Abstract
Stable Mycobacterium avium infections of several Acanthamoeba strains were characterized by increased infection resistance of recent environmental isolates and reduced infectivity in the presence of other bacteria. Exposure of M. avium in coculture with Acanthamoeba castellanii to monochloramine yielded inactivation kinetics markedly similar to those observed for A. castellanii alone.
Acanthamoebae are widely distributed in the environment (20) and generally function ecologically as predators of bacteria (23), although numerous types of bacteria resist predation (22). Acanthamoebae are very resistant to a range of disinfectants (5, 6, 8, 28), and bacteria within acanthamoebae are generally afforded extra protection (16). A notable example is the opportunistic pathogen Mycobacterium avium (10), which can survive within Acanthamoeba species trophozoites and cysts (4, 26), resulting in increased resistance to several antimicrobials (22). It has been demonstrated that many Mycobacterium spp. are able to infect the laboratory strain Acanthamoeba polyphaga (1). Acanthamoeba cultures undergo many physiological changes after several passages in the laboratory (15, 17, 21), although it is not known if prolonged cultivation of Acanthamoeba alters their capacity to be infected by M. avium. This knowledge is important for assessing the environmental relevance of associations between Acanthamoeba and M. avium. Therefore, we studied the infectivity and infection stability of M. avium with several laboratory and environmental Acanthamoeba strains for 28 days under high-nutrient (peptone-yeast extract-glucose [PYG] medium) and low-nutrient (Page's amoeba saline [PAS]) conditions.
M. avium infections in different Acanthamoeba strains.
Eight Acanthamoeba strains were studied, four of which were recently isolated from the environment (biofilm from a drinking water distribution system, forest soil, and two from marsh sediment) and four “laboratory strains” which had been passaged many times on nutrient-rich medium (see Table S1 in the supplemental material). Fresh isolates (<2 months) were passaged no more than three times and were determined to be free of endosymbionts and acid-fast stained structures, by the use of methods described previously (13). The strains were classified to genotype according to the 95% sequence similarity threshold for 18S rRNA genes (27) using standard methods (11, 13). All strains were members of sequence type T4 (24), with the exception of Acanthamoeba sp. strain F2B (type T13) and Acanthamoeba hatchetii (type 11) (GenBank accession no. FJ807647 to FJ807651) (see Fig. S1 in the supplemental material). Mycobacterium avium subsp. hominissuis 104 (2) was cultured on Middlebrook 7H9/OADC (oleic acid-albumin-dextrose-catalase) broth (Sigma-Aldrich). M. avium was added to Acanthamoeba monolayers at a multiplicity of infection of 10:1 and treated with amikacin as described previously (4). Cocultures were incubated at 20°C in the dark and were washed and treated weekly with amikacin to minimize the potential for extra-amoebal growth of M. avium, which was confirmed by daily monitoring by phase-contrast microscopy. Acid-fast staining of cocultures was performed using a modified Ziehl-Neelson staining protocol (12), and infected amoebae (>50 per time point) and intracellular mycobacteria were counted using a 100× objective microscope (Axioplan 2; Carl Zeiss Microimaging GmbH). Phenolic acridine orange fluorescence staining (25) was used in combination with confocal laser scanning microscopy (CLSM) for localization of intracellular M. avium. For mycobacterial viability assays, harvested acanthamoebae were lysed using a 3-min vortexing step with glass beads (diameter, 0.5 mm) and plated as described previously (4). The viability of M. avium cells was not affected by the bead beating treatment (data not shown).
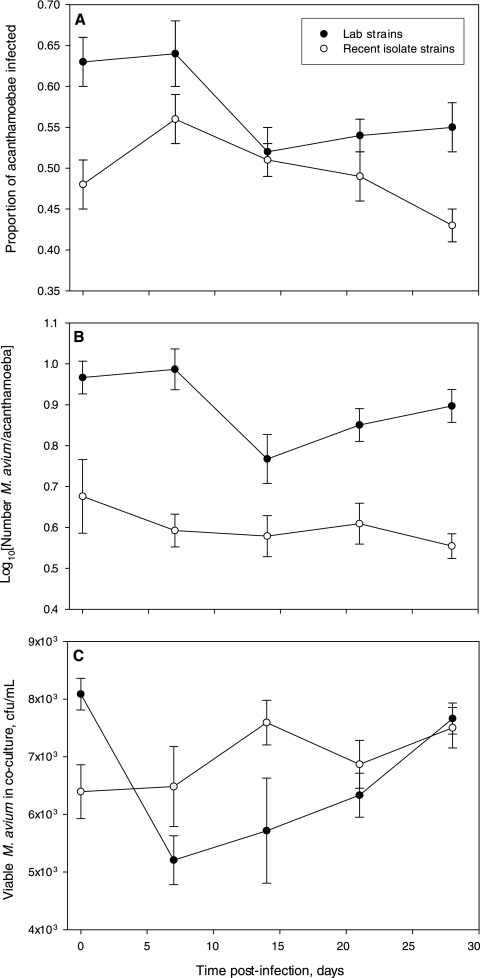
M. avium was able to infect all Acanthamoeba strains tested (see Fig. S2 in the supplemental material), with the proportion of infected amoebae (0.33 to 0.77) (Fig. 1 A) and the number of M. avium cells per infected amoeba (1.5 to 18.4) (Fig. 1B) similar to those found in previous studies (4, 26). Infections persisted in all eight Acanthamoeba strains for the duration of the 4-week experiment, and M. avium exhibited only limited net positive growth, with no statistically significant host-specific difference in M. avium viability (analysis of variance [ANOVA], P > 0.05) (Fig. 1C). Interestingly, the eight amoeba strains had significantly different susceptibilities to infection (ANOVA, P < 0.05). To test the hypothesis that recent environmental isolates were more resistant to infection, the strains were analyzed as two groups (laboratory strains versus recent isolates). Environmental isolates as a group had a significantly lower proportion of their populations infected (P < 0.05) (Fig. 1A), and each infected amoeba hosted significantly fewer M. avium cells (P < 0.05) (Fig. 1B), demonstrating for the first time that environmental isolates are indeed more resistant to M. avium. To test the effect of nutrient availability on infection dynamics, infection experiments were conducted with all the strains using either a nutrient-rich culture medium (PYG) or a nonnutrient buffer (PAS). No significant differences were observed due to the medium choice at any point in the 4-week experiment. These results indicate that nutrient availability is not critical for infectivity or infection stability and suggest that long-term stable associations between M. avium and acanthamoebae are possible in low-nutrient aquatic environments such as oligotrophic freshwater and drinking water.
FIG. 1.
Infection dynamics of Mycobacterium avium with laboratory strains (black circles) and recent environmental isolates (white circles) of Acanthamoeba. (A) Proportion of Acanthamoeba strains infected after initial infection; (B) average number of M. avium cells per infected Acanthamoeba cell over time; (C) viability of M. avium in coculture with Acanthamoeba strains over time. Data points are mean average values for four strains of Acanthamoeba (either recent environmental isolates or laboratory strains), and each strain was tested in triplicate. Viability was assessed as the number of CFU per ml of culture medium in coculture, and samples at each time point were plated in duplicate. ANOVA indicated that the variables of time and strain type were significant explanatory factors for all response variables presented (P < 0.05). Error bars indicate 95% confidence intervals.
Multispecies grazing assays.
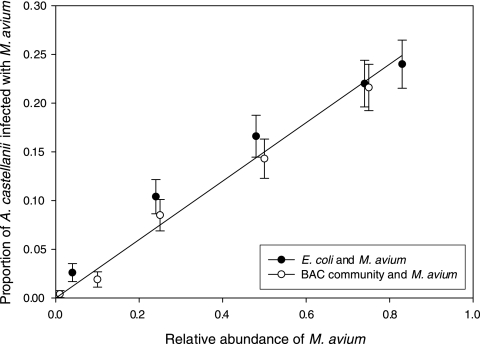
Since infections occur in the environment during grazing of acanthamoebae on bacteria, the infectivity of M. avium was examined when it was present at various relative abundances within a multispecies microbial consortium. M. avium was stained with a nontoxic stable intracellular fluorescent dye that did not inhibit bacterial growth (data not shown) according to the manufacturer's instructions (Vybrant CFDA cell tracer kit; Molecular Probes, Inc.) and then mixed with either Escherichia coli K-12 MG1665 or a microbial community from a laboratory-scale biologically active carbon (BAC) filter (described in detail elsewhere [X. Li, G. Upadhyaya, W. Yuen, J. Brown, E. Morgenroth, and L. Raskin, submitted for publication]) in several proportions (0.01 to 0.83, as biomass wet weight). Mixtures were spread evenly on nonnutrient agar plates, and A. castellanii Neff amoebae were allowed to graze for 60 h in the dark at room temperature. The numbers of total and infected acanthamoebae were then quantified using epifluorescence microscopy (10× objective). At least 300 acanthamoebae were counted for each plate, and tests were conducted in triplicate. A large reduction in infectivity (determined as the proportion of infected amoebae) was observed in the presence of other bacteria, even when M. avium was the predominant member of the consortium. When the amoebae were grazing only on M. avium, the proportion of infected amoebae was 79% (95% confidence interval [CI], 0.75 to 0.83), but when other bacteria were present, it was below 25%. This agrees with reports of reduced Legionella pneumophila infection of Acanthamoeba in the presence of other bacteria (7). Though never exceeding 25%, the proportion of infected acanthamoebae in grazing experiments was linearly dependent on the relative abundance of M. avium in the bacterial consortia (R2 = 0.97) (Fig. 2). A similar trend was observed for both M. avium spiked into E. coli and the BAC microbial community. Importantly, these results suggest that the intensity of grazing-acquired Acanthamoeba infections is determined primarily by the relative abundance of M. avium in a consortium rather than the overall community composition.
FIG. 2.
Proportion of A. castellanii Neff amoebae harboring ingested Mycobacterium avium after 60 h of grazing on two bacterial consortia. An E. coli K-12 MG1665 culture and a biologically active carbon (BAC) filter biofilm community were spiked with fluorescently labeled M. avium at proportions between 0.01 and 0.82 on a per mass basis. A linear regression line is plotted (R2 = 0.97), and error bars indicate 95% confidence intervals.
Inactivation kinetics assays.
Although survival within Acanthamoeba hosts is known to generally increase bacterial resistance to disinfection (14, 16), the relationship between Acanthamoeba and intracellular bacterial inactivation rates and dynamics is not well characterized. Therefore, inactivation kinetics during treatment with the drinking water disinfectant monochloramine were compared for M. avium in pure culture, M. avium in coculture with A. castellanii Neff, and A. castellanii Neff in pure culture. This experiment was designed to address whether inactivation of Acanthamoeba can be used by drinking water treatment professionals as an indicator for inactivation of Acanthamoeba-associated M. avium.
Monochloramine was prepared as described previously (3), and the concentration was determined before and after all inactivation experiments using the DPD titrimetric method (9). It was verified that the concentration of monochloramine did not vary significantly during the experiments (data not shown). To test the inactivation kinetics of M. avium alone, cells (107 CFU/ml) suspended in phosphate-buffered saline (PBS; pH 8.0) were exposed to 5 mg/liter monochloramine (as Cl2) for several time durations at 20°C. Monochloramine was quenched by the addition of 0.12% sodium thiosulfate, and appropriate dilutions were plated. To monitor inactivation kinetics of intracellular M. avium, infected acanthamoebae (106 cells/ml) were exposed to monochloramine, harvested, and plated as described above. Propidium iodide (PI)-based Acanthamoeba viability tests were conducted after monochloramine exposure and quenching with sodium thiosulfate using a 50-min incubation with 0.5 μM PI and CLSM quantification (excitation at 485 nm, emission at 580 nm) as previously described (S. Haider, L. Konig, A. Müller, J. Montanaro, M. Wagner, and M. Horn, unpublished data).
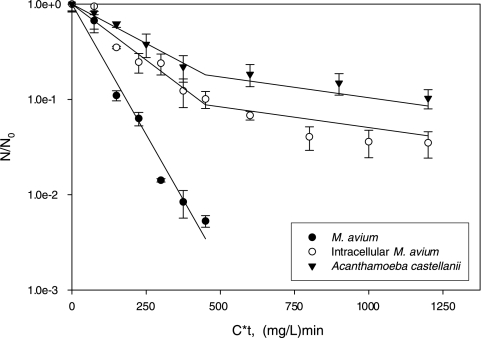
Inactivation of M. avium alone proceeded as a pseudo-first-order Chick-Watson reaction (N/N0 = e−kCt, where N/N0 is the fraction of viable cells after disinfectant exposure, C is the disinfectant concentration, and t is exposure time) (Fig. 3) (29). The rate constant (k = 0.0126 liters·mg−1·min−1, R2 = 0.97) was similar to that of a previous report under similar conditions (k = 0.0123 liters·mg−1·min−1) (19). Intracellular inactivation of both M. avium and A. castellanii Neff followed biphasic kinetics characterized by an initial rate during the first 90 min (intracellular M. avium, k = 0.0054 liters·mg−1·min−1, R2 = 0.94; A. castellanii Neff, k = 0.0038 liters·mg−1·min−1, R2 = 0.99) followed by a lower rate (intracellular M. avium, k = 0.0011 liters·mg−1·min−1, R2 = 0.77; A. castellanii Neff, k = 0.0010 liters·mg−1·min−1, R2 = 0.98). Reduced reaction rates at later points during inactivation, a phenomenon known as “tailing,” has been observed for inactivation of Acanthamoeba with disinfectants other than monochloramine (18), though not for M. avium. A salient feature of the data is that tailing begins at about the same time for intracellular M. avium and A. castellanii Neff, suggesting that mechanisms that protect A. castellanii Neff from inactivation, such as cyst formation, may also be responsible for protection of M. avium. The detailed inactivation kinetics data demonstrate for the first time the relationship between M. avium inactivation and host inactivation, suggesting that Acanthamoeba inactivation could be used as a surrogate for intracellular M. avium inactivation.
FIG. 3.
Inactivation kinetics of Mycobacterium avium in pure culture and in coculture with A. castellanii Neff (quantified using viability plating and expressed as CFU/CFU0, where CFU0 is the predisinfectant concentration of CFU), and A. castellanii Neff (quantified using viability staining and expressed as N/N0). Error bars indicate 95% confidence intervals, and inactivation model fittings are plotted (R2 = 0.77 to 0.99; see text for details).
This work demonstrates that M. avium forms stable infections in a range of Acanthamoeba strains but that recent environmental isolates are more resistant to infection. Also, the presence of other bacteria significantly reduces M. avium infectivity. Coculture with Acanthamoeba alters both the overall resistance and the dynamics of M. avium inactivation with the drinking water disinfectant monochloramine, with M. avium inactivation in coculture closely matching the inactivation characteristics of Acanthamoeba. While additional work is necessary to determine whether this correlation between amoebic host and intracellular bacterial inactivation holds for other intracellular bacterial pathogens, it is suggested to be a simple and useful conservative indicator of bacterial pathogen inactivation.
Supplementary Material
Acknowledgments
We thank Gerard Cangelosi for providing the M. avium 104 culture, Ilias Lagkouvardos for providing access to the Acanthamoeba isolates from marsh sediments and forest soil, Brian Steglitz of the City of Ann Arbor (MI) Water Treatment Plant for access to sample the drinking water distribution system, Michael Wagner for helpful discussions, and Lena König and Christian Baranyi for technical assistance.
This research was supported by NSF grant BES-0613193, and D.B. was supported by EPA STAR and Graham Environmental Sustainability Institute graduate fellowships.
Footnotes
Published ahead of print on 13 August 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adékambi, T., S. Ben Salah, M. Khlif, D. Raoult, and M. Drancourtet. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72:5974-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez, L., A. Parker, and J. Goodman. 1997. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect. Immun. 65:1916-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, D., C. Xi, and L. Raskin. 2009. Effect of growth conditions on inactivation of Escherichia coli with monochloramine. Environ. Sci. Technol. 43:884-889. [DOI] [PubMed] [Google Scholar]
- 4.Cirillo, J., S. Falkow, L. Tompkins, and L. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cursons, R. T., T. J. Brown, and E. A. Keys. 1980. Effect of disinfectants on pathogenic free-living amoebae: in axenic conditions. Appl. Environ. Microbiol. 40:62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson, M. W., and T. J. Brown. 1987. The effect of chlorine and chlorine dioxide on pathogenic free-living amoebae (PFLA) in simulated natural conditions: the presence of bacteria and organic matter. N. Z. J. Mar. Freshwater Res. 21:117-123. [Google Scholar]
- 7.Declerck, P., J. Behets, Y. Delaedt, A. Margineanu, E. Lammertyn, and F. Ollevier. 2005. Impact of non-Legionella bacteria on the uptake and intracellular replication of Legionella pneumophila in Acanthamoeba castellanii and Naegleria lovaniensis. Microb. Ecol. 50:536-549. [DOI] [PubMed] [Google Scholar]
- 8.De Jonckheere, J., and H. van de Voorde. 1976. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl. Environ. Microbiol. 31:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton, A. D., L. S. Clesceri, and A. E. Greenberg (ed.). 1995. Standard methods for the examination of water and wastewater, 19th ed. American Public Health Association, Washington, DC.
- 10.Falkinham, J. O. 2002. Nontuberculous mycobacteria in the environment. Clin. Chest Med. 23:529-551. [DOI] [PubMed] [Google Scholar]
- 11.Gast, R. J. 2006. Molecular phylogeny of a potentially parasitic dinoflagellate isolated from the solitary radiolarian, Thalassicolla nucleata. J. Eukaryot. Microbiol. 53:43-45. [DOI] [PubMed] [Google Scholar]
- 12.Giménez, D. F. 1964. Staining rickettsiae in yolk-sac cultures. Stain Technol. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 13.Heinz, E., I. Kolarov, C. Kästner, E. R. Toenshoff, M. Wagner, and M. Horn. 2007. An Acanthamoeba sp. containing two phylogenetically different bacterial endosymbionts. Environ. Microbiol. 9:1604-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard, K., and T. J. J. Inglis. 2005. Disinfection of Burkholderia pseudomallei in potable water. Water Res. 39:1085-1092. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, R., W. Heaselgrave, and S. Kilvington. 2003. Acanthamoeba polyphaga strain age and method of cyst production influence the observed efficacy of therapeutic agents and contact lens disinfectants. Antimicrob. Agents Chemother. 47:3080-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King, C. H., E. B. Shotts, Jr., R. E. Wooley, and K. G. Porter. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 54:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köhsler, M., D. Leitsch, U. Fuernkranz, M. Duchene, H. Aspoeck, and J. Walochnik. 2008. Acanthamoeba strains lose their abilities to encyst synchronously upon prolonged axenic culture. Parasitol. Res. 102:1069-1072. [DOI] [PubMed] [Google Scholar]
- 18.Loret, J.-F., M. Jousset, S. Robert, C. Anselme, G. Saucedo, F. Ribas, L. Martinez, and V. Catalan. 2008. Elimination of free-living amoebae by drinking water treatment processes. Eur. J. Water Qual. 39:37-50. [Google Scholar]
- 19.Luh, J., N. Tong, L. Raskin, and B. J. Marinas. 2008. Inactivation of Mycobacterium avium with monochloramine. Environ. Sci. Technol. 42:8051-8056. [DOI] [PubMed] [Google Scholar]
- 20.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazur, T., and E. Hadaś. 1994. The effect of the passages of Acanthamoeba strains through mice tissue on their virulence and its biochemical markers. Parasitol. Res. 80:431-434. [DOI] [PubMed] [Google Scholar]
- 22.Miltner, E. C., and L. E. Bermudez. 2000. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 44:1990-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Zaragoza, S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz-Esser, S., E. R. Toenshoff, S. Haider, E. Heinz, V. M. Hoenninger, M. Wagner, and M. Horn. 2008. Diversity of bacterial endosymbionts of environmental Acanthamoeba isolates. Appl. Environ. Microbiol. 74:5822-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smithwick, R. W., M. R. Bigbie, Jr., R. B. Ferguson, M. A. Karlix, and C. K. Wallis. 1995. Phenolic acridine orange fluorescent stain for mycobacteria. J. Clin. Microbiol. 33:2763-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinert, M., K. Birkness, E. White, B. Fields, and F. Quinn. 1998. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl. Environ. Microbiol. 64:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stothard, D. R., J. M. Schroeder-Diedrich, M. H. Awwad, R. J. Gast, D. R. Ledee, S. Rodriguez-Zaragoza, C. L. Dean, P. A. Fuerst, and T. J. Byers. 1998. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 45:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas, V., T. Bouchez, V. Nicolas, S. Robert, J. F. Loret, and Y. Lévi. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97:950-963. [DOI] [PubMed] [Google Scholar]
- 29.Watson, H. E. 1908. A note on the variation of the rate of disinfection with change in the concentration of disinfectant. J. Hyg. 8:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.