific resistance toc: |
MDRd | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzimidazoles | Dicarboximides | Anilinopyrimidines | Hydroxyanilides | Strobilurins | Carboxamides | |||||||
| 1-3 | INRA | 2007 | Champagne | France | I | − | − | − | [+] | − | − | − |
| 4 | INRA | 2007 | Versailles | France | II | − | − | − | − | − | − | − |
| 5 | INRA | 2007 | Champagne | France | II | − | − | − | − | − | − | − |
| 6 | INRA | 1994 | Champagne | France | II | + | − | − | − | − | − | − |
| 7 | INRA | 1994 | Armagnac | France | II | − | + | + | − | − | − | − |
| 8 | INRA | <1980 | Bordeaux | France | II | + | − | − | − | − | − | − |
| 9 | INRA | 2006 | Champagne | France | II | − | − | − | + | + | − | − |
| 10 | INRA | 2007 | Champagne | France | II | − | − | − | + | + | − | − |
| 11 | INRA | 2007 | Champagne | France | II | − | − | − | − | − | − | + (MDR1) |
| 12 | INRA | 2007 | Champagne | France | II | + | − | − | − | − | − | + (MDR1) |
| 13-14 | INRA | 2007 | Champagne | France | II | − | − | + | − | + | − | + (MDR1) |
| 15 | INRA | 2007 | Champagne | France | II | − | − | − | − | − | − | + (MDR2) |
| 16-17 | INRA | 2007 | Champagne | France | II | + | − | − | − | − | − | + (MDR2) |
| 18 | INRA | 2007 | Champagne | France | II | + | + | − | − | − | − | + (MDR2) |
| 19-20 | INRA | 2007 | Champagne | France | II | + | − | − | − | − | − | + (MDR3) |
| 21-22 | INRA | 2007 | Champagne | France | II | − | − | − | − | − | − | + (MDR3) |
| 23-24 | BASF | 2006 | Germany | II | − | − | − | − | − | + (CarR1) | − | |
| 25-26 | INRA | 2007 | Champagne | France | II | − | − | − | + | + | + (CarR1) | − |
| 27 | INRA | 2008 | Champagne | France | II | − | − | − | − | + | + (CarR1) | − |
| 28 | INRA | 2008 | Champagne | France | II | − | − | − | + | + | + (CarR1) | − |
| 29 | Uni. Kai | 2007 | Palatine | Germany | II | − | − | − | − | + | + (CarR1) | − |
| 30 | Uni. Kai | 2007 | Palatine | Germany | II | − | − | − | + | + | + (CarR1) | − |
| 31 | Uni. Kai | 2007 | Palatine | Germany | II | − | − | − | − | + | + (CarR1) | − |
| 32 | Uni. Kai | 2007 | Palatine | Germany | II | − | − | − | + | + | + (CarR1) | − |
| 33-35 | Uni. Kai | 2007 | Palatine | Germany | II | − | − | − | − | + | + (CarR2) | − |
| 36-44 | Uni. Kai | 2008 | Palatine | Germany | II | − | − | − | − | + | + (CarR2) | − |
| 45 | Uni. Kai | 2008 | Palatine | Germany | II | − | − | − | − | + | + (CarR3) | − |
| 46 | Uni. Kai | 2008 | Palatine | Germany | II | − | − | − | − | + | + (CarR4) | − |
| 47-48 | INRA | 2008 | Champagne | France | II | − | − | − | − | + | + (CarR5) | − |
| 49 | BASF | 2006 | Germany | II | − | − | − | − | − | + (CarR6) | − | |
| 50 | Uni. Kai | 2008 | Palatine | Germany | II | − | − | − | − | + | + (CarR6) | − |
INRA, strains collected by our group; BASF, strains provided by BASF, Limburgerhof, Germany; Uni. Kai, gift from Kaiserslautern University, Germany.
Botrytis group I and II strains were differentiated on the basis of genotype and their response to certain sterol biosynthesis inhibitors, including fenhexamid and fenpropidin (25, 46).
Specific resistance (+) is determined by an alteration in the genes encoding the target protein (see the first section of Results). In Botrytis group I, there is natural resistance ([+]) to fenhexamid (hydroxyanilides), which is probably determined by an increase in the detoxification of this compound (47). −, lack of specific resistance for a given mode of action.
Multidrug resistance (+) corresponds to positive cross-resistance between unrelated fungicides. It is determined by the overexpression of drug transporters (42). −, lack of MDR in a given isolate.
For assessment of the frequency of strobilurin resistance in representative French populations of B. cinerea, 169 isolates were collected from Champagne vineyards, where they were growing on grapevine (Vitis vinifera) or blackberry (Rubus fruticosus), at two sites—Courteron and Vandières—in September 2006 and June 2007. These strains were isolated, maintained, grown, and analyzed in the same conditions as described above. The characteristics of these samples are presented in Table SA1 in the supplemental material.
In these data sets, Botrytis group I strains were differentiated from those of Botrytis group II by culture on medium containing discriminating doses of fenhexamid (0.4 mg liter−1) and fenpropidin (0.08 mg liter−1), making use of the “natural” differences in susceptibility to these sterol biosynthesis inhibitors (SBIs) of these two groups (46). The results obtained were confirmed in molecular tests (i.e., polymorphism in genes encoding cytochrome b or subunits of SDHIs and microsatellite private alleles; see below).
Fungicides.
We tested principally respiratory inhibitors but also fungicides with other biochemical modes of action (i.e., carbendazim, fenhexamid, fludioxonil, iprodione, prochloraz, pyrimethanil, tebuconazole, and tolnaftate), which were used to differentiate between strains displaying specific and multidrug resistance (46). All the fungicides tested were of technical grade and most of them were kindly donated by manufacturers. They included boscalid, benodanil, furcarbanil, dimoxystrobin, pyraclostrobin, carbendazim, iprodione, prochloraz, pyrimethanil (BASF, Germany), methfuroxam, carboxin and its derivatives oxa-3a (3′ phenylcarboxin), oxa-3b (3′ benzylcarboxin), oxa-3c (3′ butoxycarboxin), oxa-3d (3′ hexyloxycarboxin), oxa-4a (4′ phenylcarboxin), oxa-4b (4′ butoxycarboxin) (Uniroyal), flutolanil (Nihon Nohyaku, Japan), thifluzamide (Monsanto), pyracarbolid (Hoechst, Germany), azoxystrobin, trifloxystrobin, fenpropidin, fludioxonil (Syngenta, Switzerland), tolylfluanid, fenhexamid, tebuconazole (Bayer, Germany), and fluazinam (ISK, Japan). Antimycin A, salicylhydroxamic acid (SHAM) and 2-thenoyltrifluoroacetone (TTFA) were purchased from Sigma-Aldrich. The structures of the tested carboxamides are given in Fig. SA1 in the supplemental material. These compounds were dissolved in ethanol, with the exception of iprodione and tolnaftate, which were solubilized in acetone, and then added to 50°C molten agar medium after autoclaving. The concentration of solvents in all media, including controls lacking fungicides, was 0.5% (vol/vol). At this solvent concentration in controls, assays revealed no significant change in strain growth in comparison to controls lacking ethanol or acetone (data not shown). For each respiratory inhibitor, we tested 5 to 10 concentrations, following a geometric progression with dose increments of ×2, ×2.5, or ×3, according to the fungicide and susceptibility range (46). Stock solutions, obtained after solubilizing 100 to 500 mg of inhibitor in 100 ml solvent, were stored at 4°C. Fresh dilution series were prepared for each experiment. The other fungicides were tested only at one or two discriminatory concentrations, previously chosen according to the response of known resistant phenotypes (23, 46, 61).
Fungicide susceptibility test.
The effect of fungicides was assessed on the spore germination and germ tube elongation of B. cinerea as previously described (46). For all inhibitors except carboxamides and TTFA, the medium contained 10 g glucose, 2 g K2HPO4, 2 g KH2PO4, and 12.5 g agar in 1 liter of deionized water. Previous studies (62, 66) and preliminary tests indicated that the in vitro toxicity of the SDHIs tends to be higher if the carbon source in the medium is acetate or succinate rather than glucose. We therefore replaced glucose with 4 g of sodium succinate dibasic hexahydrate (Sigma-Aldrich) in experiments involving carboxamides and TTFA. Moreover, the existence of an active alternative oxidase (68, 74) in B. cinerea, as in other fungi, made it necessary to add SHAM to in vitro tests involving inhibitors of complex III (i.e., strobilurins, antimycin A). We used a SHAM concentration of 0.5 mM. However, in our experimental conditions, this compound did not enhance carboxamide activity (data not shown).
Fungicides were added to the medium described above, which was then poured into 5.5-cm-diameter plastic petri dishes. We then dispensed 0.3 ml of a conidial suspension (200,000 conidia ml−1) onto the surface of agar plates with a pipette. The plates were incubated for 24 h in the dark at 19°C, and the percentage of spores germinating and the lengths of germ tubes (50 to 100 germinated conidia for each treatment) were estimated under a microscope, using a micrometer. Each test was replicated at least three times. Standard error was calculated and indicated an acceptable level of repeatability between replicates (data not shown).
Fungicides affecting respiration are powerful inhibitors of both germination and germ tube elongation. However, the effect of some of them, especially carboxamides, appears often transient toward germination. We therefore determined for each strain/fungicide couple an EC50 (i.e., a concentration causing a 50% decrease of fungicide in vitro efficacy) only for the germ tube elongation criterion. EC50s were determined after linear regression of the germ tube length (as percentage of control) plotted against the log10 of fungicide concentration. For a given fungicide, according to the response of susceptible (wild-type) strains, a mean EC50 could then be calculated. For each putative mutant strain, a resistance factor (RF) could be estimated as the ratio of the EC50 of the mutant to the mean EC50 of the wild type. In our experimental condition, a strain with an RF greater than or equal to 0.5 and less than 2 was considered susceptible, whereas RF values greater than 2 or less than 0.5, respectively, suggested resistance and hypersusceptibility (46).
DNA isolation.
Conidia from 1-week-old cultures on MYA medium (as described above) were used to inoculate 125 ml of the same liquid medium. The resulting suspension was cultured in an orbital shaker for 36 h at 20°C, and the fungus was then filtered through sterile muslin and rinsed in sterile water. Fungal material was frozen at −20°C for later use. DNA was extracted from this frozen material by a sarcosyl-based protocol (18).
Cytochrome b gene sequencing and molecular diagnosis of strobilurin resistance.
Primers Qo1 to Qo22 (see Table SA2 in the supplemental material) were designed based on the B. cinerea cytochrome b (cytb) gene sequence (accession number AB262969; 6,072 bp) and provided complete coverage of the entire gene for sequencing purposes. PCR was carried out with 25 ng genomic DNA, 200 μM each deoxynucleoside triphosphate (dNTP), 0.5 μM each primer, 10× titanium buffer, and 50× titanium Taq polymerase (Clontech) in a reaction volume of 50 μl. Amplifications were carried out as follows: initial denaturation at 95°C for 3 min, followed by 35 cycles at 95°C for 30 s, 60°C for 30 s, and 68°C for 1 min and a final extension at 68°C for 4 min. Fragments of the appropriate size were sequenced on both strands with a CEQ8000 Beckman Coulter sequencer. Sequences were assembled, translated, and aligned with CodonCode Aligner software (CodonCode Corporation), using the Muscle algorithm.
The cleavable amplified polymorphic sequences (CAPS) test was developed for quick and easy characterization of the G143A mutation, which is known to be the principal cause of resistance to strobilurins in many phytopathogenic fungi (21) in field populations. We digested 5 μl of unpurified PCR fragment, obtained with primers Qo13ext and Qo14ext, with the restriction enzyme SatI (isoschizomer Fnu4HI; Fermentas) at 37°C for 2 h, according to the manufacturer's recommendations. Digested fragments were visualized by electrophoresis in 2% agarose gels.
Population genetics analyses.
Only the population samples (169 isolates) collected from the Courteron and Vandières vineyards were analyzed with the Bc1, Bc2, Bc3, Bc4, Bc5, Bc6, Bc7, and Bc10 microsatellites developed by Fournier et al. (26). We assessed the allele range variability of all loci and developed a multiplex analysis procedure, with appropriate fluorochrome assignment. Multiplex PCR was first carried out as follows: multiplex PCR1 amplified Bc1 plus Bc9, multiplex PCR2 amplified Bc3 plus Bc6, multiplex PCR3 amplified Bc4 plus Bc10, and multiplex PCR4 amplified Bc2 plus Bc5 plus Bc7. We then carried out a fragment analysis of the PCR products on a CEQ8000 Beckman Coulter sequencer, as follows: one run for multiplex PCR4 products and one run for multiplex PCR1, -2, and -3 products pooled together. Diversity parameters were calculated with GenClone (http://ccmar.ualg.pt/maree/software.php?soft=genclon), Multilocus (2), and Genetix (9). We used Populations (http://bioinformatics.org/∼tryphon/populations/#ancre_fonctionnalites) software to reconstruct a tree showing the genetic relationships between the 169 individuals, using Nei's Da genetic distance (56) and a neighbor-joining clustering algorithm with 1,000 bootstrap replicates.
Sequencing of succinate dehydrogenase subunits.
Mitochondrial complex II comprises a flavoprotein (SdhA), an iron-sulfur protein (SdhB) with three iron-sulfur clusters (S1, S2, and S3), and two membrane-anchored proteins (SdhC and SdhD) (33). The SdhA and SdhB subunits form the soluble part of the complex, which has succinate dehydrogenase activity, whereas the SdhC and SdhD subunits anchor the SdhA and SdhB proteins in the mitochondrial membrane and display ubiquinone reduction activity, as previously described in Escherichia coli (75) and Saccharomyces cerevisiae (59).
The sdhB primers IpBcBeg and IpBcEnd2 (see Table SA2 in the supplemental material) were designed in a previous study (unpublished data) for NCBI accession numbers AY726618 (Botrytis group II) and AY726619 (Botrytis group I).
Other subunit sequences were obtained after tBLASTn analysis of NCBI accession numbers for Saccharomyces cerevisiae with the B. cinerea genome release from the Broad Institute (http://www.broad.mit.edu/annotation/genome/botrytis_cinerea/Home.html). This made it possible to isolate B. cinerea sequences displaying a high level of identity to the published S. cerevisiae sequences M86746, X73884, and L26333 for sdhA, sdhC, and sdhD, respectively. Sequences were retrieved from B. cinerea supercontigs 44, 143, and 4, respectively, based on Broad Institute genome release numbering. B. cinerea-specific primers were designed to bind to sdhA (primers SdhA1 to SdhA8), sdhC (primers SdhC1 to SdhC5) and sdhD (primers SdhD1 and SdhD2) (see Table SA2 in the supplemental material). PCR and sequencing protocols were as described for the cytochrome b gene, except that primers IpBcBeg and IpBcEnd2 were used with a hybridization temperature of 68°C.
RESULTS
Phenotypic characterization of fungal strains.
Among the 50 strains collected in French and German vineyards, three belonged to Botrytis group I (1 to 3) and the others to Botrytis group II (4 to 50). Resistance to botryticides was mainly found within group II (Table 1).
The three tested strains of Botrytis group I were susceptible to all fungicides tested except fenhexamid. It has been previously hypothesized that the natural tolerance of sterol C4 demethylation to this inhibitor is probably due to an increase in detoxification (47).
Some Botrytis group II strains were moderately to highly resistant to benzimidazoles, dicarboximides, anilinopyrimides, and/or fenhexamid. As previously shown, these specific resistances are determined by changes in the respective target genes of benzimidazoles (i.e., β-tubulin, a component of microtubules), dicarboximides (i.e., a histidine kinase involved in osmoregulation), and fenhexamid (i.e., a 3-keto reductase involved in sterol C4 demethylation). A similar mechanism has been proposed for anilinopyrimides, which have an unknown biochemical mode of action (23, 47). No specific resistance to fludioxonil was recorded.
In addition, several Botrytis group II strains displayed simultaneous decreases in susceptibility to various classes of fungicides and were classified into three MDR phenotypes. In all these strains, dicarboximides, anilinopyrimidines, and tolnaftate (an inhibitor of squalene epoxidase) were affected. This phenomenon extends to phenylpyrroles in MDR1 or to fenhexamid and sterol 14α-demethylase inhibitors (e.g., prochloraz, tebuconazole) in MDR2 (46). The MDR1 × MDR2 natural hybrid, called MDR3, displayed the broadest pattern of resistance (42, 47). These MDR strains are commonly found in Champagne and German vineyards. Their resistance patterns were recently found to be determined by overproduction of one to two membrane drug efflux proteins (42). Four representatives of each type were selected for this study: MDR1 (strains 11 to 14), MDR2 (strains 15 to 18), and MDR3 (strains 19 to 22) (Table 1).
Resistance to inhibitors of mitochondrial complex III.
In the presence of SHAM, low concentrations of azoxystrobin, dimoxystrobin, pyraclostrobin, trifloxystrobin, and antimycin A inhibited germ tube elongation of susceptible strains of Botrytis group II. Moreover, Botrytis group I strains were generally more susceptible than group II strains to these inhibitors of complex III, in particular to antimycin A and azoxystrobin (Table 2; see also Table SA3 in the supplemental material).
TABLE 2.
In vitro response of various B. cinerea phenotypes to fungicides affecting respiration
| Fungicides affecting respiration | EC50 (μM)a for susceptible strains of Botrytis from group: |
Resistance factorb of Botrytisgroup II strains with specific resistance to: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | Strobilurins (StrR)c | Carboxamides |
||||||
| CarR1 | CarR2 | CarR3 | CarR4 | CarR5 | CarR6 | ||||
| No. of strains | 3 | 5 | 2 | 2 | 12 | 1 | 1 | 2 | 1 |
| QoIs | |||||||||
| Azoxystrobin | 0.12 | 0.33 | >75 | 1.49 | 1.12 | ||||
| Dimoxystrobin | 0.013 | 0.014 | >100 | 1.30 | 1.00 | ||||
| Pyraclostrobin | 0.012 | 0.014 | >445 | 1.34 | 1.07 | ||||
| Trifloxystrobin | 0.006 | 0.012 | >500 | 1.40 | 0.85 | ||||
| QiI | |||||||||
| Antimycin A | 0.0047 | 0.015 | 0.64 | 1.00 | 1.04 | 1.28 | 1.03 | 1.09 | 1.22 |
| Uncoupler | |||||||||
| Fluazinam | 0.0097 | 0.026 | 1.04 | 1.08 | 1.22 | 1.67 | 1.25 | 0.92 | 1.25 |
| Multisite inhibitor | |||||||||
| Tolylfluanid | 0.067 | 0.31 | 1.85 | 1.48 | 1.10 | 1.85 | 1.39 | 1.30 | 1.06 |
| SDH inhibitor | |||||||||
| TTFA | 76.6 | 68.5 | 0.89 | 1.11 | 1.05 | 1.32 | 1.32 | 0.92 | 0.95 |
Mean EC50s calculated from three strains of Botrytis group I (1 to 3) and from five strains of Botrytis group II (4 to 8) (see Table 1).
Within Botrytis group II, for each phenotype, the resistance factor was calculated as the ratio of the EC50 of resistant strains to the mean EC50 of sensitive strains.
Mean RF values obtained with two StrR strains (9 and 10); similar values were recorded with strobilurins for the CarR strains cumulating resistance toward this class of QoIs and carboxamides (see Table 1).
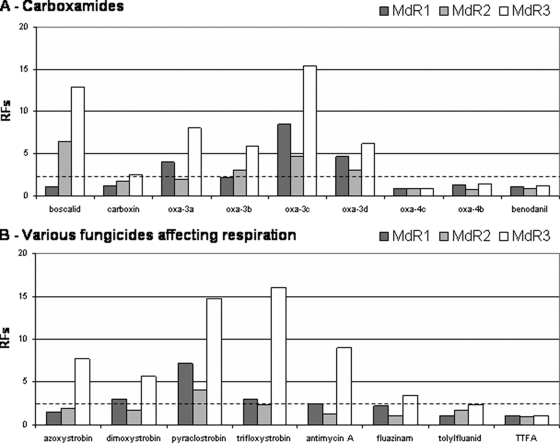
Three groups of Botrytis group II strains were defined on the basis of the EC50s recorded for pyraclostrobin: susceptible (EC50 below 0.025 μM), weakly resistant (EC50 between 0.05 and 0.3 μM), and highly resistant (EC50 > 10 μM) (see Table SA3 in the supplemental material). All the weakly resistant strains were multidrug resistant, and cross-resistance to other tested strobilurins (i.e., azoxystrobin, dimoxystrobin, trifloxystrobin) and to antimycin A was recorded only for MDR3 strains (Fig. 1). Moreover, these MDR3 strains were less susceptible toward the uncoupler fluazinam and the multisite inhibitor tolylfluanid. In strains highly resistant to pyraclostrobin, positive cross-resistance was observed with azoxystrobin, dimoxystrobin, and trifloxystrobin but not with antimycin A, fluazinam, and tolylfluanid (Table 2). These strains are referred to as StrR strains (Table 1; see also Table SA3 in the supplemental material).
FIG. 1.
In vitro responses of multidrug-resistant strains from Botrytis group II toward respiratory inhibitors. For given MDR strains, the RF equals the ratio of the EC50s of MDR strains to the mean EC50 of the wild type (see Materials and Methods). Mean RF values were calculated using four strains for each MDR category (Table 1).
Molecular analysis of cytb.
The full sequence of the mitochondrially encoded cytochrome b (characteristics in Table 3) was determined for three susceptible strains (7, 8, and 35) and three strobilurin strains (10, 25, and 32) from the Botrytis group II species. Strains 1, 2, and 3 were sequenced for comparison with the Botrytis group I species. No nucleotide polymorphism differentiating between the two Botrytis species was found in cytb sequences from the susceptible strains. Within Botrytis group II, very little polymorphism was observed in comparison to the available sequences of strains T4 and B05.10. The predicted amino acid sequence revealed high similarity to the cytochrome b sequences of other fungal species, namely, 84% identity to Aspergillus niger, 89% to Venturia inaequalis, 62% to S. cerevisiae, and 86% to Mycosphaerella graminicola. In the strobilurin-resistant strains, a mutation at codon 143 replacing a glycine residue by an alanine residue (G143A) was identified (see Table 6; Fig. 2).
TABLE 3.
Characteristics of the genes sequenced in Botrytis groups I and II
| Gene | Coverage (bp)a | Gene size (bp)b | No. of intronsc | % identityd | % similaritye | No. of polymorphismsf (%) | Accession no.g |
|---|---|---|---|---|---|---|---|
| Cytochrome b | 5,830 or 7,035 | 5,403 or 6,608 | 3 or 4 | 62 | 80 | 0 (0) | FJ217740, FJ217741, FJ217742, FJ217743, FJ217744 |
| Succinate dehydrogenase subunit A | 2,560 | 2,209 | 6 | 73 | 86 | 49 (1.9) | FJ217745, FJ217746 |
| Succinate dehydrogenase subunit B | 1,021 | 953 | 1 | 70 | 83 | 10 (1.0) | AY726618, AY726619, GQ253444, GQ253445, GQ253446, GQ253447, GQ253448, GQ253449 |
| Succinate dehydrogenase subunit C | 1,139 | 712 | 2 | 32 | 51 | 11 (1.0) | GQ253442, GQ253443 |
| Succinate dehydrogenase subunit D | 1,175 | 683 | 2 | 35 | 52 | 8 (0.7) | GQ253439, GQ253440, GQ253441 |
Size of the sequenced contig.
Size of the gene, from methionine to the stop codon, including introns.
Number of introns according to the Broad Institute prediction (B05.10 genome). For cytb, sequenced genomes (strains B05.10 and T4) don't exhibit the additional intron after codon 143.
Amino acid sequence identity between the strobilurin- or carboxamide-susceptible group II sequence and the corresponding Saccharomyces cerevisiae gene sequence.
Amino acid sequence similarity between the strobilurin- or carboxamide-susceptible group II sequence and the corresponding Saccharomyces cerevisiae gene sequence.
Number of polymorphic sites between Botrytis group II and Botrytis group I.
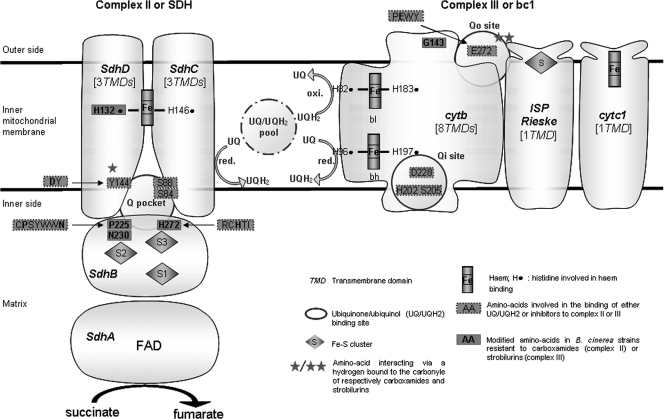
FIG. 2.
Schematic structure of mitochondrial complexes II and III indicating the main amino acids of the different subunits involved in binding of inhibitors and in resistance. Complex II contains four subunits, whereas complex III consists of 10 to 11 polypeptides. For complex III, only three main subunits (i.e., Cytb, cytochrome b; ISP Rieske, iron sulfur protein with a Rieske-type cluster; cytc1, cytochrome c1) directly involved in the electron transfer are shown. The amino acids involved in the binding of substrates (i.e., ubiquinone or ubiquinol) or of inhibitors were identified in crystallographic studies involving complex II or III from various origins (20, 28, 33, 35, 36, 59, 75). Amino acids are numbered according to B. cinerea sequences.
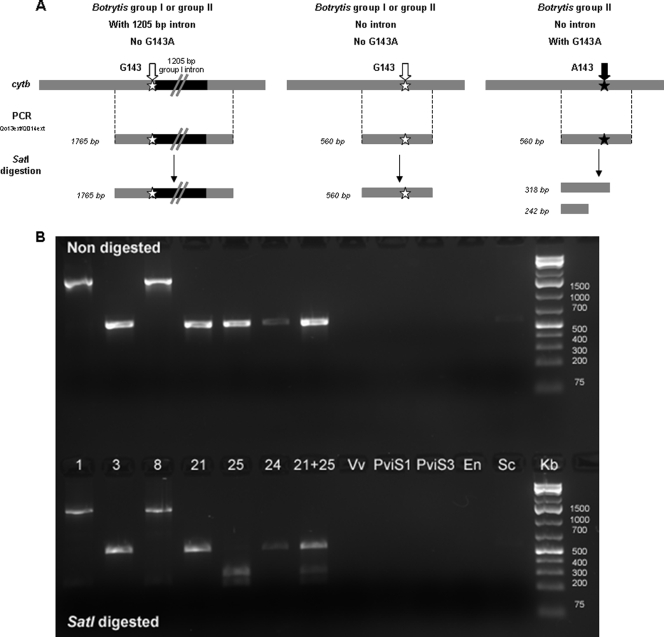
We developed a CAPS test using the SatI restriction enzyme, which recognizes, within a 560-bp fragment amplified with the Qo13ext and Qo14ext primers, the 5′-GC |GC-3′ nucleotide pattern, for rapid detection of the G143A alteration in the population data set from Courteron and Vandières. In the presence of the mutation, a typical two-band profile was observed, with fragments of 318 bp and 242 bp detected. No amplification was observed with DNA from grapevine Vitis vinifera or from several fungi and oomycetes usually present on this plant, such as Erysiphe necator, Plasmopara viticola, and S. cerevisiae, confirming the high level of specificity of this diagnostic tool (Fig. 3). This test showed the G143A substitution to be present at a frequency of 5.9% (10 isolates) in the field populations collected in Champagne. This substitution was found only in Botrytis group II strains (Table 4). Moreover, in the data set shown in Table 1, the StrR phenotype was always associated with G143A (Table 4; see also Table 6).
FIG. 3.
Molecular polymorphism of the gene encoding cytochrome b (cytb) in strains of Botrytis group I and group II. (A) Possible structure of cytb according to the presence of the 1,205-bp group I intron and/or the G143A change and possible results observed after CAPS analyses. Presence of the intron changes the length of the PCR fragment, either in Botrytis group I or II. The G143A change was found only in Botrytis group II strains without the 1,205-bp intron. The mutation in cytb is recognized by the SatI restriction enzyme and leads to a two-band pattern after digestion of the PCR fragment. (B) Ethidium bromide-stained gel of PCR fragments subjected to electrophoresis after Qo13ext/Qo14ext amplification of the cytochrome b gene in Botrytis group I and group II (first row) and after SatI digestion of these fragments (second row) (CAPS test). Strains 1 and 3, Botrytis group I with and without intron, respectively; strains 8, 21, 24, and 25, Botrytis group II. Strain 25 is strobilurin resistant (Table 1). Strain 8 exhibits an intron after codon 143. Vv, Vitis vinifera (grapevine); PviS1 and PviS3, Plasmopara viticola (downy mildew; PViS3 is strobilurin resistant); En, Erysiphe necator (powdery mildew); Sc, Saccharomyces cerevisiae (yeast).
TABLE 4.
Distribution of occurrence of a 1,205-bp intron in the cytb gene of a Botrytissp. population sample from a Champagne vineyard
| Group | Intron | No. of strains | % frequency | No. of StrR strains | Ga | G/Nb | No. of alleles/locic | Genotypic diversityd |
|---|---|---|---|---|---|---|---|---|
| B. cinerea group II | + | 13 | 7.7 | 0 | 13 | 1.00 | 5.38 | 1.000 |
| − | 129 | 76.3 | 10 | 111 | 0.86 | 10.00 | 0.997 | |
| Botrytis group I | + | 26 | 15.4 | 0 | 22 | 0.85 | 4.00 | 0.985 |
| − | 1 | 0.6 | 0 | 1 | 1.00 | 1.00 | 1.000 | |
| Total group II | 142 | 84.0 | 10 | 122 | 0.86 | 10.63 | 0.997 | |
| Total group I | 27 | 16.0 | 0 | 23 | 0.85 | 4.25 | 0.985 | |
| Total | 169 | 100 | 10 | 145 | 0.86 | 11.75 | 0.998 |
G, number of multilocus genotypes, i.e., number of unique combinations of microsatellite alleles.
G/N, frequency of unique multilocus genotypes in each category of isolates.
Mean number of microsatellite alleles per locus. Calculated with GenClone software.
Genotypic diversity calculated as the probability that two individuals taken at random have different genotypes. Calculated with Multilocus software, using 1,000 iterations.
Variable occurrence of an intron in cytb.
Regarding the size of the cytb gene, two categories were identified: strains with a 5,403-bp cytb gene and strains with a 6,608-bp sequence (Table 3). No isolate presented both fragments in the CAPS test. Sequencing revealed this difference to be due to the variable presence of a 1,205-bp intron located after codon 143 in both Botrytis group II (strains 7 and 8) and Botrytis group I (strains 1 and 2) strains (Fig. 3). This intron was not predicted in the two available Botrytis genome sequences (strains B05.10 and T4). In our population sample, 23.1% of the strains had this intron: 9.2% of Botrytis group II strains and 96.3% of Botrytis group I strains (Table 4). The G143A substitution was only found in Botrytis group II strains that did not harbor this intron.
We analyzed the evolution of intron-containing and intron-free versions of cytb in Botrytis groups I and II by investigating the relationship between the cytb genotypes and checking whether the presence of the type I intron discriminated between subpopulations. For this, field populations collected from Champagne vineyards were genotyped with the eight microsatellites listed in Table SA1 in the supplemental material. Botrytis groups I and II could be distinguished on the basis of the private allele 86 at the Bc6 locus, which was typical of Botrytis group I species. The cytb genotype was determined based on the length of the Qo13ext/Qo14ext PCR product and the result of the CAPS test. The four categories of strains (Botrytis group II with and without the intron after codon 143 and Botrytis group I with and without the intron after codon 143) were highly diverse, with more than 85% unique microsatellite profiles (G/N) and high levels of genotypic diversity. Only a few clones with identical microsatellite profiles were identified, consistent with the populations being in Hardy-Weinberg equilibrium, with recombination and/or migration occurring regularly (Table 4). As expected, pairwise calculations of the population differentiation (Fst) (71) between Botrytis group I and group II strains harboring the intron indicated high levels of differentiation between the two species (e.g., Fst = 0.488, highly significant after Bonferroni correction; a difference of the same order of magnitude was observed when Botrytis group I and group II strains were compared globally, independently of the presence of the intron). In contrast, the pairwise Fst between the two populations harboring different cytb genotypes within a single species was not significant (e.g., Fst = 0.032, not significant after Bonferroni correction, between Botrytis group II strains with and without the intron). These data indicate unrestricted gene flow between strains with intron-containing and intron-free cytb.
We calculated the Da genetic distance between all pairs of individuals and used it to reconstruct a neighbor-joining tree for distance (see Fig. SA2 in the supplemental material). The tree highlights the differentiation of strains from the two species, with the longest branch isolating the Botrytis group I strains. This is mostly due to the group I private alleles in the Bc3, Bc4, and Bc6 microsatellites (see Table SA1 in the supplemental material). Finally, within a given species, no clear differences were found between strains with and without the type I intron after codon 143, this intron being randomly distributed in different lineages.
Resistance to inhibitors of mitochondrial complex II.
Boscalid, the only carboxamide authorized for use against gray mold, prevented B. cinerea spore germination fully only at high concentrations, whereas it inhibited germ-tube elongation at low concentrations, particularly if biological tests were conducted in media containing succinate rather than glucose as the carbon source (data not shown). The tested strains of B. cinerea could be classified into categories on the basis of the EC50s for germ tube elongation. The most susceptible strains (EC50s below 0.2 μM) belonged to Botrytis group I (strains 1 to 3). The values for the wild-type (4 to 8), StrR (9 to 10), and MDR1 (11 to 14) strains in Botrytis group II were similar. Strains with EC50s between 0.5 and 2 μM included the multidrug-resistant MDR2 (15 to 18) and MDR3 (19 to 22) strains. A slight decrease in susceptibility to carboxin was observed only in MDR3 strains, whereas resistance to 3′-substituted carboxin analogs was observed for all MDR strains. Moreover, the other tested carboxamides had similar effects on wild-type and MDR strains (Fig. 3; see also Table SA3 in the supplemental material). Other strains were either moderately (EC50s of 2.5 to 10 μM) or highly (EC50s above 30 μM) resistant to boscalid. They were subdivided into various carboxamide resistance (CarR) classes on the basis of the effects in vitro of the other carboxamides from Table 5, such as the benzamides (i.e., benodanil and flutolanil) and 3′-benzyl derivative of carboxin oxa-3b. We considered hypersusceptibility when the RF was less than 0.5, normal susceptibility when the RF was greater than or equal to 0.5 and less than 2, weak resistance when the RF was greater than or equal to 2 and less than 10, moderate resistance when the RF was greater than or equal to 10 and less than 100, and strong resistance when the RF was greater than or equal to 100.
TABLE 5.
In vitro susceptibility of various B. cinerea phenotypes to carboxamide fungicides
| Carboxamides subgroupa | EC50 (μM)b for susceptible strains of Botrytis from group: |
Resistance factorc of Botrytis group II strains with specific resistance to: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | Strobilurins (StrR) | Carboxamides |
||||||
| CarR1 | CarR2 | CarR3 | CarR4 | CarR5 | CarR6 | ||||
| No. of strains | 3 | 5 | 2 | 10 | 12 | 1 | 1 | 2 | 2 |
| Pyridine | |||||||||
| Boscalid | 0.034 | 0.10 | 0.86 | 40.0 | 44.1 | 57.1 | 91.4 | >350 | >350 |
| Benzamides | |||||||||
| Flutolanil | 27.9 | 26.3 | 1.29 | 0.18 | 0.97 | 1.41 | 1.76 | 1.76 | 1.53 |
| Benodanil | 9.60 | 10.8 | 0.77 | 0.34 | 1.49 | 2.29 | 2.00 | 2.14 | 2.00 |
| Furans | |||||||||
| Furcarbanil | 6.03 | 7.44 | 1.00 | 2.06 | 2.00 | 1.56 | 4.69 | 5.00 | 0.75 |
| Methfuroxam | 13.0 | 13.1 | 0.83 | 3.67 | 2.83 | 4.00 | 4.00 | 4.00 | 5.00 |
| Thiazoles | |||||||||
| Thifluzamide | 3.80 | 7.58 | 1.25 | >5.0 | >5.0 | >5.0 | >5.0 | >5.0 | >5.0 |
| Dihydropyran | |||||||||
| Pyracarbolid | 30.9 | 28.6 | 1.05 | >3.3 | >3.3 | >3.3 | >3.3 | >3.3 | >3.3 |
| Oxathiin | |||||||||
| Carboxin | 1.30 | 2.04 | 1.15 | 7.48 | 6.56 | 2.50 | 18.8 | >21 | 3.33 |
| oxa-3a | 0.16 | 0.16 | 1.40 | 6.00 | 7.00 | 8.00 | 18.0 | 70.0 | 8.00 |
| oxa-3b | 0.12 | 0.18 | 1.33 | 8.33 | 10.0 | 1.67 | 23.3 | 41.7 | 0.83 |
| oxa-3c | 0.29 | 0.42 | 1.15 | 19.2 | 16.2 | 11.5 | 61.5 | 76.9 | 3.85 |
| oxa-3d | 0.25 | 0.40 | 1.15 | 4.62 | 3.85 | 3.85 | 11.5 | 6.15 | 1.54 |
| oxa-4ad | 4.20 | 7.37 | 1.09 | >1.1 | >1.1 | >1.1 | >1.1 | >1.1 | 0.22 |
| oxa-4b | 6.52 | 7.49 | 1.09 | 1.74 | 1.52 | 0.43 | 1.74 | 1.74 | 0.35 |
See the structure of the molecules in Fig. SA1 in the supplemental material.
Mean EC50s calculated from three strains of Botrytis group I (strains 1 to 3) and five strains of Botrytis group II (strains 4 to 8) (see Table 1).
Within Botrytis group II, for each phenotype, resistance factors were calculated as the ratio of the EC50 of resistant strains to the mean EC50 of susceptible strains.
Due to its low water solubility, this compound cannot be tested at a concentration above 8 μM.
CarR1 had moderate resistance to boscalid, weak resistance to oxa-3b, and hypersusceptibility (i.e., negative cross-resistance) to benzamides (strains 23 to 32). CarR2 had moderate resistance to boscalid, weak resistance to oxa-3b, and an absence of negative cross-resistance to benzamides (strains 33 to 44). CarR3 had moderate resistance to boscalid and normal sensitivity to oxa-3b (strain 45). CarR4 had moderate resistance to boscalid and to oxa-3b (strain 46). CarR5 had strong resistance to boscalid and moderate resistance to oxa-3b (strains 47 and 48). CarR6 had strong resistance to boscalid and normal susceptibility to oxa-3b (strains 49 and 50).
Cross-resistance between boscalid and carboxin (oxathiin derivative) occurred in all phenotypes. Experimental oxathiins, with substitutions at position 3′ in the phenyl ring (oxa3a to -c; see Fig. SA1 in the supplemental material), were 5 to 13 times more toxic to wild-type strains than carboxin. Overall resistance toward carboxamides was stronger in the CarR4 and CarR5 strains than in the CarR3 and CarR6 strains, with no cross-resistance (RFs between 0.5 and 2.0) to the 3′-benzyl derivatives oxa-3b in CarR3 and CarR6 and to oxa3d in CarR6. The two oxathiin derivatives substituted at position 4′ (oxa-4a and -b; see Fig. SA1 in the supplemental material) were less toxic than the derivatives described above and were most active against CarR6 strains (Table 5).
The other carboxamide fungicides tested (i.e., furcarbanil, methfuroxam, pyracarbolid, thifluzamide) were moderately toxic to wild-type strains (EC50 > 7 μM). Positive cross-resistance between these compounds and boscalid was recorded in all CarR strains (except for furcarbanil in CarR6 strains), generally with low RFs (Table 5).
Finally, TTFA, a noncarboxamide inhibitor of the succinate dehydrogenase complex, displayed only weak toxicity against all the B. cinerea strains tested (Table 2).
Molecular analysis of genes encoding succinate dehydrogenase subunits.
The genes encoding the four subunits, sdhA, sdhB, sdhC, and sdhD, were sequenced in the two Botrytis species, and their main characteristics are presented in Table 3.
Numerous polymorphic locations were identified in each of the four genes but only between, not within, Botrytis group I and group II strains. Most of these polymorphisms were located in the introns or flanking sequences and did not affect the amino acid sequence, except for the P40S (Table 6) substitution identified in SdhB, which may be associated with the greater susceptibility of Botrytis group I strains to boscalid (Table 5).
TABLE 6.
Alterations found in cytb, sdhB, and sdhD in various phenotypes of B. cinerea resistant to respiration inhibitors
| Strain no. | Botrytis groupa | Phenotypeb |
Molecular cytb characterization |
Alteration in: |
|||
|---|---|---|---|---|---|---|---|
| Str | Car | Intron after codon 143c | G143Ad | sdhB | sdhD | ||
| 1 | I | S | S | + | − | P40S | |
| 2 | I | S | S | + | − | P40S | |
| 3 | I | S | S | − | − | P40S | |
| 5 | II | S | S | − | − | ||
| 7 | II | S | S | + | − | ||
| 8 | II | S | S | + | − | ||
| 10 | II | R | S | − | + | ||
| 11 | II | S | S | − | − | ||
| 12 | II | S | S | − | − | ||
| 13 | II | R | S | − | + | ||
| 17 | II | S | S | − | − | ||
| 18 | II | S | S | − | − | ||
| 19 | II | S | S | − | − | ||
| 21 | II | S | S | − | − | ||
| 23 | II | S | R1 | − | − | H272Y | |
| 24 | II | S | R1 | − | − | H272Y | |
| 25 | II | R | R1 | − | + | H272Y | |
| 26 | II | R | R1 | − | + | H272Y | |
| 27 | II | R | R1 | − | + | H272Y | |
| 28 | II | R | R1 | − | + | H272Y | |
| 29 | II | R | R1 | − | + | H272Y | |
| 30 | II | R | R1 | − | + | H272Y | |
| 31 | II | R | R1 | − | + | H272Y | |
| 32 | II | R | R1 | − | + | H272Y | |
| 33 | II | R | R2 | − | + | H272R | |
| 34 | II | R | R2 | − | + | H272R | |
| 35 | II | R | R2 | − | + | H132R | |
| 36 | II | R | R2 | − | + | ||
| 37 | II | R | R2 | − | + | H272R | |
| 38 | II | R | R2 | − | + | H272R | |
| 39 | II | R | R2 | − | + | H272R | |
| 40 | II | R | R2 | − | + | H272R | |
| 41 | II | R | R2 | − | + | ||
| 42 | II | R | R2 | − | + | H272R | |
| 43 | II | R | R2 | − | + | H272R | |
| 44 | II | R | R2 | − | + | H272R | |
| 45 | II | R | R3 | − | + | P225T | |
| 46 | II | R | R4 | − | + | N230I | |
| 47 | II | R | R5 | − | + | H272L | |
| 48 | II | R | R5 | − | + | H272L | |
| 49 | II | S | R6 | − | − | P225L | |
| 50 | II | R | R6 | − | + | P225L | |
Botrytis group I and II strains were differentiated on the basis of genotype and their response to sterol biosynthesis inhibitors, including fenhexamid and fenpropidin (25, 46).
S, sensitive; R, resistant.
+, 1,205-bp intron present after codon 143; −, absence of this intron.
+, Qo13ext-Qo14ext PCR fragment digested with SatI (two-band pattern), indicating the presence of the G143A change; −, Qo13ext-Qo14ext PCR fragment undigested (single-band pattern), indicating the lack of mutation.
Within Botrytis group II, most of the changes observed occurred within the second (S2) and third (S3) cysteine-rich clusters of the iron-sulfur protein, SdhB (Table 6; Fig. 2). Isolates harboring a mutation in codon 272, affecting the third Fe-S cluster region, were the most frequent in our sampling, and three potential resistant alleles associated with different phenotypes were identified. All CarR1 strains displayed the replacement of the histidine residue by a tyrosine residue at position 272 (H272Y). The replacement of this histidine residue by an arginine residue (H272R) was observed in nine of the 12 CarR2 strains, and the H272L substitution was associated with CarR5 strains. Changes in the second cysteine-rich cluster at position 225 were observed in one CarR3 strain (P225T) and in two CarR6 strains (P225L). A third site of alteration was found in the third exon of sdhB in the CarR4 strains (N230I). The phenotype-genotype correlation was not complete for CarR2 strains: 2 strains (36 and 41) had wild-type sequences for the four subunits of succinate dehydrogenase, and strain 35 harbored the H132R substitution in the SdhD anchor subunit.
Other fungicides affecting respiration.
Low concentrations of the uncoupler fluazinam inhibited conidial germination and germ tube elongation (in media containing glucose and in the absence of SHAM). The most susceptible strains were found among Botrytis group I species and the least susceptible strains were the multidrug-resistant MDR1 and MDR3 strains. Smaller differences between these strains were observed for multisite inhibitors, such as captafol, chlorothalonil (data not shown), and tolylfluanid (Table 2; see also Table SA3 in the supplemental material; Fig. 3).
DISCUSSION
Specific resistance to strobilurins is correlated with a single mutation in cytb.
Up to 77 sites within cytb may be altered in eukaryotes and prokaryotes (10). In phytopathogenic fungi, only the F129L, G137R, or G143A substitution has been recorded in field strains resistant to strobilurins (21). In our data for B. cinerea, only the G143A alteration was found in all resistant phenotypes, either by direct sequencing or by CAPS analysis. It was identified in up to 27 phytopathogenic fungi listed by the Fungicide Resistance Action Committee (FRAC) (http://www.frac.info/frac/index.htm) and in reference 21. In susceptible strains, the glycine in position 143 probably contributes to the binding of the benzene ring linker of strobilurin fungicides to the Qo site of cytochrome b (Fig. 2) (20, 28). In our study, there was a complete correlation between the StrR phenotype and the G143A substitution. We therefore suggest that target alteration was the only mechanism of resistance operating in our data set. However, these results differ from those of a Japanese survey, which reported the absence of the typical G143A mutation from many StrR isolates collected from strawberries or citrus crops (38), consistent with an unknown mechanism of resistance, as reported for other fungi (22, 67, 70). Moreover, in our fungicide susceptibility test, strobilurins were active against susceptible strains only if SHAM was added to inhibit the alternative oxidase. This suggests that the alternative oxidase is constitutively expressed in vitro in wild-type strains, as previously reported (68), and therefore cannot mediate the resistance of StrR isolates. This finding may also account for the low field efficacy of strobilurins against gray mold and explains why they are not approved for gray mold control in vineyards. However, the fungicides of this family have been widely used to control downy mildew due to P. viticola and powdery mildew due to E. necator on grapevine, through one to three treatments per year, since their introduction in 2001 in France. As a consequence, the G143A mutation is now widespread in most French populations of P. viticola, within two different cytb haplotypes (15), resulting in poor disease control when strobilurins are used alone. In contrast, resistance associated with the same cytb alteration took longer to be selected in E. necator and was first recorded in France in 2008 (19), highlighting the dependence of the risk of resistance on the biology of the fungus. Thus, in grapevine, strobilurin resistance in B. cinerea populations may be the result of nontarget spraying unintentionally selecting the G143A alteration in cytb.
Variations in cytb structure.
Strobilurin resistance was found only in Botrytis group II strains without the 1,205-bp intron after codon 143 of cytb. This intron, frequently found in fungal mitochondrial genes, was described as a type I intron (24). Such variations in the size of the cytochrome b gene have already been reported for S. cerevisiae (58), for which a deletion of 3.4 kb has been detected in some isolates. It was recently shown that the structure of cytb, including the presence or absence of an intron after codon 143, differs between species (30, 31). In many rusts and in some Alternaria and Agrocybe spp. (55), together with Pyrenophora teres (63), an intron was found after codon 143 that prevented the selection of G143A in cytb, owing to a defect in pre-mRNA splicing leading to the creation of an intron encoding a maturase, as described in yeast (14, 51). However, other mutations conferring lower levels of resistance may be selected in these fungi, such as F129L in P. teres (30). These findings may account for the presence of the G143A substitution only in strains lacking the intron in Botrytis group II in our sample and in Californian and Chinese isolates (40) and in Japanese strains isolated from various host plants for which no fitness cost associated with strobilurin resistance could be found (7). In our population data set, G143A was not found in any of the 27 group I strains. Finally, in our data set from the Champagne vineyard, we found that the cytb 143/144 intron was carried by different lineages within each species and that it was more frequent in Botrytis group I populations than in group II populations. Within a single species, the presence of this intron does not seem to be a barrier to gene flow and should not prevent strains of different cytb genotypes from mating. Based on the findings of an ongoing phylogenetic study, Botrytis group II species may be derived from Botrytis group I species (data not shown). Recurrent independent losses of the intron from B. cinerea group II populations, according to the retro-deletion mechanism previously described in yeast (27), may have occurred on several occasions after divergence from Botrytis group I, facilitating the subsequent acquisition of the G143A substitution in these lineages, in response to fungicide selection pressure. This hypothesis could be tested by sequencing this gene in several strains from different Botrytis species.
Specific resistance to carboxamides is correlated with various mutations in sdh genes encoding Sdh subunits.
Resistance to carboxamides was first described some years ago for those effective against Basidiomycetes: field resistance to carboxin was observed in Ustilago nuda growing on barley (45) and was found to be partially dominant (57), whereas resistance to oxycarboxin was recorded in the causal agent of chrysanthemum rust, Puccinia horiana (1). More recently, resistance to boscalid has been observed in field strains of B. cinerea and of various phytopathogenic fungi from Ascomycota (65). Double resistance to these carboxamides and to strobilurins has been reported in many strains of B. cinerea (Table 1) and is also commonly found in Alternaria alternata (5). Resistance to carboxamides in field or laboratory mutants of many fungi and bacteria is generally determined by mutations in genes encoding the succinate dehydrogenase subunit SdhB, SdhC, or SdhD. These three subunits contribute to the binding of ubiquinone and carboxamide fungicides (Fig. 2) (29, 35, 36, 59, 75).
The highly conserved histidine residue (equivalent to H272 in B. cinerea) in iron sulfur cluster S3 of SdhB (Fig. 2) probably interacts with the O3 methoxy group of ubiquinone via hydrogen bonding. A similar phenomenon probably occurs with a heteroatom of most carboxamides (e.g., oxygen for oxathiin, furan, and pyran derivatives; nitrogen for pyrazole, pyridine, and thiadiazole derivatives) (35). The behavior of benzamide derivatives, which contain no such heteroatom (see Fig. SA1 in the supplemental material), remains to be determined. In many laboratory mutants of bacteria and fungi, this histidine residue has been replaced by a tyrosine, an asparagine, or a leucine residue (12, 34, 49, 53, 62, 64). More recently, field isolates of phytopathogenic fungi resistant to boscalid and carrying a mutation at this codon have been detected in populations of Corynespora cassiicola on cucurbits in Japan (H278Y) (37, 54) and of A. alternata on pistachios in the United States (H277Y/R) (6).
For Botrytis group II, we found that the three possible alleles, H272Y, H272R, and H272L, seemed to induce different phenotypes. The H272Y substitution, found within the CarR1 phenotype, is the most frequent genotype in French and German vineyards, and negative cross-resistance leads to an increase in susceptibility to the benzamides tested in this (i.e., flutolanil, benodanil) and other (i.e., fluopyram, mepronil) studies (43). This phenomenon has already been reported for some U. nuda field mutants of unknown genotype (45). These observations suggest that the binding of benzamides to succinate dehydrogenase is increased by the H272Y substitution, indirectly confirming the specific interaction of this class of carboxamides with SdhB. The other substitutions, H272R and H272L, found, respectively, in CarR2 and CarR5 strains, did not determine negative cross-resistance to any carboxamides. Moreover, the highest RFs were recorded for CarR5 strains. A similar laboratory mutant of Ustilago maydis harboring the H257L substitution was found to be highly resistant to carboxin and to display no negative cross-resistance to other carboxamides (41, 73).
All currently identified modifications affecting the second iron-sulfur complex (S2) of SdhB seem to be Botrytis specific. Most concern the proline residue in position 225, which is located within the highly conserved CPSYWWN motif. This proline residue and the two tryptophan residues probably contribute to carboxamide binding through hydrophobic interactions of the aniline group. Moreover, the tryptophan SdhB W229 seems to be a direct ligand of a carbonyl oxygen from ubiquinone or carboxamide (Fig. 2) (36, 59, 75). The substitutions P225T and P225L were observed, respectively, in CarR3 and CarR6 strains. A third possible allele, with a P225F modification at this codon, has also been identified in German isolates highly resistant to boscalid (65). In strains harboring the P225T or P225L substitution, resistance to carboxin was weak and some carboxin derivatives bearing phenyl substitutions at positions 4′ led to an increase in susceptibility. Such negative cross-resistance has also been reported for some laboratory mutants of U. maydis and Aspergillus nidulans of unknown genotype displaying moderate resistance to carboxin (72, 73). Finally, we identified, in a single CarR4 strain, a point mutation at codon 230 of SdhB (N230I) not found in the carboxamide-resistant mutants of any other fungus. The asparagine residue in position 230 of SdhB belongs to the highly conserved CPSYWWN motif described above but is not thought to be involved in carboxamide binding to SdhB (Fig. 2) (35).
The anchor subunit SdhC contributes to the binding of ubiquinone probably via hydrogen bound between its O4 carbonyl group and the highly conserved serine residue in position 84 in B. cinerea. A strong interaction of carboxin with the corresponding amino acid residue in Escherichia coli was recorded (35). Moreover, in the same domain, the highly conserved arginine (SdhC R88 in B. cinerea) contributes to the binding of ubiquinone (Fig. 2) (35, 59). Mutations in sdhC were previously found in several laboratory and field mutants (29, 39, 62). Moreover, a recent report indicated that the H134R mutation is prevalent in SdhC of A. alternata collected from pistachios (65). This histidine (position 144 in B. cinerea) is a highly conserved residue involved in the iron coordination of heme b (Fig. 2) (59, 75); a second axial histidine residue is supplied by the SdhD subunit (SdhD H132 in B. cinerea; see below).
In the anchor subunit, SdhD, the highly conserved tyrosine residue at position 144 in B. cinerea is a major binding partner for ubiquinone and carboxamides. The carbonyl group of these fungicides (see Fig. SA1 in the supplemental material) and the O1 carbonyl atom of ubiquinone interact with this tyrosine residue via hydrogen bonds (35, 36). In laboratory mutants of Paracoccus denitrificans (52) and Aspergillus oryzae (62) selected on carboxin, the conserved aspartate flanking the previous tyrosine (codon 143 in B. cinerea) was replaced either by a glycine or a glutamate (52, 62). In B. cinerea, the replacement of a histidine residue in position 132 by an arginine residue was found in one strain with the CarR2 phenotype. Moreover, the same H132R substitution was recently found in a boscalid-resistant strain of the related species Sclerotinia sclerotiorum collected on rapeseed in France (29). As stated above, the H132 residue of SdhD and the H144 residue of SdhC are involved in the iron coordination of heme b (Fig. 2). Their role in electron transfer remains unclear and they do not seem to be involved directly in ubiquinone reduction (60). However, as arginine is not considered to be involved in iron coordination, its presence (in SdhC or SdhD) may result in some rearrangement indirectly affecting the binding of carboxamides. It would be interesting to investigate the functional consequence of these substitutions in either SdhC or SdhD in fungal mutants.
More information is required to improve our understanding of the relationship between the location of mutations, their effect on subunit structure, interactions with the various carboxamides, and the fitness of the various mutants. Further studies are also required to identify the resistance mechanism occurring in the two CarR2 strains with wild-type copies of the four sdh genes.
Multiple drug resistance is not linked to target gene alteration and induces a broad spectrum of resistance.
Multidrug resistance (MDR) was demonstrated in our data set as the simultaneous resistance of organisms to a number of unrelated toxic compounds. MDR is commonly found in human fungal pathogens but has rarely been described for field strains of phytopathogenic fungi (16). In agriculture, the first case of MDR was recorded in field isolates of B. cinerea, with three possible phenotypes as a function of the fungicide resistance spectrum (48). In MDR1 strains, the ABC transporter AtrB is constitutively overexpressed due to mutations in the gene encoding a transcription factor that controls expression of the gene encoding this transporter (16, 42). In MDR2 strains, an MFS transporter has been implicated in the underlying mechanism, through the insertion of a transposon into its promoter. In MDR3, which has the broadest spectrum of fungicide resistance, both transporters are overexpressed (42). As previously reported for various classes of fungicides, the levels of resistance to respiratory inhibitors recorded in MDR strains were lower than those induced by changes in the target site. The highest levels of resistance were attributed to MDR3 strains. Based on the phenotypic responses of MDR1 and MDR2 strains, some respiratory inhibitors seem to display a particular affinity for ABC transporters (e.g., antimycin A, fluazinam, dimoxystrobin), for MFS transporters (e.g., boscalid), or for both transporters (e.g., 3′ derivatives of carboxin, pyraclostrobin, trifloxystrobin). This phenomenon applies to different extents to molecules of the same mode of action, with some fungicides, such as the carboxamide carboxin and the strobilurin azoxystrobin, little affected. Similar results have been reported for squalene epoxidase inhibitors: both MDR1 and MDR2 phenotypes are resistant to the thiocarbamate tolnaftate but susceptible to the alkylamines naftifine and terbinafine (46). The relationship between the physicochemical properties (for example, lipophilia) of the various respiratory inhibitors and their affinity to ABC or MFS transporters remains to be determined.
Respiratory inhibitors induce highly diverse resistance mechanisms in fungi, thereby complicating resistance management.
Our survey of strains from French and German vineyards showed that at least two different mechanisms—target alteration and efflux pump overexpression—are responsible for resistance to respiratory inhibitors in B. cinerea, the causal agent of gray mold. Resistance concerned either a single (specific resistance) or several (multidrug resistance) classes of fungicides. In cases of specific resistance, amino acid substitutions in the target proteins probably reduced fungicide binding. Typical qualitative resistance to strobilurins was recorded, with full cross-resistance (monogenic and monoallelic), whereas a more complex situation suggestive of quantitative resistance with partial cross-resistance was observed with carboxamides (multigenic and multiallelic). Fungicides with several modes of action (e.g., anilinopyrimidines, phenylpyrroles, dicarboximides) used to control gray mold may have been responsible for the selection of some or all of the MDR phenotypes identified (42). MDR was rarely associated with specific resistance to botryticides (Table 1), with the exception of benzimidazoles, resistance to which has been generalized throughout Champagne for more than 20 years (48). This may indicate that there is a fitness cost attached to the association of MDR with specific resistance other than to benzimidazoles. However, some combinations of specific resistances seemed to be frequent in our data set, such as multiple resistances to fenhexamid and carboxamides or strobilurins and carboxamides (Table 1). Thus, in the Champagne vineyard, which is subject to intense but diverse selective pressures, several mechanisms of resistance may have evolved independently, resulting in a complex situation in terms of fungicide resistance, which may well become increasingly difficult to manage, given the decrease in the number of available active ingredients likely to be effective (48).
Moreover, as MDR tends to result in low to moderate levels of resistance and as strobilurins do not target B. cinerea, the greatest source of concern is resistance to carboxamides. The higher diversity of genotypes recorded for B. cinerea populations than for other fungi is surprising (65). Indeed, the selection pressure in French and German vineyards was restricted to a maximum of one full-dose application of boscalid per year—the mean number of boscalid treatments per season in the Champagne vineyard varied between 0.53 at its introduction and 0.34 in 2008. Moreover, a large number of molecules with alternative modes of action are available, making it possible to vary the class of fungicide used, thereby varying selection pressure (48). As observed in several phytopathogenic fungi (6, 37), the SdhB H272Y/R genotypes predominate in the emerging carboxamide-resistant populations of B. cinerea. However, various other mutations reducing the efficacy of boscalid have nonetheless been selected in B. cinerea. This suggests that (i) not all genotypes are of equal fitness, as already shown for sdhB, sdhC, and sdhD laboratory mutants whose succinate dehydrogenase activity was differently affected (62), and (ii) new carboxamides with different biological and chemical properties, which are likely to become available soon (29), may again increase the diversity of sdh genotypes or, at least, readjust the balance in their frequencies. B. cinerea management should therefore include resistance risk assessment in populations in which carboxamide resistance is emerging. The alternation of SDHI molecules and long rotations of fungicides from different groups with different modes of action might delay the selection of carboxamide-resistant strains (48). However, it might also favor the development of MDR, the impact on field efficacy of which remains limited (61). Finally, although mixtures of inhibitors displaying negative cross-resistance have improved field efficacy in some resistance situations (e.g., mixtures of carbendazim and diethofencarb used to control carbendazim-resistant gray mold populations in the 1980s in the Champagne vineyard (44), this strategy, based for example on the combined use of boscalid and a benzamide derivative such as fluopyram (see Fig. SA1 in the supplemental material), may be less effective for carboxamides, because not all the resistant phenotypes display negative cross-resistance. The use of mixtures of SDHIs with fungicides with another mode of action may help to delay the development of this specific resistance. However, such mixtures might lead to the selection of doubly resistant mutants or MDR.
Supplementary Material
Acknowledgments
We thank Marie-Laure Panon from the Comité Interprofessionnel des Vins de Champagne (CIVC) for providing Botrytis samples and identifying plots from which strains could be collected. We thank Matthias Kretschmer and Matthias Hahn from the University of Kaiserslautern and Gerd Stammler from BASF for providing German isolates resistant to boscalid. We also thank Angélique Gautier from INRA BIOGER-CPP for excellent assistance with sequencing and François Delmotte from INRA Santé Végétale in Bordeaux for providing DNA samples from various fungi infecting grapevine. Finally, we thank Olivier Langella for help and advice about Populations software and Elisabeth Fournier, Danièle Debieu, and Sabine Fillinger for helpful comments on the manuscript.
Footnotes
Published ahead of print on 6 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abiko, K., K. Kishi, and A. Yoshioka. 1977. Occurrence of oxycarboxin-tolerant isolates of Puccinia horiana P. Hennings in Japan. Ann. Phytopathol. Soc. Japan 43:145-150. [Google Scholar]
- 2.Agapow, P. M., and A. Burt. 2001. Indices of multilocus linkage disequilibrium. Mol. Ecol. Notes 1:101-102. [Google Scholar]
- 3.Albertini, C., M. Gredt, and P. Leroux. 2003. Polymorphism of 14 alpha-demethylase gene (CYP51) in the cereal eyespot fungi Tapesia acuformis and Tapesia yallundae. Eur. J. Plant Pathol. 109:117-128. [Google Scholar]
- 4.Albertini, C., and P. Leroux. 2004. A Botrytis cinerea putative 3-keto reductase gene (ERG27) that is homologous to the mammalian 17 beta-hydroxysteroid dehydrogenase type 7 gene (17 beta-HSD7). Eur. J. Plant Pathol. 110:723-733. [Google Scholar]
- 5.Avenot, H., D. P. Morgan, and T. J. Michailides. 2008. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine (pyraclostrobin plus boscalid) fungicide in Alternaria alternata causing alternaria late blight of pistachios in California. Plant Pathol. 57:135-140. [Google Scholar]
- 6.Avenot, H. F., and T. J. Michailides. 2010. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 29:643-651. [Google Scholar]
- 7.Banno, S., K. Yamashita, F. Fukumori, K. Okada, H. Uekusa, M. Takagaki, M. Kimura, and M. Fujimura. 2009. Characterization of QoI resistance in Botrytis cinerea and identification of two types of mitochondrial cytochrome b gene. Plant Pathol. 58:120-129. [Google Scholar]
- 8.Bartlett, D. W., J. M. Clough, J. R. Godwin, A. A. Hall, M. Hamer, and B. Parr-Dobrzanski. 2002. The strobilurin fungicides. Pest Manag. Sci. 58:649-662. [DOI] [PubMed] [Google Scholar]
- 9.Belkhir, K., P. Borsa, L. Chikhi, N. Raufaste, and F. Bonhomme. 2004. Genetix 4.05: logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5171. Université de Montpellier, Montpellier, France. http://www.genetix.univ-montp2.fr/genetix/intro.htm.
- 10.Brasseur, G., A. S. Saribas, and F. Daldal. 1996. A compilation of mutations located in the cytochrome b subunit of the bacterial and mitochondrial bc(1) complex. Biochim. Biophys. Acta 1275:61-69. [DOI] [PubMed] [Google Scholar]
- 11.Brent, J. K., and D. W. Hollomon. 2007. Fungicide resistance: the assessment of risk. FRAC Monograph 2:1-28. [Google Scholar]
- 12.Broomfield, P. L., and J. A. Hargreaves. 1992. A single amino acid change in the iron-sulphur protein subunit of succinate dehydrogenase confers resistance to carboxin in Ustilago maydis. Curr. Genet. 22:117-121. [DOI] [PubMed] [Google Scholar]
- 13.Bulit, J., and B. Dubos. 1988. Botrytis bunch rot and blight, p. 13-14. In R. Pearson and A. Goheen (ed.), Compendium of grape diseases. APS Press, St. Paul, MN.
- 14.Burke, J. M. 1988. Molecular genetics of group I introns: RNA structure and protein factors required for splicing—a review. Gene 73:273-294. [DOI] [PubMed] [Google Scholar]
- 15.Chen, W. J., F. Delmotte, S. Richard-Cervera, L. Douence, C. Greif, and M. F. Corio-Costet. 2007. At least two origins of fungicide resistance in grapevine downy mildew populations. Appl. Environ. Microbiol. 73:5162-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waard, M., A. C. Andrade, K. Hayashi, H. Schoonbeek, I. Stergiopoulos, and L. Zwiers. 2006. Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag. Sci. 62:195-207. [DOI] [PubMed] [Google Scholar]
- 17.de Waard, M. A., S. G. Georgopoulos, D. W. Hollomon, H. Ishii, P. Leroux, N. N. Ragsdale, and F. J. Schwinn. 1993. Chemical control of plant diseases: problems and prospects. Annu. Rev. Phytopathol. 31:403-421. [Google Scholar]
- 18.Dellaporta, S. L., J. Wood, and J. B. Hicks. 1983. A plant DNA minipreparation: version 2. Plant Mol. Biol. Rep. 1:19-21. [Google Scholar]
- 19.Dufour, M., S. Fontaine, A. Micoud, and M. F. Corio-Costet. 2009. Real time PCR assays for quantification and differentiation of Erysiphe necator genetic groups in grapevine samples. 9th International Conference on Plant Diseases. AFPP, Tours, France.
- 20.Esser, L., B. Quinn, Y. F. Li, M. Q. Zhang, M. Elberry, L. Yu, C. A. Yu, and D. Xia. 2004. Crystallographic studies of quinol oxidation site inhibitors: a modified classification of inhibitors for the cytochrome bc(1) complex. J. Mol. Biol. 341:281-302. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Ortuno, D., J. A. Tores, A. De Vicente, and A. Perez-Garcia. 2008. Mechanisms of resistance to Qol fungicides in phytopathogenic fungi. Int. Microbiol. 11:1-9. [PubMed] [Google Scholar]
- 22.Fernandez-Ortuno, D., J. A. Tores, A. D. Vicente, and A. Perez-Garcia. 2008. Field resistance to QoI fungicides in Podosphaera fusca is not supported by typical mutations in the mitochondrial cytochrome b gene. Pest Manag. Sci. 64:694-702. [DOI] [PubMed] [Google Scholar]
- 23.Fillinger, S., P. Leroux, C. Auclair, C. Barreau, C. Al Hajj, and D. Debieu. 2008. Genetic analysis of fenhexamid-resistant field isolates of the phytopathogenic fungus Botrytis cinerea. Antimicrob. Agents Chemother. 52:3933-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher, N., and B. Meunier. 2008. Molecular basis of resistance to cytochrome bc(1) inhibitors. FEMS Yeast Res. 8:183-192. [DOI] [PubMed] [Google Scholar]
- 25.Fournier, E., T. Giraud, C. Albertini, and Y. Brygoo. 2005. Partition of the Botrytis cinerea complex in France using multiple gene genealogies. Mycologia 97:1251-1267. [DOI] [PubMed] [Google Scholar]
- 26.Fournier, E., T. Giraud, A. Loiseau, D. Vautrin, A. Estoup, M. Solignac, J. M. Cornuet, and Y. Brygoo. 2002. Characterization of nine polymorphic microsatellite loci in the fungus Botrytis cinerea (Ascomycota). Mol. Ecol. Notes 2:253-255. [Google Scholar]
- 27.Gargouri, A. 2005. The reverse transcriptase encoded by ai1 intron is active in trans in the retro-deletion of yeast mitochondrial introns. FEMS Yeast Res. 5:813-822. [DOI] [PubMed] [Google Scholar]
- 28.Gisi, U., H. Sierotzki, A. Cook, and A. McCaffery. 2002. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag. Sci. 58:859-867. [DOI] [PubMed] [Google Scholar]
- 29.Glättli, A., G. Stammler, T. Grote, and S. Schlehuber. 2010. SDH inhibitors: history, biological performance and molecular mode of action, p. 50-54. In H. W. Dehne, K. H. Kuck, P. E. Russell, and H. Lyr (ed.), Modern fungicides and antifungal compounds. 16th International Reinhardsbrunn Symposium, Firedrichroda, Germany.
- 30.Grasso, V., S. Palermo, H. Sierotzki, A. Garibaldi, and U. Gisi. 2006. Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag. Sci. 62:465-472. [DOI] [PubMed] [Google Scholar]
- 31.Grasso, V., H. Sierotzki, A. Garibaldi, and U. Gisi. 2006. Characterization of the cytochrome b gene fragment of Puccinia species responsible for the binding site of QoI fungicides. Pestic. Biochem. Physiol. 84:72-82. [Google Scholar]
- 32.Guo, Z.-J., H. Miyoshi, T. Komyoji, T. Haga, and T. Fujita. 1991. Uncoupling activity of a newly developed fungicide, fluazinam [3-chloro-N- (3-chloro-2,6-dinitro-4-trifluoromethylphenyl)-5-trifluoromethyl-2-pyridinamine]. Biochim. Biophys. Acta 1056:89-92. [Google Scholar]
- 33.Hagerhall, C. 1997. Succinate: quinone oxidoreductases—variations on a conserved theme. Biochim. Biophys. Acta 1320:107-141. [DOI] [PubMed] [Google Scholar]
- 34.Honda, Y., T. Matsuyama, T. Irie, T. Watanabe, and M. Kuwahara. 2000. Carboxin resistance transformation of the homobasidiomycete fungus Pleurotus ostreatus. Curr. Genet. 37:209-212. [DOI] [PubMed] [Google Scholar]
- 35.Horsefield, R., V. Yankovskaya, G. Sexton, W. Whittingham, K. Shiomi, S. Omura, B. Byrne, G. Cecchini, and S. Iwata. 2006. Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase)—a mechanism of electron transfer and proton conduction during ubiquinone reduction. J. Biol. Chem. 281:7309-7316. [DOI] [PubMed] [Google Scholar]
- 36.Huang, L. S., G. Sun, D. Cobessi, A. C. Wang, J. T. Shen, E. Y. Tung, V. E. Anderson, and E. A. Berry. 2006. 3-Nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 281:5965-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii, H. 2007. Fungicide development in Japan—an overview, p. 11-18. In H. W. Dehne, H. B. Deising, U. Gisi, K. H. Kuck, P. E. Russell, and H. Lyr (ed.), Modern fungicides and antifungal compounds V. 15th International Reinhardsbrunn Symposium, Friedrichroda, Germany.
- 38.Ishii, H., J. Fountaine, W. Chung, M. Kansako, K. Nishimura, K. Takahashi, and M. Oshima. 2009. Characterisation of QoI-resistant field isolates of Botrytis cinerea from citrus and strawberry. Pest Manag. Sci. 65:916-922. [DOI] [PubMed] [Google Scholar]
- 39.Ito, Y., H. Muraguchi, Y. Seshime, S. Oita, and S. Yanagi. 2004. Flutolanil and carboxin resistance in Coprinus cinereus conferred by a mutation in the cytochrome b(560) subunit of succinate dehydrogenase complex (complex II). Mol. Genet. Genomics 272:328-335. [DOI] [PubMed] [Google Scholar]
- 40.Jiang, J., L. Ding, T. J. Michailides, H. Li, and Z. Ma. 2009. Molecular characterization of field azoxystrobin-resistant isolates of Botrytis cinerea. Pestic. Biochem. Physiol. 93:72-76. [Google Scholar]
- 41.Keon, J. P. R., G. A. White, and J. A. Hargreaves. 1991. Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen Ustilago maydis. Curr. Genet. 19:475-481. [DOI] [PubMed] [Google Scholar]
- 42.Kretschmer, M., M. Leroch, A. Mosbach, A.-S. Walker, S. Fillinger, D. Mernke, H.-J. Schoonbeek, J.-M. Pradier, P. Leroux, M. A. De Waard, and M. Hahn. 2009. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 5:e1000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labourdette, G., H. Lachaise, H. Rieck, and D. Steiger. 2010. Fluopyram: efficacy and beyond on problematic diseases, p. 32-35. In H. W. Dehne, K. H. Kuck, P. E. Russell, and H. Lyr (ed.), Modern fungicides and antifungal compounds. 16th International Reinhardsbrunn Symposium, Firedrichroda, Germany.
- 44.Leroux, P. 2004. Chemical control of Botrytis and its resistance to chemical fungicides, p. 195-222. In Botrytis: biology, pathology and control. Kluwer Academic Publishers, Dordrecht, Netherlands.
- 45.Leroux, P., and G. Berthier. 1988. Resistance to carboxin and fenfuram in Ustilago nuda (Jens.) Rostr., the causal agent of barley loose smut. Crop Prot. 7:16-19. [Google Scholar]
- 46.Leroux, P., F. Chapeland, D. Desbrosses, and M. Gredt. 1999. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Prot. 18:687-697. [Google Scholar]
- 47.Leroux, P., R. Fritz, D. Debieu, C. Albertini, C. Lanen, J. Bach, M. Gredt, and F. Chapeland. 2002. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 58:876-888. [DOI] [PubMed] [Google Scholar]
- 48.Leroux, P., M. Gredt, A. S. Walker, and M. Panon. 2006. Botrytis de la vigne et neuf sortes de fongicides: distribution des souches résistantes en Champagne. Phytoma Def. Veg. 599:22-27. [Google Scholar]
- 49.Li, J., M. G. Zhou, H. X. Li, C. J. Chen, J. X. Wang, and Y. J. Zhang. 2006. A study on the molecular mechanism of resistance to amicarthiazol in Xanthomonas campestris pv. citri. Pest Manag. Sci. 62:440-445. [DOI] [PubMed] [Google Scholar]
- 50.Lyr, H. 1977. Effects of fungicides on energy production and intermediary metabolism, p. 301-332. In M. Siegel and H. D. Sisler (ed.), Antifungal compounds. Volume 2: interactions in biological and ecological systems. Dekker M, New York, NY.
- 51.Maciaszczyk, E., S. Ulaszewski, and J. Lazowska. 2004. Intragenic suppressors that restore the activity of the maturase encoded by the second intron of the Saccharomyces cerevisiae cyt b gene. Curr. Genet. 46:67-71. [DOI] [PubMed] [Google Scholar]
- 52.Matsson, M., B. A. C. Ackrell, B. Cochran, and L. Hederstedt. 1998. Carboxin resistance in Paracoccus denitrificans conferred by a mutation in the membrane-anchor domain of succinate: quinone reductase (complex II). Arch. Microbiol. 170:27-37. [DOI] [PubMed] [Google Scholar]
- 53.Matsson, M., and L. Hederstedt. 2001. The carboxin-binding site on Paracoccus denitrificans succinate: quinone reductase identified by mutations. J. Bioenerg. Biomembr. 33:99-105. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto, T. 2008. Occurrence of Corynespora cassiicola isolates resistant to boscalid on cucumber in Ibaraki Prefecture, Japan, p. 44-51. Abstracts of the 18th Symposium of Research Committee on Fungicide Resistance, April 29th 2008, Matsue, Japan.
- 55.Mouhamadou, B., C. Ferandon, G. Barroso, and J. Labarere. 2006. The mitochondrial apocytochrome b genes of two Agrocybe species suggest lateral transfers of group I homing introns among phylogenetically distant fungi. Fungal Genet. Biol. 43:135-145. [DOI] [PubMed] [Google Scholar]
- 56.Nei, M., F. Tajima, and Y. Tateno. 1983. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J. Mol. Evol. 19:153-170. [DOI] [PubMed] [Google Scholar]
- 57.Newcombe, G., and P. L. Thomas. 2000. Inheritance of carboxin resistance in a European field isolate of Ustilago nuda. Phytopathology 90:179-182. [DOI] [PubMed] [Google Scholar]
- 58.Nobrega, F. G, and A. Tzagoloff. 1980. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J. Biol. Chem. 255:9828-9837. [PubMed] [Google Scholar]
- 59.Oyedotun, K. S., and B. D. Lemire. 2004. The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase—homology modeling, cofactor docking, and molecular dynamics simulation studies. J. Biol. Chem. 279:9424-9431. [DOI] [PubMed] [Google Scholar]
- 60.Oyedotun, K. S., C. S. Sit, and B. D. Lemire. 2007. The Saccharomyces cerevisiae succinate dehydrogenase does not require heme for ubiquinone reduction. Biochim. Biophys. Acta 1767:1436-1445. [DOI] [PubMed] [Google Scholar]
- 61.Petit, A., N. Vaillant-Gaveau, A. Walker, P. Leroux, F. Baillieul, M. Panon, C. Clément, and F. Fontaine. 2010. Determinants of fenhexamid effectiveness against grey mould on grapevine: respective role of spray timing, fungicide resistance and plant defences. Crop Prot. 29:1162-1167. [Google Scholar]
- 62.Shima, Y., Y. Ito, S. Kaneko, H. Hatabayashi, Y. Watanabe, Y. Adachi, and K. Yabe. 2009. Identification of three mutant loci conferring carboxin-resistance and development of a novel transformation system in Aspergillus oryzae. Fungal Genet. Biol. 46:67-76. [DOI] [PubMed] [Google Scholar]
- 63.Sierotzki, H., R. Frey, J. Wullschleger, S. Palermo, S. Karlin, J. Godwin, and U. Gisi. 2007. Cytochrome b gene sequence and structure of Pyrenophora teres and P. tritici-repentis and implications for QoI resistance. Pest Manag. Sci. 63:225-233. [DOI] [PubMed] [Google Scholar]
- 64.Skinner, T., A. Bailey, A. Renwick, J. Keon, S. Gurr, and J. Hargreaves. 1998. A single amino acid substitution in the iron-sulphur protein subunit of succinate dehydrogenase determines resistance to carboxin in Mycosphaerella graminicola. Curr. Genet. 34:393-398. [DOI] [PubMed] [Google Scholar]
- 65.Stammler, G., B. Brix, B. Nave, R. Gold, and U. Schoefl. 2007. Studies on the biological performance of boscalid and its mode of action, p. 44-51. In H. W. Dehne, H. B. Deising, U. Gisi, K. H. Kuck, P. E. Russell, and H. Lyr (ed.), Modern fungicides and antifungal compounds V. 15th International Reinhardsbrunn Symposium, Friedrichroda, Germany.
- 66.Stammler, G., and J. Speakman. 2006. Microtiter method to test the sensitivity of Botrytis cinerea to boscalid. J. Phytopathol. 154:508-510. [Google Scholar]
- 67.Steinfeld, U., H. Sierotzki, S. Parisi, S. Poirey, and U. Gisi. 2001. Sensitivity of mitochondrial respiration to different inhibitors in Venturia inaequalis. Pest Manag. Sci. 57:787-796. [DOI] [PubMed] [Google Scholar]
- 68.Tamura, H., A. Mizutani, H. Yukioka, N. Miki, K. Ohba, and M. Masuko. 1999. Effect of the methoxyiminoacetamide fungicide, SSF129, on respiratory activity in Botrytis cinerea. Pestic. Sci. 55:681-686. [Google Scholar]
- 69.Vivier, M. A., and I. S. Pretorius. 2002. Genetically tailored grapevines for the wine industry. Trends Biotechnol. 20:472-478. [DOI] [PubMed] [Google Scholar]
- 70.Walker, A. S., C. Auclair, M. Gredt, and P. Leroux. 2009. First occurrence of resistance to strobilurin fungicides in Microdochium nivale and Microdochium majus from French naturally infected wheat grains. Pest Manag. Sci. 65:906-915. [DOI] [PubMed] [Google Scholar]
- 71.Weir, B. S., and C. C. Cockerham. 1984. Estimating F-statistics for the analysis of population-structure. Evolution 38:1358-1370. [DOI] [PubMed] [Google Scholar]
- 72.White, G. A., and S. G. Georgopoulos. 1992. Target sites of carboxamides, p. 1-29. In W. Koller (ed.), Target sites of fungicides action. CRC Press, Boca Raton, FL.
- 73.White, G. A., G. D. Thorn, and S. G. Georgopoulos. 1978. Oxathiin carboxamides highly active against carboxin-resistant succinic-dehydrogenase complexes from carboxin-selected mutants of Ustilago maydis and Aspergillus nidulans. Pestic. Biochem. Physiol. 9:165-182. [Google Scholar]
- 74.Wood, P. M., and D. W. Hollomon. 2003. A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Q(o) site of complex III. Pest Manag. Sci. 59:499-511. [DOI] [PubMed] [Google Scholar]
- 75.Yankovskaya, V., R. Horsefield, S. Tornroth, C. Luna-Chavez, H. Miyoshi, C. Leger, B. Byrne, G. Cecchini, and S. Iwata. 2003. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299:700-704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.