Abstract
Analysis of a complete set of glutamate decarboxylase (gad) mutants of Listeria monocytogenes strain LO28 (ΔgadD1, ΔgadDT1, ΔgadD2, ΔgadT2, and ΔgadD3 mutants) revealed that the ΔgadD1 mutant is impaired in its ability to tolerate exposure to both sublethal and lethal levels of the lantibiotic nisin. gadD1 is strain variable and is found only in approximately 50% of L. monocytogenes strains. Growth and survival experiments revealed that possession of gadD1 correlates with a higher degree of tolerance to nisin. Significantly, a similar finding using a gadB mutant of L. lactis IL1403 implies that this may be a general phenomenon in Gram-positive bacteria. Our findings thus suggest that the specific inhibition of GAD activity or a reduction in the levels of free glutamate may prevent the growth of otherwise resistant GAD+ bacteria in foods where low pH and/or nisin is used as a preservative.
Listeria monocytogenes is a food-borne pathogen that is the causative agent of listeriosis, an opportunistic infection associated with high rates of morbidity and mortality (18). The microorganism has also been the cause of significant commercial losses, being responsible for 71% of all recalls of food products due to bacterial contamination in the United States between 1993 and 1998 (25). The ubiquitous nature of L. monocytogenes, together with its ability to tolerate a variety of environmental extremes, including high salt concentrations and low pH, and the ability to grow at refrigeration temperatures, makes control of the bacterium in foods difficult (10). Hence it is not altogether surprising that the food industry invests considerable effort into developing strategies to prevent the survival and growth of this pathogen. One such strategy involves the utilization of bacteriocins. Bacteriocins are antimicrobial peptides produced by one bacterium that inhibit the growth of other bacteria and have been used as “natural” preservatives to control undesirable microbiota in food (5). The most extensively studied bacteriocin is nisin A (here referred to as nisin), a 34-amino-acid class I bacteriocin (lantibiotic) produced by Lactococcus lactis strains that is currently approved for use in foods in over 50 countries. Nisin functions by binding lipid II, an essential precursor of cell wall peptidoglycan biosynthesis. Binding to lipid II also facilitates the formation of pores within the cytoplasmic membrane leading to the release of ATP and other small cytoplasmic contents, resulting in depolarization of the membrane potential and ultimately cell death (13).
The molecular mechanisms employed by L. monocytogenes to cope with nisin are poorly understood. To date, loci that have been implicated in nisin tolerance include the alternative stress sigma factor SigB, the class three stress gene regulator CtsR, the two-component systems LisRK and HK1027, and a penicillin binding protein, Pbp (2, 6, 11, 15, 21). In addition, several studies have uncovered a link between the acid stress response of L. monocytogenes and nisin resistance (3, 17, 24). Several systems are employed by L. monocytogenes to withstand low pH stress, but the glutamate decarboxylase (GAD) system is probably the most important (an overview of the GAD system is in Fig. 1). Mutation of specific gad genes renders cells exquisitely sensitive to ex vivo porcine and synthetic human gastric fluid and significantly impairs growth and survival in low-pH foods (4, 7, 8). Given the link between acid and nisin resistance phenotypes, the present study was initiated in order to investigate the contribution, if any, of gad genes to the nisin tolerance of L. monocytogenes.
FIG. 1.
An overview of the L. monocytogenes glutamate decarboxylase (GAD) system. L. monocytogenes possesses five gad genes. gadD1, gadD2, and gadD3 encode decarboxylases which catalyze the conversion of glutamate to γ-amino butyrate (GABA) and carbon dioxide (CO2). gadT1 and gadT2 encode glutamate-GABA antiporters. Nisin functions by binding to lipid II, an essential precursor of cell wall peptidoglycan synthesis. Binding to lipid II facilitates the formation of pores within the cytoplasmic membrane leading to the release of ATP and may ultimately result in cell death. We suggest that under certain conditions gadD1 may contribute to intracellular ATP pools and hence tolerance of nisin.
MATERIALS AND METHODS
Media, chemicals, and growth conditions.
All L. monocytogenes strains used in this study are listed and described in Table 1. L. monocytogenes strains were grown in brain heart infusion (BHI) broth statically at 37°C. Lactococcus lactis strains were obtained from the University College Cork culture collection. L. lactis strains were grown in GM17 (M17 supplemented with 0.5% glucose) broth statically at 30°C. All media were purchased from Oxoid. Nisin powder (Sigma N5764) was resuspended in sterile high-performance liquid chromatography (HPLC) water.
TABLE 1.
L. monocytogenes strains used in this study
| L. monocytogenesstrain | Relevant characteristics | Reference |
|---|---|---|
| LO28 | wt,a serotype 1/2c | |
| ΔgadD1 mutant | LO28 wt with a deletion in gadD1 | 4 |
| ΔgadD2 mutant | LO28 wt with a deletion in gadD2 | 4 |
| ΔgadD3 mutant | LO28 wt with a deletion in gadD3 | This study |
| ΔgadT1 mutant | LO28 wt with a deletion in gadT1 | 4 |
| ΔgadT2 mutant | LO28 wt with a deletion in gadT2 | 4 |
| 748b | gadD1T1+, serotype non-4, isolated from ground beef | |
| 1191b | gadD1T1+, serotype non-4, isolated from ground turkey | |
| 1066b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1520b | gadD1T1+, serotype non-4, isolated from ground turkey | |
| 1032b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1742b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1223b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1205b | gadD1T1+, serotype non-4, isolated from ground turkey | |
| 1061b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1038b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1028b | gadD1T1+, serotype non-4, isolated from pork sausage | |
| 1121b | gadD1T1+, serotype non-4, isolated from ground beef | |
| 878b | gadD1T1 negative, serotype 4, clinical isolate | |
| 1078b | gadD1T1 negative, serotype 1/2, isolated from chicken | |
| 246b | gadD1T1 negative, serotype 4, isolated from silage | |
| 1059b | gadD1T1 negative, serotype 1/2, isolated from pork sausage | |
| 169b | gadD1T1 negative, serotype 1/2, Mercier clinical strain | |
| 2088b | gadD1T1 negative, serotype 1/2, Mercier clinical strain | |
| 241b | gadD1T1 negative, serotype 3, isolated from silage | |
| 104b | gadD1T1 negative, serotype 4, isolated from milk | |
| 83b | gadD1T1 negative, serotype 4, isolated from silage | |
| 147b | gadD1T1 negative, serotype 4, isolated from dairy enrichment | |
| 168b | gadD1T1 negative, serotype 1/2, isolated from aborted calf fetus | |
| 243b | gadD1T1 negative, serotype 1/2, isolated from silage |
Genetic manipulations.
Restriction enzymes, T4 DNA ligase, PCR reagents, and nucleic acid purification kits were purchased from Roche and used according to the manufacturers' recommendations. Colony PCRs were performed following lysis of cells with IGEPAL CA-630 (Sigma). Primers were synthesized by Sigma Genosys, and sequencing was performed by MWG Biotechnologies.
Creation of mutants.
An L. monocytogenes LO28 gadD3 in-frame deletion mutant was created using a plasmid construct generated by the splicing-by-overlap-extension procedure as previously described (4) except that β-glycerophosphate (55 mM) was added to buffer all liquid media at the plasmid-curing stage. Deletion of the 801-bp fragment of gadD3 in the ΔgadD3 mutant was confirmed by sequencing. An L. lactis IL1403 gadB insertion mutant was created by cloning 350 bp of the 5′ end of the gene into plasmid pORI19 as previously described (12).
Growth and survival experiments.
For all assays, overnight cultures were washed in one-quarter-strength Ringer's solution. For growth curves, overnight cultures were inoculated (3%) into fresh broth supplemented with nisin. Aliquots (200 μl) were added into individual wells of a 96-well plate, and cell growth was measured spectrophotometrically over 24-h periods using a temperature-controlled automatic plate reader (Spectra Max 340 spectrophotometer; Molecular Devices, Sunnyvale, CA). For survival assays, approximately 1 × 107 CFU/ml of cells was inoculated into fresh broth containing nisin and incubated at 37°C. At various time points, 100-μl aliquots were removed and viable plate counts were performed by serial dilution in one-quarter-strength Ringer's solution and plating onto agar. All growth and survival experiments were performed in triplicate from three separate overnight cultures and repeated on at least three different days.
ATP assays.
Total and extracellular levels of ATP in strains grown overnight in 10 ml BHI broth statically at 37°C were determined using the Sigma ATP bioluminescence assay kit (FL-AA) essentially by following the method described by McEntire et al. (17). Briefly, cells were washed in 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.5). To determine total ATP levels, 20 μl of cells was added to 80 μl of dimethyl sulfoxide in a test tube and mixed. Then, 5 ml of cold sterile water was added and 100 μl of the solution was used with 100 μl of the Sigma enzyme mixture. To determine extracellular ATP levels, 100 μl of bacterial cells was added to 5 ml of sterile water and 100 μl of this was used with 100 μl of the Sigma enzyme mixture. Intracellular ATP levels were calculated as the difference between total and extracellular ATP levels. ATP calibration curves were performed according to Sigma recommendations, and new standard curves were prepared each day as suggested. ATP assays were carried out on three separate overnight cultures for each strain (i.e., three biological repeats) and repeated on two separate days. Bioluminescence was measured using a Luminoskan Ascent luminometer (Thermo Scientific). To determine bacterial cell dry weight, 1 ml of each bacterial culture was washed in one-quarter-strength Ringer's solution and dried in a preweighed aluminum dish at 45°C overnight.
RESULTS AND DISCUSSION
Creation of an L. monocytogenes gadD3 deletion mutant.
L. monocytogenes strain LO28 possesses five gad genes at three separate genetic loci: gadD1T1 (formerly gadAC), gadD2T2 (formerly gadBD), and gadD3 (Fig. 2 A). The gadD genes encode decarboxylases, while the gadT genes encode glutamate-GABA antiporters (see Fig. 1). We have previously reported the mutation of four of these genes (4), but our initial efforts to mutate gadD3 proved unsuccessful. Herein we report the creation of a gadD3 deletion mutant using the same procedure as previously outlined (4) except that β-glycerophosphate (55 mM) was added to buffer all liquid media at the plasmid curing stage. This mutant completes the full set of isogenic LO28 gad mutants.
FIG. 2.
(A) Genomic organization of L. monocytogenes gad genes. This figure was drawn approximately to scale using L. monocytogenes EGDe genome sequence data. Numbers refer to the National Centre for Biotechnology Information (NCBI) annotation numbers. Arrows indicate orientation of genes. (B) Growth of L. monocytogenes LO28 wild-type and gad mutants in BHI broth supplemented with a sublethal level of nisin. Overnight cultures were washed in one-quarter-strength Ringer's solution and inoculated into BHI broth supplemented with 100 μg/ml nisin powder. Cell growth at 37°C was measured spectrophotometrically. Error bars represent standard deviations of results of triplicate experiments. (C and D) Survival of strains in BHI broth supplemented with a lethal level of nisin. Washed overnight cultures were inoculated into BHI broth supplemented with 300 μg/ml nisin and incubated with shaking at 37°C. Viable cell counts were performed at intervals (after 60 min for panel C) by serial dilution in one-quarter-strength Ringer's solution and enumeration on BHI agar. Error bars represent standard deviations of results of triplicate experiments.
Examination of the nisin tolerance of gad mutants.
Mutants were not impaired in their growth under standard laboratory conditions (BHI broth, pH 7) (data not shown). It was noted, however, that ΔgadD1 mutant reproducibly reached slightly higher optical densities (ODs) than the parent during exponential growth but the final ODs of all cultures were similar once stationary phase was reached (data not shown). In order to investigate the role, if any, of each individual gad gene in the tolerance of nisin, the growth of each mutant was compared to that of the wild-type strain in BHI broth supplemented with sublethal levels (100 μg nisin powder [Sigma]/ml) of the bacteriocin. It was observed that the ΔgadT1, ΔgadT2, ΔgadD2, and ΔgadD3 mutants behaved identically to the parent at this and all other sublethal concentrations tested. In contrast, the ΔgadD1 mutant was reproducibly more sensitive (as determined by a longer lag phase and a slightly lower growth rate) (Fig. 2B). The ability of each mutant to withstand exposure to lethal levels of nisin (300 μg nisin powder/ml) was also examined, and again the ΔgadD1 mutant exhibited enhanced sensitivity relative to the wild-type strain (two-log-greater reduction) after exposure for 60 min (Fig. 2C). A more detailed time course assay with the parent and gadD1 mutant (Fig. 2D) shows that although parental cell numbers were somewhat reduced in the presence of 300 μg powder/ml, the ΔgadD1 mutant was significantly more sensitive. Taken together, these experiments revealed that of all the gad genes only gadD1 contributes to the ability of the cell to cope with nisin.
Measurement of intracellular ATP levels in the ΔgadD1 mutant.
Although gadD1 has previously been shown to contribute to growth at mildly acidic pH (pH 5.5) (8), the addition of nisin in our experiments did not affect the pH of the broth (pH 7.4) and nisin does not affect intracellular cytoplasmic pH (3). It is therefore likely that the role of gadD1 in pH homeostasis is not responsible for the observed altered nisin phenotype. Amino acid decarboxylation has been linked to ATP biosynthesis in Lactobacillus strains (1, 14, 19), and intracellular ATP levels have been shown to be important for L. monocytogenes in surviving exposure to nisin (3). We therefore hypothesized that intracellular ATP levels may be altered in the ΔgadD1 mutant. ATP assays were performed which revealed that, under the growth conditions employed, the intracellular ATP levels were reduced in the ΔgadD1 mutant, being only approximately 60% of those of the parent (P < 0.01 as determined by Student's t test) (Fig. 3). This suggests that GadD1 contributes significantly to ATP pools within the cell.
FIG. 3.
Intracellular ATP levels of L. monocytogenes LO28 wild-type and gadD1 mutant cells. Strains were grown overnight in 10 ml BHI broth statically at 37°C. Intracellular levels of ATP were determined as described in the Materials and Methods section. Error bars represent standard deviations of results of triplicate experiments.
Correlation of nisin tolerance with the presence of gadD1.
While the other loci that have been shown to contribute to nisin tolerance in L. monocytogenes are present in all Listeria strains, the gadD1T1 operon is unusual in that it is present on an islet of five genes that is absent from a large number of L. monocytogenes strains (8, 23). We therefore hypothesized that the natural presence or absence of gadD1 may impact on the nisin tolerance of individual strains. Growth curves (12 gadD1+ and 12 gadD1-negative strains) and survival assays (5 gadD1+ and 5 gadD1-negative strains) in the presence of nisin support this hypothesis; i.e., strains possessing gadD1 grew better on average (Fig. 4 A inset) in the presence of 100 μg nisin powder/ml and were more resistant to nisin (300 μg nisin powder/ml) than those lacking the gene (Fig. 4A and B). That some strains contradicted this correlation is not totally unexpected, as the strains being compared are not isogenic, and thus another factor(s) contributing to nisin tolerance may vary, e.g., membrane charge or fluidity. While natural variation in nisin tolerance within a species has previously been reported (9, 16, 22), to our knowledge, gadD1 is the first genetic locus that has been linked to this phenomenon. Given this link, it is noteworthy that strains which possess gadD1 are more frequently isolated from foods than those lacking the gene (23). As low pH and nisin are often used together as part of the “hurdle concept” in the preservation of minimally processed foods, it is tempting to speculate that this locus may contribute to the “fitness” of L. monocytogenes strains in certain foods.
FIG. 4.
(A) Growth of L. monocytogenes gadD1-positive (squares) and gadD1-negative (circles) strains in BHI broth supplemented with a sublethal level of nisin. Overnight cultures were inoculated into BHI broth supplemented with 100 μg/ml nisin. Aliquots (200 μl) were added into individual wells of a 96-well plate, and cell growth at 37°C was measured spectrophotometrically using a temperature-controlled automatic plate reader. The strains are listed in order of descending optical density at 600 nm (OD600) after 12 h. The inset shows the mean growth of gadD1-positive and gadD1-negative strains. (B) Survival of L. monocytogenes gadD1-positive and gadD1-negative strains in BHI broth supplemented with a lethal level of nisin. Overnight cultures were inoculated into BHI broth supplemented with 300 μg/ml nisin. Viable cell counts were performed by serial dilution in one-quarter-strength Ringer's solution and enumeration on BHI agar. Error bars represent standard deviations of results of triplicate experiments.
Nisin tolerance of L. lactis strains.
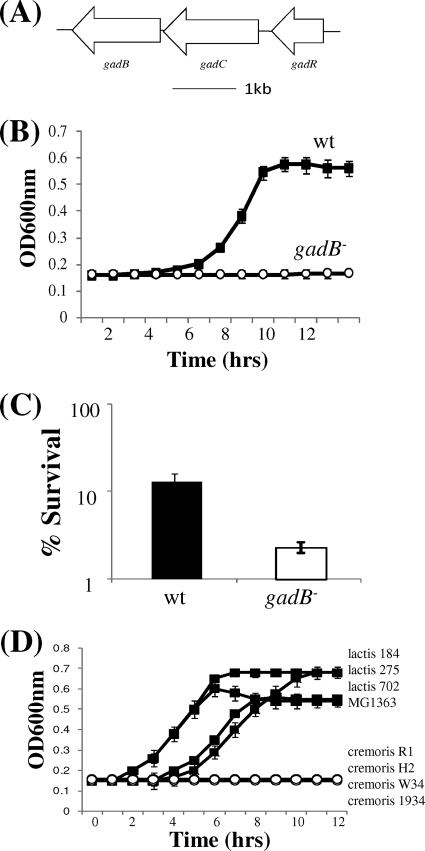
To test whether this newly established link between glutamate decarboxylase systems and nisin tolerance is specific to Listeria, or whether it holds true in other glutamate decarboxylase-positive microorganisms, we tested the sensitivity of Lactococcus lactis subsp. lactis IL1403 and a gadB mutant thereof. It should be noted that although L. monocytogenes possesses five gad genes, L. lactis subsp. lactis strains, including strain IL1403, contain only three, i.e., gadB, which encodes a glutamate decarboxylase, gadC, which encodes a transporter, and gadR, which encodes a putative regulator (Fig. 5 A). Our analysis revealed that the gadB mutant was not impaired in growth under standard laboratory conditions (GM17 broth; data not shown). However, the growth of this mutant was dramatically impaired in the presence of sublethal levels of nisin (8 μg nisin powder/ml) (P < 0.001 at 12 h as determined by Student's t test) (Fig. 5B). Survival of the mutant after 60 min in the presence of 45 μg/ml nisin powder was approximately 5-fold less than that of the parental strain (P < 0.05 as determined by Student's t test) (Fig. 5C). This result is interesting in light of the fact that GAD activity is one of the criteria used to distinguish between L. lactis subspecies. While L. lactis subsp. lactis strains are GAD+, L. lactis subsp. cremoris strains, as a consequence of inactivation of their gadB gene by a frameshift mutation, do not possess GAD activity (20). Thus, to further investigate the link between the mutation, natural or otherwise, of GAD and nisin sensitivity, a selection of L. lactis subsp. lactis and L. lactis subsp. cremoris strains was examined with a view to comparing their individual and collective sensitivity to nisin. It was observed that the former were significantly more tolerant to exposure of sublethal levels of nisin (i.e., virtually no lag phase at 8 μg/ml) whereas their L. lactis subsp. cremoris counterparts failed to grow at all (Fig. 5D). It would thus seem that the provision of innate protection against nisin as a consequence of the activity of a glutamate decarboxylase system is a common feature among GAD+ Gram-positive microorganisms. In silico analyses of publicly available genomes reveal that gad genes are present in the genomes of a wide variety of bacterial genera, including the Gram-positive food pathogens Clostridium botulinum, Clostridium perfringens, and Bacillus cereus (M. Begley, unpublished observation), and thus the outcome of future experiments to determine if these gad genes contribute to the nisin tolerance of these strains is eagerly anticipated. It should be noted that gad genes are also commonly found on the genomes of numerous Gram-negative strains. While the outer membrane of Gram-negative microorganisms provides protection against nisin, the lantibiotic is active against cells in which this barrier has been disrupted, and thus it will also be interesting to investigate the role of the GAD system in such circumstances.
FIG. 5.
(A) Genomic organization of L. lactis IL1403 gad genes. This figure was drawn approximately to scale using L. lactis IL1403 genome sequence data. Arrows indicate orientation of genes. (B) Growth of L. lactis IL1403 wild-type and gadB mutant cells in GM17 broth supplemented with a sublethal level of nisin. Overnight cultures were inoculated into GM17 broth supplemented with 8 μg/ml nisin. Aliquots (200 μl) were added into individual wells of a 96-well plate, and cell growth at 30°C was measured spectrophotometrically using a temperature-controlled automatic plate reader. Error bars represent standard deviations of results of triplicate experiments. (C) Survival of strains in GM17 broth supplemented with a lethal level of nisin. Overnight cultures were inoculated into GM17 broth supplemented with 45 μg/ml nisin and incubated statically at 30°C. Viable cell counts were performed at intervals (after 60 min) by serial dilution in one-quarter-strength Ringer's solution and enumeration on GM17 agar. Error bars represent standard deviations of results of triplicate experiments. (D) Growth of L. lactis subsp. lactis and L. lactis subsp. cremoris strains in GM17 broth supplemented with sublethal levels of nisin. Overnight cultures were inoculated into GM17 broth supplemented with 8 μg/ml nisin. Aliquots (200 μl) were added into individual wells of a 96-well plate, and cell growth at 30°C was measured spectrophotometrically using a temperature-controlled automatic plate reader. Error bars represent standard deviations of results of triplicate experiments.
In conclusion, we report the novel observation that a gene of the glutamate decarboxylase acid tolerance system, namely, gadD1, contributes to the inherent ability of L. monocytogenes to tolerate the commercially important lantibiotic nisin. In addition to uncovering this additional role for the GAD system in listerial stress responses, we have also linked gadD1 with the strain-variable nature of nisin tolerance among L. monocytogenes. Evidence is provided which reveals that this is not solely an L. monocytogenes-associated phenomenon. Thus, it would appear that targeting of the GAD system, through the specific inhibition of GAD activity or a reduction in the levels of free glutamate in foods, coupled with low pH and/or nisin may be an attractive option for food processors.
Acknowledgments
We acknowledge the funding received from the Irish Government under the National Development Plan 2000-2006. The Alimentary Pharmabiotic Centre is a research center that is funded by Science Foundation Ireland.
Footnotes
Published ahead of print on 6 August 2010.
REFERENCES
- 1.Abe, K., H. Hayashi, and P. C. Malone. 1996. Exchange of aspartate and alanine. Mechanism for development of a proton-motive force in bacteria. J. Biol. Chem. 271:3079-3084. [DOI] [PubMed] [Google Scholar]
- 2.Begley, M., C. Hill, and R. P. Ross. 2006. Tolerance of Listeria monocytogenes to cell envelope-acting antimicrobial agents is dependent on SigB. Appl. Environ. Microbiol. 72:2231-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet, M., M. M. Rafi, M. L. Chikindas, and T. J. Montville. 2006. Bioenergetic mechanism for nisin resistance, induced by the acid tolerance response of Listeria monocytogenes. Appl. Environ. Microbiol. 72:2556-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, P. D., C. G. M. Gahan, and C. Hill. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465-475. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 6.Cotter, P. D., C. M. Guinane, and C. Hill. 2002. The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob. Agents Chemother. 46:2784-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. D., K. O'Reilly, and C. Hill. 2001. Role of the glutamate decarboxylase acid resistance system in the survival of L. monocytogenes LO28 in low pH foods. J. Food Prot. 64:1362-1368. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, P. D., S. Ryan, C. G. M. Gahan, and C. Hill. 2005. Presence of GadD1 glutamate decarboxylase in selected Listeria monocytogenes strains is associated with an ability to grow at low pH. Appl. Environ. Microbiol. 71:2832-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ennahar, S., N. Deschamps, and J. Richard. 2000. Natural variation in susceptibility of Listeria strains to class IIa bacteriocins. Curr. Microbiol. 41:1-4. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi, M., and M. L. Chikindas. 2007. Listeria: a foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 113:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Gravesen, A., B. Kallipolitis, K. Holmstrøm, P. E. Høiby, M. Ramnath, and S. Knøchel. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIA bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinane, C. M., P. D. Cotter, E. M. Lawton, C. Hill, and R. P. Ross. 2007. Insertional mutagenesis to generate lantibiotic resistance in Lactococcus lactis. Appl. Environ. Microbiol. 73:4677-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasper, H. E., N. E. Kramer, J. L. Smith, J. D. Hillman, C. Zachariah, O. P. Kuipers, B. de Kruijff, and E. Breukink. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636-1637. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi, T., H. Hayashi, and K. Abe. 1997. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. Appl. Environ. Microbiol. 179:3362-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joerger, R. D., H. Chen, and K. E. Kniel. 2006. Characterization of a spontaneous, pressure-tolerant Listeria monocytogenes Scott A ctsR deletion mutant. Foodborne Pathog. Dis. 3:196-202. [DOI] [PubMed] [Google Scholar]
- 16.Katla, T., K. Naterstad, M. Vancanneyt, J. Swings, and L. Axelsson. 2003. Differences in susceptibility of Listeria monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. Appl. Environ. Microbiol. 69:4431-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEntire, J. C., G. M. Carman, and T. J. Montville. 2004. Increased ATPase activity is responsible for acid sensitivity of nisin-resistant Listeria monocytogenes ATCC 700302. Appl. Environ. Microbiol. 70:2717-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molenaar, D., J. S. Bosscher, B. Ten Brink, A. J. M. Briessen, and W. N. Konings. 1993. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 175:2864-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura, M., M. Kobayashi, and T. Okamoto. 2002. Rapid PCR-based method which can determine both phenotype and genotype of Lactococcus lactis subspecies. Appl. Environ. Microbiol. 68:2209-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer, M. E., M. Wiedmann, and K. J. Boor. 2009. Sigma B and sigma L contribute to Listeria monocytogenes 10403S response to the antimicrobial peptides SdpC and nisin. Foodborne Pathog. Dis. 6:1057-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasch, M., and S. Knochel. 1998. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA-1 and bavaricin A. Lett. Appl. Microbiol. 27:275-278. [DOI] [PubMed] [Google Scholar]
- 23.Ryan, S., M. Begley, C. Hill, and C. G. Gahan. 22 March 2010. A five-gene stress survival islet (SSI-1) that contributes to the growth of Listeria monocytogenes in suboptimal conditions. J. Appl. Microbiol. doi: 10.1111/j.1365-2672.2010.04726.x. [DOI] [PubMed]
- 24.van Schaik, W., C. G. Gahan, and C. Hill. 1999. Acid-adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotics nisin and lacticin 3147. J. Food Prot. 62:536-539. [DOI] [PubMed] [Google Scholar]
- 25.Wong, S., D. Street, S. I. Delgado, and K. C. Klontz. 2000. Recalls of food and cosmetics due to microbial contamination reported to the U.S. Food and Drug Administration. J. Food Prot. 63:1113-1116. [DOI] [PubMed] [Google Scholar]