Abstract
The food-borne pathogen Escherichia coli O157:H7 is commonly exposed to organic acid in processed and preserved foods, allowing adaptation and the development of tolerance to pH levels otherwise lethal. Since little is known about the molecular basis of adaptation of E. coli to organic acids, we studied K-12 MG1655 and O157:H7 Sakai during exposure to acetic, lactic, and hydrochloric acid at pH 5.5. This is the first analysis of the pH-dependent transcriptomic response of stationary-phase E. coli. Thirty-four genes and three intergenic regions were upregulated by both strains during exposure to all acids. This universal acid response included genes involved in oxidative, envelope, and cold stress resistance and iron and manganese uptake, as well as 10 genes of unknown function. Acidulant- and strain-specific responses were also revealed. The acidulant-specific response reflects differences in the modes of microbial inactivation, even between weak organic acids. The two strains exhibited similar responses to lactic and hydrochloric acid, while the response to acetic acid was distinct. Acidulant-dependent differences between the strains involved induction of genes involved in the heat shock response, osmoregulation, inorganic ion and nucleotide transport and metabolism, translation, and energy production. E. coli O157:H7-specific acid-inducible genes were identified, suggesting that the enterohemorrhagic E. coli strain possesses additional molecular mechanisms contributing to acid resistance that are absent in K-12. While E. coli K-12 was most resistant to lactic and hydrochloric acid, O157:H7 may have a greater ability to survive in more complex acidic environments, such as those encountered in the host and during food processing.
Some strains of Escherichia coli are capable of surviving in environments more suited to acidophiles than enterics and possess acid resistance systems that rival those of Helicobacter pylori, a species that has evolved to live in the stomach (for a review, see reference 25). The capacity to survive acid stress is an important property of E. coli as it determines its ability to survive in acidic foods and in animal or human host gastrointestinal tracts. Consequently, the acid resistance properties of this organism also influence its ability to cause disease. Acidification of food by the addition of organic acid is the primary means of preventing the growth of human pathogens in a wide range of fermented and acidified ready-to-eat foods (14). Enterohemorrhagic E. coli (EHEC), a pathotype which can cause potentially lethal sequelae, has been implicated in food-borne outbreaks involving a variety of acidic foods, such as apple cider (10), fermented sausage (15), yoghurt (62), and mayonnaise (82).
Outbreaks involving acidic foods have drawn attention to the acid tolerance response (ATR) of EHEC, in particular O157:H7. The ATR is a process induced by exposure to levels of acidity that habituates the organism and allows it to withstand lethal levels of acid (31). This response increases the ability of the pathogen to survive in acidic foods (58) and can also induce cross-protection against other environmental stresses encountered during food processing (28). Although a number of studies have investigated the molecular response of this organism to sublethal levels of acidity, they have typically focused on the use of buffering agents (morpholineethanesulfonic acid [MES]) or inorganic acids, such as hydrochloric acid (51, 61, 73, 77, 79, 83). While both inorganic and organic acids acidify the internal pH of the cell, weak organic acids also accumulate as an intracellular anion (for a review, see reference 72). Weak organic acids, being uncharged in their undissociated form, readily diffuse across the cell membrane and dissociate in the higher-pH environment of the cytosol, generating protons and the acid anion. The acid anion accumulates intracellularly, as, being charged, it cannot readily diffuse from the cell. This high anion accumulation may generate high turgor pressure and can influence free radical production, leading to severe oxidative stress. It is unlikely though that this represents the complete explanation of the mode of action of weak organic acids or that all weak organic acids operate identically to inhibit growth (68). Indeed, proteomic studies have revealed that E. coli has a unique expression profile during exposure to benzoic acid (56), lactic acid (43), and acetate and formate (54) stress. A transcriptomic study on the acetate-induced ATR has also revealed a unique gene expression signature in E. coli (5).
A deeper understanding of organic acid tolerance in E. coli would provide fundamental insight into how this organism survives a stress routinely used by the food industry and may enable control strategies to be devised. It may be possible to identify environmental conditions which prevent the expression of protective proteins, rendering the bacteria sensitive to acid.
We used a transcriptomic approach to investigate and compare the ATR of E. coli during exposure to organic lactic (L-ATR) or acetic (A-ATR) acid to that of inorganic hydrochloric acid (H-ATR). With the knowledge that strains may respond differently to environmental conditions, the gene expression responses of the laboratory strain K-12 MG1655 (11) and pathogenic strain O157:H7 Sakai (35) were investigated. The O157:H7 strain carries 1.4 Mb of sequence that is absent from the K-12 strain, most of which is horizontally transferred foreign DNA (35). We hypothesized that the EHEC pathotype may possess novel molecular mechanisms that contribute to acid resistance. Acid tolerance is strongly dependent on growth phase. Stationary-phase cultures are more acid tolerant than their exponential-phase counterparts (6, 7). In order to ensure that the response of E. coli was characterized during maximal acid resistance, and because stationary-phase bacteria are particularly significant for food microbiology (70), experiments were conducted with stationary-phase cultures.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study were E. coli K-12 MG1655 (11) (obtained from Mark Schembri, Brisbane, Australia) and O157:H7 Sakai (35) (obtained from Carlton Gyles, Guelph, Canada), designated EC2940 and EC2941 in our culture collection, respectively. Overnight cultures (18 h) were grown at 37°C in 250-ml Erlenmeyer flasks containing 100 ml of brain heart infusion broth (BHI) broth (Oxoid, Basingstoke, United Kingdom) buffered with 50 mM Tris. Media were buffered to the required pH range using Tris (and MES [see below]) in accordance with a modification to the method employed by Antón et al. (3). All cultures were tested with a model WP80 pH meter fitted with a combination pH sensor (TPS, Australia) after 18 h of growth, and only those within the pH range of 7 ± 0.2 were used in downstream experiments.
Determination of conditions required to maximally induce the stationary-phase acid tolerance response.
The conditions required to maximally induce the ATRs of each strain were determined as previously described (32). Briefly, the pH of an overnight culture was adjusted by the addition of either dl-lactic acid, acetic acid, or hydrochloric acid to an adaptation pH value of either 5.0, 5.5, or 5.8 ± 0.1 units and maintained for either 1, 2, 3, 4, 5, or 6 h. In total, for each strain, 18 combinations of adaptation pHs and incubation times were tested. The level of resistance afforded during adaptation under each of the 18 conditions was assessed using an acid survival assay, and the results were compared to each other and to that of an acid-shocked overnight culture. The method of the acid survival assay was as follows. After the required incubation time, cells were resuspended to yield viable counts of approximately 2 × 108 CFU/ml in 100 ml of fresh BHI broth buffered with 50 mM MES and acidified to a pH of 3.5 ± 0.1 with the appropriate test acidulant at 37°C. Cultures were challenged at pH 3.5, as this mimics a typical pH level for low-pH food environments. At intervals throughout incubation, samples were removed and numbers of viable bacteria determined by spread plating serial dilutions onto tryptone soya agar (TSA; Oxoid) supplemented with 0.2% (wt/vol) yeast extract and 0.2% (wt/vol) glucose (TYSG). Plates were incubated at 37°C for 24 h before enumeration of cells. All experiments were performed in triplicate from a separate overnight culture. Data at each time point were analyzed by Minitab software (Minitab 15; Minitab Inc., Minneapolis, MN) using one-way analysis of variance (ANOVA) and a post hoc Tukey test. A P value of <0.05 was assumed to be the significance level. For both strains, the conditions required to afford maximum protection against all acids was incubation at pH 5.5 for 3 h and 2 h for K-12 and O157:H7, respectively (results not shown). The final concentrations of acids required to adjust cultures of E. coli to pH 5.5 were 0.03 M acetic acid, 0.03 M dl-lactic acid, and 0.02 M hydrochloric acid.
RNA isolation and processing.
A 10-ml sample was removed from an overnight culture (18 h) to represent the reference condition. After incubation at pH 5.5 for the time required to maximally induce the ATR with the test acid, a further 10-ml sample was removed from the same culture. One-fifth of the culture volume of ice-cold phenol-ethanol solution (5:95) was added to the culture to stabilize the RNA and prevent degradation. The culture was immediately transferred to ice prior to RNA extraction. RNA samples were prepared using a Promega SV total RNA purification kit. RNA concentration was determined using a NanoPhotometer (Implen Pty.). RNA quality was determined by 16S and 23S rRNA peak examination with a Bioanalyzer 2100 (Agilent, Santa Clara, CA) using an RNA nano chip. cDNA synthesis, labeling, and hybridization to GeneChip E. coli Genome 2.0 arrays (Affymetrix, Santa Clara, CA) were performed by CSIRO Molecular and Health Technologies (Sydney, Australia).
Microarray data analysis.
The Affy package (47) of the Bioconductor software (29) was used to process raw CEL files using the robust multiarray average algorithm (RMA) (48) for normalization, background correction, and expression value calculation. Expression levels obtained from four independent biological replicates of every condition were compared using the Limma package (76) of the Bioconductor software. Elements with expression levels ≥2-fold higher or lower than the reference level and that were statistically significant (after P value adjustment by the Benjamini and Hochberg method, with an adjusted P value of ≤0.01, an average expression [A value] of ≥2, and a log odds [B value] of ≥0) were selected. However, it should be noted that <2-fold changes can also be biologically significant (44, 45). A P value of ≤0.01 was considered significant, which corresponds to a false-positive rate of 1 in 100 genes. Those genes and intergenic regions passing an even stricter P value cutoff (P ≤ 0.001) are highlighted in Table S1 of the supplemental material. A functional grouping of genes was made according to the data from the NCBI (http://www.ncbi.nlm.nih.gov/COG/). Analysis of the differentially expressed intergenic regions was conducted using sRNAMap (42).
All genes identified as differentially transcribed, along with their fold change value and a comparison to previously published microarray data, are presented in Table S1 of the supplemental material.
Validation of microarray data by qRT-PCR.
Four genes that showed significant (P value ≤ 0.01) upregulation or downregulation in the microarray experiments were selected for analysis by quantitative real-time reverse transcription-PCR (qRT-PCR). The 16S rRNA gene, rrsA, was also included for normalization within samples. Forward and reverse PCR primers for gadE were designed using Primer3 software (http://primer3.sourceforge.net/), and primer sets for oxyS (19), rpoH (16), znuA (53), and rrsA (55) were from previously published papers (see Table S2 of the supplemental material). cDNA was produced from the RNA of three biological replicates used for microarray analysis by reverse transcription of 1 μg of purified total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Tenfold dilutions (from 10−3 to 10−5) of the template cDNA were made for use in qRT-PCRs. qRT-PCR mixes contained a total volume of 25 μl consisting of 12.5 μl IQ SYBR green supermix (Bio-Rad), 1 μl MgCl2 (50 mM stock; Bio-Rad), 2 μl diluted cDNA, 0.5 μl each forward and reverse primer (25 μM stock), and 8.5 μl nuclease-free water (Ambion, Austin, TX). Real-time PCR was performed on the iCycler iQ5 multicolor real-time PCR detection system (Bio-Rad) under the following reaction conditions: 95°C for 3 min, 45 cycles consisting of 95°C for 10 s and 60°C for 30 s, and 72°C for 30 s. Melting curve analysis (55 to 81°C, 0.5°C increments for 30 s) was performed to ensure PCR specificity. The method described by Pfaffl (66) was employed to determine the expression fold changes of the target gene in cultures at the time of incubation that corresponded to maximal induction of the ATR, compared to that of the corresponding overnight culture.
Acid resistance in the presence of protein synthesis inhibitor.
In order to determine whether the increased survival of acid-adapted cultures after challenge at pH 3.5 was the result of new proteins synthesis, we compared the levels of resistance of cultures adapted in the presence and absence of a protein synthesis inhibitor. Chloramphenicol was added to a final concentration of 200 μg/ml to overnight cultures (18 h) at 37°C 10 min prior to acid adaptation. Cultures were acid adapted and challenged as described above.
Heat shock assay.
The levels of thermal tolerance of HCl-adapted cultures of K-12 and O157:H7 were determined and compared at 50°C. HCl-adapted cultures of K-12 and O157:H7 were harvested by centrifugation, washed once in an equal volume of 0.1 M phosphate buffer (pH 7), centrifuged, and resuspended in 1 ml of phosphate-buffered saline (PBS). The cell suspension was added to 100 ml of PBS preheated to 50°C to yield viable cell counts of approximately 2 × 108 CFU/ml. During incubation at 50°C, the percentage of survivors was determined at time intervals by plating dilutions directly onto TYSG plates. Viable cell counts were determined after overnight incubation at 37°C.
Microarray data accession number.
The microarray transcriptomic data were deposited at ArrayExpress (http://www.ebi.ac.uk/miamexpress/) under accession number E-TABM-912.
RESULTS
Resistance of acid-shocked and acid-adapted cultures of E. coli K-12 and O157:H7 to acetic, lactic, and hydrochloric acid.
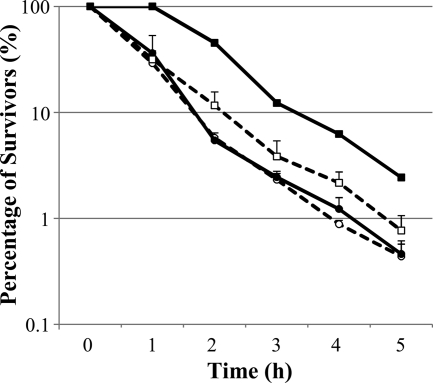
The conditions required to maximally induce the ATRs of the strains to all acidulants was experimentally determined. This involved incubation at pH 5.5 for 3 h (K-12) and for 2 h (O157:H7) (Fig. 1) and generated acid-adapted cultures that were more resistant to acid challenge at pH 3.5 than bacteria that had been grown at neutral pH prior to acid shock (Fig. 1). The acid sensitivities of the two strains were compared under these conditions. E. coli K-12 showed the greatest resistance to lactic and hydrochloric acid, while O157:H7 was most resistant to acetic acid.
FIG. 1.
Acid resistance of K-12 (•, ○) and O157:H7 (▪, □) to BHI acidified to pH 3.5 with acetic, lactic, or hydrochloric acid. The percentages of surviving cells of cultures incubated at pH 7 (acid shock) are represented by dashed lines. The percentages of surviving cells of cultures incubated at pH 5.5 (acid adapted; 3 h for K-12 and 2 h for O157:H7) in the absence and presence of chloramphenicol are represented by solid and dotted lines, respectively. The percentages of survivors were determined by plating cells on TYSG agar. Error bars represent standard errors of the means based on counts from three replicate populations; in most cases, their size was smaller than the symbol.
Comparison of the levels of resistance of cultures adapted in the presence and absence of a protein synthesis inhibitor (Fig. 1) revealed that the increased survival of acid-adapted cultures to challenge at pH 3.5 was the result of new proteins synthesis.
E. coli K-12 and O157:H7 show a universal gene expression response that involves upregulation of the oxidative, envelope, and cold shock stress responses and genes involved in iron and manganese uptake.
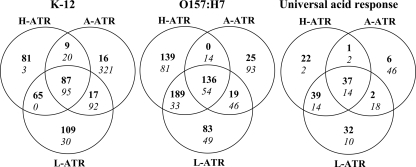
To determine the transcriptomic response of K-12 and O157:H7 to each of the three acids, RNA was extracted from cultures at the time of incubation corresponding to maximal induction of the ATR and from the corresponding overnight culture to serve as a reference. qRT-PCR experiments confirmed the trend observed in the differential expression reflected in the microarray data of four candidate genes (Table 1). The fold change detected by qRT-PCR was generally more pronounced than that detected by microarray analysis, in line with the fact that microarrays are generally less sensitive than qRT-PCR for quantification of gene expression (81). The numbers of genes and intergenic regions induced or repressed by each of the strains during exposure to each of the acids in comparison to the number induced or repressed by the reference (unadapted) culture are shown in Fig. 2.
TABLE 1.
Gene expression fold changes generated by microarray analysis and qRT-PCR for four selected genes
| Gene | Strain | Acidulant | Microarray fold changea | qRT-PCR fold change |
|---|---|---|---|---|
| gadE | K-12 | HCl | NDE | 0.4 ± 0.1 |
| Acetic acid | −4.9 | −6.4 ± 1.2 | ||
| Lactic acid | −3.9 | −5.1 ± 0.7 | ||
| O157:H7 | HCl | NDE | 0.7 ± 0.1 | |
| Acetic acid | NDE | 0.8 ± 0.1 | ||
| Lactic acid | NDE | 1.6 ± 0.4 | ||
| oxyS | K-12 | HCl | 17.1 | 4.3 ± 0.1 |
| Acetic acid | 10.5 | 2.2 ± 0.2 | ||
| Lactic acid | 18.5 | 4.8 ± 0.6 | ||
| O157:H7 | HCl | 12.1 | 3.4 ± 1.1 | |
| Acetic acid | 7.4 | 2.6 ± 0.1 | ||
| Lactic acid | 13.8 | 2.4 ± 0.3 | ||
| rpoH | K-12 | HCl | NDE | 1.8 ± 0.4 |
| Acetic acid | NDE | 0.6 ± 0.1 | ||
| Lactic acid | NDE | 0.8 ± 0.2 | ||
| O157:H7 | HCl | 3.2 | 11.8 ± 0.6 | |
| Acetic acid | NDE | 1.5 ± 0.3 | ||
| Lactic acid | 2.8 | 10.5 ± 0.4 | ||
| znuA | K-12 | HCl | NDE | 0.3 ± 0.1 |
| Acetic acid | NDE | 0.5 ± 0.2 | ||
| Lactic acid | NDE | 1.9 ± 1.5 | ||
| O157:H7 | HCl | 4.3 | 9.7 ± 1.5 | |
| Acetic acid | NDE | 0.4 ± 0.2 | ||
| Lactic acid | 3.6 | 7.8 ± 0.2 |
NDE, not differentially expressed.
FIG. 2.
Comparison of levels of genome-wide gene expression in K-12 and O157:H7 after induction of the A-ATR, L-ATR, or H-ATR. Bacteria were adapted for 3 h (K-12) and 2 h (O157:H7) in BHI acidified to pH 5.5. The numbers of differentially expressed genes (i.e., genes with a ≥2-fold difference in expression from that of the reference culture) are shown as a Venn diagram. The first two Venn diagrams compare the gene expression responses of K-12 and O157:H7 during adaptation with each of the test acidulants. In the third Venn diagram, labeled “universal acid response,” numbers of genes which are upregulated or downregulated by both strains during adaptation with each acid are compared. Numbers of upregulated genes are shown in bold, and numbers of downregulated genes are shown in italics.
Surprisingly, the acid-adapted stationary-phase cultures did not show significant upregulation of acid fitness island (AFI) genes in K-12 or O157:H7. Similarly, among the four known acid resistance systems of E. coli (25), we observed only upregulation of the lysine-dependent acid resistance system (cadB) during exposure of O157:H7 to HCl.
Under the conditions employed in this study, we identified gene expression signatures characteristic of both K-12 and O157:H7 during induction of the A-ATR, L-ATR, and H-ATR. Thirty-four genes were upregulated, including 10 FUN genes (of unknown function [41]), the small RNA oxyS, and two intergenic regions not corresponding to known sRNAs (42) (Table 2). A number of genes that protect against oxidative damage were upregulated, including katG, trxC, ahpF, grxA, and the small regulatory RNA (sRNA) oxyS. The positive modulator of RpoE envelope stress response sigma factor activity (rseC) and a poorly defined regulator of the envelope stress response (ydcQ) were upregulated, consistent with previous reports that showed acid induction of RpoE (36). Acidic pH also enhanced the expression of genes involved in iron (exbD, fepD, ydiE, hemF) and manganese (mntH) uptake and acquisition. A number of cold shock-inducible genes were induced, including cspA, encoding the major cold shock protein of E. coli, lpxP, and csdA. For both O157:H7 and K-12, oxyS, grxA, mntH, and the FUN gene yfiP were among the genes most highly expressed during adaptation to all acids.
TABLE 2.
Genes upregulated at least 2-fold in the universal acid responses of K-12 and O157:H7 during induction of the H-ATR, L-ATR, and/or A-ATRa
| ATR(s)b | COG functional categoryc | b no. (K-12) | EC no. (O157:H7) | Gened | Function(s) | Fold changee |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K-12 |
O157:H7 |
||||||||||
| H | L | A | H | L | A | ||||||
| H-ATR, L-ATR, plus | Amino acid transport and metabolism | b3870 | ECs4792 | glnA | Glutamine synthetase | 2.6 | 2.8 | 2.5 | 22.8 | 10.9 | 4.8 |
| A-ATR (universal) | Cell wall/membrane biogenesis | b2378 | ECs3258 | lpxP (ddg) | Lipid A biosynthesis, palmitoleoyl acyltransferase | 5.8 | 4.8 | 3.2 | 5.8 | 7.3 | 2.8 |
| Coenzyme transport and metabolism | b2436 | ECs3307 | hemF | Coproporphyrinogen III oxidase | 2.9 | 2.7 | 2.0 | 3.5 | 4.0 | 3.2 | |
| b2912 | ECs3782 | ygfA | Predicted ligase | 3.9 | 6.8 | 4.2 | 3.4 | 3.5 | 2.7 | ||
| Energy production and conversion | b2582 | ECs3448 | trxC | Thioredoxin 2 | 4.0 | 4.8 | 3.8 | 14.3 | 16.0 | 9.3 | |
| Inorganic ion transport and metabolism | b3005 | ECs3889 | exbD | Membrane-spanning protein in TonB-ExbB-ExbD complex | 5.1 | 4.7 | 2.5 | 7.8 | 6.4 | 3.4 | |
| b0590 | ECs0629 | fepD | Iron enterobactin transporter membrane protein | 3.3 | 2.7 | 2.4 | 2.5 | 3.0 | 2.1 | ||
| b3942 | ECs4871 | katG | Peroxidase/catalase HPIf | 2.7 | 2.7 | 2.1 | 7.8 | 7.2 | 4.7 | ||
| b2392 | ECs3271 | mntH | Manganese/divalent cation transporter | 5.3 | 9.0 | 4.5 | 15.0 | 12.7 | 6.5 | ||
| b1705 | ECs2412 | ydiE | Hypothetical conserved protein | 4.8 | 4.8 | 3.5 | 4.2 | 4.3 | 4.0 | ||
| b3345 | ECs4196 | yheN | Predicted intracellular sulfur oxidation protein | 2.2 | 2.9 | 2.0 | 3.3 | 3.6 | 3.1 | ||
| Nucleotide transport and metabolism | b3648 | ECs4523 | gmk | Guanylate kinase | 2.9 | 3.2 | 2.2 | 4.8 | 4.0 | 2.0 | |
| b2674 | ECs3537 | nrdI | Ribonucleotide reductase stimulatory protein | 2.8 | 3.5 | 2.2 | 5.4 | 5.6 | 3.0 | ||
| Posttranslational modification, protein | b0606 | ECs0645 | ahpF | Alkyl hydroperoxide reductase F52a subunit | 3.9 | 4.3 | 2.1 | 9.4 | 7.1 | 5.8 | |
| turnover, chaperones | b0849 | ECs0929 | grxA | Glutaredoxin 1 | 21.6 | 23.7 | 14.6 | 17.5 | 21.9 | 10.4 | |
| Signal transduction mechanisms | b2469 | ECs3331 | narQ | Sensory histidine kinase in two-component regulatory system with NarP (NarL) | 3.5 | 3.1 | 2.9 | 4.1 | 5.2 | 3.6 | |
| b2570 | ECs3436 | rseC | Involved in reduction of the SoxR iron-sulfur cluster | 3.6 | 3.2 | 2.4 | 3.7 | 3.0 | 2.8 | ||
| Transcription | b3556 | ECs4441 | cspA | Major cold shock protein | 4.5 | 6.4 | 3.4 | 5.8 | 7.0 | 2.8 | |
| b3162 | ECs4043 | deaD (csdA) | ATP-dependent RNA helicase | 6.2 | 5.1 | 2.7 | 6.6 | 6.4 | 2.8 | ||
| b1434 | ECs2037 | ydcN | Predicted DNA-binding transcriptional regulator | 5.6 | 3.8 | 3.8 | 4.7 | 5.5 | 2.7 | ||
| b1439 | ECs2043 | ydcR | Putative transcriptional regulator, putative ATP-binding component of a transport system | 2.9 | 2.4 | 2.1 | 3.8 | 2.9 | 2.3 | ||
| b2015 | ECs2817 | yeeY | Predicted DNA-binding transcriptional regulator | 3.4 | 5.4 | 3.5 | 2.6 | 2.8 | 2.8 | ||
| Poorly characterized or not present in | b0459 | ECs0512 | maa (ylaD) | Maltose O-acetyltransferase | 2.9 | 3.9 | 2.5 | 2.2 | 2.5 | 2.9 | |
| COGs | b4458 | ECs5566 | oxyS | RNA | 17.1 | 18.5 | 10.5 | 12.1 | 13.8 | 7.4 | |
| b1610 | ECs2316 | tus | DNA-binding inhibitor of DNA replication | 2.4 | 6.0 | 3.4 | 8.6 | 10.7 | 8.9 | ||
| b0255 | ECs0252 | yafQ | Predicted toxin of the YafQ-DinJ toxin-antitoxin system | 3.4 | 5.4 | 3.6 | 5.1 | 4.1 | 2.9 | ||
| b0802 | ECs0880 | ybiJ | Hypothetical protein | 4.3 | 5.0 | 3.4 | 6.3 | 5.4 | 2.5 | ||
| b0847 | ECs0927 | ybjL | Predicted transporter protein | 3.9 | 4.3 | 2.6 | 3.4 | 3.3 | 2.4 | ||
| b1438 | ECs2042 | ydcQ | Predicted DNA-binding transcriptional regulator | 2.4 | 2.8 | 2.3 | 3.8 | 3.7 | 2.0 | ||
| b2190 | ECs3081 | yejO | Putative ATP-binding component of a transport system | 2.1 | 2.4 | 2.2 | 2.4 | 2.9 | 2.3 | ||
| b2583 | ECs3449 | yfiP | Hypothetical conserved protein | 6.9 | 7.1 | 4.9 | 6.9 | 7.8 | 8.9 | ||
| b2603 | ECs3466 | yfiR | Hypothetical protein | 3.2 | 4.4 | 2.9 | 2.6 | 2.9 | 2.5 | ||
| b3009 | ECs3893 | yghB | Hypothetical conserved inner membrane protein | 2.3 | 3.5 | 2.4 | 3.2 | 3.4 | 2.2 | ||
| b3242 | ECs4115 | yhcR | Hypothetical protein | 3.4 | 6.7 | 3.0 | 3.3 | 5.3 | 5.2 | ||
| b1995 | ECs2792 | yoeA | Predicted disrupted hemin or colicin receptor, interrupted by IS2 and C-terminal deletion | 3.9 | 8.1 | 4.0 | 5.8 | 5.7 | 4.1 | ||
| 2 intergenic regions | |||||||||||
| H-ATR | Amino acid transport and metabolism | b2530 | ECs3396 | iscS (yfhO) | Cysteine desulfurase | 3.0 | 4.2 | ||||
| Carbohydrate transport and metabolism | b0124 | ECs0128 | gcd | Glucose dehydrogenase | 2.3 | 3.2 | |||||
| Cell wall/membrane/envelope biogenesis | b2466 | ECs3328 | ypfG | Hypothetical protein | 2.0 | 2.7 | |||||
| Defense mechanisms | b0449 | ECs0503 | mdlB | Putative multidrug transporter membrane ATP-binding component | 2.9 | 3.3 | |||||
| Energy production and conversion | b1650 | ECs2359 | nemA | N-Ethylmaleimide reductase | 2.5 | 6.8 | |||||
| b2529 | ECs3395 | iscU | Scaffold protein | 2.8 | 3.9 | ||||||
| Inorganic ion transport and metabolism | b0050 | ECs0055 | apaG | Protein associated with Co2+ and Mg2+ efflux | 2.2 | 2.4 | |||||
| b1859 | ECs2569 | znuB | High-affinity zinc transporter membrane component | 2.8 | 4.4 | ||||||
| Replication, recombination, and repair | b4361 | ECs5321 | dnaC | DNA biosynthesis protein | 2.1 | 4.2 | |||||
| Biosynthesis, transport, and catabolism of secondary metabolites | b0210 | ECs0206 | yafE | Predicted S-adenosyl-l-methionine-dependent methyltransferase | 2.8 | 2.6 | |||||
| Signal transduction mechanisms | b1129 | ECs1601 | phoQ | Sensory histidine kinase in two-component regulatory system with PhoP | 3.1 | 2.5 | |||||
| Translation, ribosomal structure and biogenesis | b0884 | ECs0969 | infA | Translation initiation factor IF-1 | 3.1 | 2.5 | |||||
| Poorly characterized or not present in | b0736 | ECs0771 | ybgC | Predicted acyl-CoAg thioesterase | 2.3 | 2.5 | |||||
| COGs | b1063 | ECs1441 | yceB | Predicted lipoprotein | 2.0 | 2.0 | |||||
| b1110 | ECs1488 | ycfJ | Hypothetical protein | 2.5 | 3.5 | ||||||
| b2528 | ECs3394 | iscA | Iron-sulfur cluster assembly protein | 2.9 | 2.1 | ||||||
| b3083 | ECs3965 | ygjN | Hypothetical conserved protein | 2.9 | 3.0 | ||||||
| b3293 | ECs4158 | yhdN | Hypothetical conserved protein | 2.2 | 3.7 | ||||||
| b4140 | ECs5121 | fxsA | Inner membrane protein | 2.0 | 2.9 | ||||||
| b4173 | ECs5149 | hflX | Predicted GTPase | 2.6 | 2.2 | ||||||
| b4360 | ECs5320 | yjjA | Hypothetical conserved protein | 2.7 | 2.7 | ||||||
| 1 intergenic region | |||||||||||
| L-ATR | Carbohydrate transport and metabolism | b1528 | ECs2135 | ydeA (sotB) | Predicted arabinose transporter | 3.2 | 3.7 | ||||
| Carbohydrate transport and metabolism, amino acid transport and metabolism | b0898 | ECs0983 | ycaD | Predicted transporter | 2.1 | 3.2 | |||||
| Cell wall/membrane/envelope biogenesis | b2142 | ECs3034 | yohK | Predicted inner membrane protein | 3.5 | 3.8 | |||||
| Cell wall/membrane/envelope biogenesis, inorganic ion transport and metabolism | b0192 | ECs0194 | nlpE (cutF) | Lipoprotein involved with copper homeostasis and adhesion | 2.1 | 2.1 | |||||
| Coenzyme transport and metabolism | b0475 | ECs0528 | hemH | Ferrochelatase | 3.2 | 8.0 | |||||
| b0630 | ECs0668 | lipB | Lipoyltransferase | 2.7 | 2.8 | ||||||
| Defense mechanisms | b0879 | ECs0965 | macB (ybjZ) | Hypothetical macrolide transporter ATP-binding/permease protein | 2.5 | 4.4 | |||||
| Inorganic ion transport and metabolism | b0150 | ECs0154 | fhuA | Ferrichrome outer membrane transporter | 2.4 | 6.4 | |||||
| b0818 | ECs0895 | ybiR | Predicted transporter | 2.1 | 2.5 | ||||||
| b4367 | ECs5327 | fhuF | Ferric iron reductase involved in ferric hydroxamate transport | 3.7 | 3.1 | ||||||
| Replication, recombination, and repair | b2509 | ECs3371 | xseA | Exodeoxyribonuclease VII large subunit | 2.0 | 2.6 | |||||
| b3397 | ECs4239 | nudE | ADP-ribose diphosphatase | 2.1 | 2.9 | ||||||
| Transcription | b0113 | ECs0117 | pdhR | DNA-binding transcriptional dual regulator | 3.2 | 6.3 | |||||
| b0959 | ECs1043 | sxy (yccR) | Hypothetical conserved protein | 2.9 | 2.4 | ||||||
| b1530 | ECs2137 | marR | DNA-binding transcriptional repressor of multiple antibiotic resistance | 3.2 | 4.1 | ||||||
| b3292 | ECs4157 | zntR | DNA-binding zinc-responsive transcriptional activator | 2.6 | 3.8 | ||||||
| b3585 | ECs4461 | yiaU | Predicted DNA-binding transcriptional regulator | 3.4 | 2.4 | ||||||
| b3755 | ECs4697 | yieP | Predicted transcriptional regulator | 2.1 | 2.2 | ||||||
| Translation, ribosomal structure and biogenesis | b3179 | ECs4058 | rrmJ | 23S rRNA methyltransferase | 2.3 | 2.7 | |||||
| Poorly characterized or not present in | b0380 | ECs0430 | yaiZ | Predicted inner membrane protein | 2.7 | 3.3 | |||||
| COGs | b0631 | ECs0669 | ybeD | Hypothetical conserved protein | 3.4 | 4.7 | |||||
| b1445 | ECs2049 | ydcX | Hypothetical protein | 2.3 | 3.2 | ||||||
| b1848 | ECs2558 | yebG | DNA damage-inducible conserved protein | 2.6 | 2.8 | ||||||
| b2141 | ECs3033 | yohJ | Hypothetical conserved inner membrane protein | 6.6 | 10.1 | ||||||
| b3238 | ECs4111 | yhcN | Hypothetical conserved protein | 2.6 | 3.6 | ||||||
| b3346 | ECs4197 | yheO | Predicted DNA-binding transcriptional regulator | 3.4 | 4.0 | ||||||
| b3536 | ECs4416 | bcsE (yhjS) | Conserved putative protease | 2.1 | 2.2 | ||||||
| b4537 | ECs5464 | yecJ | Hypothetical protein | 2.9 | 2.9 | ||||||
| b4050 | ECs5032 | pspG | Phage shock protein G | 2.3 | 2.7 | ||||||
| 3 intergenic regions | |||||||||||
| A-ATR | Inorganic ion transport and metabolism; biosynthesis, transport, and catabolism of secondary metabolites | b2211 | ECs3100 | yojI | Multidrug transporter membrane component/ATP-binding component | 2.1 | 2.1 | ||||
| Poorly characterized or not present in | b2375 | ECs3255 | yfdX | Hypothetical protein | 2.5 | 2.6 | |||||
| COGs | b2966 | ECs3842 | yqgA | Predicted inner membrane transporter protein | 2.3 | 3.2 | |||||
| 3 intergenic regions | |||||||||||
| H-ATR plus L-ATR | Carbohydrate transport and metabolism | b1280 | ECs1853 | yciM | Hypothetical conserved protein | 3.1 | 3.2 | 4.5 | 3.6 | ||
| Carbohydrate transport and metabolism, amino acid transport and metabolism | b3943 | ECs4872 | yijE | Predicted permease | 3.1 | 2.2 | 3.4 | 3.0 | |||
| Cell wall/membrane/envelope biogenesis | b2435 | ECs3306 | amiA | N-Acetylmuramoyl-l-alanine amidase I | 2.9 | 2.9 | 3.5 | 4.1 | |||
| b3967 | ECs4898 | murI | Glutamate racemase | 2.7 | 2.1 | 2.8 | 2.6 | ||||
| Cell wall/membrane/envelope biogenesis, inorganic ion transport and metabolism | b1252 | ECs1752 | tonB | Membrane-spanning protein in TonB-ExbB-ExbD complex | 2.6 | 2.7 | 5.5 | 6.0 | |||
| Coenzyme transport and metabolism | b1210 | ECs1715 | hemA | Glutamyl-tRNA reductase | 2.5 | 2.1 | 3.8 | 3.7 | |||
| b2153 | ECs3045 | folE | GTP cyclohydrolase I | 2.1 | 2.9 | 5.3 | 6.7 | ||||
| Defense mechanisms | b0448 | ECs0502 | mdlA | Putative multidrug transporter membrane ATP-binding component | 3.1 | 2.9 | 4.4 | 5.2 | |||
| Inorganic ion transport and metabolism | b3343 | ECs4194 | yheL | Predicted intracellular sulfur oxidation protein | 2.1 | 2.2 | 2.4 | 2.5 | |||
| Posttranslational modification, protein turnover, chaperones | b2527 | ECs3393 | hscB | DnaJ-like molecular chaperone specific for IscU | 3.7 | 3.0 | 2.5 | 2.5 | |||
| b2673 | ECs3536 | nrdH | Glutaredoxin-like protein | 2.2 | 4.0 | 6.9 | 5.7 | ||||
| b3414 | ECs4256 | gntY (yhgI) | Predicted gluconate transport-associated protein | 2.5 | 3.5 | 4.5 | 3.5 | ||||
| Replication, recombination, and repair | b1861 | ECs2571 | ruvA | Component of RuvABC resolvasome, regulatory subunit | 2.3 | 2.3 | 3.0 | 2.6 | |||
| b3283 | ECs4149 | yrdD | Predicted DNA topoisomerase | 2.1 | 2.2 | 3.3 | 2.5 | ||||
| b3700 | ECs4635 | recF | Recombination protein F | 3.1 | 2.3 | 2.2 | 2.2 | ||||
| Signal transduction mechanisms | b2571 | ECs3437 | rseB | Periplasmic negative regulator of RpoE | 4.6 | 3.5 | 5.1 | 4.0 | |||
| Transcription | b1422 | ECs2027 | ycdI (ydcI) | Predicted DNA-binding transcriptional regulator | 3.0 | 3.9 | 2.0 | 2.6 | |||
| b2531 | ECs3397 | iscR (yfhP) | DNA-binding transcriptional repressor | 5.0 | 3.6 | 5.8 | 4.4 | ||||
| b3067 | ECs3950 | rpoD | RNA polymerase sigma factor RpoD | 2.4 | 3.0 | 3.4 | 2.4 | ||||
| b3649 | ECs4524 | rpoZ | DNA-directed RNA polymerase subunit omega | 2.0 | 2.7 | 3.4 | 2.6 | ||||
| b3783 | ECs4716 | rho | Transcription termination factor | 2.4 | 2.4 | 3.2 | 3.0 | ||||
| b4063 | ECs5045 | soxR | Redox-sensitive transcriptional activator SoxR | 2.5 | 3.9 | 4.6 | 3.9 | ||||
| Translation, ribosomal structure and biogenesis | b1211 | ECs1716 | prfA | Peptide chain release factor 1 | 2.7 | 2.4 | 3.6 | 3.3 | |||
| b2532 | ECs3398 | trmJ (yfhQ) | Predicted methyltransferase | 2.0 | 2.2 | 2.5 | 2.2 | ||||
| b3282 | ECs4148 | rimN (yrdC) | Predicted ribosome maturation factor | 2.0 | 2.0 | 2.9 | 2.7 | ||||
| Poorly characterized or not present in | b0006 | ECs0006 | yaaA | Hypothetical conserved protein | 2.4 | 3.2 | 7.6 | 11.8 | |||
| COGs | b0224 | ECs0251 | yafK | Hypothetical conserved protein | 2.8 | 2.6 | 2.5 | 2.5 | |||
| b1016 | ECs1263 | efeU | Putative cytochrome | 6.4 | 4.6 | 3.7 | 4.4 | ||||
| b1847 | ECs2557 | yebF | Hypothetical lipoprotein | 2.2 | 3.0 | 4.5 | 3.3 | ||||
| b2152 | ECs3044 | yeiB | Hypothetical conserved inner membrane protein | 3.4 | 3.3 | 4.0 | 5.0 | ||||
| b3096 | ECs3978 | yqjB | Hypothetical conserved protein | 2.6 | 2.5 | 2.9 | 2.5 | ||||
| b3280 | ECs4146 | yrdB | Hypothetical conserved protein | 2.3 | 3.1 | 2.7 | 2.3 | ||||
| b3546 | ECs4425 | eptB (yhjW) | Phosphoethanolamine transferase | 2.4 | 2.5 | 2.6 | 4.4 | ||||
| b3618 | ECs4496 | htrL | Hypothetical protein | 2.5 | 2.7 | 2.6 | 3.6 | ||||
| b4060 | ECs5042 | yjcB | Predicted inner membrane protein | 3.5 | 5.8 | 5.6 | 7.3 | ||||
| b4218 | ECs5196 | ytfL | Predicted inner membrane transport protein | 2.3 | 2.4 | 2.8 | 2.8 | ||||
| 3 intergenic regions | |||||||||||
| H-ATR plus A-ATR | Inorganic ion transport and metabolism | b3344 | ECs4195 | yheM | Predicted intracellular sulfur oxidation protein | 2.4 | 2.2 | 2.3 | 2.3 | ||
| L-ATR plus A-ATR | Defense mechanisms | b3241 | ECs4114 | aaeA (yhcQ) | p-Hydroxybenzoic acid efflux system component | 3.5 | 2.4 | 5.4 | 5.2 | ||
| Poorly characterized or not present in COGs | b2181 | ECs3073 | yejG | Hypothetical protein | 4.9 | 3.3 | 5.1 | 2.8 | |||
For more detail, refer to Table S1 in the supplemental material.
Genes and intergenic regions were upregulated during induction of the specified ATRs by both K-12 and O157:H7. For example, genes and intergenic regions listed under “H-ATR plus L-ATR” were upregulated by K-12 and O157:H7 during induction of the H-ATR and L-ATR.
COG, cluster of orthologous groups.
Genes in parentheses are alternate names.
H, H-ATR; L, L-ATR; A, A-ATR.
HPI, hydroperoxidase I.
CoA, coenzyme A.
Membrane-bound systems for electron transport were downregulated by both strains during exposure to all acids and included most members of the atp operon (atpEFHAG), encoding F1Fo ATP synthase (32), which imports H+ during oxidative respiration. In the present study, frdB is universally downregulated by both strains, as are other members of the operon during induction of the A-ATR, L-ATR, and/or H-ATR (see Table S1 of the supplemental material). Previous studies have also reported a downregulation of genes at low pH encoding components of fumarate reductase (61), in line with the fact that mixed-acid fermentation leads to the accumulation of a mixture of acidic end products.
In summary, under the conditions employed in this study, we identified a universal acid response which was characterized by upregulation of genes involved in the oxidative, envelope, and cold shock stress responses and in iron and manganese uptake.
E. coli K-12 and O157:H7 elicit acidulant-specific gene expression responses during induction of the A-ATR, L-ATR, and H-ATR.
Apart from the universal acid response elicited by both strains during exposure to all acids, an acidulant-specific response was observed. An additional 62 genes and intergenic regions were upregulated by both strains during induction of the H-ATR (Table 2). Genes involved in functions previously described as part of the universal acid response were upregulated, including genes involved in oxidative stress resistance (soxR, nrdH, iscR), maintenance of the integrity of the cell membrane (yciM), and iron uptake and acquisition (tonB, efeU, hemA). We also observed increased expression of genes involved in zinc uptake (znuB), multidrug efflux (mdlA, mdlB), and DNA damage repair (ruvA, recF) and genes encoding protein chaperones (hscB, iscS, iscU, iscA, gntY/yhgI). Both strains also upregulated the housekeeping sigma factor (rpoD) and the RpoE-negative regulator, rseB.
The responses of E. coli K-12 and O157:H7 to lactic and hydrochloric acid show a high degree of overlap.
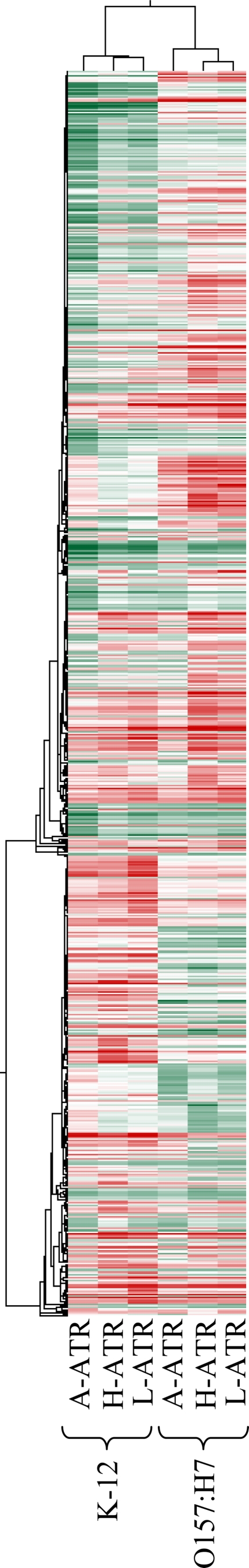
The L-ATR and H-ATR of O157:H7 showed some similarities, and this overlap was also observed for K-12 (Fig. 3 ). An additional 73 genes and intergenic regions were upregulated during exposure of both strains to lactic acid, and of these, 53% (39/73) were also upregulated during adaptation of both strains to HCl (Table 2). Among those genes commonly upregulated during induction of the L-ATR and H-ATR were those involved in the universal acid response, including oxidative stress resistance, iron uptake and acquisition, multidrug efflux, and DNA damage repair, those encoding protein chaperones, and those encoding sigma factors and their regulators (rpoD, rseB). Genes upregulated during induction of the L-ATR and not the H-ATR included those involved in similar physiological functions, such as iron acquisition and utilization (fhuA, fhuF, hemH), multidrug resistance (marR, macB/ybjZ), and DNA damage repair (xseA, yebG). A number of genes that were uniquely expressed during induction of the L-ATR are known to be induced at low pH. These include zntR, the activator of zinc export (52), the predicted permease bcsE (52, 61), and the predicted DNA-binding transcriptional regulator yieP (36). We also observed upregulation of aaeA and nlpE, which are involved in aromatic carboxylic acid efflux and protection of the outer membrane, respectively.
FIG. 3.
Hierarchical cluster analysis of gene expression in K-12 and O157:H7 after induction of the A-ATR, L-ATR, or H-ATR. The hierarchical cluster analysis was performed in GeneSpringGX with the Pearson correlation. Green and red indicate decreased and increased RNA levels, respectively, relative to that of the reference culture.
The high degree of overlap in the gene expression responses elicited by E. coli during induction of the H-ATR and L-ATR suggests that similar mechanisms are responsible for adaptive tolerance to these acids.
Only a small amount of universal acid resistance genes are unique to the A-ATR.
Induction of the A-ATR resulted in increased transcript levels of a small number of genes in both strains (Fig. 2). The universal acid response included the upregulation of six genes and three intergenic regions, including genes involved in multidrug (yojI) and aromatic carboxylic acid (aaeA) efflux (Table 2).
Strain-specific responses to acetic, lactic, and hydrochloric acid stress.
In addition to the 37 genes and intergenic regions upregulated by both strains during exposure to all acids that defined the universal acid response, a strain-specific response was observed with a further 50 and 99 genes and intergenic regions upregulated by K-12 (87 total) and O157:H7 (136 total), respectively (Fig. 2). Importantly, most of the acid-regulated genes belong to the same functional categories as the universal acid response (see Table S1 in the supplemental material). The transcriptomic data indicate that both strains experience oxidative stress during exposure to the three acids. The acid-induced oxidative stress generated a strain-specific response, with O157:H7 increasing the transcript levels of a number of genes not upregulated by K-12 (gor, yhjA, ahpC, nrdH, trxB). Similarly, we observed a strain-specific response in the upregulation of genes involved in DNA damage repair and protein misfolding in K-12 (pphB) and O157:H7 (xseA, ECs1953, ydjQ, xthA, degP, ibpB). Interestingly, we observed an induction of iron and zinc acquisition and storage genes in response to the three acids in O157:H7 (fhuA, fepB, entC, entE, fes, fitA, ECs5531, znuB, znuC) that was stronger than in K-12.
Eighteen of the elements that were uniquely upregulated by K-12 are termed K-12 specific, as they were absent from the O157:H7 genome and include genes involved in iron uptake and homeostasis (fecI, ryhB) and DNA damage repair (cho) and genes encoding predicted and hypothetical proteins (ybbC, ybfB, yfcO, yfjL, ymgD). Seventeen of the elements uniquely upregulated by O157:H7 are defined as unique to O157:H7 and are absent from the K-12 genome; they include genes involved in protection against oxidative damage (ECs1120), iron transport and metabolism (ECs3917, ECs4380), and DNA damage repair (ECs2447, ECs5242) and FUN genes (ECs0239, ECs0549, ECs1067, ECs1068, ECs1317, ECs1815). In total, O157:H7 upregulated 30 FUN genes, some of which have previously been reported to be upregulated at low pH. These include yejG (61), yebF (83, 86), yheO (36, 61), and yhcN, which has been reported to be one of the most strongly induced genes at acidic pH (52, 61). However, the majority have not previously been associated with acid conditions.
The E. coli O157:H7-specific H-ATR involved upregulation of RpoH- and RpoE-dependent stress response genes and virulence genes.
In addition to the universal acid response elicited by both strains during induction of the H-ATR, a strain-specific response was observed (see Table S1 in the supplemental material). The O157:H7-specific H-ATR included upregulation of genes encoding the heat shock sigma factor (rpoH) and the extracytoplasmic stress response sigma factor (rpoE), which responds to the effects of heat shock and other stresses that impact membrane and periplasmic proteins. Transcript levels increased for 35 genes belonging to the RpoH regulon (64) and 10 genes belonging to the RpoE regulon (20, 71). In addition, genes involved in functions in line with those of the heat shock and extracytoplasmic stress responses were upregulated, including those involved in the maintenance of cell envelope integrity (tolQ, tolR), DNA damage repair (uvrA, uvrB, uvrC, uvrY, ruvB, mfd), and protein turnover and those encoding chaperones (msrB/yeaA, grpE, ybbN, ybiY, hslO, clpS, clpA). Interestingly, HCl-adapted cultures of O157:H7 also survived heat shock challenge at 50°C better than K-12 HCl-adapted cultures (Fig. 4).
FIG. 4.
Resistance of K-12 (•, ○) and O157:H7 (▪, □) to heat shock challenge at 50°C. The percentages of survivors of cultures incubated at pH 7 (acid shock) and at pH 5.5 (acid adapted; 3 h for K-12 and 2 h for O157:H7) are represented by dashed lines and solid lines, respectively. The percentages of survivors were determined by plating cells on TYSG agar. Error bars represent standard errors of the means based on counts from three replicate populations.
The O157:H7-specific response also involved upregulation of the expression of genes involved in lysine-dependent acid resistance system 4 (cadB), oxidative-stress resistance (soxS), osmoregulation (proP, proB), and multidrug efflux (amiD/ybjR, macB/ybjZ, marR, marA, marB, mdtH/yceL, mdlB). Adaptation of O157:H7 to HCl resulted in increased transcript levels of 55 genes unique to O157:H7, including a number of virulence genes, namely, those associated with Shiga toxin production (stx1A), hemolysin expression (hha), and O-antigen production (wzy).
The O157:H7-specific H-ATR also involved upregulation of a number of genes encoding predicted and putative regulatory proteins (ECs1087, ECs1069, ECs1556, ydhB, ycfQ, ydfH, ECs1941, feoC/yhgG, yggD, ychA, ybaQ, yfeR, ECs4598). Strain differences were observed in transcript levels of major regulators of metabolism. E. coli O157:H7 upregulated the DNA-binding transcriptional regulator required for fermentation and anaerobic respiration (fnr) and the sucrose operon repressor (ECs3244), while K-12 upregulated the transcriptional repressor of d-galactose metabolism (galR).
Comparison of the O157:H7-specific response to hydrochloric and lactic acid.
E. coli K-12 and O157:H7 also displayed a strain-specific response to lactic acid (see Table S1 in the supplemental material). The strain-specific L-ATR of O157:H7 involved the upregulation of 79% (249/317) of those genes and intergenic regions upregulated during induction of the H-ATR (Fig. 2). In keeping with the trend observed in the transcriptomic response of O157:H7 to hydrochloric acid, we observed upregulation of the rpoH-encoded heat shock response sigma factor, genes for major regulators of metabolism, genes encoding chaperones, and genes involved in oxidative-stress resistance, osmoregulation, multidrug efflux, DNA damage repair and protein turnover, Shiga toxin production, and maintenance of cell envelope integrity. Of the genes unique to O157:H7 which were upregulated during induction of the L-ATR, 59% (23/39) were also upregulated during induction of the H-ATR. Fifty-one genes and intergenic regions were uniquely upregulated by O157:H7 during induction of the L-ATR (see Table S1 of the supplemental material). Genes involved in functions distinct from those of the H-ATR included those encoding predicted diguanylate cyclases (yneF, yeaJ) and components of two independent glutathione-regulated potassium efflux systems (kefB, kefG) which play a role in protecting the cell from electrophile toxicity. The extent of overlap in the strain-specific gene expression responses of K-12 to HCl and lactic acid was not quite as marked as that for O157:H7, with 45% (76/168) of those genes and intergenic regions that were upregulated during induction of the H-ATR also upregulated in the L-ATR. Surprisingly, the K-12-specific L-ATR included upregulation of the transcriptional repressor of the GAD system (gadW) and the downregulation of two genes under its regulation (gadA, gadB).
The E. coli K-12-specific A-ATR involved decreased expression of genes involved in nucleotide transport and metabolism, translation, energy production, and stress protection.
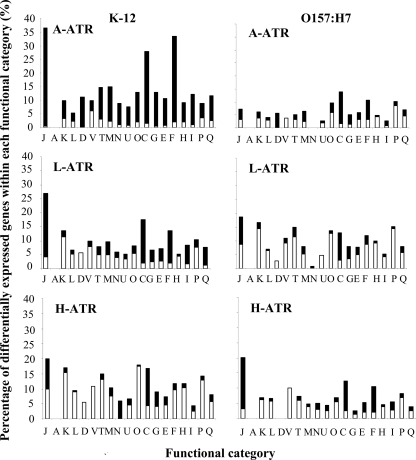
The number of genes and intergenic regions downregulated by K-12 during induction of the A-ATR was greater than double that downregulated by O157:H7 (Fig. 2; see Table S1 in the supplemental material). In comparison to that of O157:H7, the A-ATR of K-12 included the downregulation of a large percentage of genes involved in nucleotide transport and metabolism, translation, and energy production (Fig. 5). Interestingly, K-12 downregulated a number of genes within the AFI (slp, hdeB, hdeA, gadE, mdtE, gadA). However, a K-12 gadE mutant was observed to be more sensitive than the wild type during acetic acid challenge at pH 3.5 (results not shown), indicating that gene products of the AFI are required under these conditions.
FIG. 5.
Functional groups of E. coli genes that are differentially expressed during induction of the A-ATR, L-ATR, or H-ATR. Bars indicate percentages of genes in each group that showed significant changes in expression in K-12 and O157:H7 after adaptation for 3 and 2 h, respectively, in BHI, acidified to pH 5.5 with acetic, lactic, or hydrochloric acid (see Table S1 in the supplemental material). The white bars show the percentages of genes upregulated, and the black bars show the percentages of genes downregulated. Genes were divided into functional categories according to the NCBI (http://www.ncbi.nlm.nih.gov/COG/). Functional categories are abbreviated as follows: J, translation, ribosomal structure and biogenesis; A, RNA processing and modification; K, transcription; L, replication, recombination, and repair; D, cell cycle control, cell division, and chromosome partitioning; V, defense mechanisms; T, signal transduction mechanisms; M, cell wall/membrane/envelope biogenesis; N, cell motility; U, intracellular trafficking and secretion; O, posttranslational modification, protein turnover, and chaperoning; C, energy production and conversion; G, carbohydrate transport and metabolism; E, amino acid transport and metabolism; F, nucleotide transport and metabolism genes; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; and Q, secondary-metabolite biosynthesis, transport, and catabolism.
E. coli K-12 also downregulated genes involved in conferring protection against oxidative stress (katE, sodB, sodC, soxS, pqiB), osmotic stress (osmE), and DNA damage (dps) and genes encoding global stress response regulators (rpoS, uspA), protein chaperones (groS, groL, skp/hlpA, cbpA, hchA/yedU), and multidrug efflux proteins (mdtE). Moreover, the rpoS transcript was downregulated in K-12 by 3- and 2-fold during induction of the A-ATR and L-ATR, respectively.
Acid induction of intergenic regions.
The GeneChip E. coli Genome 2.0 array includes probe sets for intergenic regions of the K-12 MG1655 genome. Intergenic regions can encode sRNAs (1, 4, 60). We observed the upregulation of intergenic regions by both strains (see Table S1 in the supplemental material). E. coli K-12 upregulated a number of intergenic regions during induction of the H-ATR (35), L-ATR (53), and A-ATR (24). Of these, 15 were universally upregulated by all three acids. E. coli O157:H7 upregulated intergenic regions during induction of the H-ATR (31), L-ATR (33), and A-ATR (18). Seven O157:H7-induced intergenic regions were universally upregulated by all three acids. A BLAST search of all intergenic regions upregulated in this study against identified sRNAs from 70 microbial genomes contained within the sRNAMap database identified intergenic regions that contained sRNAs. During induction of the H-ATR in O157:H7 and the A-ATR, L-ATR, and H-ATR in K-12, intergenic regions highly homologous to the E. coli K-12 MG1655 sRNA C0362 were upregulated. The H-ATR and L-ATR of O157:H7 included upregulation of an intergenic region with a sequence match to the 64-bp E. coli K-12 sRNA rydC.
DISCUSSION
All whole-genome profiling studies that have investigated the effect of sublethal pH on E. coli have focused on the response of exponential-phase cultures to acid (61, 73, 77, 79, 83). However, in the natural environment, bacteria are normally in stationary phase (70), and stationary-phase cells exhibit pH-dependent acid tolerance, which further increases acid resistance (12, 13). Our study is unique in characterizing the whole-genome responses of stationary-phase E. coli during adaptation to organic and inorganic acids, reflecting physiological states of bacteria in food systems or food manufacturing or processing environments.
We have discovered that E. coli shows an acidulant and strain-specific ATR to acetic, lactic, and hydrochloric acids. Our data revealed a high level of similarity in the L-ATR and H-ATR of K-12, while the A-ATR was quite distinct. This was also the case for O157:H7, with approximately 70% of the genes that are upregulated in response to lactic acid also induced by HCl. This trend in the expression response to acetic, lactic, and hydrochloric acids has also been observed in the ATR of Salmonella and the response of acid-adapted and -shocked cultures of E. coli when challenged at pH 3.5 (unpublished data). The most likely reason for the similarity in the responses elicited by E. coli to lactic and hydrochloric acid is that these treatments merely result in acidification of the cytoplasm through the accumulation of protons, whereas treatment with acetic acid also results in intracellular accumulation of the anion. The distinct changes in gene expression observed during induction of the A-ATR reflect the additional changes required to remove this anion, such as those involved in the “acetate switch” (85). While there was not a heavy representation of genes involved in the “acetate switch” upregulated by either strain during induction of the A-ATR, these elements are already highly expressed in stationary phase (85).
Although the mechanisms of microbial inactivation by inorganic and organic acids are different (72), we discovered a core set of 34 genes, plus the oxyS sRNA, and two other intergenic regions that showed a universal acid response in both strains during adaptation to all acids. The identification of this universal response suggests physiological changes that are caused by mildly acidic pH, irrespective of acidulant type. Upregulated genes included those involved in protection against envelope and oxidative stress, consistent with the interaction of several stress responses with pH stress and pH resistance (26, 75). Low pH is predicted to amplify the toxicity of oxygen radicals, and a strong connection between acid and oxidative stress has previously been reported for the gene expression response of E. coli grown at pH 5 (61). Corresponding with previous reports in which acidic pH enhanced the expression of transporters, particularly for metal cations, such as iron (36), both strains increased transcript levels of genes involved in manganese and iron transport. Iron is an essential cofactor for the functions of several enzymes involved in alternative energetic pathways and may play a role in the antioxidative response (23). The coregulation of genes involved in manganese and iron transport with those involved in acid resistance may relate to the requirement for both elements by bacterial pathogens once inside the host body, where manganese and iron are in limiting amounts. A number of cold shock-associated genes were acid inducible, suggesting an association between the acid and cold shock responses. During low-temperature stress, csdA (50) and cspA (49) play important roles in protein synthesis, and the palmitoleoyl acyltransferase encoded by lpxP has been suggested to confer a selective advantage by making the outer membrane a more effective barrier to harmful chemicals (80). However, further studies are required to determine the biological significance of this finding, as the activities of these elements may be regulated at several levels. For example, cspA mRNA rapidly degrades at temperatures greater than 30°C (33). Another universal response of K-12 and O157:H7 to acid stress was the enhanced expression of multidrug transporters which have previously been reported to be acid inducible in E. coli (36). It is now understood that these efflux pumps play a role in physiological functions apart from drug efflux (67). Indeed, a multidrug resistance transporter confers extreme alkaline pH resistance to E. coli (57), and multidrug transporters may play a role in acid stress resistance (36). Interestingly, certain drug efflux pumps showed acidulant-specific upregulation. E. coli possesses 5 families of translocases which mediate drug extrusion with different specificities (63). Both K-12 and O157:H7 expressed only macB, a member of the ABC (ATP-binding cassette) family, during exposure to lactic acid but not to other acids. E. coli O157:H7 expressed only yjiO, a member of the MF (major facilitator) family, during induction of the L-ATR and A-ATR. This suggests that drug efflux pumps with certain substrate specificities were upregulated; it does not simply reflect a general response to stress.
The universal acid response of K-12 and O157:H7 involved upregulation of a number of genes involved in DNA damage repair and encoding protein chaperones, reflecting the fact that DNA damage and protein misfolding can occur as a result of oxidative and acid stress (27). It is possible that the link between acid and oxidative stress observed in the transcriptomic responses of both strains may contribute to the disparity in their acid resistance phenotypes. It has previously been reported that the O157:H7 Sakai strain is significantly more sensitive than K-12 MG1655 to oxidative stress (M. Goldberg, personal communication). A major part of the toxicity of oxidative stress can be attributed to DNA and protein damage caused by generation of OH radicals through the iron-mediated Fenton reaction (46). E. coli O157:H7 Sakai has been shown to possess an intrinsically higher level of intracellular iron than K-12 MG1655, and it was hypothesized that this renders O157:H7 Sakai more sensitive to oxidative stress due to the higher level of OH radicals generated via the Fenton reaction (M. Goldberg, personal communication).
We have identified a number of interesting strain differences. However, when considering the basis of strain variation in acid resistance, we note that our transcriptomic approach does not highlight genes which are constitutively expressed and are not acid regulated. During induction of the A-ATR, and to a lesser extent the L-ATR, the K-12-specific response involved downregulation of a large percentage of genes involved in nucleotide transport and metabolism, translation, and energy production and conversion. This pattern resembles the profile of a population of persister cells. Persister cells are dormant cells that have a low level of translation and exhibit increased tolerance to antibiotics, toxic metal ions, and other antimicrobial agents (74). This low level of translation conserves energy and amino acids under stress conditions (30). Toxin-antitoxin systems in E. coli (78) are predicted to participate actively in the persister phenotype, and K-12 increases expression of a number of genes encoding components of toxin-antitoxin modules during induction of the A-ATR (yafQ, yafO) and L-ATR (yafQ, yafO, yoeB, yefM, chpA).
During induction of the A-ATR, the K-12-specific response also involved downregulation of genes involved in protection against acid stress, oxidative stress, osmotic stress, and DNA damage and genes encoding global stress response regulators, protein chaperones, and multidrug efflux proteins. Further work is required to determine whether these acetic acid-treated cultures are in a persister-type state and whether they exhibit increased resistance to other environmental stresses. This general decrease in gene expression also led to a decrease in expression of rpoS during induction of the A-ATR (3-fold) and L-ATR (2-fold). RpoS is the master regulator of the general stress response in E. coli and is believed to be the most important sigma factor for adaptation to, and survival under, nonoptimal conditions (37). However, the significance of this finding remains to be determined, as the cellular levels of RpoS are regulated at the level of transcription, translation, and posttranslational processing (38).
The O157:H7-specific response involved the upregulation of a number of elements involved in stress resistance and ancillary functions during induction of the H-ATR and L-ATR, including upregulation of the genes encoding heat shock response sigma factors, osmoregulatory genes involved in proline accumulation, and a Shiga toxin production gene. This indicates that O157:H7 may possess a greater capacity than K-12 to survive acidic environments in which low pH is associated with other environmental stresses. Indeed, in this study, we have demonstrated that HCl-adapted O157:H7 is more resistant to heat shock challenge than HCl-adapted K-12. It has been suggested that EHEC strains have greater acid resistance than other E. coli strains (6, 9, 13). However, the present study and previous reports (8, 22) indicate that EHEC strains are no more acid resistant than generic E. coli strains. An intriguing possibility is that the enhanced ability of this pathotype to survive acid stress in the food processing and host body environments may reflect a better ability to combat more-complex acidic environments than nonpathogenic E. coli.
Distinct differences were also noted between the strains in their modes of osmoregulation. Trehalose is as an important osmoprotectant and stress protectant in E. coli. Upregulation of the trehalose biosynthetic operon (otsBA) during exposure to HCl at acidic pH has previously been described (52, 83). During acid exposure, neither K-12 nor O157:H7 upregulated the otsBA operon, probably because the operon is already highly induced in stationary phase (39). Accumulation of the osmoprotectant trehalose has been reported for Saccharomyces cerevisiae during exposure to organic acid (18). Our data showed a K-12-specific increase in expression of the repressor (treR) of the trehalose degradative enzymes (treB, treC) during exposure to all acids. In contrast, induction of the L-ATR and H-ATR caused O157:H7-specific upregulation of the major facilitator superfamily transporter involved in accumulation of the osmoprotectant proline (proP).
Another interesting O157:H7 strain-specific difference was the increased expression of transporters for zinc during exposure to all acids. Of those O157:H7 Sakai genes encoding zinc-containing proteins (34), yodA was upregulated during adaptation of O157:H7 to all acids. YodA is induced under conditions of cadmium or oxidative stress and is proposed to be a generalized stress factor and a periplasmic partner of an unknown ABC transporter in E. coli (21). The observed increase in yodA expression in O157:H7 Sakai during induction of the ATR may explain the concomitant increase in expression of zinc transporters.
Many of the metabolic rearrangements triggered by acidic pH are consistent with previous reports. The pyruvate dehydrogenase complex encoded by pdhR is upregulated by O157:H7 during exposure to all acids and during induction of the L-ATR in K-12. PdhR plays a key role in the metabolic interconnection between glycolysis and the citric acid cycle and is an important regulator for the steady-state maintenance of the central metabolism for energy production in response to changes in external environmental conditions (65). PdhR has also been identified as a positive regulator of the fecA iron import operon (24), and iron uptake was a function universally upregulated by both strains during exposure to all acids. Our findings fit with previous studies which showed that several components of the citric acid cycle of E. coli were acid repressed (36, 61, 83). Because sugar fermentation generates short-chain acids that lead to further acidification of the cell (61), it was not surprising that both strains upregulated transcriptional repressors of genes involved in sugar metabolism. Comparably with previous reports that members of the maltose regulon are strongly repressed by acid, during exposure of exponential-phase bacteria to all acids, we observed a K-12-specific upregulation of the transcriptional repressor of the maltose operon (MalI) at stationary phase. Similarly, induction of the H-ATR and L-ATR induced K-12-specific upregulation of a repressor of d-galactose metabolism and O157:H7-specific upregulation of a repressor of sucrose metabolism.
A number of genes involved in amino acid biosynthesis were acid induced, perhaps reflecting the limiting levels of amino acids in stationary-phase cultures requiring de novo synthesis of amino acids. In addition, environmental stress can trigger the production of amino acids, as is the case with arginine where acid stress, oxidative damage, and growth under other suboptimal conditions can trigger the synthesis and transport of this amino acid (17, 59, 69, 84). Previous studies have shown that stressed cells may decrease aerobic respiration in favor of a more fermentative and/or anaerobic respiration-based energy metabolism (83), and we observed an O157:H7-specific increase in transcript levels of fnr during exposure to lactic and hydrochloric acid.
Our experiments with stationary-phase E. coli at pH 5.5 identified a large number of genes not previously known to be regulated by pH, many of which were FUN. In addition, we observed O157:H7-specific upregulation of a large number of poorly characterized genes unique to O157:H7 Sakai, raising the exciting possibility that these genes encode additional molecular mechanisms which contribute to the relative acid resistance of this EHEC strain. Further characterization of these genes may reveal proteins required for acid resistance. These may be potential targets for novel interventions or may shed light on the physiology of E. coli by conferring novel abilities or previously unsuspected properties (41).
In addition, a large number of intergenic regions were observed to be differentially expressed by both strains, a few of which encode or are highly homologous to identified sRNAs. While little is known about the sRNA C0362 since it was identified in 2003 (40), rydC is involved in the repression of the yejABEF-encoded ABC permease and is thought to contribute to optimal adaptation of some enterobacteria to environmental conditions (2). sRNAs are important regulators of bacterial expression, and identification and investigation of the significance of the intergenic regions which display differential expression are likely to provide insight into how E. coli survives acid stress.
This study is the first to demonstrate and characterize the acidulant- and pathotype-specific transcriptomic responses of E. coli to organic and inorganic acids during stationary phase and to identify a universal acid response. The discovery of a strain-specific response may shed light on the observed differences in strain prevalence and persistence in certain food-related environments. Further characterization of the role of some of the most highly expressed FUN genes and intergenic regions in the ATR of E. coli K-12 and O157:H7 will also be important. Future work will aid in identifying those systems specifically involved in mounting the stationary-phase ATR by deciphering whether each of the observed transcriptional responses is part of a considered programmed response to acid stress or more akin to a panic attack that is induced regardless of the stress experienced. We anticipate that this information will facilitate the knowledge-based enhancement of current interventions or the development of new means by which the food industry can eliminate or control this pathogen.
Supplementary Material
Acknowledgments
We thank Mark Schembri and Carlton Gyles for provision of strains and Martin Goldberg for help with analysis of the COG (cluster of orthologous groups) functional categories. We also thank Scott Chandry, Belinda Chapman, and anonymous reviewers for constructive comments.
We acknowledge funding from a BBSRC Core Strategic Grant to Jay C. D. Hinton.
Footnotes
Published ahead of print on 13 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Altuvia, S. 2004. Regulatory small RNAs: the key to coordinating global regulatory circuits. J. Bacteriol. 186:6679-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antal, M., V. Bordeau, V. Douchin, and B. Felden. 2005. A small bacterial RNA regulates a putative ABC transporter. J. Biol. Chem. 280:7901-7908. [DOI] [PubMed] [Google Scholar]
- 3.Antón, J., A. Oren, S. Benlloch, F. Rodríguez-Valera, R. Amann, and R. Rosselló-Mora. 2002. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Microbiol. 52:485-491. [DOI] [PubMed] [Google Scholar]
- 4.Argaman, L., R. Hershberg, J. Vogel, G. Bejerano, E. G. H. Wagner, H. Margalit, and S. Altuvia. 2001. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 11:941-950. [DOI] [PubMed] [Google Scholar]
- 5.Arnold, C. N., J. McElhanon, A. Lee, R. Leonhart, and D. A. Siegele. 2001. Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J. Bacteriol. 183:2178-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold, K. W., and C. W. Kaspar. 1995. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:2037-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin, M. M., and A. R. Datta. 1995. Acid tolerance of enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 61:1669-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, E. D., G. A. Barkocy-Gallagher, and G. R. Siragusa. 2004. Stationary phase acid resistance and injury of recent bovine Escherichia coli O157 and non-O157 biotype I Escherichia coli isolates. J. Food Prot. 67:583-590. [DOI] [PubMed] [Google Scholar]
- 9.Berry, E. D., and C. N. Cutter. 2000. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microbiol. 66:1493-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 11.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan, R. L., and S. G. Edelson. 1996. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl. Environ. Microbiol. 62:4009-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchanan, R. L., and S. G. Edelson. 1999. pH-dependent stationary-phase acid resistance response of enterohemorrhagic Escherichia coli in the presence of various acidulants. J. Food Prot. 62:211-218. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan, R. L., S. G. Edelson, and G. Boyd. 1999. Effects of pH and acid resistance on the radiation resistance of enterohemorrhagic Escherichia coli. J. Food Prot. 62:219-228. [DOI] [PubMed] [Google Scholar]
- 15.Cameron, S., C. Walker, and M. Beers. 1995. Enterohemorrhagic Escherichia coli outbreak in South Australia associated with the consumption of Mettwurst. Commun. Dis. Intell. 19:70-71. [Google Scholar]
- 16.Carruthers, M. D., and C. Minion. 2009. Transcriptome analysis of Escherichia coli O157:H7 EDL933 during heat shock. FEMS Microbiol. Lett. 295:96-102. [DOI] [PubMed] [Google Scholar]
- 17.Castanie-Cornet, M.-P., T. A. Penfound, D. Smith, J. F. Elliott, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng, L., J. Moghraby, and P. W. Piper. 1999. Weak organic acid treatment causes a trehalose accumulation in low-pH cultures of Saccharomyces cerevisiae, not displayed by the more preservative-resistant Zygosaccharomyces bailii. FEMS Microbiol. Lett. 170:89-95. [DOI] [PubMed] [Google Scholar]
- 19.Crépin, S., M. G. Lamarche, P. Garneau, J. Séguin, J. Proulx, C. M. Dozois, and J. Harel. 2008. Genome-wide transcriptional response of an avian pathogenic Escherichia coli (APEC) pst mutant. BMC Genomics 9:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli sigma E regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 21.David, G., K. Blondeau, M. Schiltz, S. Penel, and A. Lewit-Bentley. 2003. YodA from Escherichia coli is a metal-binding, lipocalin-like protein. J. Biol. Chem. 278:43728-43735. [DOI] [PubMed] [Google Scholar]
- 22.Duffy, L. L., F. H. Grau, and P. B. Vanderlinde. 2000. Acid resistance of enterohaemorrhagic and generic Escherichia coli associated with foodborne disease and meat. Int. J. Food Microbiol. 60:83-89. [DOI] [PubMed] [Google Scholar]
- 23.Echave, P., J. Tamarit, E. Cabiscol, and J. Ros. 2003. Novel antioxidant role of alcohol dehydrogenase E from Escherichia coli. J. Biol. Chem. 278:30193-30198. [DOI] [PubMed] [Google Scholar]
- 24.Faith, J. J., B. Hayete, J. T. Thaden, I. Mogno, J. Wierzbowski, G. Cottarel, S. Kasif, J. J. Collins, and T. S. Gardner. 2007. Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. PLoS. Biol. 5:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster, J. W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2:898-907. [DOI] [PubMed] [Google Scholar]
- 26.Foster, J. W. 2000. Microbial responses to acid stress, p. 99-115. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, DC.
- 27.Foster, J. W., and B. Bearson. 1994. Acid-sensitive mutants of Salmonella typhimurium identified through a dinitrophenol lethal screening strategy. J. Bacteriol. 176:2596-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garren, D. M., M. A. Harrison, and S. M. Russell. 1998. Acid tolerance and acid shock response of Escherichia coli O157:H7 and non-O157:H7 isolates provide cross protection to sodium lactate and sodium chloride. J. Food Prot. 61:158-161. [DOI] [PubMed] [Google Scholar]
- 29.Gentleman, R., V. Carey, D. Bates, B. Bolstad, M. Dettling, S. Dudoit, B. Ellis, L. Gautier, Y. Ge, J. Gentry, K. Hornik, T. Hothorn, W. Huber, S. Iacus, R. Irizarry, F. Leisch, C. Li, M. Maechler, A. Rossini, G. Sawitzki, C. Smith, G. Smyth, L. Tierney, J. Yang, and J. Zhang. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerdes, K., S. K. Christensen, and A. Lobner-Olesen. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 31.Goodson, M., and R. J. Rowbury. 1989. Habituation to normally lethal acidity by prior growth of Escherichia coli at a sub-lethal acid pH value. Lett. Appl. Microbiol. 8:77-79. [Google Scholar]
- 32.Greenacre, E. J., T. F. Brocklehurst, C. R. Waspe, D. R. Wilson, and P. D. G. Wilson. 2003. Salmonella enterica serovar Typhimurium and Listeria monocytogenes acid tolerance response induced by organic acids at 20°C: optimization and modeling. Appl. Environ. Microbiol. 69:3945-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hankins, J. S., C. Zappavigna, A. Prud'homme-Genereux, and G. A. Mackie. 2007. Role of RNA structure and susceptibility to RNase E in regulation of a cold shock mRNA, cspA mRNA. J. Bacteriol. 189:4353-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hantke, K. 2005. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8:196-202. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C.-G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Eschelichia coli O157:H7 and genomic comparison with a laboratory strain, K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 36.Hayes, E. T., J. C. Wilks, P. Sanfilippo, E. Yohannes, D. P. Tate, B. D. Jones, M. D. Radmacher, S. S. Bondurant, and J. L. Slonczewski. 2006. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol. 6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
- 38.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hengge-Aronis, R., W. Klein, R. Lange, M. Rimmele, and W. Boos. 1991. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J. Bacteriol. 173:7918-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hershberg, R., S. Altuvia, and H. Margalit. 2003. A survey of small RNA-encoding genes in Escherichia coli. Nucleic Acids Res. 31:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinton, J. C. D. 1997. The Escherichia coli genome sequence: the end of an era or the start of the FUN? Mol. Microbiol. 26:417-422. [DOI] [PubMed] [Google Scholar]
- 42.Huang, H.-Y., H.-Y. Chang, C.-H. Chou, C.-P. Tseng, S.-Y. Ho, C.-D. Yang, Y.-W. Ju, and H.-D. Huang. 2009. sRNAMap: genomic maps for small non-coding RNAs, their regulators and their targets in microbial genomes. Nucleic Acids Res. 37:D150-D154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang, Y.-J., T.-Y. Tsai, and T.-M. Pan. 2007. Physiological response and protein expression under acid stress of Escherichia coli O157:H7 TWC01 isolated from Taiwan. J. Agric. Food Chem. 55:7182-7191. [DOI] [PubMed] [Google Scholar]
- 44.Hughes, T. R., C. J. Roberts, H. Dai, A. R. Jones, M. R. Meyer, D. Slade, J. Burchard, S. Dow, T. R. Ward, M. J. Kidd, S. H. Friend, and M. J. Marton. 2000. Widespread aneuploidy revealed by DNA microarray expression profiling. Nat. Genet. 25:333-337. [DOI] [PubMed] [Google Scholar]
- 45.Ichikawa, J. K., A. Norris, M. G. Bangera, G. K. Geiss, A. B. van 't Wout, R. E. Bumgarner, and S. Lory. 2000. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. U. S. A. 97:9659-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395-418. [DOI] [PubMed] [Google Scholar]
- 47.Irizarry, R. A., L. Gautier, and L. M. Cope. 2003. An R package for analyses of Affymetrix oligonucleotide arrays, p. 102-119. In G. Parmigiani, E. S. Garrett, R. A. Irizarry, and S. L. Zeger (ed.), The analysis of gene expression data: methods and software. Springer-Verlag London, London, United Kingdom.
- 48.Irizarry, R. A., B. Hobbs, F. Collin, Y. D. Beazer-Barclay, K. J. Antonellis, U. Scherf, and T. P. Speed. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249-264. [DOI] [PubMed] [Google Scholar]
- 49.Jiang, W., Y. Hou, and M. Inouye. 1997. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272:196-202. [DOI] [PubMed] [Google Scholar]
- 50.Jones, P. G., M. Mitta, Y. Kim, W. Jiang, and M. Inouye. 1996. Cold shock induces a major ribosome-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 93:76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jordan, K. N., L. Oxford, and C. P. O'Byrne. 1999. Survival of low-pH stress by Escherichia coli O157:H7: correlation between alterations in the cell envelope and increased acid tolerance. Appl. Environ. Microbiol. 65:3048-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kannan, G., J. C. Wilks, D. M. Fitzgerald, B. D. Jones, S. S. BonDurant, and J. L. Slonczewski. 2008. Rapid acid treatment of Escherichia coli: transcriptomic response and recovery. BMC Microbiol. 8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kershaw, C. J., N. L. Brown, C. Constantinidou, M. D. Patel, and J. L. Hobman. 2005. The expression profile of Escherichia coli K-12 in response to minimal, optimal and excess copper concentrations. Microbiology 151:1187-1198. [DOI] [PubMed] [Google Scholar]
- 54.Kirkpatrick, C., L. M. Maurer, N. E. Oyelakin, Y. N. Yoncheva, R. Maurer, and J. L. Slonczewski. 2001. Acetate and formate stress: opposite responses in the proteome of Escherichia coli. J. Bacteriol. 183:6466-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi, A., H. Hirakawa, T. Hirata, K. Nishino, and A. Yamaguchi. 2006. Growth phase-dependent expression of drug exporters in Escherichia coli and its contribution to drug tolerance. J. Bacteriol. 188:5693-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambert, L. A., K. Abshire, D. Blankenhorn, and J. L. Slonczewski. 1997. Proteins induced in Escherichia coli by benzoic acid. J. Bacteriol. 179:7595-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewinson, O., E. Padan, and E. Bibi. 2004. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. U. S. A. 101:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leyer, G. J., L. L. Wang, and E. A. Johnson. 1995. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl. Environ. Microbiol. 61:3752-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin, J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177:4097-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masse, E., N. Majdalani, and S. Gottesman. 2001. Regulatory roles for small RNAs in bacteria. Curr. Opin. Microbiol. 6:120-124. [DOI] [PubMed] [Google Scholar]
- 61.Maurer, L. M., E. Yohannes, S. S. BonDurant, M. Radmacher, and J. L. Slonczewski. 2005. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 187:304-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan, D., C. P. Newman, D. N. Hutchinson, A. M. Walker, B. Rowe, and F. Majid. 1993. Verotoxin producing Escherichia coli O157 infections associated with consumption of yogurt. Epidemiol. Infect. 111:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nonaka, G., M. Blankschien, C. Herman, C. A. Gross, and V. A. Rhodius. 2006. Regulon and promoter analysis of the E. coli heat-shock factor, σ32, reveals a multifaceted cellular response to heat stress. Genes Dev. 20:1776-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogasawara, H., Y. Ishida, K. Yamada, K. Yamamoto, and A. Ishihama. 2007. PdhR (pyruvate dehydrogenase complex regulator) controls the respiratory electron transport system in Escherichia coli. J. Bacteriol. 189:5534-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piddock, L. J. V. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629-636. [DOI] [PubMed] [Google Scholar]
- 68.Piper, P., C. Ortiz Calderon, K. Hatzixanthis, and M. Mollapour. 2001. Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147:2635-2642. [DOI] [PubMed] [Google Scholar]
- 69.Price, S. B., C. M. Cheng, C. W. Kaspar, J. C. Wright, F. J. DeGraves, T. A. Penfound, M. P. Castanie-Cornet, and J. W. Foster. 2000. Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl. Environ. Microbiol. 66:632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rees, C. E. D., C. E. R. Dodd, P. T. Gibson, I. R. Booth, and G. S. A. B. Stewart. 1995. The significance of bacteria in stationary phase to food microbiology. Int. J. Food Microbiol. 28:263-275. [DOI] [PubMed] [Google Scholar]
- 71.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli σE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 72.Russell, J. B. 1992. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J. Appl. Microbiol. 73:363-370. [Google Scholar]
- 73.Šeputienė, V., K. Sužiedėlis, and E. Sužiedelieṅė. 2004. Gene expression profiling of the low-pH response in Escherichia coli. Biologija 3:6-12. [Google Scholar]
- 74.Shah, D., Z. Zhang, A. Khodursky, N. Kaldalu, K. Kurg, and K. Lewis. 2006. Persisters: a distinct physiological state of E. coli. BMC Microbiol. 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slonczewski, J. L., and J. W. Foster. 1996. pH-related genes and survival at extreme pH, p. 1539-1552. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 76.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article3. [DOI] [PubMed] [Google Scholar]
- 77.Stancik, L. M., D. M. Stancik, B. Schmidt, D. M. Barnhart, Y. N. Yoncheva, and J. L. Slonczewski. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184:4246-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsilibaris, V., G. Maenhaut-Michel, N. Mine, and L. Van Melderen. 2007. What is the benefit to Escherichia coli of having multiple toxin-antitoxin systems in its genome? J. Bacteriol. 189:6101-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tucker, D. L., N. Tucker, and T. Conway. 2002. Gene expression profiling of the pH response in Escherichia coli. J. Bacteriol. 184:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vorachek-Warren, M. K., S. Ramirez, R. J. Cotter, and C. R. H. Raetz. 2002. A triple mutant of Escherichia coli lacking secondary acyl chains on lipid A. J. Biol. Chem. 277:14194-14205. [DOI] [PubMed] [Google Scholar]
- 81.Wang, S., K. Deng, S. Zaremba, X. Deng, C. Lin, Q. Wang, M. L. Tortorello, and W. Zhang. 2009. Transcriptomic response of Escherichia coli O157:H7 to oxidative stress. Appl. Environ. Microbiol. 75:6110-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weagant, S. D., J. L. Bryant, and D. H. Bark. 1994. Survival of Escherichia coli O157:H7 in mayonnaise-based sauces at room and refrigerated temperatures. J. Food Prot. 57:629-631. [DOI] [PubMed] [Google Scholar]
- 83.Weber, H., T. Polen, J. Heuveling, V. F. Wendisch, and R. Hengge. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weerasinghe, J. P., T. Dong, M. R. Schertzberg, M. G. Kirchhof, Y. Sun, and H. E. Schellhorn. 2006. Stationary phase expression of the arginine biosynthetic operon argCBH in Escherichia coli. BMC Microbiol. 6:1471-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang, G., S. Brokx, and J. H. Weiner. 2005. Extracellular accumulation of recombinant proteins fused to the carrier protein YebF in Escherichia coli. Nat. Biotechnol. 24:100-104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.