Abstract
Botulinum neurotoxin (BoNT) serotype B (BoNT/B) is one of the serotypes of BoNT that causes deadly human botulism, though it is used clinically for treatment of many neuromuscular diseases. BoNT/B is produced by Clostridium botulinum, and it is secreted along with a group of neurotoxin-associated proteins (NAPs) in the form of a BoNT/B complex. The complex dissociates into a 150-kDa holotoxin and NAPs at alkaline pHs. The 150-kDa BoNT/B holotoxin can be nicked to produce a 50-kDa domain referred to as the light chain (LC) and a 100-kDa heavy chain, with the former possessing a unique endopeptidase activity. The two chains remain linked through a disulfide bond that can be reduced to separate the two chains. The endopeptidase activity is present in all three forms of the toxin (complex, purified BoNT/B holotoxin, and separated light chain), which are used by different researchers to develop detection methods and screen for inhibitors. In this research, the endopeptidase activities of the three forms, for the first time, were compared under the same conditions. The results show that enzyme activities of the three forms differ significantly and are largely dependent on nicking and disulfide reduction conditions. Under the conditions used, LC had the highest level of activity, and the complex had the lowest. The activity was enhanced by nicking of BoNT/B holotoxin and was enhanced even more by dithiothreitol (DTT) reduction after nicking. This information is useful for understanding the properties of BoNT endopeptidases and for comparing the efficacies of different inhibitors when they are tested with different forms of BoNT endopeptidase.
Botulinum neurotoxins (BoNTs) produced by Clostridium botulinum are the most toxic substances known to humans and block the release of neurotransmitters, resulting in flaccid muscle paralysis. There are seven serotypes of BoNT, designated A to G, which are serologically distinct. An antitoxin against one serotype does not work on other serotypes. Different BoNT serotypes differ in their amino acid sequences, their substrates, or cleavage sites on the same substrate. Of the seven serotypes, BoNT type A (BoNT/A), BoNT/B, BoNT/E, and BoNT/F are known to cause human botulism (9). The extreme lethality of BoNTs makes them potent bioterror agents. BoNT/A and BoNT/B are two serotypes which have been approved by the Food and Drug Administration (FDA) for cosmetic purposes and for treatment of a wide range of neuromuscular diseases, including cervical dystonia (3).
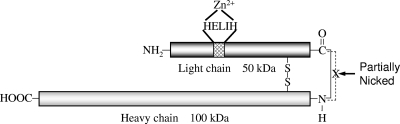
Like other BoNT serotypes, BoNT/B is secreted by the bacteria as a complex of the holotoxin and several nontoxic proteins called neurotoxin-associated proteins (NAPs). The NAPs protect the holotoxin from harsh environmental conditions, such as the high temperature, low pH, and multiple proteases present in the gastrointestinal tract (14, 17). The holotoxin, of about 150 kDa, can be obtained by removing the non-covalently bound accessory proteins with ion-exchange chromatography. The 150-kDa polypeptide chain consists of a 100-kDa heavy chain (HC) and a 50-kDa light chain (LC), which are synthesized as a single polypeptide chain but nicked by endogenous or exogenous proteases and remain linked through a disulfide bond (Fig. 1). The HC binds the receptors on neuronal cells and helps translocate the LC into the cell. The BoNT/B LC cleaves the vesicle-associated membrane protein (VAMP), also called synaptobrevin. VAMP is necessary for the docking and fusion of synaptic vesicles to plasma membrane at the neuromuscular junctions for neurotransmitter release. Once the VAMP is cleaved, the neurotransmitters in synaptic vesicles cannot be released, resulting in flaccid paralysis that can be fatal.
FIG. 1.
Schematic diagram of BoNT/B pure toxin. Dark gray, light chain; light gray, heavy chain; hatch-marked box, the active site of the toxin. The 50-kDa light chain and 100-kDa heavy chain are linked through a disulfide bridge as well as a covalent bond. The latter is partially nicked by bacterial proteases before the toxin is secreted.
Strains producing BoNT/B can be nonproteolytic or proteolytic (4). BoNT/B from nonproteolytic strains occurs as a single polypeptide chain of 150 kDa. BoNT/B secreted by proteolytic strains is a mixture of the single polypeptide chain and a dichain in which the peptide bond linking the HC and LC has been nicked by proteases produced by the bacteria (Fig. 1). The single polypeptide chain in both nonproteolytic and proteolytic cultures can be converted to the dichain form through in vitro trypsinization. The HC and LC in the dichain can be further separated by breaking the disulfide bond with a reducing agent such as dithiothreitol (DTT) and treating it with chaotropic reagents such as urea (10).
The complex, holotoxin, and LC are three different forms of BoNT/B with endopeptidase activity, although LC is the only active unit in all three forms. The complex is the native form of the toxin, which causes botulism. It is also the main component of the only licensed drug with BoNT/B currently available (2). The complex, holotoxin, and LC of BoNT/B have all been extensively used to develop methods to detect this serotype or to screen for inhibitors against the toxin (1, 5, 7, 8, 13, 15, 16). Since different forms of the toxin were used by different researchers, it is difficult to compare the sensitivities of different detection methods or the efficacies of different inhibitors. Therefore, in this study, the activities of BoNT/B complex, holotoxin, and LC were compared under the same conditions for the first time. The results suggest that the endopeptidase activity with a peptide substrate varies substantially depending on whether BoNT/B is used in its native complex form, its isolated holotoxin form, or a separated LC form. The LC form was the most active form of the endopeptidase under the conditions used.
MATERIALS AND METHODS
Materials.
BoNT/B complex and holotoxin from Clostridium botulinum strain Okra and recombinant BoNT/B LC were kindly provided by BB Tech (North Dartmouth, MA). The concentrations of proteins were determined with a bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL). Molar concentrations were calculated by using molecular masses of 550, 150, and 50 kDa for BoNT/B complex (13), holotoxin, and LC, respectively. VAMPtide, a synthetic peptide containing the native cleavage site for BoNT/B, was purchased from List Biological Laboratories (Campbell, CA). All other chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Determination of the endopeptidase activity of BoNT/B.
A 10 nM concentration of BoNT/B complex, purified holotoxin, or LC in 20 mM HEPES buffer, pH 6, with 0.1% Tween 20 was mixed with 4.2 μM VAMPtide in a 96-well Costar microplate (Corning Life Science, Corning, NY). The peptide substrate was intramolecularly quenched over the cleavage site of BoNT/B by fluorescence resonance energy transfer (FRET). The fluorophore was o-aminobenzoic acid (o-Abz), and the acceptor chromophore was 2,4-dinitrophenyl (Dnp). Once the VAMPtide was cleaved by BoNT/B, the fluorescence signal increased because of a reduced quenching effect. Fluorescence intensity was measured with a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 321 nm and an emission wavelength of 418 nm. When needed, 0.04 nmol BoNT/B complex or holotoxin in 20 mM HEPES buffer, pH 8, with 5 mM CaCl2 was subjected to limited proteolysis, or nicking, by the addition of 7.0 or 2.3 μg/ml trypsin, respectively, and incubated at 37°C. After 30 min, the reaction was stopped by the addition of 1 mM phenylmethylsulfonyl fluoride (PMSF). Part of nicked protein sample was assayed with VAMPtide directly, while the rest was tested after the addition of 20 mM DTT. Fluorescence of VAMPtide with buffer but not BoNT/B was measured simultaneously as a control. Cleavage of VAMPtide by BoNT/B was calculated as the difference between the fluorescence signals of a sample and the control in relation to the signal of the control. The cleavage rate was the change of cleavage per minute. Each time, the enzyme was mixed with substrate in three different microplate wells and data were averaged. All experiments were repeated on at least two different occasions, and a representative of them is reported. For every sample that was tested for endopeptidase activity, an aliquot was assayed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
SDS-PAGE.
Protein samples were mixed with sample loading buffer (1 M Tris buffer, pH 6.8, and 2% SDS) for nonreducing SDS-PAGE. For reducing SDS-PAGE, 100 mM DTT was added in addition to the sample loading buffer. Both reduced and nonreduced samples were heated at 95°C for 5 min before being loaded on 4 to 12% gels on an XCell SureLock Mini Cell (Invitrogen, Carlsbad, CA). After Coomassie blue staining and destaining, the images of gels were captured with a gel imaging and analysis system (Eastman Kodak Co., Rochester, NY). Results reported are representative of at least two replicates.
Statistical analysis.
All data reported for the VAMPtide assay are the means of triplicates. Statistical analysis was carried out using SAS software (SAS Institute Inc., Cary, NC).
RESULTS AND DISCUSSION
Endopeptidase activity of BoNT/B LC.
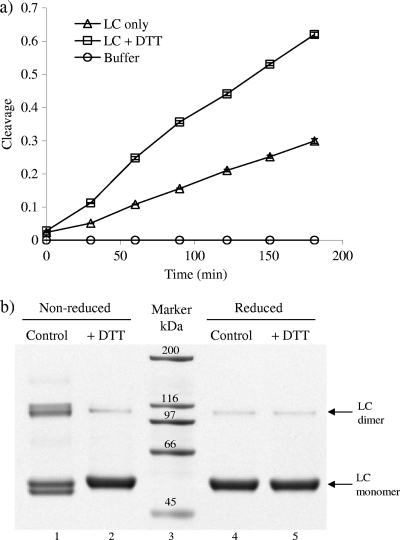
When VAMPtide was incubated with BoNT/B LC at 37°C in the absence of DTT, the fluorescence signal increased gradually over a 3-h time span (Fig. 2 a). In the meantime, the fluorescence of VAMPtide incubated with buffer hardly changed, indicating that the increase of fluorescence signal was due to the cleavage of VAMPtide by the LC. With DTT added, the cleavage of VAMPtide by BoNT/B LC occurred more rapidly. After 30 min of the reaction, the amount of cleavage in the presence of DTT was double the amount in the absence of DTT. The difference between the two further increased with extended incubation. The difference in the endopeptidase activities of BoNT/B LC with DTT and LC without DTT is related to the structure of the LC. Recombinant LC of BoNTs exists as a homodimer (12) under nonreducing conditions. As shown on an SDS-PAGE gel, in addition to the LC band at about 52 kDa, there was also a band at about 100 kDa, corresponding to the dimer, in the absence of DTT (Fig. 2b, lane 1). With 20 mM DTT added, the band corresponding to the dimer became weaker, while the monomer band was much stronger (Fig. 2b, lane 2), indicating that the dimer had been reduced to the monomer. The doublets observed at both 52 kDa and 100 kDa under nonreducing conditions entirely disappeared upon treatment with DTT. These doublets are likely to originate from differential shapes of the monomeric and dimeric forms of BoNT/B LC, presumably from differential intrachain disulfide bond formation. There are a number of cysteine residues in BoNT/B LC which could be responsible for the intrachain disulfide bond formation. Increasing the concentration of DTT up to 100 mM did not seem to break the remaining dimer any further (Fig. 2b, lanes 4 and 5). This is consistent with the results obtained by other researchers (6) who demonstrated that some dimers remain intact even under reducing conditions. Steric hindrance may make the substrate binding cleft and active site less accessible in the dimer; thus, the cleavage of VAMPtide by BoNT/B LC was slow without DTT (Fig. 2a). In the presence of DTT, the homodimer was converted into monomers (Fig. 2b), and it was presumed to become easier for the substrate peptide to access the active site of LC. Therefore, the cleavage reaction took place more rapidly.
FIG. 2.
BoNT/B LC under different conditions. (a) Cleavage of VAMPtide by BoNT/B LC left untreated or treated with 20 mM DTT. The cleavage difference between the fluorescence signals of a sample and the control in relation to the signal of the control. (b) SDS-PAGE of BoNT/B LC before and after being treated with 20 mM DTT. Both samples were subjected to nonreducing SDS-PAGE and reducing SDS-PAGE with 100 mM DTT.
Endopeptidase activity of BoNT/B holotoxin.
The purified BoNT/B holotoxin naturally consists of both the single-chain and dichain forms (Fig. 1). Both the single chain and the dichain appeared on the nonreducing SDS-PAGE gel as a single band at about 150 kDa (Fig. 3 b, lane 1). With reducing SDS-PAGE that had 100 mM DTT in the sample buffer, in addition to the 150-kDa band, two very weak bands appeared at about 100 and 50 kDa (Fig. 3b, lane 5), and these were more visible with silver staining (15), which has higher sensitivity than Coomassie blue staining. These two bands corresponded to the HC and LC of BoNT/B that originated from the dichain in the holotoxin and were separated by the treatment with 100 mM DTT in the sample buffer for SDS-PAGE. The 150-kDa band in reducing SDS-PAGE (Fig. 3b, lane 5) is attributed to the single chain in BoNT/B holotoxin. Even though DTT reduces the disulfide bond between the HC and LC under reducing conditions, the covalent bond linking the two chains is intact in the single chain (Fig. 1), thus showing a protein band at about 150 kDa. The 150-kDa band, even upon reduction of the disulfide bond (Fig. 3b, lane 5), had an intensity similar to that without reduction of the disulfide bond (Fig. 3b, lane 1), and the bands corresponding to the HC and LC upon disulfide reduction (Fig. 3b, lane 5) were very weak, indicating that in its natural form, the purified holotoxin contained mostly single chain (>99%). After treatment with trypsin, the covalent linkage connecting the HC and LC in the single-chain molecules was proteolytically nicked, while the disulfide bond stayed intact. As a result, the molecules remained at 150 kDa in nonreducing SDS-PAGE (Fig. 3b, lane 2). With the presence of 100 mM DTT in reducing SDS-PAGE, the disulfide bond broke down, resulting in the separation of HC and LC on the electrophoresis gel, with concurrent disappearance of the 150-kDa band of BoNT/B (Fig. 3b, lane 6).
FIG. 3.
Effects of nicking and DTT on the properties of purified BoNT/B holotoxin. (a) Endopeptidase activity of BoNT/B holotoxin on VAMPtide before and after being nicked and treated with 20 mM DTT. (b) SDS-PAGE of BoNT/B holotoxin under different conditions. Three different samples, namely, untreated, nicked, and nicked and DTT-treated BoNT/B holotoxin, were subjected to nonreducing SDS-PAGE and reducing SDS-PAGE with 100 mM DTT.
Like LC, purified BoNT/B holotoxin also cleaved VAMPtide during 3-h incubation (Fig. 3a), irrespective of nicking and the treatment with DTT. However, nicking or DTT treatment enhanced the activity of purified BoNT/B holotoxin (Fig. 3a). The cleavage reaction was slow initially, and then it became rapid, as indicated by the cleavage rate being higher during the second 30 min than during the first 30-min period (Table 1). This was especially true for the purified BoNT/B holotoxin control without nicking and DTT treatment, which hardly showed any activity within the first 30 min. During the second 30 min, the cleavage rate for the untreated BoNT/B holotoxin control was about 10% lower than that for the nicked toxin and 70% lower than that for the nicked and disulfide bond-reduced toxin (Table 1). Considering that there was a very small amount of the dichain form present in the natural preparation of the holotoxin (Fig. 3b, lanes 1 and 5), it is possible that unnicked BoNT/B single chain also accounted for some of the activity of the purified BoNT/B holotoxin. The accessibility of the endopeptidase active site for the substrate is likely to be limited in the BoNT/B single chain due to the presence of the HC domain through the disulfide bond. As shown in the crystal structure of BoNT/B holotoxin (18), Zn2+ and the active site are located in a deep pocket in the LC (Fig. 4). Part of the HC forms a belt which occludes the opening of the pocket, interfering with the substrate approaching the active site (Fig. 4). Once the purified BoNT/B holotoxin was subjected to nicking by trypsin, the LC in the BoNT/B single chain became more accessible to the substrates, giving rise to increased endopeptidase activity. As a control for nicking of the single chain by trypsin, the same amount of trypsin was added to the same volume of buffer without toxin, and after 30 min of incubation at 37°C, PMSF was added to all samples to inhibit trypsin. As shown in Fig. 3a, the trypsin control sample obtained thereof did not cause much change in the fluorescence signal; hence, the increase in the fluorescence of other samples was attributed to the nicked holotoxin rather than trypsin itself. This also shows that trypsin does not generate a false-positive signal in the presence of PMSF in the VAMPtide assay. Adding DTT to nicked holotoxin further disconnected the LC from the HC, and thus, there was more cleavage of the substrate (Fig. 3a). The positive effect of nicking on the endopeptidase activity of BoNT/B was also reported by other researchers using an immunoassay (7).
TABLE 1.
Cleavage rates of different forms of BoNT/B
| Endopeptidase | Mean cleavage rate ± SE (10−3 min−1)a |
|
|---|---|---|
| First 30 min | Second 30 min | |
| BoNT/B LC | 1.16 ± 0.01 b | 1.88 ± 0.18 b |
| BoNT/B LC plus DTT | 2.80 ± 0.44 c | 4.52 ± 0.43 d |
| BoNT/B holotoxin | — | 0.77 ± 0.33 a |
| Nicked BoNT/B holotoxin | 0.58 ± 0.08 a | 0.85 ± 0.31 a |
| Nicked BoNT/B holotoxin plus DTT | 0.60 ± 0.19 a | 2.50 ± 0.37 b |
| BoNT/B complex | — | — |
| Nicked BoNT/B complex | — | 0.56 ± 0.02 a |
| Nicked BoNT/B complex plus DTT | 1.19 ± 0.08 b | 3.52 ± 0.69 c |
Values within a column that do not share a common letter differ significantly (P < 0.05). —, cleavage not detected.
FIG. 4.
Crystal structure of BoNT/B holotoxin. The LC is shown as blue. The Zn2+ (red)-containing active site (yellow) is occluded by the presence of the belt extended from the HC (green ribbon).
Endopeptidase activity of BoNT/B complex.
The reaction progress curves of VAMPtide cleaved by native BoNT/B complex (Fig. 5 a) are similar to those corresponding to the purified holotoxin (Fig. 3a). The lowest level of activity of the complex was observed under conditions in which the single chain was not nicked and the disulfide bond was not reduced. Cleavage was hardly observed within the first 60 min (Table 1). Even after 3 h, the cleavage of VAMPtide by unnicked and non-DTT-treated BoNT/B complex was limited (Fig. 5a). Nicking and DTT treatment enhanced the endopeptidase activity of the BoNT/B complex substantially, about seven times higher than the control level in 3 h, while this treatment corresponded to a value about four times higher than the control value in the case of purified BoNT/B holotoxin (Fig. 3a). Before being nicked, BoNT/B holotoxin in the complex stayed mostly as a 150-kDa single chain, while a very small amount of dichain was converted into the 100-kDa HC and 50-kDa LC in reducing SDS-PAGE (Fig. 5b, lane 5). After the nicking, the 150-kDa band became lighter in nonreducing SDS-PAGE (Fig. 5b, lane 2). It is possible that some of the 150-kDa BoNT/B toxin molecules in the complex were proteolytically hydrolyzed by trypsin at sites other than the nicking site (Fig. 1), giving rise to molecules of sizes less than 150 kDa. However, no clear bands were visible to prove this. Another possibility is that after the nicking, there was a heterogeneous combination of different conformations (resulting from differential intermolecular disulfide formation) of the nicked holotoxin as well as degraded neurotoxin binding protein (originally at about 120 kDa) (Fig. 5b, lanes 1 and 5), as indicated by diffused bands and smear in nonreducing SDS-PAGE in the 100- to 150-kDa region (Fig. 3b, lane 2, and Fig. 5b, lane 2). The 150-kDa band for nicked single chain in nonreducing SDS-PAGE (Fig. 5b, lane 2) was not observed under reducing SDS-PAGE gel conditions (Fig. 5b, lane 6); instead, bands corresponding to BoNT/B HC (approximately 100 kDa) and LC (approximately 50 kDa) appeared with the expected band intensities. This is consistent with the enhanced endopeptidase activity of the BoNT/B complex that was observed upon nicking, presumably because the cleavage of the covalent bond linking BoNT/B HC and LC results in more flexibility for the LC to interact with the substrate. For the same reason, DTT treatment after nicking further separated the BoNT/B HC and LC (Fig. 5b, lane 3) and enhanced the activity of the BoNT/B complex (Fig. 5a).
FIG. 5.
Effects of nicking and DTT on the properties of BoNT/B complex. (a) Endopeptidase activity of BoNT/B complex on VAMPtide before and after being nicked and treated with 20 mM DTT. (b) SDS-PAGE of BoNT/B complex under different conditions. Three different samples, namely, untreated, nicked, and nicked and DTT-treated BoNT/B complex, were subjected to nonreducing SDS-PAGE and reducing SDS-PAGE with 100 mM DTT.
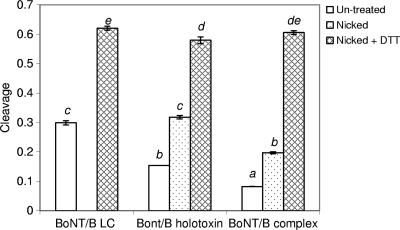
The endopeptidase activities of the three different forms of BoNT/B differ considerably and are affected by nicking and disulfide reduction conditions (Fig. 6). For the originally prepared BoNT/B LC, purified holotoxin, and complex before any treatment, such as nicking and DTT reduction, only the LC showed some activity within the first 30 min, with a cleavage rate of 1.16 × 10−3 min−1 (Table 1). Holotoxin and complex activities were hardly observed during the same time span. The cleavage rate increased to 1.88 × 10−3 min−1 for nontreated LC within the second 30 min, while the nontreated holotoxin started to cleave the substrate with a rate of 0.77 × 10−3 min−1; the complex still showed no activity. Due to the different cleavage rates, after 3 h of the reaction, non-DTT-treated BoNT/B LC (dimer) showed the highest level of activity, followed by purified holotoxin, and then the complex (Fig. 6). The activity level of nontreated BoNT/B LC being higher than that of holotoxin can be explained by the differences in their structures. Nonreduced LC exists as a homodimer (Fig. 7). Its crystal structure shows that for both monomers, the active site is not blocked by the presence of the other monomer. However, in nontreated holotoxin, part of the HC belt hangs above the open pocket in which the active site is located (Fig. 4), making it difficult for the substrate to access, thus showing a lower level of activity than the LC dimer (Fig. 6). Though dimerization does not affect availability of the LC catalytic site, it can reduce the flexibility of LC molecules to move around and to coordinate with the substrate for the enzymatic reaction to occur. Therefore, once the LC dimer is reduced, each monomer becomes completely free, providing more flexibility to interact with the substrate. As a result, DTT-reduced LC was notably more active than its dimeric form (Fig. 6).
FIG. 6.
Cleavage of VAMPtide by BoNT/B LC, purified holotoxin, and complex under different conditions after 3 h of the reaction. DTT was added to LC directly but was added to holotoxin and complex after they were nicked. Bars not sharing a common letter differ significantly (P < 0.05).
FIG. 7.
Crystal structure of BoNT/B LC (Protein Data Bank accession code 2ETF). The LC exists as a homodimer in its natural form. The two monomers are shown in blue and green, the active site is shown in yellow, and Zn2+ is shown in red. The active sites on both monomeric units are not affected by each other.
After the BoNT/B holotoxin and complex were nicked, both of their activities increased. The cleavage rate for purified holotoxin increased by about 10% during the second 30 min of the cleavage reaction after it was nicked (Table 1). Nicking also enabled the BoNT/B complex to cleave the substrate during the second 30 min, with a rate of 0.56 × 10−3 min−1. Though the cleavage rate increased for both the holotoxin and complex after they were nicked, the cleavage rate for the holotoxin was higher (Table 1), and after 3 h, the holotoxin appeared to be more active than the complex, with the former's activity similar to that of the BoNT/B LC under nonreduced conditions (Fig. 6). After further reduction with DTT, all three forms of BoNT/B endopeptidase became even more active. The cleavage rate of DTT-reduced BoNT/B LC was 2.4 times higher than the cleavage rate of the nontreated LC control during the second 30 min of the cleavage reaction (Table 1). For the holotoxin and complex, though nicking increased the cleavage rate, the influence of DTT reduction after nicking was even greater. For example, the cleavage rate for purified holotoxin within the second 30 min of the reaction became 1.1 times higher after nicking and 3.3 times higher with DTT reduction after nicking (Table 1). It seems that the NAPs in the BoNT/B complex not only protect the toxin from harsh environments but also delay its cleavage of the substrate by decreasing the accessibility of the active site for the substrate. Nontreated BoNT/B complex did not show any cleavage within the first 60 min. Even after it was nicked, its activity was not observed until the second 30 min of incubation with the substrate. With DTT reduction as well as nicking, the activity of complex was enhanced considerably. While the cleavage rate during the second 30 min of the reaction was lower for the nicked complex (0.56 × 10−3 min−1) than for nicked holotoxin (0.85 × 10−3 min−1), with the reduction after nicking, the cleavage rate for the complex became higher (3.52 × 10−3 min−1) than that for the holotoxin (2.50 × 10−3 min−1). After 3 h of cleavage, the three forms of BoNT/B endopeptidase showed similar activities (Fig. 6). These results clearly show that the activities of different forms of BoNT/B are highly dependent on reaction conditions which affect the protein structure. This information is useful for understanding the present discrepancies in regard to the activity and inhibition of BoNT/B due to the different forms of active BoNT/B used in different studies (6, 11). For example, it has been reported that the Km for BoNT/B LC without DTT treatment is about 0.08 mM and its kcat is about 40 s−1 (6, 11). Nonnicked BoNT/B holotoxin has a Km of 0.3 mM and a kcat of 23.8 s−1 (16). The catalytic efficiency of nonnicked holotoxin being lower than that of nontreated LC matches what was observed in this study (Fig. 6). The VAMPtide assay used here has been correlated with cell-based assays for the holotoxin, and the results obtained were similar for tests in vitro and in vivo (15). It is expected that in a comparison of the activities of the three different forms of BoNT/B in vivo, results can be different from those presented here due to the physiological conditions and delivery mechanisms involved in vivo. Though LC has been widely used in vitro for assay development and inhibitor screening, assaying its endopeptidase activity under in vivo conditions has remained a challenge.
Conclusions.
Endopeptidase activities of the three forms of BoNT/B, namely, complex, holotoxin, and LC, were, for the first time, evaluated using the same method and under the same conditions. Given the same concentration, the three different forms of BoNT/B differed significantly in their activities. For each individual form, the activity was enhanced by nicking and DTT reduction. Although these results were obtained with BoNT/B, variations in the activities of different forms and the influence of nicking and reducing are expected to be applicable to other types of BoNTs as well. Comparison between the activities of different forms of BoNT is important for understanding the efficacy of BoNT as a therapeutic agent or its toxicity as a poison. The differences in the activities of different forms of BoNT and under different conditions should also be considered when comparing the sensitivities of different detection methods in diagnostics and in evaluation of the effectiveness of inhibitors when different forms of BoNT are used. This information is also useful for optimizing the conditions for detection or inhibitor-screening purposes. This study also demonstrated a rapid fluorescence method for assaying all forms of BoNT/B. This method allows for real-time measurement of active BoNT/B, and there is no need to separate cleaved substrate and noncleaved substrate. It is especially useful as a routine high-throughput screening method without the need for specifically developed antibodies and complicated equipment.
Acknowledgments
This work is funded by the United States Department of Homeland Security (grant AB070214TET-01) and the National Institutes of Health (grant 1U01A1078070-01).
Footnotes
Published ahead of print on 6 August 2010.
REFERENCES
- 1.Adler, M., J. D. Nicholson, F. Cornille, and B. E. Hackley, Jr. 1998. Efficacy of a novel metalloprotease inhibitor on botulinum neurotoxin B activity. FEBS Lett. 429:234-238. [DOI] [PubMed] [Google Scholar]
- 2.Callaway, J. E. 2004. Botulinum toxin type B (Myobloc): pharmacology and biochemistry. Clin. Dermatol. 22:23-28. [DOI] [PubMed] [Google Scholar]
- 3.Comella, C. L., J. Jankovic, K. M. Shannon, J. Tsui, M. Swenson, S. Leurgans, and W. Fan. 2005. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology 65:1423-1429. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta, B. R., and H. Sugiyama. 1976. Molecular forms of neurotoxins in proteolytic Clostridium botulinum type B cultures. Infect. Immun. 14:680-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, E. R., J. M. Sutton, A. Gravett, and C. C. Shone. 2005. Analysis of the substrate recognition domain determinants of botulinum type B toxin using phage display. Toxicon 46:446-453. [DOI] [PubMed] [Google Scholar]
- 6.Gilsdorf, J., N. Gul, and L. A. Smith. 2006. Expression, purification, and characterization of Clostridium botulinum type B light chain. Protein Expr. Purif. 46:256-267. [DOI] [PubMed] [Google Scholar]
- 7.Hallis, B., B. A. James, and C. C. Shone. 1996. Development of novel assays for botulinum type A and B neurotoxins based on their endopeptidase activities. J. Clin. Microbiol. 34:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalb, S. R., H. Moura, A. E. Boyer, L. G. McWilliams, J. L. Pirkle, and J. R. Barr. 2006. The use of Endopep-MS for the detection of botulinum toxins A, B, E, and F in serum and stool samples. Anal. Biochem. 351:84-92. [DOI] [PubMed] [Google Scholar]
- 9.Poulain, B. 2008. How do the botulinum neurotoxins block neurotransmitter release: from botulism to the molecular mechanism of action. Botulinum J. 1:14-87. [Google Scholar]
- 10.Sathyamoorthy, V., and B. R. DasGupta. 1985. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J. Biol. Chem. 260:10461-10466. [PubMed] [Google Scholar]
- 11.Schmidt, J. J., and R. G. Stafford. 2003. Fluorigenic substrates for the protease activities of botulinum neurotoxins serotypes A, B, and F. Appl. Environ. Microbiol. 69:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segelke, B., M. Knapp, S. Kadkhodayan, R. Balhorn, and B. Rupp. 2004. Crystal structure of Clostridium botulinum neurotoxin protease in a product-bound state: evidence for noncanonical zinc protease activity. Proc. Natl. Acad. Sci. U. S. A. 101:6888-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma, S. K., J. L. Ferreira, B. S. Eblen, and R. C. Whiting. 2006. Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labeled antibodies. Appl. Environ. Microbiol. 72:1231-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma, S. K., and B. R. Singh. 2004. Enhancement of the endopeptidase activity of purified botulinum neurotoxins A and E by an isolated component of the native neurotoxin associated proteins. Biochemistry 43:4791-4798. [DOI] [PubMed] [Google Scholar]
- 15.Shi, X., G. E. Garcia, M. P. Nambiar, and R. K. Gordon. 2008. Un-nicked BoNT/B activity in human SHSY-5Y neuronal cells. J. Cell. Biochem. 105:129-135. [DOI] [PubMed] [Google Scholar]
- 16.Shone, C. C., and A. K. Roberts. 1994. Peptide substrate specificity and properties of the zinc-endopeptidase activity of botulinum type B neurotoxin. Eur. J. Biochem. 225:263-270. [DOI] [PubMed] [Google Scholar]
- 17.Sugii, S., I. Ohishi, and G. Sakaguchi. 1977. Intestinal absorption of botulinum toxins of different molecular sizes in rats. Infect. Immun. 17:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swaminathan, S., and S. Eswaramoorthy. 2000. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol. 7:693-699. [DOI] [PubMed] [Google Scholar]