Abstract
Family 43 glycoside hydrolases (GH43s) are known to exhibit various activities involved in hemicellulose hydrolysis. Thus, these enzymes contribute to efficient plant cell wall degradation, a topic of much interest for biofuel production. In this study, we characterized a unique GH43 protein from Fibrobacter succinogenes S85. The recombinant protein showed α-l-arabinofuranosidase activity, specifically with arabinoxylan. The enzyme is, therefore, an arabinoxylan arabinofuranohydrolase (AXH). The F. succinogenes AXH (FSUAXH1) is a modular protein that is composed of a signal peptide, a GH43 catalytic module, a unique β-sandwich module (XX domain), a family 6 carbohydrate-binding module (CBM6), and F. succinogenes-specific paralogous module 1 (FPm-1). Truncational analysis and site-directed mutagenesis of the protein revealed that the GH43 domain/XX domain constitute a new form of carbohydrate-binding module and that residue Y484 in the XX domain is essential for binding to arabinoxylan, although protein structural analyses may be required to confirm some of the observations. Kinetic studies demonstrated that the Y484A mutation leads to a higher kcat for a truncated derivative of FSUAXH1 composed of only the GH43 catalytic module and the XX domain. However, an increase in the Km for arabinoxylan led to a 3-fold decrease in catalytic efficiency. Based on the knowledge that most XX domains are found only in GH43 proteins, the evolutionary relationships within the GH43 family were investigated. These analyses showed that in GH43 members with a XX domain, the two modules have coevolved and that the length of a loop within the XX domain may serve as an important determinant of substrate specificity.
The plant cell wall is composed of a variety of polysaccharides and is the most abundant source of renewable biomass on our planet. There is an increasing effort to convert the cellulosic component to alcohols that can serve as biofuels. A critical step in this process is the enzymatic hydrolysis to release easily fermentable monomeric sugars, such as glucose and xylose, from the complex polysaccharides. However, the conversion of plant cell wall polysaccharides to biofuels is still far from being an ideal cost-effective process (53). Increasing the yields of enzymes during gene expression and bio-prospecting for enzymes with higher catalytic efficiencies are two strategies that can reduce the cost of production of biofuels. Ruminant animals have coevolved with a microbial consortium that harnesses enzymatic hydrolysis to release fermentable sugars from plant cell wall polysaccharides. The released sugars are subsequently fermented by the microbes to short-chain fatty acids that serve as the main energy source of the host (14, 33). Therefore, the genomes of plant cell wall-degrading microbes in the rumen represent a rich source of highly active plant cell wall-degrading enzymes. In addition, a better understanding of the strategies utilized by ruminal plant cell wall-degrading microorganisms should enhance rational design of enzymes with novel functions and/or improved activities through genetic engineering.
The enzymes at the core of microbial plant cell wall degradation are the glycoside hydrolases (GHs). GHs frequently display a variety of modular structures. In addition to the catalytic domain, the most commonly observed module in glycoside hydrolases is the carbohydrate-binding module (CBM), which is known to enhance the accessibility of GHs to their appropriate polysaccharide substrates. Currently, there are 115 GH families and 59 CBM families in the carbohydrate active enzyme database (CAZy) (10), and combinations of these modules provide functional diversities to GHs.
Hemicellulose is the second most abundant sugar polymer in the plant cell wall, and due to its heterogenous structure, it requires a set of at least five enzymes for its saccharification (12). The family 43 glycoside hydrolases (GH43s) are hemicellulolytic enzymes. They exhibit β-1,4-xylosidase (EC 3.2.1.37), β-1,3-xylosidase (EC 3.2.1.72), α-l-arabinofuranosidase (EC 3.2.1.55), arabinanase (EC 3.2.1.99), xylanase (EC 3.2.1.8), and galactan 1,3-β-galactosidase (EC 3.2.1.145) activities. Recent biophysical studies have revealed domain organizations and catalytic mechanisms in this family (3, 8, 9, 43, 65, 73). Based on their domain organization, these proteins are grouped into three different types. The first group includes 1,5-α-l-arabinanases from Cellvibrio japonicus (43), Bacillus thermodenitrificans (73), and Geobacillus stearothermophilus (3), and these proteins are composed of a single GH43 catalytic domain. The second group includes an arabinoxylan arabinofuranohydrolase enzyme from Bacillus subtilis (BsAXH-m2,3) and, in addition to the GH43 module, the proteins in this group have a family 6 carbohydrate-binding module (CBM6) at their C termini (65). The third group, which includes a β-xylosidase/α-l-arabinofuranosidase from the rumen bacterium Selenomonas ruminantium (SXA) (9) and a β-xylosidase from Geobacillus stearothermophilus (XynB3) (8), possesses in addition to the GH43 modules a C-terminally appended β-sandwich fold structure composed of approximately 200 amino acid residues. GH43 proteins of similar organization as SXA and XynB3 abound in the protein databases, and they are thought to form a cluster of orthologous group of proteins (COG) with β-xylosidase as their functional annotation. The large CBM-like β-sandwich structure in these proteins, however, lacks detailed biochemical characterization. Therefore, one of the aims of this study was to use both truncational and mutational analyses to probe the role of this module in the function of its associated GH43 module.
Fibrobacter succinogenes S85 is a highly active cellulolytic ruminal bacterium (15). Interestingly, the genome of this bacterium also codes for many hemicellulolytic enzymes, despite its limited utilization of hemicellulose (41). To gain insight into this unusual metabolism, we have been studying a hemicellulolytic gene cluster that encodes more than 10 hemicellulose-targeting enzymes in the genome of F. succinogenes S85 (74). In this study, it is demonstrated that a GH43 modular protein (FSU2269) in the cluster (see Fig. S1 in the supplemental material) is an arabinoxylan arabinofuranohydrolase (AXH), which has been named FSUAXH1. Furthermore, the truncational and biochemical studies of this enzyme suggest that the unique β-sandwich domain (XX domain), which shares significant homology with the β-sandwich domains of SXA and XynB3, is important for binding to arabinoxylan. Since the majority of XX domains are only observed in GH43 proteins, we probed the relationship between the two different structural folds. The data presented here demonstrate interdependence between the two folds for substrate binding and suggest discovery of a new form of carbohydrate-binding module, likely composed of the interface between the GH43 module and the XX domain.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Fibrobacter succinogenes subsp. succinogenes S85 was obtained from a culture collection at the Department of Animal Sciences, University of Illinois at Urbana-Champaign (42). F. succinogenes S85 was grown in a synthetic medium (49) under anaerobic conditions. Gene manipulation and plasmid construction were performed in Escherichia coli JM109 (Stratagene, La Jolla, CA). E. coli BL21(DE3) CodonPlus RIPL (Stratagene) was used for gene expression. The E. coli cells were grown aerobically at 37°C in lysogeny broth medium supplemented with appropriate antibiotics.
Gene cloning, expression, and protein purification.
The genomic DNA of F. succinogenes S85 was extracted using a DNeasy tissue kit (Qiagen, Hilden, Germany). The genes of wild-type (WT) FSUAXH1 and its truncational mutant proteins (TM1 to TM7) were amplified from genomic DNA by PCR using Prime STAR HS DNA polymerase (Takara Bio, Otsu, Japan). The primer pairs used for amplifying the WT and its truncated derivatives, TM1, TM2, TM3, TM4, TM5, TM6, and TM7, were F1/R1, F1/R2, F1/R3, F1/R4, F2/R3, F2/R1, F3/R1, and F3/R5 (Table 1; see also Fig. 2, below). Since it is expected that the functional FSUAXH1 protein produced by F. succinogenes cells will lack the predicted signal peptide, which is required for secretion, it was removed by PCR amplification. For the construction of expression vectors, the pET-46 Ek/LIC cloning kit was utilized (Novagen, San Diego, CA). All genes encoding derivatives of FSUAXH1 were sequenced to confirm the integrity of the coding sequence after cloning into the expression vector. The gene expression vectors for FSUAXH1 or its truncated derivatives were introduced individually into E. coli BL21(DE3) CodonPlus RIPL competent cells, and the proteins were expressed as described in our previous study (74). All proteins were purified through the HisTrap FF column (GE Healthcare, Piscataway, NJ). For TM2-W473A, HitrapQ anion exchange chromatography (GE Healthcare) was added to the routine purification step. The buffer was exchanged to 50 mM Na2HPO4-HCl, pH 7.5, and 100 mM NaCl by use of a desalting column (HiPrep 26/10 desalting; GE Healthcare). All columns used in the protein purification steps were fit to an ÄKTAxpress system (GE Healthcare).
TABLE 1.
Primers used in this study
| Primer | Sequence | Expt type |
|---|---|---|
| F1 | 5′-GACGACGACAAGATGGCCGTTAAGGTCAATAACCCG-3′a | Cloning |
| F2 | 5′-GACGACGACAAGATGGGCTACGGCATGGTCACGAGTGAC-3′a | Cloning |
| F2A | 5′-GACGACGACAAGATGGGCAAGTGGTATGCCCTCCTGTTCC-3′a | Cloning |
| F2B | 5′-GACGACGACAAGATGGGTCCGGTTGGCCGTATGTCG-3′a | Cloning |
| F2C | 5′-GACGACGACAAGATGGGATCCAAGGCTCCCTCGACG-3′a | Cloning |
| F3 | 5′-GACGACGACAAGATG GGCGAAAACTGCCCTGCAAATGC-3′a | Cloning |
| R1 | 5′-GAGGAGAAGCCCGGTTACTTTGTTACGGATAGGCGGTGGG-3′a | Cloning |
| R2 | 5′-GAGGAGAAGCCCGGTTAAGCGTTTGCGCCCTTCACGAACG-3′a | Cloning |
| R3 | 5′-GAGGAGAAGCCCGGTTAGCCGTCGAGATAAATTTCATCGTTCAC-3′a | Cloning |
| R4 | 5′-GAGGAGAAGCCCGGTTAGCCCGGGAGCGGAGATTCCGG-3′a | Cloning |
| R5 | 5′-GAGGAGAAGCCCGGTTAGCCAACAATGGGATCCGGATCCG-3′a | Cloning |
| W337A | 5′-GGCGAACTTGCTCTCGAAGCGCAGTTCAACCATAACCCT-3′b | Mutagenesis |
| W348A | 5′-CCATAACCCTGATAACAAAAACGCGAGCTTGTCTGCAAATCCGGG-3′b | Mutagenesis |
| Y465A | 5′-CCGTGGTACTGCATATTTCGCTTACAGCACCGATGGTAGC-3′b | Mutagenesis |
| Y466A | 5′-GGTACTGCATATTTCTATGCCAGCACCGATGGTAGCTCTTGG-3′b | Mutagenesis |
| W473A | 5′-GCACCGATGGTAGCTCTGCGAAAAAGATTGGCAACGATG-3′b | Mutagenesis |
| Y484A | 5′-GGCAACGATGTGAAGCTCAATGCTGACCTCCACATGTTCGTG-3′b | Mutagenesis |
| Y507A | 5′-CCAAGCAGGCGGGCGGCGCTGCAGACTTCGACTGGTTC-3′b | Mutagenesis |
| D148A | 5′-GCAGCTCCCGTTCTACCATGCCCCTTCTTTGTTCTTTGATGAC-3′b | Mutagenesis |
Nucleotides incorporated for exonuclease digestion are underlined.
Nucleotides corresponding to the substituted amino acids in the mutagenesis experiments are underlined.
Bioinformatics analysis.
The genome sequence of F. succinogenes S85 was determined by the North American Consortium for Genomics of Fibrolytic Ruminal Bacteria in collaboration with The Institute for Genomic Research (TIGR) (FibRumba database; http://www.jcvi.org/rumenomics). The Biocyc database collection was also used as a resource for searches of the genome of F. succinogenes S85 (http://biocyc.org/FSUC59374/). A functional domain search was performed to determine the protein family and domain organization by using the Pfam search server (http://www.sanger.ac.uk/Software/Pfam) and NCBI BLAST server (http://www.ncbi.nlm.nih.gov/BLAST). Prediction of signal peptides was performed by using LipoP 1.0 server (http://www.cbs.dtu.dk/services/LipoP). The secondary structure of FSUAXH1 was predicted by using the Advanced Protein Secondary Structure Prediction server (http://imtech.res.in/raghava/apssp). Multiple amino acid sequences were aligned with ClustalW (http://www.ebi.ac.uk/clustalw), and the guide tree was obtained based on the neighbor-joining method using the p-distance model in the DNA Data Bank of Japan (DDBJ; http://clustalw.ddbj.nig.ac.jp/top-e.html). The TreeView software (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html) was utilized to visualize the tree. PDB files were analyzed by the UCSF Chimera molecular graphics program (http://www.cgl.ucsf.edu/chimera). The homology model of the FSUAXH1 TM2 protein was created by using the ModWeb online server (http://modbase.compbio.ucsf.edu/ModWeb20-html/modweb.html).
Enzyme assays and substrate specificity determination.
The specific activity of FSUAXH1 WT was determined at 39°C in a buffer composed of 50 mM Na2HPO4-HCl (pH 7.5) and 100 mM NaCl. The WT protein (5 nM) was incubated with 5 mg/ml of the following polysaccharides: wheat arabinoxylan with low viscosity, sugar beet arabinan, larch arabinogalactan, tamarind seed xyloglucan, konjac glucomannan (Megazyme, Bray, Ireland), oatspelt xylan (Sigma-Aldrich, St. Louis, MO), and carboxymethyl cellulose with an average molecular mass of 90 kDa (Acros Organics, Bridgewater, NJ), and the reducing ends were quantified with a para-hydroxybenzoic acid hydrazide (pHBAH) assay (36). For para-nitrophenol (pNP)-adducted α-l-arabinopyranoside, α-l-arabinofuranoside, β-d-fucopyranoside, β-l-fucopyranoside, α-d-galactopyranoside, β-d-galactopyranoside, α-d-glucopyranoside, β-d-glucopyranoside, β-d-maltopyranoside, α-d-maltopyranoside, α-d-mannopyranoside, β-d-mannopyranoside, α-l-rhamnopyranoside, and β-d-xylopyranoside (Sigma-Aldrich), the enzyme (5 nM) was incubated with a 2 mM concentration of these synthetic substrates, and the production of pNP was continuously monitored at the wavelength of 400 nm. The specific activity was calculated from the initial catalytic rate with linearity. High-performance anion exchange chromatography with pulsed amperometric detection (HPAEC/PAD) was utilized for the identification of the hydrolytic products of arabinoxylan by the WT enzyme. The HPAEC/PAD analysis was carried out on a System Gold high-performance liquid chromatography instrument (Beckman Coulter, Fullerton, CA) equipped with a CarboPac PA1 guard column (4 by 50 mm) and a CarboPac PA1 analytical column (4 by 250 mm) from Dionex Corporation (Sunnyvale, CA) and a Coulochem III electrochemical detector (ESA Biosciences, Chelmsford, MA). Arabinose, xylose and xylo-oligosaccharides (Megazyme) were used as standards. These saccharides were resolved by using a mobile phase of 100 mM NaOH with a linear gradient from 0 to 300 mM sodium acetate (NaOAc) for 30 min (13).
pH and temperature profiles for enzyme activity.
The pH effect on the WT protein was examined at 39°C in the presence of 50 mM citrate-NaOH (pH 3.5 to 6.0), 50 mM Na2HPO4-HCl (pH 6.0 to 8.0), and bicine-NaOH (pH 8.0 to 9.0). The temperature profile was determined in 50 mM Na2HPO4-HCl, pH 7.5, and 100 mM NaCl at temperatures between 20°C and 55°C with 5°C increments. The WT protein (5 nM) was incubated with 5 mg/ml of arabinoxylan under each condition, and the initial rate was determined. The pHBAH assay was applied for the measurement of reducing sugar ends released.
Kinetic analysis.
A 5 nM concentration of protein was incubated with various concentrations of arabinoxylan, and the released arabinose was quantified with the pHBAH assay. Initial rates were plotted against arabinoxylan concentrations, and the kinetic parameters were determined by the Michaelis-Menten equation utilizing Graph Pad Prism v5.01 (GraphPad Software, San Diego, CA).
Affinity gel electrophoresis.
Qualitative analysis to determine binding of FSUAXH1 proteins to arabinoxylan was performed by affinity gel electrophoresis as described by Tomme et al. (59). Proteins were resolved on a nondenaturing 5% polyacrylamide gel with and without 0.1% (wt/vol) arabinoxylan. The electrophoresis was carried out at 80 V at room temperature. Proteins were visualized by staining with Coomassie brilliant blue G-250. A high molecular weight (HMW) calibration kit for native gel electrophoresis (GE Healthcare) was used for the marker.
ITC.
For isothermal titration calorimetry (ITC), measurements were made at 25°C by using a VP-ITC calorimeter (MicroCal Inc., Northhampton, MA) following the manufacturer's recommended procedures. To minimize the heat of dilution between protein and ligand samples, purified proteins were dialyzed against 50 mM Na2HPO4-HCl buffer (pH 7.5) with a 10-kDa cutoff dialysis tube, and ligands were dissolved in the exterior buffer in the dialysis. The protein sample (50 μM), in an ∼1.5-ml reaction cell, was injected with 5 μl, followed by 28 successive 10-μl aliquots of ligand (1.2 mg/ml arabinoxylan, 2 mM arabinose, or 2 mM xylohexaose) at 300-s intervals. Data were fit by nonlinear regression using a single-site model (MicroCal Origin), and thermodynamic parameters were calculated using the Gibbs free energy equation (ΔG = ΔH − TΔS) and ΔG = −RT lnKa, where R is the gas constant and T is temperature. From the results of our affinity gel electrophoresis and ITC, the number of binding sites was presumed to be one. The molar concentration of binding sites on arabinoxylan was determined by altering the ligand concentration in the model fitting until the number of binding sites on the protein (n) was equal to one, as proposed by Szabó et al. (57).
Site-directed mutagenesis.
Site-directed mutagenesis was carried out using the QuikChange multisite-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. Primers used in the site-directed mutagenesis study are presented in Table 1.
CD spectra.
Determination of circular dichroism (CD) spectra for the FSUAXH1 TM2 protein and its site-directed mutant proteins was carried out using a J-815 circular dichroism spectropolarimeter (Jasco, Tokyo, Japan). Protein samples were prepared at a concentration of 0.1 mg/ml in 20 mM NaH2PO4 buffer (pH 7.5) (29). For the measurements, a quartz cell with a path length of 0.1 cm was utilized. CD scans were carried out at 25°C from 190 nm to 260 nm at a speed of 50 nm/min with a 0.1-nm wavelength pitch, with five accumulations. Data files were analyzed on the DICHROWEB online server (http://www.cryst.bbk.ac.uk/cdweb/html/home.html) using the CDSSTR algorithm with reference set 4, which is optimized for analysis of data recorded in the range of 190 nm to 240 nm (37).
Synthesis, cloning, expression, purification, and enzyme assays of XynB3 and SXA.
The genes for XynB3 from Geobacillus stearothermophilus (GenBank accession number ABI49959) and that for SXA from Selenomonas ruminantium (GenBank accession number AAB97967) were commercially synthesized with codon optimization for expression in E. coli cells (Integrated DNA Technologies, Coralville, IA). The primer pairs used for amplifying XynB3 and SXA were XynB3-F/XynB3-R and SXA-F/SXA-R (see Table S1 in the supplemental material). The construction of the expression plasmids, the purification of the gene products, and the assays for the enzymes followed the methods described for the FSUAXH1 wild-type protein.
Cloning, expression, and substrate binding of FPm-1 homologs.
The primers used for cloning the FPm-1 modules appended to FSUAXH1, FSU2263, FSU2265, and FSU2272 are shown in Table S1 of the supplemental material. The cloning, expression, purification of the recombinant proteins and the binding activities were the same as described earlier for FSUAxe6B (74).
Nucleotide sequence accession number.
The nucleotide sequence of the gene encoding FSUAXH1 can be found in the GenBank database under accession number ACX75362.
RESULTS
Characterization of the catalytic activities of FSU2269.
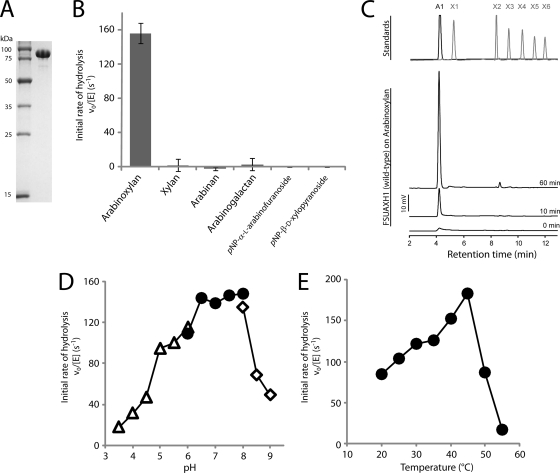
Based on amino acid sequence homology, the FSU2269 protein of Fibrobacter succinogenes S85 was determined to be a member of the GH43 family. The GH43 proteins are known to exhibit activities including β-xylosidase (EC 3.2.1.37), β-1,3-xylosidase (EC 3.2.1.72), α-l-arabinofuranosidase (EC 3.2.1.55), arabinanase (EC 3.2.1.99), xylanase (EC 3.2.1.8), and galactan 1,3-β-galactosidase (EC 3.2.1.145). To determine the enzymatic activities of FSU2269, the protein was overexpressed in E. coli and purified to near homogeneity (Fig. 1 A). The recombinant protein migrated between the 100-kDa and 75-kDa markers, which was in agreement with the molecular mass (84.3 kDa) predicted from the polypeptide sequence. Hydrolysis of the general substrates for GH43 enzymes (arabinoxylan, xylan, arabinan, arabinogalactan, pNP-α-l-arabinofuranoside, and pNP-β-d-xylopyranoside) was tested by incubating each substrate with FSU2269, and the specific activities were determined based on the reducing sugar ends or the amount of pNP released as end products. The protein exhibited very high enzymatic activity on arabinoxylan (Fig. 1B). To gain further insights into the catalytic activity of FSU2269, additional activity screening was carried out with carboxymethyl cellulose, xyloglucan, glucomannan, and other pNP-adducted substrates as described in Materials and Methods. No activities were detected with these substrates (data not shown). To identify the precise linkage in arabinoxylan that is cleaved by FSU2269, the degradation products were analyzed by HPAEC/PAD. As shown in Fig. 1C, arabinose accumulated in the reaction mixture in a time-dependent manner. These results indicated that FSU2269 cleaves the arabinose linked to the β-1,4-xylose backbone of arabinoxylan, and therefore the enzyme is an α-l-arabinofuranosidase, i.e., an enzyme that cleaves the α-1,2, α-1,3, and/or α-1,5 linkage(s) in hemicelluloses containing arabinose. The type A forms of these enzymes are active on small compounds such as pNP-α-l-arabinofuranoside and arabino-oligosaccharides. In addition to these substrates, the type B enzymes can also hydrolyze polymeric substrates such as branched arabinan and arabinoxylan (45). Arabinoxylan arabinofuranohydrolases (AXHs) are members of type B enzymes; however, they are specific for arabinoxylan hydrolysis (7, 28, 30, 31, 54, 64, 66, 67). Therefore, based on this nomenclature, we designated FSU2269, which showed activity only on arabinoxylan, as Fibrobacter succinogenes arabinoxylan arabinofuranohydrolase, or FSUAXH1.
FIG. 1.
FSUAXH1 (FSU2269) catalyzes the cleavage of arabinose moieties from arabinoxylan. (A) SDS-PAGE showing purified recombinant FSUAXH1 wild-type protein. The protein was purified as described in Materials and Methods, and 2.5 μg of protein was loaded on a 12.5% polyacrylamide gel and stained with Coomassie brilliant blue G-250. (B) Catalytic activities of FSUAXH1. Substrates in sodium phosphate buffer were incubated with FSUAXH1, and the initial catalytic rates were determined. Bars are shown with standard errors for three independent experiments. (C) Time course of cleavage of arabinose from arabinoxylan by FSUAXH1. Arabinoxylan (0.5% [wt/vol]) in sodium phosphate buffer was incubated with 5 nM FSUAXH1 for 0, 10, and 60 min, and the products of hydrolysis were analyzed by HPAEC/PAD. Standards of arabinose (A1), xylose (X1), and xylooligosaccharides (X2 to X6) were resolved under the same conditions, and the retention times are shown in the upper panel. (D) Effect of pH on hydrolysis of arabinoxylan by FSUAXH1. Reactions were carried out at 39°C in 50 mM citrate-NaOH (open triangles; pH 3.5 to 6.0), 50 mM Na2HPO4-HCl (closed circles; pH 6.0 to 8.0), and bicine-NaOH (open diamonds; pH 8.0 to 9.0). (E) Effect of temperature on hydrolysis of arabinoxylan by FSUAXH1. Reactions were performed in 50 mM Na2HPO4-HCl (pH 7.5) and 100 mM NaCl buffer at temperatures ranging from 20°C to 55°C.
Optimum pH and temperature of FSUAXH1.
The pH and temperature optima of FSUAXH1 were investigated. The enzyme exhibited maximal activity in the pH range of 6.5 to pH 8.0 (Fig. 1D). Therefore, we employed a buffer at pH 7.5 for all of the experiments in this study, and at this pH the optimum temperature for activity of FSUAXH1 was 45°C (Fig. 1E).
Carbohydrate-binding activity of FSUAXH1 and delineation of the binding module.
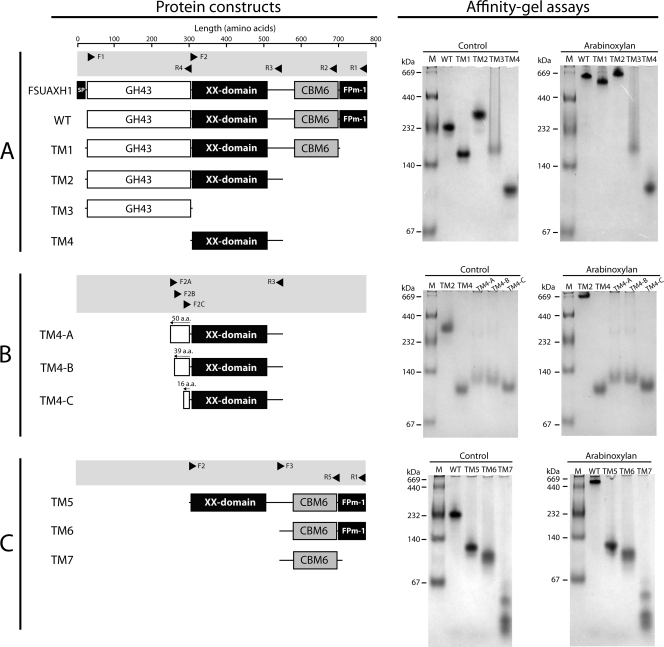
FSUAXH1 is a modular protein (Fig. 2 A), composed of a signal peptide, a GH43 catalytic module, a β-sandwich-shaped unassigned domain (XX domain), a CBM6, and FPm-1. A member of FPm-1 was recently assigned a function as a carbohydrate-binding module (74). Since arabinoxylan serves as a good substrate for catalysis by FSUAXH1, it was investigated as a substrate for carbohydrate binding by its CBM6. As shown in Fig. 2A, incorporating arabinoxylan into the native gel at 0.1% (wt/vol) retarded the mobility of FSUAXH1, showing that the protein binds to soluble arabinoxylan (Fig. 2A, WT affinity gel electrophoresis). To delineate the binding module(s) in FSUAXH1, a gene truncation strategy was utilized. The truncated proteins were made by selecting glycines or alanines in loop regions, determined by secondary structure prediction, as terminal amino acids for the protein constructs. As shown in Fig. 2A, if the construct devoid of the predicted signal peptide (SP) is considered the WT polypeptide, four truncated derivatives (TM1, TM2, TM3, and TM4) were initially made. All truncated derivatives of FSUAXH1 were successfully expressed in E. coli as soluble proteins and purified. Affinity gel electrophoresis was then performed with FSUAXH1 WT, TM1, TM2, TM3, and TM4 to delineate the region harboring the carbohydrate-binding activity. Surprisingly, the results showed that truncating the two CBMs, i.e., CBM6 and FPm-1, did not abolish arabinoxylan binding by FSUAXH1. On the other hand, TM3 and TM4 completely lost their binding activities. We reasoned that the XX domain may harbor the CBM that binds to arabinoxylan. Thus, further truncational mutant proteins that extended the length of the polypeptide were made in an attempt to delineate a functional CBM construct of the XX domain. However, all constructs (Fig. 2B) resulted in polypeptides incapable of binding to arabinoxylan. The carbohydrate-binding activity, therefore, appears to be derived from both the GH43 module and the XX domain. To determine the residues that may be important for carbohydrate-binding activity in TM2 (Fig. 2A), conserved large aromatic residues, known to be critical for carbohydrate binding, were identified. Special emphasis was placed on the XX domain, which harbors mostly β-sheets according to published structures (8, 9). The polypeptides of the top nine BLAST hits, using the XX domain of FSUAXH1 for the search, were selected and aligned with the XX domain of FSUAXH1 (Fig. 3 A). Among the conserved aromatic residues, tryptophans (W337, W348, and W473) and tyrosines (Y465, Y466, Y484, and Y507), which generally play a critical role in binding by forming hydrophobic and stacking interactions with the sugars in the carbohydrate polymer (5), were selected for mutagenesis. Each of the listed residues was replaced with alanine in the TM2 derivative (Fig. 2A) by site-directed mutagenesis. Hence, the following mutants of TM2 were made: TM2-W337A, TM2-W348A, TM2-Y465A, TM2-Y466A, TM2-W473A, TM2-Y484A, and TM2-Y507A. The W473A mutant protein was mostly observed in inclusion bodies in E. coli cells. However, the other mutant proteins were highly expressed and were also soluble. All mutants, including W473A, were purified and examined by affinity gel electrophoresis for the ability to bind to arabinoxylan. As shown in Fig. 3B, except for TM2-Y484A, which completely lost the capacity to bind to arabinoxylan, the other mutants retained the carbohydrate-binding property. The results, therefore, suggested that residue Y484 is essential for binding to arabinoxylan, and furthermore the XX domain is a component of the CBM that recognizes soluble arabinoxylan. CD spectral data for the TM2 and TM2-Y484A proteins were similar, suggesting that the inability of the mutant to bind to arabinoxylan was not due to major changes in secondary structural elements triggered by the mutation (see Table S2 in the supplemental material).
FIG. 2.
Truncational analysis to delineate the arabinoxylan-binding region in FSUAXH1. (Left panels) Schematic representations of the truncated constructs of FSUAXH1. The primers used for the amplification of the genes are shown above the schematic for the protein constructs, and the primer nucleotide sequences are presented in Table 1. The arrowheads display the 5′ to 3′ direction of oligonucleotides. (Right panels) Qualitative binding analysis results for FSUAXH1 and its truncational derivatives by affinity gel electrophoresis. Two micrograms of each protein was resolved on a nondenaturing 5% polyacrylamide gel containing 0% (control) or 0.1% (wt/vol) arabinoxylan and stained with Coomassie brilliant blue G-250. The reason for the slower migration of TM2 (in the control) is currently unknown.
FIG. 3.
Site-directed mutational analysis of conserved large aromatic residues in domain XX, in order to determine residues important for carbohydrate binding. (A) Amino acid sequence alignments of the FSUAXH1 XX domain and its homologous peptides. The amino acid sequences were obtained through an NCBI BLAST search and aligned with that of domain XX of FSUAXH1 by utilizing ClustalW. The conserved tyrosines (Y) and tryptophans (W) are indicated by arrows. The amino acid numbers are based on the numbering of the FSUAXH1 polypeptide. The NCBI accession numbers (source of organism) were as follows: YP_001181179 (Caldicellulosiruptor saccharolyticus DSM 8903), YP_002572107 (Anaerocellum thermophilum DSM 6725), ZP_02421660 (Eubacterium siraeum DSM 15702), ZP_03678241 (Bacteroides cellulosilyticus DSM 14838), ZP_03013018 (Bacteroides intestinalis DSM 17393), ZP_03458529 (Bacteroides eggerthii DSM 20697), YP_001196205 (Flavobacterium johnsoniae UW101), YP_678646 (Cytophaga hutchinsonii ATCC 33406), and YP_003012971 (Paenibacillus sp. JDR-2). The output files were entered into the BoxShade version 3.21 program (http://www.ch.embnet.org/software/BOX_form.html), with the fraction of sequences that must agree for shading set at 1.0. The conserved amino acids are shaded black, and similar amino acids are shaded gray. (B) Binding assay for FSUAXH1 TM2 and its site-directed mutant proteins. The proteins (1 μg/lane) were resolved on a nondenaturing 5% polyacrylamide gel containing 0% (control) or 0.1% (wt/vol) arabinoxylan and stained with Coomassie brilliant blue G-250.
Quantitative binding studies to delineate the CBM in FSUAXH1.
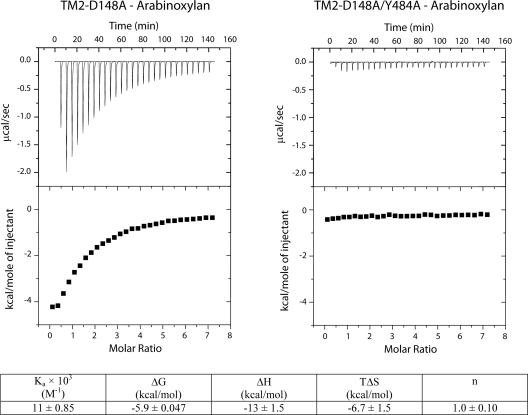
ITC, a quantitative approach, was used to further investigate the location of the CBM in FSUAXH1. Recent reports based on biochemical (50) and structural (8) studies on a GH43 protein revealed that an aspartate located in this module contributes to binding and orientation of the ligand during catalysis. The aspartate is conserved in FSUAXH1 as D148. Site-directed mutagenesis was, therefore, used to generate a D148A mutant of FSUAXH1 TM2 (TM2-D148A), and as shown in Table 2, this mutation abolished detectable enzymatic activity, while TM2 exhibited activity similar to the WT. The TM2-D148A mutant, which should be severely impaired in terms of heat generation due to catalysis, was used in ITC experiments to determine heat generation contributed by its noncatalytic binding, i.e., heat emanating from the binding of the CBM to the substrate. In addition, a double mutant (TM2-D148A/Y484A) protein was created. Since Y484A, as demonstrated in Fig. 3B, loses the function of the CBM, we reasoned that comparison of TM2-D148A to TM2 D148A/Y484A should allow determination of the contribution of Y484 in carbohydrate binding by the TM2 construct. The TM2-D148A migrated slightly faster in an arabinoxylan gel than TM2, suggesting some differences in the two derivatives of FSUAXH1. However, TM2-D148A still retained binding to arabinoxylan, whereas the double mutant (TM2-D148A/Y484A) lacked binding to this substrate (see Fig. S2 in the supplemental material). In the ITC studies, there was clearly heat evolution in the titration of arabinoxylan against TM2-D148A, indicating binding of the protein to the ligand (Fig. 4). In contrast, TM2-D148A/Y484A exhibited only slight heat generation, which likely represented the heat of dilution, and thus showed that this mutant protein no longer interacts with arabinoxylan (Fig. 4). These results indicate that residue Y484 is critical for binding to arabinoxylan, in agreement with the results obtained with the native gel assays (Fig. 3B). In agreement with this finding, Y484 is not conserved in SXA and XynB3 (see Fig. 6A), which lacks binding activity to arabinoxylan (data not shown). The binding parameters of TM2 were calculated from the data obtained by subtracting the raw data of TM2-D148A/Y484A from that of TM2-D148A. The binding parameters, ΔH and TΔS, between arabinoxylan and the TM2 carbohydrate-binding region, are negative, and thus the thermodynamic interaction is driven by a favorable enthalpy (ΔH < 0) and a compensating unfavorable entropy (TΔS < 0) with a net result of a favorable ΔG (Fig. 4). Arabinose and xylohexaose, constituents of arabinoxylan, were tested as ligands; however, no binding signals were detected (data not shown). These results suggest that the binding region recognizes some unique linkages within soluble arabinoxylan.
TABLE 2.
Kinetic parameters of FSUAXH1 proteinsa
| FSUAXH1 protein form | kcat (s−1) | Km (mg/ml) | kcat/Km [s−1 (mg/ml)−1] |
|---|---|---|---|
| WT | 240 ± 23 | 4.1 ± 1.0 | 58 ± 16 |
| TM1 | 360 ± 40 | 5.4 ± 1.6 | 67 ± 21 |
| TM2 | 310 ± 21 | 3.4 ± 0.70 | 89 ± 19 |
| TM2-Y484A | 630 ± 120 | 24 ± 7.1 | 27 ± 10 |
| TM2-D148A | NDb | ||
| TM2-D148A/Y484A | ND | ||
| TM3 | ND |
Data are means ± standard errors based on the nonlinear regression model.
ND, not detected; activity was below the detection limit of the assay.
FIG. 4.
ITC analysis of FSUAXH1 TM2 mutants with soluble wheat arabinxylan as ligand. The ITC experiments were performed with an initial titration of 5 μl followed by 28 successive 10-μl aliquots of ligand (1.2 mg/ml arabinoxylan) into 50 μM protein sample at 25°C. The binding parameters shown in the figure were derived by subtracting the results for TM2-D148A/Y484A from those for TM2-D148A. The data should, therefore, provide estimates of the binding parameters of the CBM activity within TM2. The molar ratio of ligand indicates the concentration of binding sites on arabinoxylan. Affinity gel electrophoresis for the proteins is presented in Fig. S2 of the supplemental material.
Effect of attenuation of substrate binding on catalytic efficiency of FSUAXH1.
In order to investigate the role of carbohydrate binding for TM2 to the catalytic activity of the enzyme, kinetics studies for the FSUAXH1 TM2 protein and its substrate-binding-deficient mutant, TM2-Y484A, were performed. Using arabinoxylan as a substrate, the initial rate for release of arabinose was fit to the Michaelis-Menten equation. The TM2 protein showed a turnover rate of 310 s−1 and a Km of 3.4 mg/ml, resulting in a kcat/Km of 89 s−1 (mg/ml)−1. Although the TM2-Y484A mutant exhibited an increased turnover rate (630 s−1), the Km of the mutant protein for arabinoxylan also increased about 7-fold (Km, 24 mg/ml), leading to a lower catalytic efficiency (kcat/Km, 27 s−1 [mg/ml]−1) than that of TM2 (Table 2). These results clearly showed the importance of having an intact XX domain for the function of FSUAXH1. The kinetic parameters of FSUAXH1 WT and TM1 (Table 2) were also determined. In this case, the truncational mutant did not exhibit the large differences observed with TM2 and its mutant, TM2-Y484A.
Phylogenetic analysis of GH43 domains.
A critical residue, Y484, for arabinoxylan binding by FSUAXH1 is located in its XX domain. To investigate the distribution of the XX domain in known polypeptide sequences, especially GH43 module-carrying polypeptides, the amino acid sequence of the GH43 module of FSUAXH1 was used to search the protein databases of Pfam and NCBI. Interestingly, different modular organizations were observed for the retrieved polypeptides. The GH43 modules were phylogenetically analyzed. However, since there is a large number of this family of proteins in the database (>950 entries in the CAZy database [http://www.cazy.org/]), only proteins with Enzyme Commission (EC) numbers in addition to 10 GH43 proteins in the genome of F. succinogenes S85 (FSU0192, FSU2262, FSU2263, FSU2264, FSU2269 or FSUAXH1, FSU2274, FSU2517, FSU2520, FSU3191, and FSU3192) were used in our analysis. The phylogenetic tree is shown in Fig. 5 A. On examination of the domain architectures of the different clusters in the GH43 protein tree, a unique clustering was observed. In cluster A, we found only polypeptides that have the XX domain appended C-terminally to the GH43 module (Fig. 5A). In cluster B, except for one protein, all polypeptides were composed of only the GH43 catalytic module, and in cluster C, the proteins were a mixture of those with only a GH43 catalytic module and another type that has a CBM6 at the C-terminal end of the GH43 module. From the phylogenetic analysis, it can be concluded that the most frequently seen modules in association with the GH43 catalytic modules are the CBM family 6 and the XX domains. Therefore, based on the presence or absence of these domains, the GH43 proteins can be classified into four types, as shown in Fig. 5B. These are type I (most members have a GH43 stand-alone module), type II (GH43 module fused at its C terminus to a CBM6), type III (GH43 module fused at its C terminus to an XX domain), and type IV (a fusion derived from types I to III).
FIG. 5.
Diversity of GH43 modules or proteins. (A) Unrooted phylogenetic tree of GH43 proteins. The eukaryotic and bacterial GH43 proteins with EC numbers in the CAZy database and GH43 proteins from F. succinogenes S85 obtained from the FibRumba database were used to generate the phylogenetic tree. GH43 domains of the retrieved proteins were aligned by utilizing ClustalW. The sequence alignments are shown in Fig. S3 of the supplemental material. The GenBank accession numbers (source of protein) were as follows: ACE84667 (Cellvibrio japonicus Ueda107) (39), BAC68753 (Streptomyces avermitilis MA-4680) (21), BAA90772 (Streptomyces chartreusis GS901) (38), BAF98235 (Vibrio sp. XY-214) (60), AAF66622 (Azospirillum irakense KBC1), CAB13699 (Bacillus subtilis subsp. subtilis ATCC 6051) (7), AAB08024 (Bacteroides ovatus V975), AAO75476 (Bacteroides thetaiotaomicron VPI-5482), AAO67499 (Bifidobacterium adolescentis DSM20083) (64), AAA63610 (Butyrivibrio fibrisolvens GS113) (61), AAB87371 (Caldicellulosiruptor saccharolyticus), BAA02527 (Clostridium stercorarium F-9) (48), CAD48310 (Clostridium stercorarium NCIMB 11745) (2), AAB97967 (Selenomonas ruminantium GA192) (25), ACF39706 (uncultured bacterium) (70), ABB92159 (uncultured bacterium) (68), BAB07402 (Bacillus halodurans C-125) (52), AAC97375 (Bacillus pumilus PLS) (34), CAA29235 (Bacillus pumilus IPO) (72), AAC27699 (Bacillus sp. KK-1) (26), AAB41091 (Bacillus subtilis), BAC87941 (Clostridium stercorarium F-9) (55), ABI49959 (Geobacillus stearothermophilus T-6) (50), ABC75004 (Geobacillus thermoleovorans IT-08) (69), CAA89208 (Prevotella bryantii B14) (16), BAA20372 (Bacillus subtilis IFO3134), CAA99586 (Bacillus subtilis subsp. subtilis 168T+) (35), CAB15969 (Bacillus subtilis subsp. subtilis 168T+) (23), ACE73676 (Geobacillus stearothermophilus T-6) (3), BAB64339 (Bacillus thermodenitrificans TS-3) (58), ABN51896 (Clostridium thermocellum ATCC 27405) (20), BAC69820 (Streptomyces avermitilis MA-4680) (19), AAB95326 (Caldicellulosiruptor sp. Rt69B.1), AAD30363 (Caldicellulosiruptor sp. Tok7B.1), CAA40378 (Paenibacillus polymyxa) (17), AAG27441 (Aspergillus aculeatus 101.43) (51), EAA58736 (Aspergillus nidulans FGSC A4) (4), EAA58810 (Aspergillus nidulans FGSC A4) (4), AAA32682 (Aspergillus niger) (46), CAK49041 (Aspergillus niger) (46), BAD89094 (Penicillium chrysogenum 31B), BAD15018 (Penicillium chrysogenum 31B) (47), BAE55732 (Aspergillus oryzae RIB40) (56), AAC67554 (Cochliobolus carbonum) (71), XP_391644 (Gibberella zeae PH-1), BAC75546 (Penicillium herquei IFO 4674) (24), XP_391670 (Gibberella zeae PH-1), CAL81199 (Humicola insolens DSM 18000) (54), ACP50519 (Penicillium purpurogenum MYA-38), BAH29957 (Irpex lacteus NBRC5367) (32), and BAD98241 (Phanerochaete chrysosporium) (22). The identifications of the proteins from F. succinogenes S85 were as denoted in the FibRumba database (http://www.jcvi.org/rumenomics). An abbreviation for the EC number(s) for each protein is shown in the square brackets. The abbreviations are as follows: [8], EC 3.2.1.8 (xylanase); [37], EC 3.2.1.37 (β-1,4-xylosidase); [55], EC 3.2.1.55 (α-l-arabinofuranosidase); [72], EC 3.2.1.72 (β-1,3-xylosidase); [99], EC 3.2.1.99 (arabinanase); [145], EC 3.2.1.145 (galactan 1,3-β-galactosidase). When there is biochemical evidence for the enzymatic activity, the numbers in the square brackets denoting the particular activity are underlined, and the references are included in the legend above, after the GenBank accession numbers. Bar, 0.1 amino acid substitutions per single site. The bootstrap values are shown at the branch points. Differences in domain organizations of proteins are represented by the different colors: green (type I), blue (type II), red (type III), and black (type IV). (B) Classification of GH43 proteins based on the presence or absence of the commonly associated modules (CBM6 or XX domain). The GH43 proteins were grouped into type I (composed mostly of GH43 stand-alone module), type II (GH43 module fused at the C terminus to a CBM6), type III (GH43 module fused at the C terminus to an XX domain), and type IV (a more complex GH43 protein with a modular architecture outside types I, II, and III). Only the GH43 module was used in the phylogenetic analysis in panel A.
Biochemical analysis of SXA and XynB3 proteins.
The genes for XynB3 and SXA were commercially synthesized and expressed and the gene products were purified. In the enzymatic assay for synthetic small substrates (pNP-β-d-xylopyranoside and α-l-arabinofuranosidase), while FSUAXH1 TM2 did not show detectable catalytic activities with these substrates, XynB3 and SXA were able to release pNP from both substrates (see Fig. S4A in the supplemental material). In contrast, SXA and XynB3 lacked detectable activity when the polysaccharide wheat arabinoxylan was used as the substrate (see Fig. S4B).
The FPm-1 domain of FSUAXH1 binds to insoluble xylan.
It was reported that the FPm-1 domain of an F. succinogenes S85 acetyl xylan esterase binds to insoluble oatspelt xylan (74). Since most of the genes in the F. succinogenes S85 hemicellulose-targeting gene cluster encode FPm-1-containing gene products (see Fig. S1A in the supplemental material), we investigated the capacity of the FPm-1 domain of FSUAXH1 (Fig. 2) to bind to either cellulose (Avicel PH-101) or insoluble xylan. In addition to the FPm-1 of FSUAXH1, FPm-1 homologs from three different genes in the gene cluster were cloned, expressed, and purified for substrate-binding analysis. Similar to the previously characterized FPm-1 from the acetyl xylan esterase, the homologs in FSUAXH1 and all other expressed FPm-1 peptides bound to insoluble xylan and not to cellulose (data not shown).
DISCUSSION
In the present study, the structure/function of a modular GH43 protein encoded in the F. succinogenes S85 hemicellulose-targeting gene cluster (see Fig. S1A in the supplemental material) was analyzed through delineating its modules and investigating their contributions to catalytic and binding activities. The F. succinogenes S85 GH43 protein exhibited AXH activity and was designated FSUAXH1.
Two crystal structures of type III GH43 proteins, a β-xylosidase from Geobacillus stearothermophilus (XynB3; GenBank accession number ABI49959) (8) and a β-xylosidase from Selenomonas ruminantium (SXA; GenBank accession number AAB97967) (9), have been solved. Therefore, these structures provide important insights into the functional relationship between the GH43 catalytic module and the XX domain found to constitute a novel carbohydrate-binding module in the present study. Brüx and coworkers (8) observed that a loop (residues 494 to 507) (Fig. 6A) in the XynB3 β-sandwich domain (XX domain) intrudes into the β-propeller domain (catalytic domain) (Fig. 6B). The authors noted that the loop is positioned in the vicinity of the active site pocket and suggested that the loop restricts the size of the substrate, leading to the exo mode of catalysis by the enzyme, with xylo-oligomers serving as substrates (8). In contrast, in this study, we clearly showed that FSUAXH1, a type III GH43 protein (Fig. 5B), is specifically active on soluble arabinoxylan, a polymeric substrate. Although XynB3 and SXA exhibited β-xylosidase and α-l-arabinofuranosidase activities on simple substrates (pNP-β-d-xylopyranoside and pNP-α-l-arabinofuranoside), unlike FSUAXH1, the two enzymes lacked activity on wheat arabinoxylan, a more complex substrate. It is likely that the region around the loop is key to the differences observed in the substrate specificities between XynB3, SXA, and FSUAXH1. In accordance with this observation, the amino acid sequence alignment of XynB3, SXA, and FSUAXH1 showed a gap (four missing amino acids) in FSUAXH1 within the loop region compared to XynB3 and SXA (Fig. 6A). In addition to this gap, three amino acid residues N-terminal to the loop are also missing. Thus, the loop in FSUAXH1 is likely to be shorter than those of XynB3 and SXA. More importantly, the amino acid residue Y484, located in the XX domain of FSUAXH1 and determined in this study as being critical to arabinoxylan binding, is present in the region corresponding to the aforementioned loop in XynB3. Further evidence that this region is important for substrate binding and likely substrate specificity is demonstrated by the results obtained for the TM3 construct of FSUAXH1. This truncated derivative contains the GH43 catalytic module (Fig. 2A) and failed to bind to the polymeric substrate and also exhibited no detectable catalytic activity (Table 2). Thus, the juxtaposition of the shorter loop in the β-sandwich (XX domain) to the side of the β-propeller (GH43) may play an important role for a polysaccharide serving as substrate for FSUAXH1 (Fig. 6B).
FIG. 6.
Comparisons of amino acid sequences and three-dimensional (3D) structures of the GH43 proteins harboring the XX domain. (A) Amino acid sequence alignment of SXA, XynB3, and FSUAXH1. Sequences were aligned with ClustalW, and the output files were entered into the BoxShade version 3.21 program, with the fraction of sequences that must agree for shading set at 1.0. The conserved amino acids are shaded black, while similar amino acids are shaded gray. The Y484 residue of FSUAXH1, which was demonstrated as being critical to binding to arabinoxylan in this study, is marked by an asterisk. Phylogenetic positions of the three proteins (cluster A) are also shown in Fig. 5A. (B) 3D structures of GH43 proteins with an XX domain. The 3D structures of XynB3 and SXA were obtained from the Protein Data Bank (http://www.pdb.org/pdb/home/home.do). The 3D homology model of FSUAXH1 TM2 was created based on the crystal structure of the β-xylosidase from Bacillus halodurans C-125 (PDB file, 1YRZ). Regions corresponding to the loop in XynB3 (494 to 507) that may impact substrate specificity are highlighted in magenta. The side chain of the Y484 residue in FSUAXH1 TM2 is indicated with an arrow.
Based on our phylogenetic analysis, we postulate that three different lineages (clusters A, B, and C) of GH43 modules have evolved from an ancestral module (Fig. 5A). The members of cluster A have coevolved with or acquired a module designated in the present study as the XX domain, and the enzymatic activities presented by this group are β-1,4-xylosidase and α-l-arabinofuranosidase. Members of cluster B are shorter polypeptides and most of them are composed of only a GH43 module, and almost invariably they encode arabinanases. Cluster C, on the other hand, presents more diversity, with some of the polypeptides composed of only the GH43 module, whereas others have acquired a CBM6, which may or may not be related to the CBM6 rarely seen appended to the XX domain associated with members of cluster A. Interestingly, AAB87371, which we designated type IV, has two GH43 domains, one at the N-terminal half and the other at the C-terminal half of the polypeptide (Fig. 5B). The N-terminally and C-terminally located modules appear to derive from cluster A and cluster C of our phylogenetic tree, respectively. It is likely that the AAB87371 polypeptide evolved through the fusion of two genes. The gene at the N-terminal half encoded a type III protein, and the gene at the C-terminal half encoded a type II protein (Fig. 5B). However, a CBM4_9 is inserted at the junction between the GH43 module and XX domain. Other than this unusual organization, the XX domain found in the current protein databases are always C-terminally juxtaposed to a cluster A GH43, hence suggesting coevolution of the two modules. It is noteworthy that on the phylogenetic tree, although XynB3, SXA, and FSUAXH1 were members of cluster A, XynB3 and SXA clustered on the same branch while FSUAXH1 was located on a different branch.
Structural and thermodynamic studies on CBM6 (1, 6, 11, 18, 44, 62, 63) have shown that this module by itself is functionally active. It would be interesting to compare the biochemistry of a cluster A GH43 fused directly at the C terminus to a CBM6 with a typical cluster A GH43 polypeptide (GH43 module/XX domain). However, as can be deduced from Fig. 5A and B, the former organization is rare, supporting the hypothesis of the coevolution of the XX domain with its unique GH43 module (Fig. 5). It is also of significance that unlike the XX domain, which is limited to association with GH43 modules, CBM6s are observed in association with diverse glycoside hydrolase and carbohydrate esterase families (1, 40).
The roles of the CBM6 and FPm-1 domains in the hydrolysis of arabinoxylan by FSUAXH1 were not characterized in detail in this study. However, the truncational analysis depicted in Fig. 2 shows that the CBM6 and the FPm-1 regions do not participate in the binding of soluble arabinoxylan, which was identified as a substrate for FSUAXH1 in this study. By demonstrating that the FSUAXH1 FPm-1 binds to insoluble xylan, it is reasonable to suggest that FSUAXH1 is equipped to recognize both insoluble xylan through its FPm-1 module and soluble xylan through the interface of the GH43/XX domain, leading to a very efficient enzyme.
A relevant question here is: why is a bacterium that only partially uses hemicellulose and its products of hydrolysis equipped to degrade this structural plant cell wall polysaccharide? The finding of a hemicellulose-targeting gene cluster and the demonstration of functional activities previously by our group and by the Forsberg group (27, 74) of acetylxylan esterases, and now in this report an α-l-arabinofuranosidase, suggest the model depicted in Fig. S1B in the supplemental material. In the cow rumen the bacterium encounters lignocellulose, a complex structure with cellulose, which represents the preferred substrates of the bacterium, protected by hemicellulose. This highly efficient cellulose degrader has, therefore, acquired the versatility to degrade hemicellulose (but not fully utilize it), but rather to access the cellulose, which serves as its main carbon and energy source. Despite the availability of the F. succinogenes S85 genome, still little is understood of how it releases fermentable sugars from the crystalline cellulose Avicel. Thus, to further our understanding of the enigmatic mechanism by which F. succinogenes S85 derives energy from crystalline cellulose, our laboratory is currently using transcriptomic analyses, based on microarrays and RNAseq, to search for clues. The current report, including the model presented in Fig. S1B of the supplemental material, however, sheds much light on the unusual and economically useful metabolism of this bacterium.
Supplementary Material
Acknowledgments
This research was supported by a grant from the Energy Biosciences Institute.
We thank Dylan Dodd, Yejun Han, Michael Iakiviak, Young-Hwan Moon, and Xiaoyun Su of the Energy Biosciences Institute for scientific discussions.
Footnotes
Published ahead of print on 13 August 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abbott, D. W., E. Ficko-Blean, A. L. van Bueren, A. Rogowski, A. Cartmell, P. M. Coutinho, B. Henrissat, H. J. Gilbert, and A. B. Boraston. 2009. Analysis of the structural and functional diversity of plant cell wall specific family 6 carbohydrate binding modules. Biochemistry 48:10395-10404. [DOI] [PubMed] [Google Scholar]
- 2.Adelsberger, H., C. Hertel, E. Glawischnig, V. V. Zverlov, and W. H. Schwarz. 2004. Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150:2257-2266. [DOI] [PubMed] [Google Scholar]
- 3.Alhassid, A., A. Ben-David, O. Tabachnikov, D. Libster, E. Naveh, G. Zolotnitsky, Y. Shoham, and G. Shoham. 2009. Crystal structure of an inverting GH 43 1,5-α-L-arabinanase from Geobacillus stearothermophilus complexed with its substrate. Biochem. J. 422:73-82. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, S., P. Vasu, S. Persson, A. J. Mort, and C. R. Somerville. 2006. Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc. Natl. Acad. Sci. U. S. A. 103:11417-11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boraston, A. B., D. N. Bolam, H. J. Gilbert, and G. J. Davies. 2004. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem. J. 382:769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boraston, A. B., V. Notenboom, R. A. Warren, D. G. Kilburn, D. R. Rose, and G. Davies. 2003. Structure and ligand binding of carbohydrate-binding module CsCBM6-3 reveals similarities with fucose-specific lectins and “galactose-binding” domains. J. Mol. Biol. 327:659-669. [DOI] [PubMed] [Google Scholar]
- 7.Bourgois, T. M., V. Van Craeyveld, S. Van Campenhout, C. M. Courtin, J. A. Delcour, J. Robben, and G. Volckaert. 2007. Recombinant expression and characterization of XynD from Bacillus subtilis subsp. subtilis ATCC 6051: a GH 43 arabinoxylan arabinofuranohydrolase. Appl. Microbiol. Biotechnol. 75:1309-1317. [DOI] [PubMed] [Google Scholar]
- 8.Brüx, C., A. Ben-David, D. Shallom-Shezifi, M. Leon, K. Niefind, G. Shoham, Y. Shoham, and D. Schomburg. 2006. The structure of an inverting GH43 β-xylosidase from Geobacillus stearothermophilus with its substrate reveals the role of the three catalytic residues. J. Mol. Biol. 359:97-109. [DOI] [PubMed] [Google Scholar]
- 9.Brunzelle, J. S., D. B. Jordan, D. R. McCaslin, A. Olczak, and Z. Wawrzak. 2008. Structure of the two-subsite β-D-xylosidase from Selenomonas ruminantium in complex with 1,3-bis[tris(hydroxymethyl)methylamino]propane. Arch. Biochem. Biophys. 474:157-166. [DOI] [PubMed] [Google Scholar]
- 10.Cantarel, B. L., P. M. Coutinho, C. Rancurel, T. Bernard, V. Lombard, and B. Henrissat. 2009. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37:D233-D238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czjzek, M., D. N. Bolam, A. Mosbah, J. Allouch, C. M. G. A. Fontes, L. M. A. Ferreira, O. Bornet, V. Zamboni, H. Darbon, N. L. Smith, G. W. Black, B. Henrissat, and H. J. Gilbert. 2001. The location of the ligand-binding site of carbohydrate-binding modules that have evolved from a common sequence is not conserved. J. Biol. Chem. 276:48580-48587. [DOI] [PubMed] [Google Scholar]
- 12.Dodd, D., and I. K. O. Cann. 2009. Enzymatic deconstruction of xylan for biofuel production. GCB Bioenergy 1:2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodd, D., S. Kiyonari, R. I. Mackie, and I. K. O. Cann. 2010. Functional diversity of four glycoside hydrolase family 3 enzymes from the rumen bacterium Prevotella bryantii B14. J. Bacteriol. 192:2335-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flint, H. J., E. A. Bayer, M. T. Rincon, R. Lamed, and B. A. White. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6:121-131. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg, C. W., B. Crosby, and D. Y. Thomas. 1986. Potential for manipulation of the rumen fermentation through the use of recombinant DNA techniques. J. Anim. Sci. 63:310-325. [DOI] [PubMed] [Google Scholar]
- 16.Gasparic, A., J. Martin, A. S. Daniel, and H. J. Flint. 1995. A xylan hydrolase gene cluster in Prevotella ruminicola B14: sequence relationships, synergistic interactions, and oxygen sensitivity of a novel enzyme with exoxylanase and β-(1,4)-xylosidase activities. Appl. Environ. Microbiol. 61:2958-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosalbes, M. J., J. A. Pérez-González, R. González, and A. Navarro. 1991. Two β-glycanase genes are clustered in Bacillus polymyxa: molecular cloning, expression, and sequence analysis of genes encoding a xylanase and an endo-β-(1,3)-(1,4)-glucanase. J. Bacteriol. 173:7705-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henshaw, J., A. Horne-Bitschy, A. L. van Bueren, V. A. Money, D. N. Bolam, M. Czjzek, N. A. Ekborg, R. M. Weiner, S. W. Hutcheson, G. J. Davies, A. B. Boraston, and H. J. Gilbert. 2006. Family 6 carbohydrate binding modules in β-agarases display exquisite selectivity for the non-reducing termini of agarose chains. J. Biol. Chem. 281:17099-17107. [DOI] [PubMed] [Google Scholar]
- 19.Ichinose, H., T. Kotake, Y. Tsumuraya, and S. Kaneko. 2006. Characterization of an exo-β-1,3-D-galactanase from Streptomyces avermitilis NBRC14893 acting on arabinogalactan-proteins. Biosci. Biotechnol. Biochem. 70:2745-2750. [DOI] [PubMed] [Google Scholar]
- 20.Ichinose, H., A. Kuno, T. Kotake, M. Yoshida, K. Sakka, J. Hirabayashi, Y. Tsumuraya, and S. Kaneko. 2006. Characterization of an exo-β-1,3-galactanase from Clostridium thermocellum. Appl. Environ. Microbiol. 72:3515-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichinose, H., M. Yoshida, Z. Fujimoto, and S. Kaneko. 2008. Characterization of a modular enzyme of exo-1,5-α-L-arabinofuranosidase and arabinan binding module from Streptomyces avermitilis NBRC14893. Appl. Microbiol. Biotechnol. 80:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichinose, H., M. Yoshida, T. Kotake, A. Kuno, K. Igarashi, Y. Tsumuraya, M. Samejima, J. Hirabayashi, H. Kobayashi, and S. Kaneko. 2005. An exo-β-1,3-galactanase having a novel β-1,3-galactan-binding module from Phanerochaete chrysosporium. J. Biol. Chem. 280:25820-25829. [DOI] [PubMed] [Google Scholar]
- 23.Inácio, J. M., and I. de Sá-Nogueira. 2008. Characterization of abn2 (yxiA), encoding a Bacillus subtilis GH43 arabinanase, Abn2, and its role in arabino-polysaccharide degradation. J. Bacteriol. 190:4272-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito, T., E. Yokoyama, H. Sato, M. Ujita, T. Funaguma, K. Furukawa, and A. Hara. 2003. Xylosidases associated with the cell surface of Penicillium herquei IFO 4674. J. Biosci. Bioeng. 96:354-359. [DOI] [PubMed] [Google Scholar]
- 25.Jordan, D. B., X. L. Li, C. A. Dunlap, T. R. Whitehead, and M. A. Cotta. 2007. Structure-function relationships of a catalytically efficient β-D-xylosidase. Appl. Biochem. Biotechnol. 141:51-76. [DOI] [PubMed] [Google Scholar]
- 26.Jung, K. H., C. C. Yong, J. C. Lee, S. H. Park, and K. H. Yoon. 1998. Purification and characterization of the Bacillus sp. KK-1 β-xylosidase from a recombinant Escherichia coli. J. Microbiol. Biotechnol. 8:258-263. [Google Scholar]
- 27.Kam, D. K., H. S. Jun, J. K. Ha, G. D. Inglis, and C. W. Forsberg. 2005. Characteristics of adjacent family 6 acetylxylan esterases from Fibrobacter succinogenes and the interaction with the Xyn10E xylanase in hydrolysis of acetylated xylan. Can. J. Microbiol. 51:821-832. [DOI] [PubMed] [Google Scholar]
- 28.Kellett, L. E., D. M. Poole, L. M. Ferreira, A. J. Durrant, G. P. Hazlewood, and H. J. Gilbert. 1990. Xylanase B and an arabinofuranosidase from Pseudomonas fluorescens subsp. cellulosa contain identical cellulose-binding domains and are encoded by adjacent genes. Biochem. J. 272:369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, S. M., T. J. Jess, and N. C. Price. 2005. How to study proteins by circular dichroism. Biochim. Biophys. Acta 1751:119-139. [DOI] [PubMed] [Google Scholar]
- 30.Kormelink, F. J. M., M. J. F. Searle-Van Leeuwen, T. M. Wood, and A. G. J. Voragen. 1991. (1,4)-β-D-Arabinoxylan arabinofuranohydrolase: a novel enzyme in the bioconversion of arabinoxylan. Appl. Microbiol. Biotechnol. 35:231-232. [Google Scholar]
- 31.Kormelink, F. J. M., M. J. F. Searle-Van Leeuwen, T. M. Wood, and A. G. J. Voragen. 1991. Purification and characterization of a (1,4)-β-D-arabinoxylan arabinofuranohydrolase from Aspergillus awamori. Appl. Microbiol. Biotechnol. 35:753-758. [Google Scholar]
- 32.Kotake, T., K. Kitazawa, R. Takata, K. Okabe, H. Ichinose, S. Kaneko, and Y. Tsumuraya. 2009. Molecular cloning and expression in Pichia pastoris of a Irpex lacteus exo-β-(1→3)-galactanase gene. Biosci. Biotechnol. Biochem. 73:2303-2309. [DOI] [PubMed] [Google Scholar]
- 33.Krause, D. O., S. E. Denman, R. I. Mackie, M. Morrison, A. L. Rae, G. T. Attwood, and C. S. McSweeney. 2003. Opportunities to improve fiber degradation in the rumen: microbiology, ecology, and genomics. FEMS Microbiol. Rev. 27:663-693. [DOI] [PubMed] [Google Scholar]
- 34.La Grange, D. C., I. S. Pretorius, and W. H. van Zyl. 1997. Cloning of the Bacillus pumilus β-xylosidase gene (xynB) and its expression in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 47:262-266. [DOI] [PubMed] [Google Scholar]
- 35.Leal, T. F., and I. de Sa-Nogueira. 2004. Purification, characterization and functional analysis of an endo-arabinanase (AbnA) from Bacillus subtilis. FEMS Microbiol. Lett. 241:41-48. [DOI] [PubMed] [Google Scholar]
- 36.Lever, M. 1972. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47:273-279. [DOI] [PubMed] [Google Scholar]
- 37.Lobley, A., L. Whitmore, and B. A. Wallace. 2002. DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics 18:211-212. [DOI] [PubMed] [Google Scholar]
- 38.Matsuo, N., S. Kaneko, A. Kuno, H. Kobayashi, and I. Kusakabe. 2000. Purification, characterization and gene cloning of two α-L-arabinofuranosidases from Streptomyces chartreusis GS901. Biochem. J. 346:9-15. [PMC free article] [PubMed] [Google Scholar]
- 39.McKie, V. A., G. W. Black, S. J. Millward-Sadler, G. P. Hazlewood, J. I. Laurie, and H. J. Gilbert. 1997. Arabinanase A from Pseudomonas fluorescens subsp. cellulosa exhibits both an endo- and an exo- mode of action. Biochem. J. 323:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michel, G., T. Barbeyron, B. Kloareg, and M. Czjzek. 2009. The family 6 carbohydrate-binding modules have coevolved with their appended catalytic modules toward similar substrate specificity. Glycobiology 19:615-623. [DOI] [PubMed] [Google Scholar]
- 41.Miron, J., and D. Ben-Ghedalia. 1993. Digestion of cell-wall monosaccharides of ryegrass and alfalfa hays by the ruminal bacteria Fibrobacter succinogenes and Butyrivibrio fibrisolvens. Can. J. Microbiol. 39:780-786. [DOI] [PubMed] [Google Scholar]
- 42.Montgomery, L., B. Flesher, and D. Stahl. 1988. Transfer of Bacteroides succinogenes (Hungate) to Fibrobacter gen. nov. as Fibrobacter succinogenes comb. nov. and description of Fibrobacter Intestinalis sp. nov. Int. J. Syst. Bacteriol. 38:430-435. [Google Scholar]
- 43.Nurizzo, D., J. P. Turkenburg, S. J. Charnock, S. M. Roberts, E. J. Dodson, V. A. McKie, E. J. Taylor, H. J. Gilbert, and G. J. Davies. 2002. Cellvibrio japonicus α-L-arabinanase 43A has a novel five-blade β-propeller fold. Nat. Struct. Biol. 9:665-668. [DOI] [PubMed] [Google Scholar]
- 44.Pires, V. M. R., J. L. Henshaw, J. A. M. Prates, D. N. Bolam, L. M. A. Ferreira, C. M. G. A. Fontes, B. Henrissat, A. Planas, H. J. Gilbert, and M. Czjzek. 2004. The crystal structure of the family 6 carbohydrate binding module from Cellvibrio mixtus endoglucanase 5A in complex with oligosaccharides reveals two distinct binding sites with different ligand specificities. J. Biol. Chem. 279:21560-21568. [DOI] [PubMed] [Google Scholar]
- 45.Pitson, S. M., A. G. Voragen, and G. Beldman. 1996. Stereochemical course of hydrolysis catalyzed by arabinofuranosyl hydrolases. FEBS Lett. 398:7-11. [DOI] [PubMed] [Google Scholar]
- 46.Rombouts, F. M., A. G. J. Voragen, M. F. Searle-van Leeuwen, C. C. J. M. Geraeds, H. A. Schols, and W. Pilnik. 1988. The arabinanases of Aspergillus niger: purification and characterization of two α-L-arabinofuranosidases and an endo-1,5-α-L-arabinanase. Carbohydr. Polym. 9:25-47. [Google Scholar]
- 47.Sakamoto, T., H. Ihara, S. Kozaki, and H. Kawasaki. 2003. A cold-adapted endo-arabinanase from Penicillium chrysogenum. Biochim. Biophys. Acta 1624:70-75. [DOI] [PubMed] [Google Scholar]
- 48.Sakka, K., K. Yoshikawa, Y. Kojima, S. Karita, K. Ohmiya, and K. Shimada. 1993. Nucleotide sequence of the Clostridium stercorarium xylA gene encoding a bifunctional protein with β-D-xylosidase and α-L-arabinofuranosidase activities, and properties of the translated product. Biosci. Biotechnol. Biochem. 57:268-272. [DOI] [PubMed] [Google Scholar]
- 49.Scott, H. W., and B. A. Dehority. 1965. Vitamin requirements of several cellulolytic rumen bacteria. J. Bacteriol. 89:1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shallom, D., M. Leon, T. Bravman, A. Ben-David, G. Zaide, V. Belakhov, G. Shoham, D. Schomburg, T. Baasov, and Y. Shoham. 2005. Biochemical characterization and identification of the catalytic residues of a family 43 β-D-xylosidase from Geobacillus stearothermophilus T-6. Biochemistry 44:387-397. [DOI] [PubMed] [Google Scholar]
- 51.Skjøt, M., S. Kauppinen, L. V. Kofod, C. Fuglsang, M. Pauly, H. Dalbøge, and L. N. Andersen. 2001. Functional cloning of an endo-arabinanase from Aspergillus aculeatus and its heterologous expression in A. oryzae and tobacco. Mol. Genet. Genomics 265:913-921. [DOI] [PubMed] [Google Scholar]
- 52.Smaali, I., C. Remond, and M. J. O'Donohue. 2006. Expression in Escherichia coli and characterization of β-xylosidases GH39 and GH-43 from Bacillus halodurans C-125. Appl. Microbiol. Biotechnol. 73:582-590. [DOI] [PubMed] [Google Scholar]
- 53.Somerville, C. 2007. Biofuels. Curr. Biol. 17:R115-R119. [DOI] [PubMed] [Google Scholar]
- 54.Sørensen, H. R., C. T. J.ørgensen, C. H. Hansen, C. I. J.ørgensen, S. Pedersen, and A. S. Meyer. 2006. A novel GH43 α-L-arabinofuranosidase from Humicola insolens: mode of action and synergy with GH51 α-L-arabinofuranosidases on wheat arabinoxylan. Appl. Microbiol. Biotechnol. 73:850-861. [DOI] [PubMed] [Google Scholar]
- 55.Suryani, T. Kimura, K. Sakka, and K. Ohmiya. 2004. Sequencing and expression of the gene encoding the Clostridium stercorarium β-xylosidase Xyl43B in Escherichia coli. Biosci. Biotechnol. Biochem. 68:609-614. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, S., M. Fukuoka, H. Ookuchi, M. Sano, K. Ozeki, E. Nagayoshi, Y. Takii, M. Matsushita, S. Tada, K. Kusumoto, and Y. Kashiwagi. 2010. Characterization of Aspergillus oryzae glycoside hydrolase family 43 β-xylosidase expressed in Escherichia coli. J. Biosci. Bioeng. 109:115-117. [DOI] [PubMed] [Google Scholar]
- 57.Szabó, L., S. Jamal, H. Xie, S. J. Charnock, D. N. Bolam, H. J. Gilbert, and G. J. Davies. 2001. Structure of a family 15 carbohydrate-binding module in complex with xylopentaose. Evidence that xylan binds in an approximate 3-fold helical conformation. J. Biol. Chem. 276:49061-49065. [DOI] [PubMed] [Google Scholar]
- 58.Takao, M., A. Yamaguchi, K. Yoshikawa, T. Terashita, and T. Sakai. 2002. Molecular cloning of the gene encoding thermostable endo-1,5-α-L-arabinase of Bacillus thermodenitrificans TS-3 and its expression in Bacillus subtilis. Biosci. Biotechnol. Biochem. 66:430-433. [DOI] [PubMed] [Google Scholar]
- 59.Tomme, P., A. Boraston, J. M. Kormos, R. A. Warren, and D. G. Kilburn. 2000. Affinity electrophoresis for the identification and characterization of soluble sugar binding by carbohydrate-binding modules. Enzyme Microb. Technol. 27:453-458. [DOI] [PubMed] [Google Scholar]
- 60.Umemoto, Y., R. Onishi, and T. Araki. 2008. Cloning of a novel gene encoding β-1,3-xylosidase from a marine bacterium, Vibrio sp. strain XY-214, and characterization of the gene product. Appl. Environ. Microbiol. 74:305-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Utt, E. A., C. K. Eddy, K. F. Keshav, and L. O. Ingram. 1991. Sequencing and expression of the Butyrivibrio fibrisolvens xylB gene encoding a novel bifunctional protein with β-D-xylosidase and α-L-arabinofuranosidase activities. Appl. Environ. Microbiol. 57:1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Bueren, A. L., and A. B. Boraston. 2004. Binding sub-site dissection of a carbohydrate-binding module reveals the contribution of entropy to oligosaccharide recognition at “non-primary” binding subsites. J. Mol. Biol. 340:869-879. [DOI] [PubMed] [Google Scholar]
- 63.van Bueren, A. L., C. Morland, H. J. Gilbert, and A. B. Boraston. 2005. Family 6 carbohydrate binding modules recognize the non-reducing end of β-1,3-linked glucans by presenting a unique ligand binding surface. J. Biol. Chem. 280:530-537. [DOI] [PubMed] [Google Scholar]
- 64.van den Broek, L. A. M., R. M. Lloyd, G. Beldman, J. C. Verdoes, B. V. McCleary, and A. G. J. Voragen. 2005. Cloning and characterization of arabinoxylan arabinofuranohydrolase-D3 (AXHd3) from Bifidobacterium adolescentis DSM20083. Appl. Microbiol. Biotechnol. 67:641-647. [DOI] [PubMed] [Google Scholar]
- 65.Vandermarliere, E., T. M. Bourgois, M. D. Winn, S. van Campenhout, G. Volckaert, J. A. Delcour, S. V. Strelkov, A. Rabijns, and C. M. Courtin. 2009. Structural analysis of a glycoside hydrolase family 43 arabinoxylan arabinofuranohydrolase in complex with xylotetraose reveals a different binding mechanism compared with other members of the same family. Biochem. J. 418:39-47. [DOI] [PubMed] [Google Scholar]
- 66.Van Laere, K. M. J., C. H. L. Voragen, T. Kroef, L. A. M. Van den Broek, G. Beldman, and A. G. J. Voragen. 1999. Purification and mode of action of two different arabinoxylan arabinofuranohydrolases from Bifidobacterium adolescentis DSM 20083. Appl. Microbiol. Biotechnol. 51:606-613. [Google Scholar]
- 67.Van Laere, K. M. J., G. Beldman, and A. G. J. Voragen. 1997. A new arabinofuranohydrolase from Bifidobacterium adolescentis able to remove arabinosyl residues from double-substituted xylose units in arabinoxylan. Appl. Microbiol. Biotechnol. 47:231-235. [DOI] [PubMed] [Google Scholar]
- 68.Wagschal, K., D. Franqui-Espiet, C. C. Lee, R. E. Kibblewhite-Accinelli, G. H. Robertson, and D. W. S. Wong. 2007. Genetic and biochemical characterization of an α-L-arabinofuranosidase isolated from a compost starter mixture. Enzyme Microb. Technol. 40:747-753. [Google Scholar]
- 69.Wagschal, K., C. Heng, C. C. Lee, G. H. Robertson, W. J. Orts, and D. W. S. Wong. 2009. Purification and characterization of a glycoside hydrolase family 43 β-xylosidase from Geobacillus thermoleovorans IT-08. Appl. Biochem. Biotechnol. 155:304-313. [DOI] [PubMed] [Google Scholar]
- 70.Wagschal, K., C. Heng, C. C. Lee, and D. W. Wong. 2009. Biochemical characterization of a novel dual-function arabinofuranosidase/xylosidase isolated from a compost starter mixture. Appl. Microbiol. Biotechnol. 81:855-863. [DOI] [PubMed] [Google Scholar]
- 71.Wegener, S., R. F. Ransom, and J. D. Walton. 1999. A unique eukaryotic β-xylosidase gene from the phytopathogenic fungus Cochliobolus carbonum. Microbiology 145:1089-1095. [DOI] [PubMed] [Google Scholar]
- 72.Xu, W. Z., Y. Shima, S. Negoro, and I. Urabe. 1991. Sequence and properties of β-xylosidase from Bacillus pumilus IPO. Contradiction of the previous nucleotide sequence. Eur. J. Biochem. 202:1197-1203. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi, A., T. Tada, K. Wada, T. Nakaniwa, T. Kitatani, Y. Sogabe, M. Takao, T. Sakai, and K. Nishimura. 2005. Structural basis for thermostability of endo-1,5-α-L-arabinanase from Bacillus thermodenitrificans TS-3. J. Biochem. 137:587-592. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida, S., R. I. Mackie, and I. K. O. Cann. 2010. Biochemical and domain analyses of FSUAxe6B, a modular acetyl xylan esterase, identify a unique carbohydrate binding module in Fibrobacter succinogenes S85. J. Bacteriol. 192:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.