Abstract
Here we show that the C-terminal domain of LuxR activates the transcription of Aliivibrio fischeri luxICDABEG in Escherichia coli SKB178 gro+ and E. coli OFB1111 groEL673 strains to the same level. Using affinity chromatography, we showed that GroEL binds to the N-terminal domain of LuxR, pointing to a GroEL/GroES requirement for the folding of the N-terminal domain of LuxR.
In the marine bacterium Aliivibrio fischeri, the expression of lux genes is regulated by the LuxI-LuxR system, which determines the intensity of the bioluminescence of growing cells depending on the density of the cell population (quorum sensing) (8, 9, 17). LuxR is a quorum-sensing transcriptional regulator of the luxICDABEG operon (8, 9, 13). Upon binding with an autoinducer (AI), the LuxR protein acquires the ability to form a complex with the lux box and to activate luxICDABEG operon transcription (6, 7, 21, 22). It was demonstrated that rpoH and groE mutants with the entire lux system of A. fischeri show a significant decrease in bioluminescence; it was proposed that the GroEL/GroES chaperonins participate in the folding of LuxR (1, 5). The GroEL/GroES chaperonin-folding chamber is an encapsulated space with a hydrophilic wall where many cellular proteins acquire their native state (12).
During extraction using affinity chromatography, the glutathione S-transferase (GST)-LuxR fusion protein and chaperonin GroEL coelute (15). Interestingly, both GroEL and cochaperonin GroES were previously shown to enhance the accumulation of the soluble LuxR-like protein TraR, a quorum-sensing transcription factor from Agrobacterium tumefaciens (3).
The LuxR protein consists of two domains: the 88-amino-acid (aa) C-terminal domain (CTD), which is responsible for contacts of the protein with the lux box in DNA (the DNA-binding domain), and the 162-aa N-terminal domain (NTD), which determines its binding with the AI (4, 11, 20). Here we tested the hypothesis that GroEL/GroES chaperonins are required for the folding of the NTD of LuxR.
The Escherichia coli strains and plasmids used in this study are listed in Table 1.
TABLE 1.
E. coli strains and plasmids used
| Strain or plasmid | Relevant characteristicsa | Reference(s) |
|---|---|---|
| Strains | ||
| SKB178 | F−galE sup gro+ | 25 |
| OFB1111 | groE673 Gly173Asp Gly337Asp (others markers the same as SKB178) | 25 |
| TG-1 | thi-1 supE44 hsdΔ5 Δ(lac-proAB) [F′ traD36 proAB+lacIqlacZΔM15] | 20 |
| Plasmids | ||
| pF1 | pBR322 with a 16-kb BamHI fragment (luxR luxICDABE) of A. fischeri strain MJ-1 (AF170104); Apr | 24 |
| pGEX-KG | Plasmid vector for the synthesis of the GST fusions, ColE1 replicon; Apr | 10 |
| pGEX-LuxR | pGEX-KG vector containing the gst-luxR fusion gene; Apr | 10, 16 |
| pDEW201 | The replication origin of pBR322; promoterless P. luminescens luxCDABE genes; Apr | 23 |
| pVFR1 | pDEW201 with a 1-kb fragment of A. fischeri DNA (luxR and Pl and Pr promoters), Apr; the luxCDABE cassette of P. luminescens controlled by Pr promoter | 16 |
| pLuxR | pUC19 with luxR under the lac promoter; Apr | This study |
| pLuxRΔN | pTZ57R with a 5′-region-deleted luxR gene encoding the LuxR CTD (88 aa), under lac promoter; Apr | This study |
| pGEX-LuxRΔN | pGEX-KG with a 5′-region-deleted fragment of luxR gene fused with gst; Apr | This study |
| pGEX-LuxRΔC | pGEX-KG with a 3′-region-deleted fragment of the gst-luxR fusion; Apr | This study |
| pOM | pACYC184 with a BamHI/NruI fragment of A. fischeri DNA from pF1 (luxICDABEG under the Pr promoter and lux-regulatory DNA between luxR and luxI [without luxR]); Cmr | This study |
Apr, ampicillin resistant; Cmr, chloramphenicol resistant.
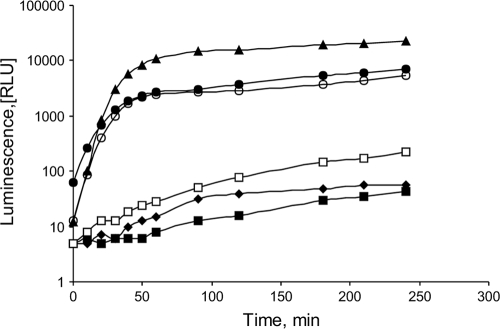
In order to determine the effect of the GroEL chaperonin on LuxR and CTD activities, we transformed pLuxR or pLuxRΔN in E. coli SKB178 gro+ and OFB1111 groEL673 strains containing pOM plasmid. The pLuxR plasmid contains the entire luxR gene; the pLuxRΔN plasmid contains a 5′-region-deleted luxR gene encoding only the CTD of LuxR; both genes are transcribed from the lac promoter. In E. coli SKB178 gro+(pOM) cells, the addition of the pLuxR or pLuxRΔN plasmid leads to a rise in the intensity of bioluminescence due to activation of the Pr promoter of the luxICDABEG operon (Fig. 1).
FIG. 1.
Effect of groEL mutation on activities of the full-length LuxR and its CTD. RLU, relative luminescence units. The OD was controlled before and after each experiment. All strains contained pOM. Data for SKB178 gro+(pUC19) (filled diamonds), OFB1111 groEL673(pUC19) (filled squares), SKB178 gro+(pLuxR) (filled triangles), OFB1111 groEL673(pLuxR) (open squares), SKB178 gro+(pLuxRΔN) (open circles), and OFB1111 groEL673(pLuxRΔN) (filled circles) are shown.
Single colonies of cells grown overnight on solid medium at 37°C were transferred to Tris buffer and washed twice by centrifugation. Then, the cells were resuspended in 200 μl of L broth (20) and grown at 22°C without agitation.
Bioluminescence of cells was measured at room temperature in 200 μl of cell suspension by an LMA01 luminometer (Beckman). The intensity of the bioluminescence dramatically decreased in E. coli OFB1111 groEL673 cells containing full-length LuxR. However, in E. coli SKB178 gro+ or OFB1111 groEL673 cells with the pLuxRΔN plasmid, the groEL673 mutation had no influence on the level of bioluminescence. Thus, chaperonin GroEL is likely to be necessary for the folding of the active full-length LuxR but not for the folding of its CTD.
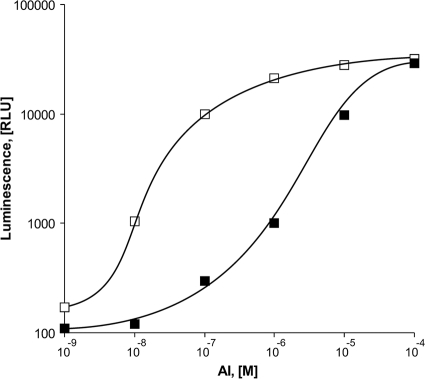
Plasmid pVFR1 contains a 1-kb DNA fragment of A. fischeri including luxR and Pl/Pr promoters along with a luxCDABE cassette of Photorhabdus luminescens. In this model, the transcription of luxCDABE from Pr is initiated after the addition of AI to the medium. Overnight bacterial inoculates with an optical density (OD) equal to 0.01 were grown in LB media in the presence of ampicillin (100 μg/ml) with aeration at 28°C until the OD reached 0.4 to 0.5. AI was added after 30 min of incubation at 42°C, which allowed for inactivation of any endogenous LuxR. After AI addition, cells were incubated at 22°C with no mixing. After 1 h, samples were collected for the measurement of bioluminescence. As shown in Fig. 2, in E. coli OFB1111 groEL673 cells, the defect of GroEL may be compensated by an increase of the AI level in the medium. In the SKB178 gro+ strain, bioluminescence is initiated at 10− 9 M AI, while OFB1111 groEL673 cells require at least 10−7 M AI.
FIG. 2.
Effects of AI concentrations in the media on the peak bioluminescence intensities of SKB178 gro+(pVFR1) (open squares) and OFB1111 groEL673(pVFR1) (filled squares).
We constructed the gst-fused variants of luxR and 5′-region-deleted luxR genes. The resulting fusion proteins, GST-LuxR and GST CTD, maintained their ability to activate in vivo the lux operon of A. fischeri. However, only GST-LuxR retained its ability to activate lux operon expression in the presence of AI (data not shown).
E. coli TG-1 cells were transformed with pGEX-LuxR, pGEX-LuxRΔN, or pGEX- LuxRΔC and grown in LB medium supplemented with ampicillin until the mid-exponential phase (OD, 0.5 to 0.6). The promoter (Plac) was induced with 1 mM IPTG (isopropyl β-d-thiogalactopyranoside). Cells were incubated for 2 h at 37°C and then 12 h at 18°C. Cells were collected by centrifugation, disrupted by sonication in 1× phosphate-buffered saline (PBS; 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) with supplemental 0.5% Triton X-100, and centrifuged to obtain cell extract. GST-containing proteins were purified by affinity chromatography on glutathione Sepharose (Novagen) by using a reduced glutathione solution as an eluent (10, 15).
Electrophoresis of proteins in 12% polyacrylamide gel (PAG) was performed under denaturing conditions (with SDS) according to the method of Laemmli (14).
Proteins isolated from the SDS-PAGE gels were identified by mass spectrometry. Samples for matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS) were prepared as described previously (19). MS was carried out after trypsin digestion in a Reflex III unit (Bruker) at the Orekhovich Institute of Biomedical Chemistry (Moscow, Russia). The resulting mass fingerprints were identified by using the Swiss-Prot database (2).
The GST, GST-LuxR, GST-NTD, and GST-CTD proteins were extracted by affinity chromatography using glutathione Sepharose columns. The molecular masses of the proteins were estimated by SDS electrophoresis in 12% gel (Fig. 3). In addition to the bands corresponding to GST-LuxR (54 kDa) and GST-NTD (45 kDa), one 60-kDa band was detected (Fig. 3, lanes 2 and 3). The 60-kDa protein was analyzed by mass spectrometry. According to the distribution and compositions of peptide fingerprints and comparison with the Swiss-Prot database (2), the 60-kDa protein was identified as chaperonin GroEL. Meanwhile, during affinity extraction of the deletion mutant, the GST-CTD protein was not accompanied by GroEL (Fig. 3, lane 1).
FIG. 3.
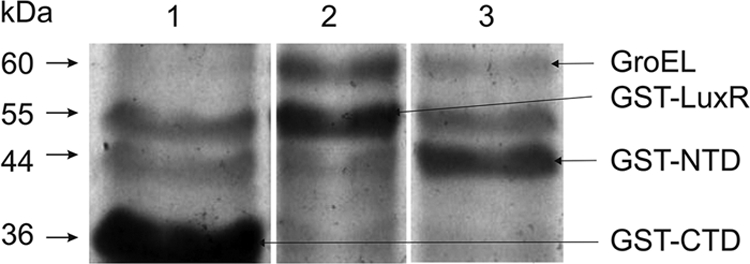
SDS electrophoresis, in 12% gel, of the protein fractions obtained by affinity chromatography on a column with glutathione Sepharose. Proteins were isolated from E. coli TG-1 containing pGEX-LuxRΔN (lane 1), pGEX-LuxR (lane 2), or pGEX-LuxRΔC (lane 3). The bands of glycerol kinase (56.2 kDa; lanes 1 and 3) and of elongation factor EF-Tu (43.3 kDa; lanes 1 and 2) are present also (according to mass-spectrometry analysis, these bands correspond to the glycerol kinase and EF-Tu). These proteins accompanied the GST protein purified using gentle washing conditions.
Our results provide evidence for the notion that the GroEL/GroES chaperonins participate in the folding of the LuxR protein through binding to the NTD, while the CTD of LuxR does not require GroEL/GroES for its folding. Interestingly, at high concentrations of AI (10−5 M and higher), the groEL673 defect is completely compensated (Fig. 2). As AI forms a complex with the N-terminal domain of LuxR, one can assume that the formation of soluble LuxR takes place either simultaneously with the process of its synthesis on the ribosome after the binding of its N-terminal domain to already available AI or later, after the binding of the GroEL/GroES to the same domain.
Here we showed that the NTD of LuxR is a target for the GroEL/GroES chaperonins participating in the folding of the LuxR protein. In our previous study, we showed that the NTD of LuxR is targeted by Lon protease (18). Taken together, these data indicate that the NTD of LuxR may play a role both in quorum sensing and in the recognition of the stress signals.
Acknowledgments
We thank S. Z. Mindlin for the SKB178 gro+ and groEL673 strains.
This work was supported by the Federal Agency for Education of Russia, state contract no. 02.740.11.5034.
Footnotes
Published ahead of print on 20 August 2010.
REFERENCES
- 1.Adar, Y. Y., M. Simaan, and S. Ulitzur. 1992. Formation of the LuxR protein in the Vibrio fischeri lux system is controlled by HtpR through the GroESL proteins. J. Bacteriol. 174:7138-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairoch, A., and R. Apweiler. 2000. The SWISS-PROT protein sequence database and its supplement TrEMBL 2000. Nucleic Acids Res. 28:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai, Y., and S. G. Winans. 2009. The chaperone GroESL enhances the accumulation of soluble, active TraR protein, a quorum-sensing transcription factor from Agrobacterium tumefaciens. J. Bacteriol. 191:3706-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, S. H., and E. P. Greenberg. 1991. The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc. Natl. Acad. Sci. U. S. A. 88:11115-11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolan, K. M., and E. P. Greenberg. 1992. Evidence that GroEL, not sigma 32, is involved in transcriptional regulation of the Vibrio fischeri luminescence genes in Escherichia coli. J. Bacteriol. 174:5132-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nelson, and N. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 7.Engebrecht, J., and M. Silverman. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. U. S. A. 81:4154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 10.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 11.Hanzelka, B., and E. P. Greenberg. 1995. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol. 177:815-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwich, A. L., A. C. Apetri, and W. A. Fenton. 2009. The GroEL/ES cis cavity as a passive anti-aggregation device. FEBS Lett. 583:2654-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Manukhov, I. V., V. Y. Kotova, and G. B. Zavil'gel'skii. 2006. Role of GroEL/GroES chaperonin system and Lon protease in regulation of expression Vibrio fischeri lux genes in Escherichia coli cells. Mol. Biol. (Moscow) 40:277-283. [PubMed] [Google Scholar]
- 16.Manukhov, I. V., V. Y. Kotova, and G. B. Zavil'gel'skii. 2006. Involvement of host factors in the regulation of the Vibrio fischeri lux operon in Escherichia coli. Mikrobiologiia (Moscow) 75:525-531. [PubMed] [Google Scholar]
- 17.Meighen, E. A. 1991. Molecular biology of bacterial bioluminescence. Microbiol. Rev. 55:123-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mel'kina, O. E., I. V. Manukhov, and G. B. Zavilgelsky. 2010. The C-terminal domain of the Vibrio fischeri transcription activator LuxR is not essential for degradation by Lon protease. Mol. Biol. (Moscow) 44:454-457. [PubMed] [Google Scholar]
- 19.Patterson, S. D., and R. Aebersold. 1995. Mass-spectrometric approaches for the identification of gel-separated proteins. Electrophoresis 16:1791-1814. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Stevens, A. M., K. M. Dolan, and E. P. Greenberg. 1994. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc. Natl. Acad. Sci. U. S. A. 91:12619-12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbanowski, M. L., C. P. Lostroh, and E. P. Greenberg. 2004. Reversible acyl-homoserine lactone binding to purified Vibrio fischeri LuxR protein. J. Bacteriol. 186:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dyk, T., and R. A. Rosson. 1998. Photorhabdus luminescens luxCDABE promoter probe vectors, p. 85-95. In R. A. LaRossa (ed.), Methods in molecular biology, vol. 102. Humana Press Inc., Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 24.Zavil'gel'skiĭ, G. B., and I. V. Manukhov. 1994. Lon-protease participates in the regulation of transcription of the Lux-operon of Vibrio fischeri. Genetika 30:337-341. [PubMed] [Google Scholar]
- 25.Zeilstra-Ryalls, J., O. Fayet, L. Baird, and C. Georgopoulos. 1993. Sequence analysis and phenotypic characterization of groEL mutations that block lambda and T4 bacteriophage growth. J. Bacteriol. 175:1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]