Abstract
We report development of a genetic system for making targeted gene knockouts in Clostridium thermocellum, a thermophilic anaerobic bacterium that rapidly solubilizes cellulose. A toxic uracil analog, 5-fluoroorotic acid (5-FOA), was used to select for deletion of the pyrF gene. The ΔpyrF strain is a uracil auxotroph that could be restored to a prototroph via ectopic expression of pyrF from a plasmid, providing a positive genetic selection. Furthermore, 5-FOA was used to select against plasmid-expressed pyrF, creating a negative selection for plasmid loss. This technology was used to delete a gene involved in organic acid production, namely pta, which encodes the enzyme phosphotransacetylase. The C. thermocellum Δpta strain did not produce acetate. These results are the first examples of targeted homologous recombination and metabolic engineering in C. thermocellum, a microbe that holds an exciting and promising future in the biofuel industry and development of sustainable energy resources.
Conversion of cellulosic biomass using saccharolytic fermentative microorganisms without the addition of purified cellulase and hemicellulase enzymes is a promising approach for low-cost production of renewable fuels and chemicals (22, 23). Thermophilic, cellulolytic bacteria are one departure point for development of microorganisms with the requisite capabilities for such consolidated bioprocessing (CBP), with Clostridium thermocellum being exemplary in this regard. As reviewed elsewhere (6, 22), C. thermocellum is a Gram-positive organism able to ferment cellulose and products of cellulose solubilization to ethanol, acetic acid, lactic acid, formic acid, hydrogen, and CO2. C. thermocellum appears to be a cellulose-utilizing specialist (6, 8) and produces a multienzyme cellulose-solubilizing complex termed a cellulosome (2, 3, 9).
Metabolic engineering is required in order to increase the yield of ethanol or other desired products from mixed-product fermentation, such as that carried out by Clostridium thermocellum. Comprehensive work directed to this end has been carried out with genetically tractable organisms, such as Escherichia coli, resulting in high or near-theoretical yields achieved for ethanol (35, 36), other native products (21, 25), and nonnative products (7, 12). In these organisms, genetic systems involving both positive and negative selection markers have been employed in order to facilitate reuse of the same marker and to develop marker-free strains. One prominent system in the category involves use of the gene encoding the enzyme orotidine 5-phosphate decarboxylase (PyrF) (4, 11, 20, 27-29, 39). PyrF participates in de novo pyrimidine biosynthesis but is also a target for the antimetabolite 5-fluoroorotic acid (5-FOA) (4). Thus, cells lacking pyrF are uracil auxotrophs and resistant to 5-FOA, creating an opportunity whereby ectopic expression of pyrF can be selected or counterselected (4).
Reliable genetic tractability has been elusive for Clostridium species. Prior to this report, the only Clostridia species in which gene deletion via homologous recombination has been demonstrated are Clostridium acetobutylicum, Clostridium perfringens, and Clostridium septicum. In the first organism, the use of a replicating plasmid for transformation followed by selection and screening for plasmid segregation resulted in a single clone that when analyzed contained a disruption in the gene of interest but not by the expected recombination events (13). The last two organisms have either an unusually high transformation frequency or feasibility for acquiring DNA from E. coli via conjugation, allowing the use of suicide plasmids (1, 16, 19). By comparison, the recently reported method of C. thermocellum transformation consists of a complex and cumbersome electroporation protocol using a custom pulse delivery system (37, 38). In our hands, efficiency of the C. thermocellum electrotransformation system does not compare with that of typical model organisms and does not enable the use of nonreplicating plasmids as a means of gene manipulation. Alternatively, group II intron technology has been used to inactivate gene targets in clostridia that were previously characterized as genetically intractable, but systems described to date have a temperature restriction that make such approaches incompatible with thermophilic clostridia (14, 15, 34).
The only C. thermocellum mutant characterized genetically was isolated following a random mutagenesis and enrichment for cells that did not adhere to cellulose (43). The random mutagenesis approach is limited, in the sense that it does not lend itself well to reverse genetics, as many desired mutations lack selectable or screenable phenotypes. For instance, attempts have been made, with little success, to isolate saccharolytic thermophiles containing lesions in the pta-ack operon responsible for acetate production by selective enrichment using antimetabolites (26). In contrast, the creation of a Thermoanaerobacterium saccharolyticum Δpta-ack strain has been achieved using selectable markers that serve as a proxy for the events leading to targeted gene deletion (32). Motivated by the potential of microbial cellulose processing and the attributes of C. thermocellum, we undertook to develop a gene deletion system based on the pyrF gene.
MATERIALS AND METHODS
Media, culture conditions, and reagents.
C. thermocellum DSM 1313 was cultured in DSM 122 broth (37) with addition of 50 mM MOPS (morpholinepropanesulfonic acid) and 10 mM sodium citrate, herein referred to as rich medium. Cellobiose or Avicel was added at 5 g/liter unless otherwise noted, and 0.8% Difco agar was used for solid medium preparation. A uracil-free version of the rich medium, herein referred to as defined medium, omitted yeast extract and contained 3 μg/ml pyridoxamine hydrochloride, 0.3 μg/ml biotin, 0.6 μg/ml p-aminobenzoic acid, and 0.3 μg/ml vitamin B12. Alternatively, MJ medium (17), which contains urea instead of ammonium sulfate as a nitrogen source, was used as a uracil-free chemically defined medium. No difference was seen between MJ and the uracil-free version of DSM 122 when performing genetic selections. C. thermocellum was grown anaerobically at 55°C unless otherwise noted. E. coli was grown aerobically at 37°C in LB broth (Fischer Scientific). Saccharomyces cerevisiae was grown in YPD medium or synthetic uracil dropout medium (MP Biomedicals). For C. thermocellum, thiamphenicol (Tm) was used in a range from 3 to 48 μg/ml as indicated, at 55°C (at 60°C and above, nonspecific growth was observed, indicating a potential decrease in the stability of Tm at elevated temperatures). For E. coli, 20 μg/ml chloramphenicol (Cm) and 15 μg/ml ampicillin (Ap) were used. When appropriate, 40 μg/ml uracil was used to supplement C. thermocellum growth. Where indicated, 500 μg/ml 5-FOA was added (Zymo Research). All reagents, unless noted, were purchased from Sigma-Aldrich.
Molecular cloning and plasmid construction.
Primer design for amplification of DNA from C. thermocellum 1313 was based on the available C. thermocellum ATCC 27405 genome (http://genome.jgi-psf.org/cloth/cloth.home.html). The oligonucleotides and the plasmids/strains used in this study are listed in Tables 1 and 2, respectively. The 5′and 3′ flanking regions (∼1 kb) of pyrF and pta were amplified and assembled using yeast gap repair cloning to create gene deletion plasmids (30). The pyrF and pta deletion vectors (pMU769 and pMU1162, respectively) contained cat (encoding chloramphenicol acetyltransferase) expressed from the C. thermocellum gapDH promoter (gapDHp) positioned between the 5′ and 3′ flanking regions (see Fig. 3A). The pyrF complementing construct (pMU612) contained pyrF expressed from the C. thermocellum cellobiose phosphorylase gene (cbp) promoter (cbpp). All DNA manipulations and cloning procedures were performed per the method of Maniatis et al. (24). For maps of vectors and details of vector constructions, see Fig. SA1 in the supplemental material.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| X00105 | ACTCCGCGGCCGGGAAGCCGATCTCGGCTTGAACGAATTGGGATCCTCTAGAGTCGACCT |
| X00106 | GGTCGATGTTTGATGTTATGGAGCAGCAACGGAAAGCAGAGCAATGTGGAATTGGGAACG |
| X00109 | CCTGATAAATGCTTCAATAATATTGAAAAAGGAAGAGTATCCAGTATTCTGACATGGGTG |
| X00110 | CTTTTCTCTTCCAATTGTCTAAATCAATTTTATTAAAGTTCATTAATATCGCCTCCTATTG |
| X00967 | GGCGGAATTCGGAGGCTTACTTGTCTGCTT |
| X00968 | GGCGAGATCTGTTGTGTGGAATTGTGAGCG |
| X00969 | GGCGGAATTCGGTTTCTTAGACGTCAGGTG |
| X00970 | GGCGAGATCTTGACGCTCAGTGGAACGAAA |
| X00973 | AGGTCGACTCTAGAGGATCCCCGGGTACCGAGCTCGAATTTAATATCGCCTCCTATTG |
| X00974 | AAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGATCGCTTGCCTGTAACTTAC |
| X01840 | ATCAGTTATTACCCACTTTTCGGGGAAATGTGCGCGGAACCCATTGCGAAGATCTGGACC |
| X01841 | GCACTGTTAATATCGTCCCTAGGGTCTAATCCTACAACGG |
| X01842 | CCGTTGTAGGATTAGACCCTAGGGACGATATTAACAGTGC |
| X01843 | GCAGTGAGCGCAACGCAATTAATGTGAGTTAGCTCACCGTCGTTCATGTTTCTGTGG |
| X01893 | GGCGGGTACCCGTCGTTCATGTTTCTGTGG |
| X02004 | CATGAGTGCTGCAATGGGAG |
| X02005 | TATCCTTGTACCTTACGCC |
| X02051 | CTAATAACCCTCATGTCAAG |
| X01905 | GGCGTCTAGACCATTGCGAAGATCTGGACC |
| X03061 | AAGAAACCATTATTATCATGACATTAACCTATAAAAAAGGTCTAGAGAGTCGTGACTAAGAACGTC |
| X03062 | CGTTTCACTTCTGAGTTCGCGAATTCAAGAACGCAGGATTGAATAGAG |
| X03063 | CTCTATTCAATCCTGCGTTCTTGAATTCGCGAACTCAGAAGTGAAACG |
| X03064 | GAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTTCTAGAACAAGGGTTACCGGAATTTAC |
| X03079 | CAAATAAACGAAAATTTTAAGGAGGACGAAAGATGTTTATTGATACATTAATTGAAAAGATTA |
| X03080 | ACCCGGATAACTTCTTTTTGTCATACCTTGCTTTATTACTTCCTGTCTCGCAACGCACTG |
| X03852 | CAGTGAGGCACCTATCTCAGCGATCTGTCAATTCGCGGCCGCGAAAAGTGGGTAATAACTG |
| X03853 | CTGCAGGTCGACTCTAGAGGATCCCCGGGTACCGCGGCCGCGATCGCTTGCCTGTAACTTAC |
| X03886 | ATTACGCCAAGCTTGCATGCCCTGCAGGTCGACTCTAGAGCGTCGTTCATGTTTCTGTGG |
| X03889 | TGTAAGTTACAGGCAAGCGATCGCGGCCGCGGTACCCGGGCCATTGCGAAGATCTGGACC |
| X03896 | AAGGATAATCCTTCCGTTGTAGGATTAGACCCTACTAGTAGCCCTTCAAACTTCCCAAAG |
| X03897 | CACTAGGGCTCGCCTTTGGGAAGTTTGAAGGGCTACTAGTAGGGTCTAATCCTACAACGG |
| X03898 | CTTTTTTAAAAGTCAATCCCGTTTGTTGAACTACTGTACAAGGGACGATATTAACAGTGC |
| X03899 | TGTCTCGCAACGCACTGTTAATATCGTCCCTTGTACAGTAGTTCAACAAACGGGATTGAC |
| X05109 | TGTAAGTTACAGGCAAGCGATCGCGGCCGCGGTACCCGGGCAGGCAAAGTCCAACTATGC |
| X05110 | CTTTGGGAAGTTTGAAGGGCTGCGGCTTTAATAACCCTCAG |
| X05111 | CTGAGGGTTATTAAAGCCGCAGCCCTTCAAACTTCCCAAAG |
| X05112 | GACATATTGAGCCTGAACCGGTAGTTCAACAAACGGGATTGAC |
| X05113 | GTCAATCCCGTTTGTTGAACTACCGGTTCAGGCTCAATATGTC |
| X05114 | ATTACGCCAAGCTTGCATGCCCTGCAGGTCGACTCTAGAGTGTCATCTCCCTTTTCTGCG |
| X05850 | GCTTCTTAAGACAGGAAAGC |
TABLE 2.
Plasmids and strains used in this study
| Plasmid or strain | Description and relevant characteristicse | Source or reference |
|---|---|---|
| Plasmids | ||
| pUC19 | General purpose cloning vector, Ap | NEBa |
| pMU102 | pMU104, region between the FokI and EcoRI sites has been deleted, Cm | This study |
| pMU104 | pNW33N, E. coli-C. thermocellum shuttle vector, Cm | BGSCb |
| pMU110 | pMQ87, S. cerevisiae-E. coli shuttle vector, Ura+, Gm | This study |
| pMU111 | pMU110 with aac1 (Gm) replaced by cat from pMU104, Ura+, Cm | This study |
| pMU113 | pMU111 with C. thermocellum gapDHp driving cat, Ura+, Cm | This study |
| pMU245 | E. coli-C. thermocellum cloning vector, Ap | This study |
| pMU357 | S. cerevisiae-E. coli-C. thermocellum shuttle vector for expressing genes in C. thermocellum, C. thermocellum gapDHp, Ura+, Cm | This study |
| pMU440 | S. cerevisiae-E. coli-C. thermocellum shuttle vector, C. thermocellum ΔpyrF cassette, Ura+, Cm | This study |
| pMU482 | E. coli-C. thermocellum shuttle vector, C. thermocellum ΔpyrF cassette, Cm | This study |
| pMU582 | pMU110, C. thermocellum cbp promoter, cbp gene, T1T2 terminator, Ura+, Gm | This study |
| pMU597 | pMU582, C. thermocellum cbp gene replaced by C. thermocellum pyrF gene, creating cbpp-pyrF cassette, Ura+, Gm | This study |
| pMU612 | pMU102 containing cbpp-pyrF cassette, Cm | This study |
| pMU749 | pMU245, CEN6/ARSH4, ura3, S. cerevisiae-E. coli-C. thermocellum vector, Ura+, Ap | This study |
| pMU769 | pMU749 with ΔpyrF::gapDHp-cat cassette, Ura+, Ap, Cm | This study |
| pMU1016 | pMU749 with Δpta::gapDHp-cat cassette, Ura+, Ap, Cm | This study |
| pMU1162 | pMU1016 with cbpp-pyrF cassette, Ura+, Ap, Cm | This study |
| Strains | ||
| E. coli Top10 | Cloning strain | Invitrogenc |
| S. cerevisiae InvSC1 | Ura3− for gap repair cloning | Invitrogenc |
| C. thermocellum M0003 | Wild-type C. thermocellum DSM 1313 | DSMZd |
| C. thermocellum M0970 | DSM 1313 ΔpyrF | This study |
| C. thermocellum M0971 | DSM 1313 ΔpyrF Δpta::gapDHp-cat | This study |
| C. thermocellum M1061 | DSM 1313 ΔpyrF pMU612 | This study |
| C. thermocellum M1062 | DSM 1313 ΔpyrF pMU102 | This study |
New England Biolabs, Ipswich, MA.
Bacillus Genetic Stock Center; http://www.bgsc.org/.
Carlsbad, CA.
Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Germany.
Ap, ampicillin; Cm, chloramphenicol; Gm, gentamicin.
Transformation of C. thermocellum and selection of mutants.
The transformation protocol used was modified substantially from the previously reported protocol (37). The pulse generator was custom built and utilized a solid-state insulated-gate bipolar transistor (IGBT) instead of a power tetrode as the high-voltage switch (Infineon; part no. FZ200R65KF2). The device was charged with a high-voltage power supply from Emco (part no. F101). The charge was stored in an 8-kV, 32-μF capacitor made by General Atomics (part no. 39742). Pulse duration and interval were controlled by an arbitrary function generator (Tektronix; part no. AFG3101). All manipulations were done under anaerobic conditions. Cultures were grown to mid-log phase (optical density at 600 nm [OD600] of 0.4 to 0.8) in rich medium and harvested by centrifugation (2,200 × g for 12 to 14 min). Cells were washed twice in autoclaved, deionized water, and the final pellet was resuspended in 200 μl deionized water. For each transformation, 20 μl of cell suspension was added, along with 1 to 8 μg of plasmid DNA, to a 0.1-cm gap electroporation cuvette (Fisher Scientific). A series of 60 square pulses were applied to the sample (see Fig. SA2, showing a pulse diagram, in the supplemental material). The period of the pulses was 300 μs, and the amplitude was 1.9 kV, resulting in an applied field strength of 19 kV/cm. After pulsing, cells were recovered overnight (15 to 18 h) at 51°C in 3 to 5 ml rich medium. For liquid selection, recovered cultures were inoculated (10% [vol/vol]) into either rich medium supplemented with 3 to 6 μg/ml thiamphenicol (Tm) or uracil-free MJ medium when selecting for uracil prototrophy. For selecting transformants on solid medium, the recovery cultures were plated by mixing them in rich medium containing agar and 3 to 6 μg/ml Tm or MJ medium containing agar followed by a short incubation at room temperature in the anaerobic chamber to allow medium solidification before incubation of the plates at 55°C. To select ΔpyrF mutants, transformants were grown in 3 μg/ml Tm. The cultures were then diluted to approximately 108 cells/ml, and 100 μl of the diluted culture was plated as agar suspensions in rich medium containing 5-FOA. 5-FOA-resistant colonies were screened by PCR using primers X02004 and X02005 (Table 1), which anneal outside the regions of homology used to delete pyrF (Fig. 1A). More than 99% of the colonies screened were pyrF mutants that resulted from homologous recombination events as opposed to spontaneous mutations (see Results for more details). The pyrF::gapDHp-cat mutants were isolated as described in Results, and the same primer set was used to screen ΔpyrF::gapDHp-cat mutants.
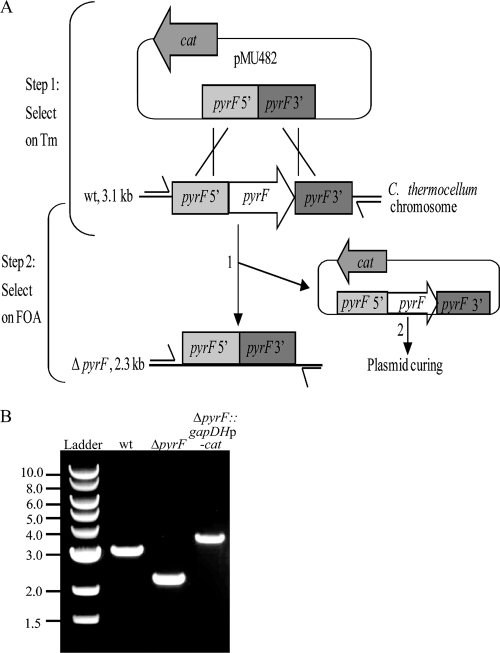
FIG. 1.
Creation of a C. thermocellum ΔpyrF strain. (A) Relevant features of the pyrF deletion vector, pMU482, are the positive marker for transformation (cat) and the flanking regions of homology that target deletion of pyrF (pyrF 5′ and pyrF 3′). The chromosomal pyrF locus contains the pyrF gene in the context of the flanking DNA. Step 1, plating on Tm allows selection of transformants harboring pMU482; step 2, plating on 5-FOA selects for deletion of pyrF from chromosome and insertion into pMU482 (numbered 1) and loss of pMU482 containing pyrF (numbered 2). Primers used for PCR are indicated by the one-sided arrows, and the sizes of the expected amplicons are shown to the left. (B) DNA gel showing colony PCR results using primers X02204 and X02205 on wild-type (lane 2), ΔpyrF (lane 3), and ΔpyrF::gapDHp-cat (lane 4) strains. The numbers on the left indicate the band sizes in kb for the NEB 1-kb ladder used as a marker (lane 1).
To select pta::gapDHp-cat mutants, the ΔpyrF strain transformed with pMU1162 was grown in 5 ml of rich medium supplemented with 6 to 12 μg/ml Tm or in MJ medium for about 14 to 16 h. Various volumes of the cultures (ranging from 10 μl to 1 ml) were plated as agar suspensions of rich medium containing 5-FOA and 48 μg/ml Tm. Resistant colonies were screened by PCR using primers X02051 and X05980 (Table 1), which anneal outside the regions of homology used to delete pta (see Fig. 3A).
Fermentation conditions and analytical methods.
Batch fermentations in anaerobic tubes with the wild-type (WT), ΔpyrF, and Δpta::gapDHp-cat strains were performed at 55°C in rich medium under a nitrogen atmosphere utilizing cellobiose or Avicel as the primary carbon source. The fermentation products were analyzed using high-performance liquid chromatography (HPLC) as previously described (32). Growth rate measurements were performed in a 200-μl volume in a 96-well plate at 55°C. The optical density at 600 nm was read by a Powerwave XS plate reader customized by the manufacturer to incubate up to 68°C (BioTek). The plates were shaken continuously and read at 3-min intervals. Each sample was measured in quadruplicate. The specific growth rate (μ) was determined by measuring the slope of the natural log-transformed OD readings. A 2-h sliding window of OD readings between 0.08 and 1.00 was used for determination of the maximum growth rate, μmax. In all cases, the R2 value was greater than 0.99.
RESULTS
Isolation of a C. thermocellum ΔpyrF strain.
A schematic depicting events leading to deletion of pyrF is shown in Fig. 1A. The current transformation efficiency of C. thermocellum does not allow use of nonreplicating plasmids for genetic manipulation. Thus, to delete pyrF, WT C. thermocellum was transformed with a replicating allelic exchange vector, pMU482, using Tm selection (step 1). Single colonies representing transformants were propagated in liquid medium with Tm selection prior to plating on 5-FOA (step 2). As a control, the WT strain harboring an empty vector encoding Tm resistance, pMU102, was subjected to the same selective pressures. After 2 days of incubation, a substantial number of CFU resulted with pMU482, whereas only occasional spontaneous 5-FOA-resistant mutants arose with pMU102.
5-FOA-resistant colonies were screened by PCR using primers that anneal outside the regions of homology used to delete pyrF (Fig. 1A). As expected, in the presence of pMU482, a number of 5-FOA resistant colonies exhibited double recombination events, leading to the deletion of pyrF. These events were easily detected using diagnostic PCR, in which the expected amplicon for the WT is 3.1 kb and that for the ΔpyrF strain is 2.3 kb (Fig. 1A and B). The pyrF locus was sequenced to confirm gene deletion.
Characterization and complementation of the C. thermocellum ΔpyrF strain.
A pyrF-complementing plasmid, pMU612, was constructed that contained the native C. thermocellum pyrF gene under the control of the native C. thermocellum cellobiose phosphorylase gene (cbp) promoter (cbpp). Either pMU612 or pMU102 was transformed into the ΔpyrF strain to evaluate known pyrF phenotypes, for example, 5-FOA sensitivity/resistance and uracil auxotrophy/prototrophy. Plasmid-free versions of the WT strain and the ΔpyrF strain were used as controls. All strains were tested on four variations of solid media: a rich medium, with and without supplementation of 5-FOA, and a uracil-free defined medium, with and without supplementation of uracil. The numbers of CFU are listed in Table 3. As expected, the wild-type strain was sensitive to 5-FOA, whereas the ΔpyrF strain was resistant. The occurrence of spontaneous 5-FOA resistance in the WT background was 0.3%. Additionally, growth of the ΔpyrF strain was severely impaired in the defined medium lacking uracil, and addition of uracil supported normal growth. These results indicated that pyrF can be used as both a positive and a negative selection marker in C. thermocellum.
TABLE 3.
Phenotype and complementation of the ΔpyrF strain
| Strain or genotype | CFUa |
|||
|---|---|---|---|---|
| Rich medium | Rich medium + 5-FOA | Defined medium | Defined medium + uracil | |
| WT | 1.0 × 107 | 3.0 × 104 | 1.8 × 107 | 1.2 × 107 |
| ΔpyrF | 6.9 × 107 | 5.7 × 107 | 1.0 × 103 | 2.7 × 107 |
| ΔpyrF pMU102 | 4.7 × 107 | 4.0 × 107 | 4.0 × 105 | 7.0 × 107 |
| ΔpyrF pMU612 | 2.3 × 107 | 1.0 × 107 | 3.7 × 107 | 5.1 × 107 |
Each strain was dilution plated on the indicated medium, and the number of CFU resulting after 48 h is shown from a representative data set of three independent experiments. The phenotypes of the ΔpyrF strain were consistent with respect to uracil auxotrophy and 5-FOA resistance in all of the experiments, whereas the overall number of CFU varied based on the plating efficiency.
Comparisons made with the ΔpyrF strain containing either pMU102 or pMU612 further validated the use of pyrF-based selections. The ΔpyrF strain containing pMU102 was resistant to 5-FOA and a uracil auxotroph mimicking the parental ΔpyrF strain (Table 3). The ΔpyrF strain containing pMU612 was complemented, with respect to uracil prototrophy, as indicated by its ability to grow on a defined medium lacking uracil (Table 3). This confirms that pyrF is functionally expressed from the cbp promoter on pMU612. Surprisingly, the complemented strain was as resistant to 5-FOA as the ΔpyrF strain. Since the occurrence of spontaneous 5-FOA-resistant mutants was low, the likely explanation for this finding is that selection of plasmid loss in the presence of 5-FOA is unusually high. However, an alternative possibility is that the absence of antibiotic selection might be responsible for plasmid loss. To investigate this, colonies representing the ΔpyrF strain harboring pMU612 that appeared in the presence of 5-FOA were screened for the presence of the plasmid. Twenty colonies were tested, and none contained pMU612. In contrast, 19 out of 20 colonies representing the ΔpyrF strain harboring pMU102 plated on 5-FOA contained the control plasmid, indicating that lack of antibiotic selection alone is insufficient to achieve high rates of plasmid loss. Collectively, the results shown in Table 3 indicated that pyrF can be used as a negative selection to identify plasmid loss in C. thermocellum.
Optimization of the cat marker for use in single copy on the chromosome.
In order to create a marked mutation, a positive selection was needed to select for a chromosomal integration event and a negative selection was needed to select for loss of the replicating knockout plasmid. The latter component can be achieved using the ΔpyrF strain and ectopic expression of pyrF from a plasmid. To achieve the former, the cat marker, which provides Tm resistance at thermophilic temperatures from a multicopy plasmid (33), was investigated for its ability to provide Tm resistance when harbored in a single copy on the chromosome at the pyrF locus. An allelic replacement vector, pMU769, was constructed to delete the pyrF gene and replace it with cat controlled by the native glyceraldehyde 3-phosphate dehydrogenase gene (gapDH) promoter of C. thermocellum. The vector contained gapDHp-cat elements positioned between 5′ and 3′ pyrF flanking DNA. To replace pyrF with gapDHp-cat, C. thermocellum transformants containing pMU769 were subjected to two simultaneous selections in liquid rich medium. Thiamphenicol was used to select for plasmid-encoded gapDHp-cat, while 5-FOA was used to select against chromosomal pryF. Recovered cultures were evaluated by PCR using primers that anneal upstream and downstream of pyrF. Using these conditions, replacing the pyrF gene with gapDHp-cat would increase the PCR amplicon size by ∼300 bp compared to the WT. The result of this is shown in Fig. 1B (compare lanes 2 and 4). The strain represented in lane 4 is a plasmid-free ΔpyrF::gapDHp-cat strain that was resistant to Tm concentrations of up to 48 μg/ml. This informed us that cat expressed from the gapDH promoter was functional in a single copy on the C. thermocellum chromosome and could be used as a marker for allele replacement.
Deletion of the C. thermocellum pta gene using pyrF and cat selection.
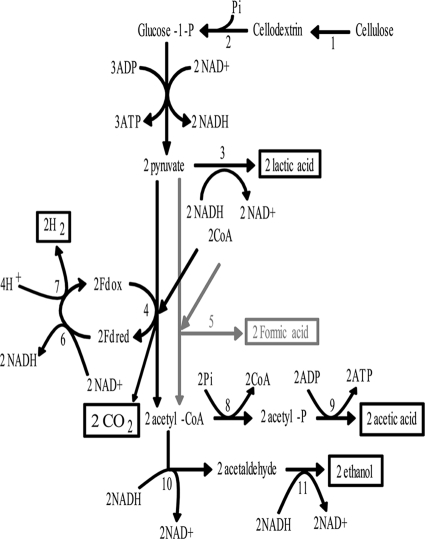
Mixed acid fermentation of C. thermocellum involves coproduction of lactic acid, acetic acid, formic acid, and ethanol (Fig. 2). For C. thermocellum strain DSM 1313, acetic acid is the major coproduct that needs to be eliminated to create a strain with increased ethanol yield. The production of acetic acid from acetyl coenzyme A (CoA) involves two enzymatic activities that are catalyzed by Pta and Ack.
FIG. 2.
Mixed acid fermentation of C. thermocellum. The primary fermentation products are highlighted in black boxes. Formic acid, highlighted in a gray box, is a fermentation end product that has been observed with C. thermocellum but was not under the conditions used in this study. The following key intermediates and enzymatic steps are noted: 1, extracellular cellulose hydrolysis; 2, intracellular phosphorolytic cleavage of cellodextrins by cellobiose phosphorylase; 3, lactate dehydrogenase; 4, pyruvate ferredoxin oxidoreductase; 5, pyruvate formate lyase; 6, ferredoxin NAD+(H) oxidoreductase; 7, ferredoxin-dependent hydrogenase; 8, phosphotransacetylase; 9, acetate kinase; 10, acetaldehyde dehydrogenase; 11, alcohol dehydrogenase.
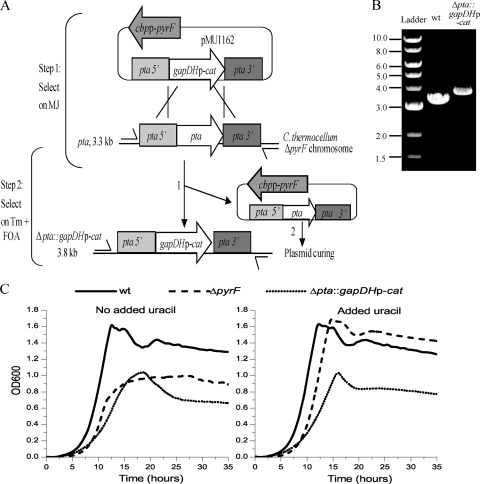
The scheme used to replace pta with cat expressed from the gapDH promoter in the C. thermocellum ΔpyrF background is shown in Fig. 3 A. MJ medium lacking uracil was used to select ΔpyrF mutant clones restored to uracil prototrophy as a result of being transformed with pMU1162 (Fig. 3A, step 1). Single colonies representing transformants were propagated in liquid medium with Tm selection prior to plating on Tm plus 5-FOA (Fig. 3A, step 2). Colonies resistant to both Tm and 5-FOA were screened by PCR using primers that anneal outside the regions of homology used to delete pta (Fig. 3A). More than 75% of the colonies screened indicated the occurrence of homologous recombination events at the pta locus. Clones in which pta was replaced by the gapDHp-cat cassette were discernible by a 0.5-kb increase in the size of the amplicon. For simplicity, the pta mutants generated in the ΔpyrF background strain are designated the Δpta::gapDHp-cat strain hereinafter (excluding the background strain, with the ΔpyrF mutant genotype). The expected amplicon was 3.3 kb for the WT and 3.8 kb for the Δpta::gapDHp-cat strain (Fig. 3A and B). The pta locus was sequenced to confirm allele replacement.
FIG. 3.
Creation of a C. thermocellum Δpta::gapDHp-cat strain. (A) Relevant features of the pta deletion vector, pMU1162, are the dual selection cassette for transformation and plasmid loss (cbpp-pyrF) and the allelic replacement cassette containing gapDHp-cat between the flanking regions (pta 5′ and pta 3′). The chromosomal pta locus contains the pta gene in the context of the flanking DNA. Step 1, plating on MJ allows selection of transformants harboring pMU1162; step 2, plating on Tm plus 5-FOA selects replacement of pta with the gapDHP-cat cassette and insertion of pta into pMU1162 (numbered 1) and loss of pyrF-containing plasmid (numbered 2). Primers used for PCR are indicated by the one-sided arrows, and the sizes of the expected amplicons are shown to the left. (B) DNA image showing the results of colony PCR using primers X02051 and X02099 on the wild type (lane 2) and a putative Δpta::gapDHp-cat strain (lane 3). The numbers on the left indicate the band sizes in kb for the NEB 1-kb ladder used as a marker (lane 1). (C) Growth analysis of the WT, ΔpyrF, and Δpta::gapDHp-cat strains in rich medium with or without supplementation of uracil. The graphs indicate the OD600 of each culture over time. The μmax for the WT, ΔpyrF, and Δpta::gapDHp-cat strains were 0.55 h−1, 0.58 h−1, and 0.38 h−1, respectively, and did not change significantly upon addition of uracil.
Growth analysis and fermentation profile of the Δpta::gapDHp-cat strain.
The growth of the Δpta::gapDHp-cat strain was compared to those of the WT and ΔpyrF strains in rich medium, with and without uracil supplementation. Although initial rates of growth of the ΔpyrF and WT strains were similar (Fig. 3C), the ΔpyrF strain slowed abruptly at an OD of ∼0.7, while the WT continued to grow until it reached an OD of ∼1.6 (Fig. 3C), suggesting that the rich medium was uracil limited. Supplementing the medium with an additional 40 μg/ml uracil eliminated the growth defect of the ΔpyrF strain and resulted in a growth curve that was indistinguishable from that of the WT strain (Fig. 3C). Even with additional uracil supplementation to compensate for the ΔpyrF mutation, the maximum specific growth rate (μmax) of the Δpta::gapDHp-cat strain was about one-third lower than that of either the WT or the ΔpyrF strain, and the final OD was also reduced (Fig. 3C). This indicates that the growth defect of the Δpta::gapDHp-cat strain is distinct from the growth defect of the ΔpyrF strain and is a result of the pta mutation.
End product analysis was performed on batch fermentations started at pH 7.0 with 5 g/liter cellobiose as the primary carbon source under anaerobic conditions with a nitrogen atmosphere and 80-ml working volume. As shown in Fig. 4 A, after 48 h of fermentation, the WT and ΔpyrF strains produced about 1 g/liter acetic acid, whereas the acetic acid production of the Δpta::gapDHp-cat strain was indistinguishable from background levels (average, 0.03 g/liter). All three strains produced comparable amounts of ethanol and lactic acid. Due to the growth defect of the Δpta::gapDHp-cat strain, a 96-h sample point was taken, but acetate levels did not change, measuring 0.031 g/liter. The average dry cell masses for the WT, ΔpyrF, and Δpta::gapDHp-cat strains were 0.54 g, 0. 54 g, and 0.35 g, respectively, indicating that the Δpta::gapDHp-cat strain made about one-third less biomass than the WT and ΔpyrF strains. This was expected, based on the growth defect of the Δpta::gapDHp-cat strain observed previously (Fig. 3C).
FIG. 4.
Batch fermentation analysis of the Δpta::gapDHp-cat strain at 55°C in rich medium supplemented with uracil. (A) Measurement of lactic acid, acetic acid, and ethanol resulting from the consumption of 5 g/liter cellobiose in 48 hours by the WT, ΔpyrF, and Δpta::gapDHp-cat strains. Error bars represent data from triplicate fermentations. (B) Measurement of lactic acid, acetic acid, and ethanol resulting from the consumption of 5 g/liter Avicel in 48 hours by the wild-type, ΔpyrF, and Δpta::gapDHp-cat strains. Error bars represent data from triplicate fermentations.
The elimination of the acetic acid pathway is expected to cause a decrease in ATP gained per mole of glucose consumed (22, 32). Cellulosome synthesis is considered to be an ATP-expensive process. Therefore, we examined the cellulolytic capacity of the Δpta::gapDHp-cat strain and determined the fermentation product profile on Avicel, a refined, crystalline cellulose. Batch fermentations were performed starting at pH 7.0 using 5 g/liter Avicel as the primary carbon source under anaerobic conditions with a nitrogen atmosphere and 80-ml working volume. As seen in Fig. 4B, after 48 h of fermentation the WT and the parental ΔpyrF strain both produced about 1 g/liter acetic acid, whereas the Δpta::gapDHp-cat strain produced ∼0.03 g/liter acetic acid. All three strains produced comparable levels of ethanol and lactic acid. Even after 96 h of fermentation, the Δpta::gapDHp-cat strain did not produce more than 0.03 g/liter acetate. Avicel is a solid substrate, and nonhydrolyzed material can be estimated by measuring the dry weight of the fermentation. Table 4 shows that the average dry weights for all of the strains at the end of 96 h of fermentation were comparable, and this translates into ∼89% Avicel hydrolysis. However, the fermentation dry weight does not distinguish residual Avicel from dry cell mass. For Avicel fermentations, the latter can be estimated by assuming that the dry cell mass produced from 5 g/liter Avicel is similar to that produced from 5 g/liter cellobiose (see above). Using this logic, all strains showed >95% solubilization of Avicel. Therefore, despite a growth defect, the cellulose hydrolysis capacity of the Δpta::gapDHp-cat strain was comparable to that of the WT strain.
TABLE 4.
Dry weight and Avicel conversion profile of the Δpta::gapDHp-cat strain
| Strain or genotype | DW (g/liter)a | % Avicel conversion |
|
|---|---|---|---|
| DW-based calculationb | Dry cell mass-based calculationc | ||
| WT | 0.532 (0.133) | 89.4 | 100.1 |
| ΔpyrF | 0.508 (0.034) | 89.8 | 100.7 |
| Δpta::gapDHp-cat | 0.537 (0.033) | 89.3 | 95.9 |
The average dry weight (DW) from triplicate samples of each strain at the end of 96 h of fermentation on Avicel is indicated with the standard deviation (SD).
The percent conversion of Avicel is calculated based on the starting Avicel concentration (5 g/liter) and the final DW, which includes residual Avicel and dry cell weight.
The percent conversion of Avicel is calculated based on the starting Avicel concentration (5 g/liter) and the estimated final Avicel concentration. We assumed that the dry cell mass for each strain after 96 h of fermentation is roughly equivalent to that obtained from cells that have consumed 5 g/liter cellobiose (data reported in the text). Therefore, the estimated final Avicel concentration was calculated by subtracting dry cell mass from DW.
DISCUSSION
To create a gene deletion system for C. thermocellum, which has low transformation efficiency and requires replicating plasmids, we needed to develop multiple positive and negative selectable markers to allow selection of both allele replacement and loss of the replicating plasmid used to deliver the allelic replacement cassette. Key factors responsible for achieving this result involved exploiting the native capacity of the host in question. To this end, we utilized the native gapDH promoter to express cat and confer resistance to thiamphenicol. In addition, C. thermocellum was sensitive to the antimetabolite 5-FOA, allowing us to create, as in other model systems, a native dual selection marker around pyrF (4). The use of native elements when developing a genetic system serves to constrain the experimental space by eliminating functional uncertainty, allowing focus on other areas such as transformation.
In this report we have simplified electroporation-based transformation of C. thermocellum and obtained reliable and reproducible results. In contrast to the original protocol (37, 38), the generation of competent cells has been simplified such that the isoniacin incubation, cellobiose solution wash, and custom-built electroporation cuvettes are no longer required. Best results are obtained with a custom pulse generator, but success can be achieved with a commercial device (the latter being the scope of a separate study). Furthermore, the gene deletion system we developed utilizes replicating plasmids and does not rely on high-efficiency transformation. Development of the pyrF-based genetic selections, in addition to the reliable electrotransformation protocol, removes a significant barrier to routine transformation and genetic manipulation of C. thermocellum.
The genetic tools described in this report were applied in the context of metabolic engineering to delete pta and create a C. thermocellum strain that does not produce acetate. This is a major metabolic perturbation, and like various bacteria in which the acetate production pathway has been eliminated, the C. thermocellum pta mutant displayed growth defects (10, 18, 32, 42). Adaptation and evolutionary approaches have been shown to ameliorate this effect but have not been explored in this study (10, 18, 32, 42). Thus, it is unclear if the growth defects associated with the C. thermocellum Δpta strain are stable or part of a cellular adjustment process associated with redirecting carbon and energy flux. Possible explanations for growth limitation include redox imbalance and/or perturbation in acetyl-CoA flux (5, 40). The fate of acetyl-CoA is related to the pool of reduced ferredoxin, which if used to make hydrogen results in acetate production (31). Elimination of the ability to make acetate requires the cell to utilize alternate pathways to oxidize ferredoxin. If such pathways are present, strain adaptation and evolution should improve growth. However, if they are lacking from the system, further metabolic engineering would be required to provide such an activity. The other significant phenotype of the C. thermocellum Δpta strain was, in spite of a growth defect, that the cellulolytic capability was not compromised. This implies that the ATP yield associated with acetate production is not essential for cellulosome production and is consistent with an ATP-conserving model of oligosaccharide uptake (41). According to this model, upon cellulose hydrolysis, C. thermocellum preferentially assimilates cellodextrins up to 4 subunits as opposed to cellobiose, which is the primary cellulose hydrolysis end product of other cellulolytic organisms, such as Trichoderma reesei. This allows more hexose units to be transported into the cell per unit ATP. Additionally, C. thermocellum expresses cellobiose and cellodextrin phosphorylases that can use inorganic phosphate to phosphorolytically cleave β-glucan bonds, generating glucose-1-phosphate in the process. These aspects of cellulose utilization allow C. thermocellum to conserve ATP per hexose monomer consumed and more than compensate for the ATP-expensive process of cellulase synthesis.
Unlike the T. saccharolyticum pta-ack mutant, the C. thermocellum Δpta strain did not exhibit significantly increased ethanol production (32). Moreover, the C. thermocellum Δpta strain showed a notable difference in lactic acid production when fermenting Avicel versus cellobiose compared to the wild-type strain (Fig. 4). These results might be an indication of fundamental differences between the carbon fluxes through pyruvate in these two organisms. Future effort in developing a C. thermocellum homoethanologenic strain would involve deletion of lactate dehydrogenase, carbon flux analysis, and adaptation/evolution efforts. Nevertheless, the genetic system developed here demonstrates a step toward engineering a C. thermocellum homoethanologenic strain.
The recalcitrance of cellulose is the major challenge to utilizing lignocellulosic biomass as a feedstock. In this regard, C. thermocellum is now a unique platform, in that it is highly cellulolytic and amenable to genetic manipulation. The work presented here is a foundation for future metabolic engineering that will enable C. thermocellum to serve as a biocatalyst for the production of cellulosic fuels and chemicals with high yield and titer. The same technology can be used to explore the regulation of cellulosome synthesis and the roles of various structural and functional components of this multiprotein complex, providing a better understanding of how this fascinating microbe hydrolyzes cellulose. Advancements in these areas, facilitated by the described genetic tools, opens the way for further development of C. thermocellum and related CBP organisms as biocatalysts in the conversion of lignocellulosic biomass to sustainable fuels and chemicals.
Supplementary Material
Acknowledgments
This research was supported by Mascoma Corporation, a grant from the BioEnergy Science Center (BESC), and Oak Ridge National Laboratory, a U.S. Department of Energy (DOE) Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Footnotes
Published ahead of print on 6 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, E. A., J.-P. Belaich, Y. Shoham, and R. Lamed. 2004. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58:521-554. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, E. A., E. Setter, and R. Lamed. 1985. Organization and distribution of the cellulosome in Clostridium thermocellum. J. Bacteriol. 163:552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke, J. D., F. Croute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 5.Chang, D.-E., S. Shin, J.-S. Rhee, and J.-G. Pan. 1999. Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme A flux for growth and survival. J. Bacteriol. 181:6656-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demain, A. L., M. Newcomb, and J. H. D. Wu. 2005. Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev. 69:124-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer, W. R., and J. C. Liao. 2001. Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol. Prog. 17:57-61. [DOI] [PubMed] [Google Scholar]
- 8.Freier, D., C. P. Mothershed, and J. Wiegel. 1988. Characterization of Clostridium thermocellum JW20. Appl. Environ. Microbiol. 54:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold, N. D., and V. J. J. Martin. 2007. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J. Bacteriol. 189:6787-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green, E. M., Z. L. Boynton, L. M. Harris, F. B. Rudolph, E. T. Papoutsakis, and G. N. Bennett. 1996. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079-2086. [DOI] [PubMed] [Google Scholar]
- 11.Haas, L. O., J. M. Cregg, and M. A. Gleeson. 1990. Development of an integrative DNA transformation system for the yeast Candida tropicalis. J. Bacteriol. 172:4571-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanai, T., S. Atsumi, and J. C. Liao. 2007. Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl. Environ. Microbiol. 73:7814-7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris, L. M., N. E. Welker, and E. T. Papoutsakis. 2002. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J. Bacteriol. 184:3586-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heap, J. T., S. A. Kuehnea, M. Ehsaana, S. T. Cartmana, C. M. Cooksleya, J. C. Scotta, and N. P. Minton. 2010. The ClosTron: mutagenesis in Clostridium refined and streamlined. J. Microbiol. Methods 80:49-55. [DOI] [PubMed] [Google Scholar]
- 15.Heap, J. T., O. J. Pennington, S. T. Cartman, G. P. Cartera, and N. P. Minton. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70:452-464. [DOI] [PubMed] [Google Scholar]
- 16.Heap, J. T., T. C. Stephen, J. P. Oliver, M. C. Clare, C. S. Jamie, B. Ben, A. B. David, and P. M. Nigel. 2009. Development of genetic knock-out systems for clostridia, p. 179-198. In H. Brüggemann and G. Gottschalk (ed.), Clostridia: molecular biology in the post-genomic era. Caister Academic Press, Norfolk, United Kingdom.
- 17.Johnson, E. A., A. Madia, and A. L. Demain. 1981. Chemically defined minimal medium for growth of the anaerobic cellulolytic thermophile Clostridium thermocellum. Appl. Environ. Microbiol. 41:1060-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakuda, H., K. Shiroishi, K. Hosono, and S. Ichihara. 1994. Construction of Pta-Ack pathway deletion mutants of Escherichia coli and characteristic growth profiles of the mutants in a rich medium. Biosci. Biotechnol. Biochem. 58:2232-2235. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy, C. L., E. O. Krejany, L. F. Young, J. R. O'Connor, M. M. Awad, R. L. Boyd, J. J. Emmins, D. Lyras, and J. I. Rood. 2005. The alpha toxin of Clostridium septicum is essential for virulence. Mol. Microbiol. 57:1357-1366. [DOI] [PubMed] [Google Scholar]
- 20.Kondo, S., A. Yamagishi, and T. Oshima. 1991. Positive selection for uracil auxotrophs of the sulfur-dependent thermophilic archaebacterium Sulfolobus acidocaldarius by use of 5-fluoroorotic acid. J. Bacteriol. 173:7698-7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, H., G. N. Bennett, and K.-Y. San. 2005. Metabolic engineering of aerobic succinate production systems in Escherichia coli to improve process productivity and achieve the maximum theoretical succinate yield. Metab. Eng. 7:116-127. [DOI] [PubMed] [Google Scholar]
- 22.Lynd, L. R., P. J. Weimer, W. H. van Zyl, and I. S. Pretorius. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66:506-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynd, L. R., W. H. van Zyl, J. E. McBride, and M. Laser. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16:577-583. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 25.Patnaik, R., and J. C. Liao. 1994. Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl. Environ. Microbiol. 60:3903-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothstein, D. M. 1986. Clostridium thermosaccharolyticum strain deficient in acetate production. J. Bacteriol. 165:319-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl. Environ. Microbiol. 71:3889-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 185:210-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider, J. C., A. F. Jenings, D. M. Mun, P. M. McGovern, and L. C. Chew. 2005. Auxotrophic markers pyrF and proC can replace antibiotic markers on protein production plasmids in high-cell-density Pseudomonas fluorescens fermentation. Biotechnol. Prog. 21:343-348. [DOI] [PubMed] [Google Scholar]
- 30.Shanks, R. M. Q., N. C. Caiazza, S. M. Hinsa, C. M. Toutain, and G. A. O'Toole. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl. Environ. Microbiol. 72:5027-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw, A. J., D. A. Hogsett, and L. R. Lynd. 2009. Identification of the [FeFe]-hydrogenase responsible for hydrogen generation in Thermoanaerobacterium saccharolyticum and demonstration of increased ethanol yield via hydrogenase knockout. J. Bacteriol. 191:6457-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw, A. J., K. K. Podkaminer, S. G. Desai, J. S. Bardsley, S. R. Rogers, P. G. Thorne, D. A. Hogsett, and L. R. Lynd. 2008. Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. Proc. Natl. Acad. Sci. U. S. A. 105:13769-13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soutschek-Bauer, E., W. Scholz, E. Grill, and W. L. Staudenbauer. 1987. Thermostability and superhelicity of plasmid DNA in Bacillus stearothermophilus. Mol. Gen. Genet. 209:575-579. [DOI] [PubMed] [Google Scholar]
- 34.Tolonen, A. C., A. C. Chilaka, and G. M. Church. 2009. Targeted gene inactivation in Clostridium phytofermentans shows that cellulose degradation requires the family 9 hydrolase Cphy3367. Mol. Microbiol. 74:1300-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinh, C. T., and F. Srienc. 2009. Metabolic engineering of Escherichia coli for efficient conversion of glycerol to ethanol. Appl. Environ. Microbiol. 75:6696-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinh, C. T., P. Unrean, and F. Srienc. 2008. Minimal Escherichia coli cell for the most efficient production of ethanol from hexoses and pentoses. Appl. Environ. Microbiol. 74:3634-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyurin, M. V., S. G. Desai, and L. R. Lynd. 2004. Electrotransformation of Clostridium thermocellum. Appl. Environ. Microbiol. 70:883-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyurin, M. V., C. R. Sullivan, and L. R. Lynd. 2005. Role of spontaneous current oscillations during high-efficiency electrotransformation of thermophilic anaerobes. Appl. Environ. Microbiol. 71:8069-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagishi, A., T. Tanimoto, T. Suzuki, and T. Oshima. 1996. Pyrimidine biosynthesis genes (pyrE and pyrF) of an extreme thermophile, Thermus thermophilus. Appl. Environ. Microbiol. 62:2191-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, Y.-T., G. N. Bennett, and K.-Y. San. 1999. Effect of inactivation of nuo and ackA-pta on redistribution of metabolic fluxes in Escherichia coli. Biotechnol. Bioeng. 65:291-297. [PubMed] [Google Scholar]
- 41.Zhang, Y.-H. P., and L. R. Lynd. 2005. Cellulose utilization by Clostridium thermocellum: bioenergetics and hydrolysis product assimilation. Proc. Natl. Acad. Sci. U. S. A. 102:7321-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, Y., X. Chen, T. Chen, and X. Zhao. 2007. Enhancement of riboflavin production by overexpression of acetolactate synthase in a pta mutant of Bacillus subtilis. FEMS Microbiol. Lett. 266:224-230. [DOI] [PubMed] [Google Scholar]
- 43.Zverlov, V. V., M. Klupp, J. Krauss, and W. H. Schwarz. 2008. Mutations in the scaffoldin gene, cipA, of Clostridium thermocellum with impaired cellulosome formation and cellulose hydrolysis: insertions of a new transposable element, IS1447, and implications for cellulase synergism on crystalline cellulose. J. Bacteriol. 190:4321-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.