Abstract
This study aims to model the effects of acid and osmotic shifts on the intermediate lag time of Listeria monocytogenes at 10°C in a growth medium. The model was developed from data from a previous study (C. I. A. Belessi, Y. Le Marc, S. I. Merkouri, A. S. Gounadaki, S. Schvartzman, K. Jordan, E. H. Drosinos, and P. N. Skandamis, submitted for publication) on the effects of osmotic and pH shifts on the kinetics of L. monocytogenes. The predictive ability of the model was assessed on new data in milk. The effects of shifts were modeled through the dependence of the parameter h0 (“work to be done” prior to growth) induced on the magnitude of the shift and/or the stringency of the new environmental conditions. For shifts across the boundary, the lag time was found to be affected by the length of time for which the microorganisms were kept at growth-inhibiting conditions. The predicted concentrations of L. monocytogenes in milk were overestimated when the effects of this shift were not taken into account. The model proved to be suitable to describe the effects of osmotic and acid shifts observed both within the growth domain and across the growth boundaries of L. monocytogenes.
The lag phase of a microorganism is usually seen as a period of transition from an initial physiological state to the state of balanced growth. The duration of the lag phase, denoted by lag in what follows, depends on the amount of work to be carried out by the cells prior to exponential growth and the rate at which this work is undertaken (6, 13). According to Baranyi and Roberts (2), the “work to be done” is proportional to h0, the product of the lag time and the rate at which the work is carried out. Robinson et al. (13) pointed out that there is no direct way to measure this rate, and it is often assumed that it is equal to the specific growth rate characteristic of the growth conditions (2). Some authors use the relative lag time (RLT) (7, 8) as a replacement for the “work to be done” h0 parameter. In fact the two concepts appear to be very similar, RLT and h0 being proportional to each other.
The transient phase following inoculation is commonly called the initial lag phase. Many authors (6, 7, 14, 17) observed that subsequent abrupt changes in the environmental conditions (temperature, pH, and water activity [aw]) during the growth phase were able to induce a so-called “intermediate” lag phase. In other words, abrupt changes cause extra “work to be done” that cells have to perform before reinitiating their growth. Most of the studies on intermediate lag times have focused on abrupt thermal changes. For example, some authors have proposed models for the effects of temperature shifts on the lag time of Escherichia coli (14) and Lactobacillus plantarum (17). Using the data of Whiting and Bagi (15), for Listeria monocytogenes, Delignette-Muller et al. (3) highlighted a linear relationship between the “work to be done” and the magnitude and direction of the temperature shifts. Less attention has been given to the effects of acid and osmotic shifts, although such shifts pose a higher energetic burden to the cells than temperature shifts, especially around the growth boundaries (C. I. A. Belessi, Y. Le Marc, S. I. Merkouri, A. S. Gounadaki, S. Schvartzman, K. Jordan, E. H. Drosinos, and P. N. Skandamis, submitted for publication). Generally, the “work to be done” increases with the magnitude of the shifts applied (3, 7) and the cells in exponential phase are more sensitive to abrupt shifts than those in stationary phase (7). Muñoz-Cuevas et al. (9) proposed a model for the lag time of L. monocytogenes induced by temperature and water activity downshifts within the growth region. For osmotic shifts, the authors found that the “work to be done” was related not only to the magnitude of the shift and but also to the level of the environmental factors (temperature and water activity) after the shift.
In most of the available modeling packages, predictions in dynamic environments are based on the assumption that when the environmental conditions change, the specific growth rate changes instantaneously relative to the new conditions. The intermediate lag times caused by abrupt changes in the environmental conditions are commonly neglected in the models. Besides, the models usually ignore the effects of shifts across the growth boundary and the duration of the period the cells spend above the growth/no-growth boundary on the physiological state of the cells. However, such abrupt shifts are important, as they may occur for example during fermentation and ripening of dairy products (10, 11) or during cross-contamination (e.g., when L. monocytogenes is accidently transferred to a different environment). The aim of this work was to develop a model to describe the effects of such abrupt shifts (within the growth range or across the growth boundary) on the possibly induced intermediate lag time of L. monocytogenes. The analysis here is based on the data from a previous study (Belessi et al., submitted) on the effects of acid and osmotic shifts on the kinetics of L. monocytogenes at 10°C. We also used new data in milk to explore the possibility of integrating the proposed approach in a generic growth model.
MATERIALS AND METHODS
Inoculum and preparation of media.
L. monocytogenes C5 (serotype 4b) isolated from a farm environment was kindly provided by Teagasc, Dairy Products Research Centre, Ireland, and used throughout this study. A subculture of this microorganism was incubated at 37°C in a tryptic soy broth (TSB) supplemented with 0.6% yeast extract (TSBYE). The second subculture was grown at the same temperature for 16 h, in order to achieve late exponential phase for the cells. Experiments were conducted in TSBYE at various pH and water activity values, adjusted by the addition of lactic acid (dl-lactic acid, 85% [wt/wt]; Sigma-Aldrich, Ltd., Greece) and 10 N NaOH (sodium hydroxide pellets; Panreac, Spain) and NaCl to 100 ml TSBYE. pH was measured with a pH electrode (pH 691; Metrohm, Zofingen, Switzerland). The final pH values were 7.0, 6.0, 5.8, 5.5, 5.3, 5.1, and 4.9. Taking into account the presence of 0.5% NaCl in commercial formulation of TSB, the final salt concentrations of the media tested were 0.5, 2, 5, 8, 10.5, and 12.5%.
Experimental conditions. (i) Osmotic shifts within the growth range.
L. monocytogenes was inoculated at 102 to 103 CFU/ml in growth media (pH 7) at 2, 5, 8, and 10.5% NaCl and incubated at 10°C until the late exponential phase-early stationary phase (ca. 8 log CFU/ml) was reached. Aliquots (1 ml) were then serially diluted in maximum recovery diluent (MRD) and transferred to growth-permitting levels of aw (2, 5, 8, and 10.5% NaCl).
(ii) pH shifts within the growth range.
L. monocytogenes was grown at pH (0.5% NaCl) at 10°C at pH values of 5.3, 5.5, 5.8, 6, and 7 until the late exponential to early stationary phase. As for the aw experiments, aliquots (1 ml) were diluted in MRD and transferred to different pH conditions (pHs ranging from 5.1 to 6).
(iii) Shifts across the growth boundaries.
L. monocytogenes was transferred to media under growth-prohibiting conditions, namely, pH 4.9 (0.5% NaCl) and a salt concentration of 12.5% (pH 7.0) for the pH and aw experiments, respectively. The cells were habituated for 1, 5, or 10 days and then transferred to growth-permitting levels (pH 5.1 to 7 for pH experiments and 0.5 to 10.5% NaCl for aw experiments).
(iv) Validation data in milk.
The growth of L. monocytogenes C5 in milk at 10°C was measured under fluctuating conditions of pH and NaCl concentrations. Reductions of pH and increases of NaCl during the experiments were obtained by addition of lactic acid and NaCl. Increase of pH and reduction of NaCl concentrations were performed by mixing inoculated and noninoculated milk at a 1:1 ratio. The 2-fold reduction of the L. monocytogenes induced was not taken into account for simulations as such a reduction is negligible in logarithmic scale. Five profiles were tested. In profiles 1 and 2, abrupt osmotic shifts were applied within the growth boundary. In profile 3, osmotic shift was applied from 2.5% to 12.5% NaCl (no-growth condition). After 4 days at 12.5% NaCl, the cells were transferred to 6% NaCl (growth condition). Acid shifts across the growth limits were applied for profile 4: after 70 h, the cells were transferred from pH 6 to pH 4.9, kept at pH 4.9 for 5 days, and transferred to milk at pH 5.8. In profile 5, both osmotic and pH shifts were applied, sequentially and/or simultaneously.
Bacterial enumeration.
For broth and milk experiments, samples were plated on tryptone soya agar supplemented with 0.6% yeast extract (TSAYE; Biolife, Italy). The plates were incubated at 37°C for 24 h.
Model development.
The model was developed based on the set of equations of Baranyi and Roberts (2) and previous experimental observations (Belessi et al., submitted). This model can be written as follows:
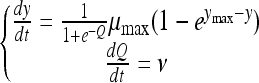 |
(1) |
with the initial conditions y(0) = y0 and Q(0) = Q0, where y(t) is the natural logarithm of the bacterial concentration at time t, Q(t) is a quantification of the actual physiological state of the cells, μmax is the specific growth rate characteristic of the actual environment (temperature, pH, and water activity), and ymax is the natural logarithm of the maximum concentration of the microorganism. Note that pH and aw can change dynamically with time, due to the osmotic and acid shifts applied. The lag time does not appear directly in equation 1. In this model, the lag is a derived parameter determined partly by the actual environment, partly by the initial physiological state of the cells, reflected by Q0. As the cells gradually adjust to their new environment, Q(t) increases linearly at a certain rate, ν. To make the curve-fitting procedure more stable, Baranyi and Roberts (2) proposed a transformation of Q0:
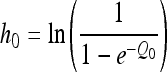 |
(2) |
Using the terminology of Robinson et al. (13), h0 may be regarded as a quantity proportional to the “work to be done” by the bacterial cells to adapt to their new environment before commencing exponential growth. As stated above, it was demonstrated mathematically that h0 is the product of the lag time and the rate of adaptation, ν:
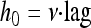 |
(3) |
In the commonly used version of the model of Baranyi and Roberts, the adaptation rate is assumed to be the same as the cells' specific growth rate characteristic of the exponential phase in the actual environment: i.e., ν = μmax.
Let hs be the amount of “work to be done” due to the shift, prior to regrowth. For shifts back and forth across the growth boundary, let hs be the amount of work the cells still need to carry out just after the shift up to growth-permitting conditions. Let Qs be the value of Q corresponding to the amount of work (hs) induced by the shift. According to equation 2, Qs can be expressed as a function of hs:
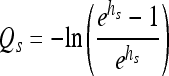 |
(4) |
Shifts within the growth range.
When the bacterial population is subjected to a sudden shift, the system is reset with the new value Qs, corresponding to the work to be done, hs, induced by the shift. After the shift, the cells were assumed to adapt themselves at the rate ν = μmax.
For osmotic and pH shifts within the growth range, it has been observed (Belessi et al., submitted) that shifts to levels more favorable for growth usually do not induce intermediate lag times. Therefore, only aw and pH downshifts with magnitudes |Δaw| ≥ 0.02 and |ΔpH| ≥ 0.2 were assumed to induce intermediate lag.
The dependence of hs on the shift in water activity was modeled as
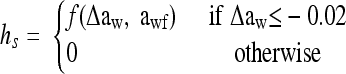 |
(5) |
where Δaw is the shift magnitude, awf is the water activity after shift, and f is a linear function of Δaw and awf extended with cross-product terms.
For acid shifts within the growth range, it was found that hs dramatically increased for downshifts close to the growth-limiting conditions (Belessi et al. submitted). These data also suggest that hs depends not only on the magnitude of the shift but also on how close the initial and final conditions are to the limiting conditions. Based on these observations, we propose the following model:
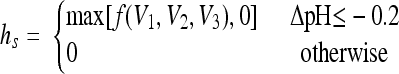 |
(6) |
where
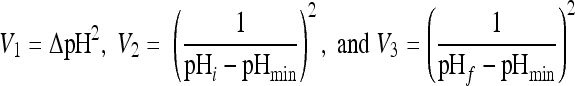 |
with ΔpH = pHf − pHi indicating the magnitude of shift, where pHi and pHf are the pHs before and after shift, pHmin is the minimum theoretical pH for growth, and f is a quadratic polynomial function.
Shifts across the growth boundary.
For osmotic shifts back and forth across the growth/no-growth interface, the authors showed that the “work to be done” decreases with the time length for which the cells were held at 12.5% NaCl. In the case of pH shifts, on the contrary, the amount of work increases with the time of habituation at pH 4.9. These findings suggest that the cells are able to carry out at least some of the “work to be done” during habituation at low aw, while the period at low pH induces negative effects on the physiological state of the cells (Belessi et al., submitted). It was also observed that the magnitude of shifts up to growth-permitting conditions also affects the lag time, with the work to be done being more important when the new conditions after shifts are close to the growth boundaries. Based on these considerations, we assumed the following. (i) The shift from growth to no-growth conditions (pH 4.9 or 12.5% NaCl) can be described through “resetting” the parameter Q to a new value, Q1. (ii) The physiological state, Q, of the cells is affected during the stress applied at a rate, ν, depending on the experimental conditions during the stress. Either the cells are able to carry out some work at a certain rate (ν > 0) or the stress conditions continue to damage the cells (ν < 0). Therefore, we hypothesized that when conditions do not allow bacterial growth, the parameter ν in equation 1 is not equal to μmax (which is zero in this region):
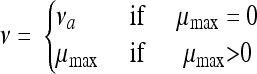 |
(7) |
(iii) Finally, the “work to be done” is also dependent on the new conditions after the shift. The closer the new conditions are to the growth boundary, the greater is the amount of work to be carried out (Belessi et al., submitted). To describe this, it was assumed that the adaptation work due to new growth conditions, h2, can be expressed as
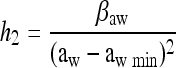 |
(8) |
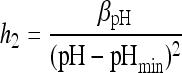 |
(9) |
where 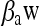 , and βpH are parameters, aw and pH are levels of water activity and pH after the shift, and aw min and pHmin are the theoretical minimum water activity and pH for growth, respectively.
, and βpH are parameters, aw and pH are levels of water activity and pH after the shift, and aw min and pHmin are the theoretical minimum water activity and pH for growth, respectively.
Like Muñoz-Cuevas et al. (9), we assumed that the total amount of work to be done will be the “unfinished work” just before the shift plus the new work, h2, caused by the shift to the growth conditions. Let hbs be the “work to be done” just before the shift. The total “work to be done” after the shift hs is therefore assumed to be
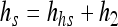 |
(10) |
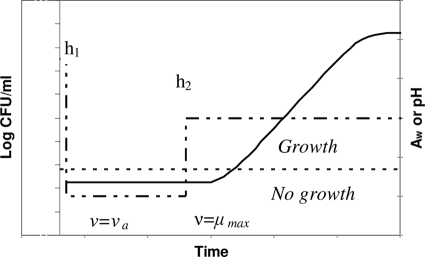
After the shift, Q is readjusted to a new value Qs corresponding to the work hs. The basis of the model for shifts across the growth boundary is summarized in Fig. 1.
FIG. 1.
Conceptual basis of the model for the lag time of L. monocytogenes in response to aw or pH shifts (dashed line) back and forth across the growth boundary (dotted line). The downshift to no-growth conditions induces “work to be done,” h1. The work to be done is carried out or increases at the rate ν = νa. The upshift to growing conditions also adds a supplementary workload, h2, which depends on the new conditions after the shift. Once the cells are in the growth region, adaptation is carried out at the rate ν = μmax.
Growth rate model.
The effects of the environment on μmax were described by a cardinal-type model. It has been observed that such models may fail to describe the bacterial growth rate near the growth limits and may predict strictly positive values for μmax for some no-growth conditions (4). As some shifts were applied across or near the growth boundaries, we included in the model an interaction term, ξ, proposed by Le Marc et al. (4) and Mejlhom and Dalgaard (5) in order to improve the description of the interactions between environmental factors at the growth limits. The model is written as
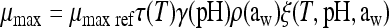 |
(11) |
where μmax ref is the maximum growth rate at a reference temperature of 25°C (the other conditions being optimum for growth). ρ(T), γ(pH), and ρ(aw) characterize the specific effects of the temperature, pH, and water activity, respectively. For these effects, the equations proposed by Mejlhom and Dalgaard (5) were used:
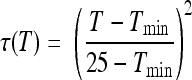 |
(12) |
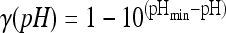 |
(13) |
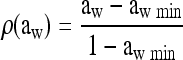 |
(14) |
The interaction term ξ was also calculated as proposed by Mejlhom and Dalgaard (5). For Tmin, we used a value of −2.7°C (1). aw- and pH-related parameters (i.e., theoretical minimum aw and pH for growth) were estimated using the experimental growth curves submitted for publication by Belessi et al. μmax ref was estimated from a reference growth curve of L. monocytogenes in milk.
Fitting procedures. (i) Shifts within the growth range.
The growth curves obtained after the applied shifts were fitted with the model of Baranyi and Roberts (2) using DMFit 2.0 (IFR, Norwich, United Kingdom [http://www.ifr.bbsrc.ac.uk/Safety/DMFit/]). For each experimental condition, hs was calculated by multiplying the estimated specific growth rate and the lag time. Equations 5 and 6 relating hs to the magnitude of the shifts were fitted with the Essential Regression and Experimental Design, version 2.2, a Microsoft Excel add-in available at http://www.geocities.com/SiliconValley/Network/1032/. A backward-elimination procedure was adopted to eliminate the insignificant terms.
(ii) aw shifts across the growth boundaries.
The procedure we used to identify the parameters related to the aw shifts across the growth boundary is based on the following observations and assumptions. (i) For all experimental conditions, the cells are first grown under the same experimental conditions (pH 7.0 and 0.5% NaCl) and then submitted to the same shift (to 12.5% NaCl). Therefore, it is expected that the bacterial population has the same physiological state, Q1, after the shift from 0.5% NaCl to 12.5% NaCl for all experiments. (ii) The rate of adaptation/injury, ν2, is assumed to depend only on the environmental conditions (temperature, pH, and aw). Consequently, as the cells are initially exposed to the same stressing conditions (12.5% NaCl), ν2 is assumed to have the same value for all experiments.
(iii) Model fitting.
The fitting was carried out in two stages. First, the 12 growth curves obtained after the shifts from no-growth to growth conditions (see Table 5) were fitted individually in order to estimate for each curve the specific growth rate μmax(i) and the maximum bacterial concentration ymax(i), then all of the curves were refitted by the model defined by equations 1, 7, and 8. In this second fit, μmax(i) and ymax(i) were fixed to the values obtained in the individual fits. The logarithm of the initial concentration, y0(i), was kept constant, equal to the measured values at the end of the stress (12.5% NaCl). aw minwas fixed to the value estimated for the growth model. In this fit, only Q1 (physiological state after the first shift to 12.5% NaCl), νs (rate of adaptation/injury during stress), and 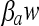 from equation 8 were estimated. During the fitting procedure, all of these parameters were constrained to take the same value (common to all 12 experimental conditions).
from equation 8 were estimated. During the fitting procedure, all of these parameters were constrained to take the same value (common to all 12 experimental conditions).
(iv) pH shifts across the growth boundaries.
A similar procedure was employed to estimate Q1, νa, and βpH in the case of pH shifts across the growth boundary using 18 curves obtained after shifts (see Table 6). pHmin was constrained to the value estimated in the growth rate model. In both cases (osmotic and acid shifts), DMFit was used for the individual fits, whereas the second fit was performed by using a nonlinear regression module (NLINFIT, Matlab 2007a; The MathWorks).
(v) Comparison of observations and global fit results.
The values of hs (“work to be done” just after shift) and the corresponding lag time (hs/μmax) obtained from the global fit were compared with the observed “work to be done” and lag times. The mean absolute difference (MAD) between observations and results derived from the global fits and the root mean square error (RMSE) were used to assess the goodness of fit.
Prediction of the growth of L. monocytogenes under dynamic conditions of pH and water activity in milk.
The system consisting of equations 1 and 7 to 11 was solved numerically by a Runge-Kutta method (ODE23, Matlab 2007b; The MathWorks). The NaCl concentration (% [wt/vol]) was converted to aw using the following equation of Resnik and Chirife (12):
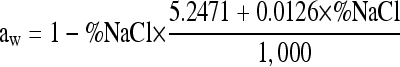 |
(15) |
Shifts within the growth range.
Shifts above the growth boundaries were applied for profiles 1, 2, and 5. When a shift occurred, Q was reset according to equations 4, 5, and 6.
Shifts across the growth boundaries.
Shifts across the growth/no-growth boundary were applied during profiles 3 to 5.
Profile 3: osmotic shifts.
When the NaCl concentration was shifted to 12.5%, Q was reset to the value estimated for Q1 in the “global” fit. For the adaptation rate during a period at 12.5% NaCl and the work to be done, h2, after the shift to growth conditions, we used values estimated for equations 7 and 8.
Profile 4: pH shifts.
The values used for Q1, νs, and h2 were those estimated in the global fit for pH shifts (equations 7 and 9).
Profile 5: combined aw and pH shifts.
After the combined pH and aw shift, Q1 was calculated according to the sum of an additional “work to be done” for a pH and for a water activity shift. Based on a higher contribution of aw to the interaction term ξ, we used the adaptation rate νa estimated for osmotic shifts. For the simultaneous acid and osmotic shift from no-growth conditions to growth conditions, we assumed that the total amount of “work to be done” induced by the shifts to be the sum of the two values of h2 related to the aw and pH shifts (equations 7 and 8, respectively). Small variations in pH (|ΔpH| ≤ 0.2) which were observed throughout the experiments were assumed not to induce any intermediate lag time. The effects of those variations on the bacterial growth rate were, however, taken into account through equation 11. The mean absolute difference between the observed and predicted bacterial concentrations was used as a criterion to compare predictions performed with and without taking into account the effects of shifts.
RESULTS
Growth rate model.
Parameter estimates of fitted equations 13 and 14 and their standard errors are shown in Table 1. Values of 4.96 and 0.915 were estimated for pHmin (pH controlled by addition of lactic acid) and aw min, respectively. The good fit of the model is evidenced by the high correlation coefficient (R2adj = 0.95), the low standard errors of the estimates, and the low standard error of the fit (Table 1).
TABLE 1.
Estimated values of the parameters of the growth rate model
| Parameter | Estimate | SE |
|---|---|---|
| pHmin | 4.96 | 0.01 |
| aw min | 0.915 | 0.002 |
| SE of fit | 0.015 |
Shifts within the growth range.
Tables 2 and 3 show the observed and fitted values for the “work to be done,” hs, consecutive to the tested downshifts. After eliminating the insignificant terms, the fitted equations 5 and 6 for osmotic and pH downshifts, respectively, are
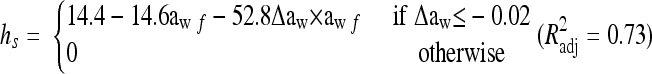 |
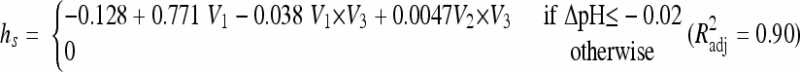 |
For both aw and pH downshifts, there is a good agreement between the observed and predicted values of lag times and hs. For osmotic shifts, the MAD between the observed intermediate lag time and values derived from fitted equation 5 is 8 h and the RMSE is 9.6 h. For pH shifts, values of 12.2 and 24.7 were obtained for MAD and RMSE, respectively.
TABLE 2.
Comparison between the observed values for hs and lag induced by osmotic downshifts and the values derived from the fit of equation 5
| Initial % of NaCl | Final % of NaCl | μmax (h−1) | Observed value |
Value derived from fitted equation 5 |
||
|---|---|---|---|---|---|---|
| hs | Lag (h) | hs | Lag (h) | |||
| 2 | 5 | 0.094 | 0.9 | 9.9 | 1.3 | 13.9 |
| 8 | 0.057 | 2.7 | 47.3 | 2.6 | 45.3 | |
| 10.5 | 0.030 | 3.6 | 122.7 | 3.8 | 128.9 | |
| 5 | 8 | 0.053 | 2.2 | 42.1 | 1.6 | 30.0 |
| 10.5 | 0.034 | 3.1 | 91.7 | 2.8 | 84.3 | |
| 8 | 10.5 | 0.025 | 1.4 | 55.2 | 1.8 | 72.5 |
TABLE 3.
Comparison between the observed values for hs and lag induced by pH downshifts and the values derived from the fit of equation 6
| Initial pH | Final pH | μmax (h−1) | Observed value |
Value derived from fitted equation 6 |
||
|---|---|---|---|---|---|---|
| hs | Lag (h) | hs | Lag (h) | |||
| 7 | 6 | 0.117 | 0 | 0 | 0 | 0 |
| 5.8 | 0.111 | 0.5 | 4.3 | 0.8 | 7.1 | |
| 5.5 | 0.010 | 4.3 | 43.4 | 4.4 | 44 | |
| 5.1 | 0.034 | 4.0 | 115.9 | 3.9 | 114.9 | |
| 6 | 5.8 | 0.101 | 0 | 0 | 0.1 | 0.9 |
| 5.5 | 0.097 | 0.7 | 7.1 | 0 | 0 | |
| 5.3 | 0.073 | 0 | 0 | 0 | 0 | |
| 5.1 | 0.027 | 0 | 0 | 0 | 0 | |
| 5.8 | 5.5 | 0.083 | 0 | 0 | 0.1 | 1.3 |
| 5.3 | 0.070 | 0 | 0 | 0.05 | 0.7 | |
| 5.1 | 0.031 | 0 | 0 | 0.7 | 23.9 | |
| 5.3 | 0.065 | 1.25 | 19.2 | 0.6 | 8.8 | |
| 5.5 | 5.1 | 0.036 | 0 | 0 | 2.6 | 72.5 |
| 5.3 | 0.052 | 6 | 116.0 | 3.4 | 65.8 | |
| 6 | 0.117 | 0 | 0 | 0 | 0 | |
| 5.3 | 5.8 | 0.111 | 0.5 | 4.3 | 0.8 | 7.1 |
Shifts across the growth boundaries.
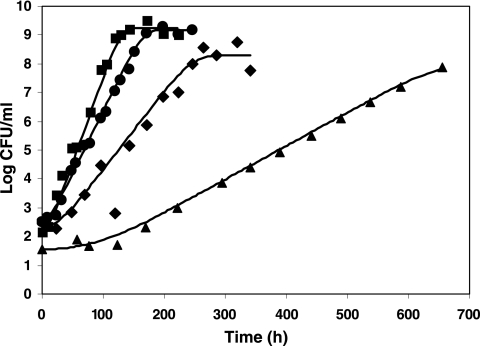
Figures 2 and 3 show examples of comparison between the fitted curves and the observed log counts after osmotic and pH shifts back and forth across the growth boundary. Estimated parameters for Q1, νa, 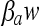 and βpH are shown in Table 4. Values of Q1 of −1.15 and 1.04 were estimated for the osmotic and pH shocks, respectively (corresponding to h1 = 1.43 and h1 = 0.25 “work to be done” values, respectively). As expected, the estimates of νa are positive for the osmotic shifts (νa = 0.016) and negative for the pH shifts (νa = −0.017). Table 5 presents the comparison between the lag times observed after the osmotic shifts (from no-growth to growth conditions) and the values deduced from the fitted model (equations 7, 8, 10, and 11). Table 5 exhibits two general patterns: an increase of the “work to be done” and lag time with decreasing water activity and a decrease of the lag as a function of the time of habituation. For example, for shifts from 12.5% to 10.5% NaCl, the lag time decreases from 93.7 h (1 day of habituation at 12.5% NaCl) to 51.7 h (5 days at 12.5% NaCl) and 43.8 h (10 days at 12.5% NaCl). This trend is correctly described by the model, the lag times resulting from the “global fit” values being 91.1, 58.0, and 47.4 h, respectively. The good accordance between the observed and fitted values is demonstrated by the MAD (7.8 h) and the RMSE (3.7 h) values. However, for one experimental condition (5 days of habituation at 12.5% NaCl), the observed lag time is 54.7 h, whereas the “fitted” value is 14 h. It should be noted that this observed value does not follow the general patterns observed in Table 5, as the “work to be done” observed for this condition at 8% NaCl was higher than the workload at 10.5% NaCl.
and βpH are shown in Table 4. Values of Q1 of −1.15 and 1.04 were estimated for the osmotic and pH shocks, respectively (corresponding to h1 = 1.43 and h1 = 0.25 “work to be done” values, respectively). As expected, the estimates of νa are positive for the osmotic shifts (νa = 0.016) and negative for the pH shifts (νa = −0.017). Table 5 presents the comparison between the lag times observed after the osmotic shifts (from no-growth to growth conditions) and the values deduced from the fitted model (equations 7, 8, 10, and 11). Table 5 exhibits two general patterns: an increase of the “work to be done” and lag time with decreasing water activity and a decrease of the lag as a function of the time of habituation. For example, for shifts from 12.5% to 10.5% NaCl, the lag time decreases from 93.7 h (1 day of habituation at 12.5% NaCl) to 51.7 h (5 days at 12.5% NaCl) and 43.8 h (10 days at 12.5% NaCl). This trend is correctly described by the model, the lag times resulting from the “global fit” values being 91.1, 58.0, and 47.4 h, respectively. The good accordance between the observed and fitted values is demonstrated by the MAD (7.8 h) and the RMSE (3.7 h) values. However, for one experimental condition (5 days of habituation at 12.5% NaCl), the observed lag time is 54.7 h, whereas the “fitted” value is 14 h. It should be noted that this observed value does not follow the general patterns observed in Table 5, as the “work to be done” observed for this condition at 8% NaCl was higher than the workload at 10.5% NaCl.
FIG. 2.
Growth of L. monocytogenes after 1 day of habituation at 12.5% NaCl at 10°C, with water activity levels of 2 (▪), 5 (•), and 8 (⧫) and 10.5% NaCl (▴). Shown is the comparison between experimental values and results from the global fit (continuous lines).
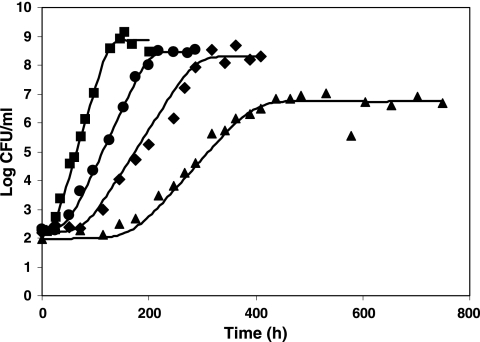
FIG. 3.
Growth of L. monocytogenes at 10°C after 10 days of habituation at pH 4.9 at pH values of 7 (▪), 5.5 (•), 5.3 (⧫), and 5.1 (▴). Shown is a comparison between experimental values and results from the global fit (continuous lines).
TABLE 4.
Estimated values and 95% confidence intervals of Q1, νs, and β for the osmotic and pH shifts across the growth/no-growth interface
| Parameter | Estimated value (95% confidence interval) |
|
|---|---|---|
| Osmotic shift | pH shift | |
| Q1 | −1.15 (−1.46 to −0.86) | 1.04 (0.47 to 1.59) |
| νa | 0.016 (0.015 to 0.017) | −0.017 (−0.019 to −0.014) |
| β | 0.0012 (0.0009 to 0.0014) | 0.80 (0.73 to 0.88) |
TABLE 5.
Comparison between the observed values for hs and lag and the values derived from the global fita
| No. of days at 12.5% NaCl | NaCl (%) | μmax (h−1) | Observed value |
Value derived from global fit |
||
|---|---|---|---|---|---|---|
| hs | Lag (h) | hs | Lag (h) | |||
| 1 | 2 | 0.137 | 0 | 0 | 1.3 | 9.5 |
| 5 | 0.100 | 0.97 | 9.7 | 1.39 | 13.9 | |
| 8 | 0.063 | 2.11 | 33.4 | 1.62 | 25.7 | |
| 10.5 | 0.027 | 2.52 | 93.7 | 2.45 | 91.1 | |
| 5 | 2 | 0.135 | 0 | 0 | 0.52 | 3.9 |
| 5 | 0.103 | 0.54 | 5.2 | 0.62 | 6 | |
| 8 | 0.060 | 3.28 | 54.7 | 0.84 | 14 | |
| 10.5 | 0.029 | 1.49 | 51.7 | 1.67 | 58 | |
| 10 | 2 | 0.143 | 0 | 0 | 0.21 | 1.5 |
| 5 | 0.104 | 0 | 0 | 0.3 | 2.9 | |
| 8 | 0.055 | 0 | 0 | 0.53 | 9.6 | |
| 10.5 | 0.029 | 1.26 | 43.8 | 1.36 | 47.4 | |
Osmotic shifts across the growth boundary.
For pH shifts, the comparison between observed lag times and model results is presented in Table 6. A good correspondence between observations and the model fit (equations 7, 9, 10, and 11) can be observed: the absolute mean difference between observed and lag times resulting from the “global fit” is 9.8 h, and the RMSE is 12.9 h. Notably, the model was able to describe the observed trends: a decrease of the “work to be done” as the amplitude of the shift increases and an increase of this work with the duration of the period at pH 4.9. As the bacterial growth rate shows great variability near the growth limits, an increasing “work to be done” hs (lag·μmax) does not always imply an increase of the lag time. For example, after 5 days at pH 4.9, the specific rate at pH 5.1 is 0.023 h−1, whereas after 10 days of habituation, the corresponding rate at the same final pH is 0.048 h−1. Even if the observed lag is shorter after 10 days than after 5 days of habituation at pH 4.9, the “work to be done” is higher after 10 days. A good performance of the fitted model was also observed for the extremely long lag phases occurring at pH 5.1 (fitted values of 244 and 164 h against observed values of 271 and 167 h, respectively).
TABLE 6.
Comparison between the observed values for hs and lag and the values derived from the global fita
| Time at pH 4.9 (days) | pH | μmax (h−1) | Observed value |
Value derived from global fit |
||
|---|---|---|---|---|---|---|
| hs | Lag (h) | hs | Lag (h) | |||
| 1 | 7 | 0.129 | 0 | 0 | 0.58 | 4.5 |
| 6.2 | 0.115 | 0 | 0 | 0.78 | 6.8 | |
| 6 | 0.127 | 1.62 | 12.7 | 0.9 | 7.1 | |
| 5.8 | 0.117 | 1.64 | 14.1 | 1.09 | 9.3 | |
| 5.6 | 0.067 | 0 | 0 | 1.42 | 21.1 | |
| 5.4 | 0.082 | 1.65 | 20.18 | 2.07 | 25.2 | |
| 5 | 7 | 0.1342 | 0 | 0 | 1.45 | 10.8 |
| 6 | 0.1141 | 0 | 0 | 1.77 | 15.5 | |
| 5.8 | 0.1197 | 2.52 | 21.03 | 1.96 | 16.4 | |
| 5.5 | 0.088 | 2.45 | 27.8 | 2.55 | 29.0 | |
| 5.3 | 0.065 | 5.35 | 82.3 | 3.53 | 54.3 | |
| 5.1 | 0.0234 | 5.72 | 244.5 | 6.33 | 270.5 | |
| 10 | 7 | 0.1456 | 2.45 | 16.8 | 3.20 | 22.0 |
| 6 | 0.1379 | 3.53 | 25.6 | 3.52 | 25.5 | |
| 5.8 | 0.1102 | 2.16 | 19.6 | 3.71 | 33.7 | |
| 5.5 | 0.0928 | 4.02 | 43.3 | 4.30 | 46.3 | |
| 5.3 | 0.0676 | 6.50 | 96.2 | 5.28 | 78.1 | |
| 5.1 | 0.0483 | 7.95 | 164.5 | 8.07 | 167.1 | |
pH shifts across the growth boundary.
Validation experiments in milk.
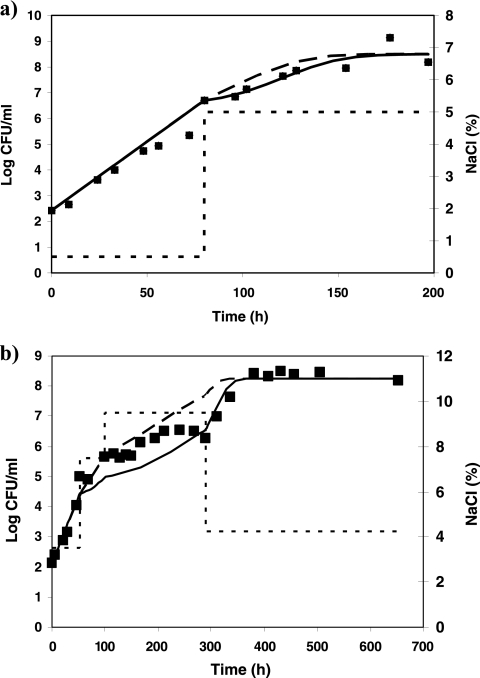
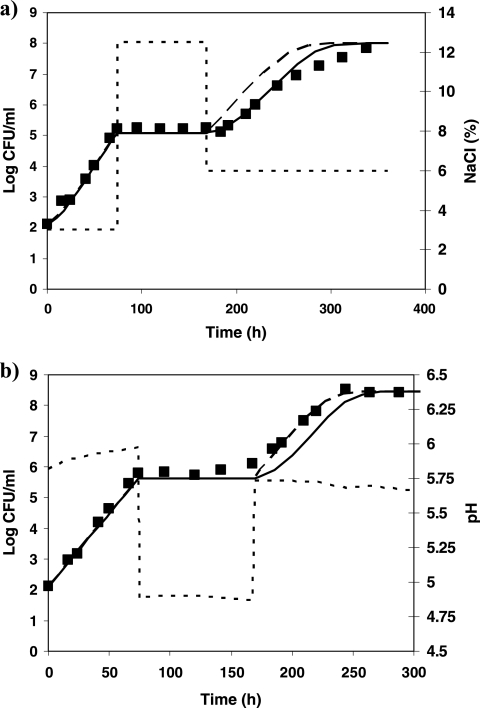
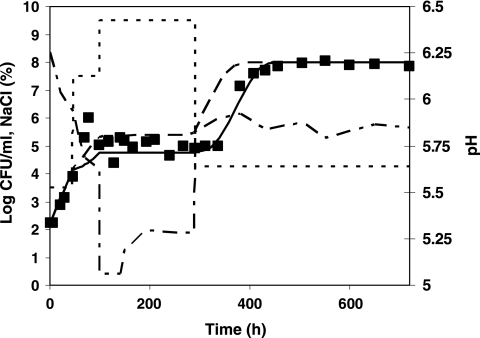
A value of 0.84 h−1 was estimated for μmax ref (value derived from a growth curve of L. monocytogenes in milk). Comparisons between observed and predicted growth kinetics of L. monocytogenes for profiles 1 to 5 are shown in Fig. 4 to 6. The overall results show a good agreement of the model predictions: MAD values range from 0.1 (profile 5) to 0.35 log10 CFU/ml (profile 2). For profiles 1 and 2, the predictions correlate well with the observed log counts (Fig. 4). Taking into account the effects of shifts in the predictions provides better predictions in both cases: for profile 1, MAD is 0.2 log10 CFU/ml when effects of shifts are considered against 0.4 log10 CFU/ml when they are not. For profile 2, MAD values of 0.35 and 0.4 log10 CFU/ml are obtained for predictions with and without considering the shifts, respectively. For profile 3, the osmotic shifts across the growth/no-growth interface clearly result in a lag phase prior to regrowth, which is described accurately by the model (Fig. 5a). In profile 4, after 3 days of growth at pH 5.8, the pH was shifted down to 4.9, and after 4 days, it was shifted up to 5.8. In this case, the shifts did not induce a lag phase, the population of L. monocytogenes starting to grow as soon as the pH was shifted up to growth conditions. This observation in milk is not consistent with observations made in broth for similar conditions. In TSBYE, 5 days of habituation at 4.9 followed by a shift to pH 5.8 induced a lag time of 21 h (Table 6). As the model was developed based on experiments in TSBYE, an intermediate lag time is predicted for those conditions and consequently, the bacterial concentration at the end of the experiment is underestimated (Fig. 5b). In profile 5, aw and pH shifts were applied simultaneously. This situation is an extrapolation of the model as the water activity and pH have been studied independently in the model fitting. The combination of the pH and water activity levels occurring after the simultaneous shifts prevented growth of L. monocytogenes, although when examined individually, such levels are growth supporting. The inclusion of the interaction term ξ allows the accurate prediction of no growth at those conditions. The lag time observed after the shift to growth conditions was also correctly predicted by the model (Fig. 6). When the effects of this shift are not taken into account, the predicted bacterial concentrations are overestimated by values up to 2.3 log10 CFU/ml.
FIG. 4.
Growth of L. monocytogenes (▪) at 10°C in milk under various conditions of water activity (dotted line), including shifts within the growth range. Shown is a comparison between the model predictions with (continuous line) and without (dashed line) taking into account the effects of sudden shifts. (a) Profile 1; (b) profile 2.
FIG. 5.
Growth of L. monocytogenes (▪) at 10°C in milk under various conditions of water activity or pH (dotted line), including shifts across the growth/no-growth boundary. Shown is a comparison between the model predictions with (continuous lines) and without (dashed lines) taking into account the effects of sudden shifts. (a) Profile 3; (b) profile 4.
FIG. 6.
Growth of L. monocytogenes (▪) at 10°C in milk under simultaneously varying conditions of pH (dotted and dashed line) and water activity (dotted line), including shifts across the growth boundary (profile 5). Shown is a comparison between the model predictions with (continuous line) and without (dashed line) taking into account the effects of sudden shifts.
DISCUSSION
This study supports the findings of Muñoz-Cuevas et al. (9) according to which, the workload induced by shifts within the growth range does not only depend on the magnitude of shift but also on the environmental conditions after the shifts. For our data, the nonlinear model proposed by these authors for the osmotic downshifts underestimated the work to be done induced by the shifts. This can be due to either the difference between the strains and growth media used in the two studies or to the difference between the physiological states of the cells at the time of shifts (shifts applied on an exponentially growing population, whereas, for our study, the shifts were applied when L. monocytogenes reached the late exponential-early stationary phase).
The mechanisms involved in osmotic and acid shifts appear to be different. Osmotic shifts within the growth range usually induce a greater amount of “work” prior to regrowth than pH shifts. Acidic shifts within the growth domain do not usually induce long intermediate lag times unless the previous and current conditions are near the growth limits. Differences appear between the pH and osmotic shifts back and forth across the growth boundary, with the adaptation/injury being strictly positive for aw but negative for pH shifts. The effects of shifts back and forth across the growth boundary could be modeled using only three parameters: Q1, νs, and β. To our best knowledge, this is the first attempt to model the intermediate lag times induced by osmotic or pH shifts back and forth across the growth/no-growth interface.
In the usual form of the model of Baranyi and Roberts (2), the cells are assumed to carry out the work to be done at a rate ν = μmax. Under no-growth conditions, μmax is zero, which implies that the physiological state of the cells remains constant. However, our results suggest that if it is still hypothesized that ν = μmax in the growth region, then the physiological state of the cells is affected by the environmental conditions in the no-growth region. In this study, we suggested the use of different values for ν, according to the level of the environmental factors (growth or no-growth area).
As we cannot directly measure Q (which quantifies the physiological state of the cells), it is not straightforward to estimate the parameters for the effects of the downshift across the growth boundary, the habituation during time at pH 4.9 or 12.5% NaCl, and the conditions after the shift back to the growth region. To identify these parameters, we used the fact that some of them are common to all or at least some experiments and fit the growth curves altogether. To obtain a convergence to an optimum solution, the growth rate of each curve had to be fixed to the value indentified by the fit of individual growth curves. This was due to the fact that the parameter μmax not only describes the specific growth rate but also describes the rate of adaptation of the cells during the lag phase after the shift back to growth conditions.
This work supports the idea of integrating the “intermediate lag time” in predictive modeling approaches. Intermediate lag times observed after shifts within the growth range or back and forth across the growth/no-growth interface can reach values up to 116 h (Table 3). To our knowledge, this intermediate lag time is not taken into account by any of the commonly used predictive software packages. Validation results in milk showed that the approach developed could be successfully integrated into previously existing growth models. In all but one case (profile 4), the inclusion of the intermediate-lag-time model improves the quality of predictions. Predictions with and without inclusion of the interaction term ξ are identical for profiles 1 and 3. For profile 2, the inclusion of the term ξ slightly improved the quality of the prediction when the environmental conditions were close to the growth boundary (9.5% NaCl). For profile 5, the term ξ allows the description of the absence of growth at 10°C, pH ∼5 to 5.2 (with lactic acid used as an acidulant), with 9.5% NaCl, although the conditions of pH and water activity taken individually would allow the growth of L. monocytogenes (pH > pHmin and aw > aw min).
For profile 4, after the second osmotic shift at t = 100 h, experimental conditions become close to the growth boundaries and the growth of L. monocytogenes becomes more erratic (Fig. 4b). The observed cessation of growth might be due to the stringent conditions and the high bacterial concentrations and the inclusion of an equation for the effects of pH and aw on ymax might improve model predictions for this experiment.
Further investigation is required to assess both the applicability of the model and some of the assumptions made for the predictions. For example, the effects of aw shifts within the growth range have been studied at optimal pH and the effects of pH shifts at optimal water activity. More experimental data would be desirable on the effects of osmotic and acidic shifts at more stringent pH and aw conditions too. Besides, in the validation experiments, shifts were applied only once L. monocytogenes had started to grow and not during the initial lag phase. Additional work would be needed to investigate the ability of the model to predict the extra “work to be done” when shifts are applied during the initial lag phase. We also assumed that the effects of simultaneous or sequential water activity and pH shifts on the amount of “work to be done” were additive. This assumption was successfully applied to describe the behavior of L. monocytogenes under shifts across the growth boundaries (profile 5; Fig. 6). However, it has been noticed that in some cases, when the simultaneous shifts of two factors affected a population already in lag phase, the amount of work was greater than expected from the addition of the individual effects (9). Further work is needed to assess the effects of simultaneous shifts when applied within the growth boundaries. To describe the adaptation/injury during the habituation under no-growth conditions, we used two values of νa: one positive for osmotic stress and the other one negative for pH stress. Combined pH and osmotic stresses were not tested in the model development. For profile 5, when simultaneous pH and aw downshifts to no-growth conditions are applied in milk, we assumed that the appropriate value of νa could be selected according to the contribution of pH and aw to the interaction term ξ. The validity of this assumption needs additional investigation.
It was shown by several studies (e.g., Xantiakos et al. [16]) that L. monocytogenes grows faster in broth than in milk. This is taken into account in the growth rate model through the parameter μmax ref which was estimated from a growth curve of L. monocytogenes in milk. However, the intermediate-lag-time parameters used for prediction purposes in milk were all estimated from broth experiments. From the available experimental data, we could not establish the conditions under which intermediate lag time estimated in broth can be extrapolated to food experiments.
Under some specific conditions, food contents may provide the bacterial cells some protection against pH or osmotic shifts, which could lead to an underestimation of the growth of the pathogen. This could explain the discrepancy between observations and predictions for profile 4 (Fig. 5b). Besides, our experiments were performed at 10°C so the effect of temperature on the above model can be the subject of further studies.
Conclusion.
A model was proposed for the effects of shifts both within the growth region and across the growth boundaries of L. monocytogenes on the intermediate lag time of L. monocytogenes at 10°C. The first validation experiments in milk showed that it is possible to incorporate the developed approach in existing growth models. Some additional work would be desirable to verify some of the assumptions made and to test whether the model can be extrapolated to other temperatures.
Footnotes
Published ahead of print on 30 July 2010.
REFERENCES
- 1.Augustin, J. C., and V. Carlier. 2000. Mathematical modelling of the growth rate and lag time for Listeria monocytogenes. Int. J. Food Microbiol. 56:29-51. [DOI] [PubMed] [Google Scholar]
- 2.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 3.Delignette-Muller, M. L., F. Baty, M. Cornu, and H. Bergis. 2005. Modelling the effect of a temperature shift on the lag phase duration of Listeria monocytogenes. Int. J. Food Microbiol. 100:77-84. [DOI] [PubMed] [Google Scholar]
- 4.Le Marc, Y., V. Huchet, C. M. Bourgeois, J. P. Guyonnet, P. Mafart, and D. Thuault. 2002. Modelling the growth kinetics of Listeria as a function of temperature, pH and organic acid concentration. Int. J. Food Microbiol. 73:219-237. [DOI] [PubMed] [Google Scholar]
- 5.Mejlholm, O., and P. Dalgaard. 2007. Modeling and predicting the growth boundary of Listeria monocytogenes in lightly preserved seafood. J. Food Prot. 70:70-74. [DOI] [PubMed] [Google Scholar]
- 6.Mellefont, L. A., T. A. McMeekin, and T. Ross. 2003. The effect of abrupt osmotic shifts on the lag phase duration of foodborne bacteria. Int. J. Food Microbiol. 83:281-293. [DOI] [PubMed] [Google Scholar]
- 7.Mellefont, L. A., T. A. McMeekin, and T. Ross. 2004. The effect of abrupt osmotic shifts on the lag phase duration of physiologically distinct populations of Salmonella typhimurium. Int. J. Food Microbiol. 99:111-120. [DOI] [PubMed] [Google Scholar]
- 8.Mellefont, L. A., T. A. McMeekin, and T. Ross. 2005. Viable count estimates of lag time responses for Salmonella typhimurium M48 subjected to abrupt osmotic shifts. Int. J. Food Microbiol. 105:399-410. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz-Cuevas, M., P. S. Fernández, S. George, and C. Pin. 2010. Modeling the lag period and exponential growth of Listeria monocytogenes under conditions of fluctuating temperature and water activity values. Appl. Environ. Microbiol. 76:2908-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papageorgiou, D. K., and E. H. Marth. 1989. Behavior of Listeria monocytogenes at 4 and 22°C in whey and skim milk containing 6 or 12% sodium chloride. J. Food Prot. 52:625-630. [DOI] [PubMed] [Google Scholar]
- 11.Ramsaran, H., J. Chen, B. Brunke, A. Hill, and M. W. Griffiths. 1998. Survival of bioluminescent Listeria monocytogenes and Escherichia coli O157:H7 in soft cheeses. J. Dairy Sci. 81:1810-1817. [DOI] [PubMed] [Google Scholar]
- 12.Resnik, S. L., and J. Chirife. 1988. Proposed theoretical aw values at various temperatures for selected solutions to be used as reference sources in the range of microbial growth. J. Food Prot. 51:419-423. [DOI] [PubMed] [Google Scholar]
- 13.Robinson, T. P., M. J. Ocio, A. Kaloti, and B. M. Mackey. 1998. The effect of the growth environment on the lag phase of Listeria monocytogenes. Int. J. Food Microbiol. 44:83-92. [DOI] [PubMed] [Google Scholar]
- 14.Swinnen, I. A. M., K. Bernaerts, K. Gysemans, and J. F. Van Impe. 2005. Quantifying microbial lag phenomena due to a sudden rise in temperature: a systematic macroscopic study. Int. J. Food Microbiol. 100:85-96. [DOI] [PubMed] [Google Scholar]
- 15.Whiting, R. C., and L. K. Bagi. 2002. Modelling the lag phase of Listeria monocytogenes. Int. J. Food Microbiol. 73:291-295. [DOI] [PubMed] [Google Scholar]
- 16.Xanthiakos, K., D. Simos, A. S. Angelidis, G. J. E. Nychas, and K. Koutsoumanis. 2006. Dynamic modeling of Listeria monocytogenes growth in pasteurized milk. J. Appl. Microbiol. 100:1289-1298. [DOI] [PubMed] [Google Scholar]
- 17.Zwietering, M. H., J. C. de Wit, H. G. A. M. Cuppers, and K. Van't Riet. 1994. Modeling of bacterial growth with shifts in temperature. Appl. Environ. Microbiol. 60:204-213. [DOI] [PMC free article] [PubMed] [Google Scholar]