Abstract
The epidermal growth factor (EGF)-ErbB-mitogen-activated protein kinase (MAPK) transcription signaling pathway is altered in many types of carcinomas, and this pathway can be regulated by new protein-protein interactions. Vaccinia-related kinase (VRK) proteins are Ser-Thr kinases that regulate several signal transduction pathways. In this work, we study the effect of VRK2 on MAPK signaling using breast cancer as a model. High levels of VRK2 inhibit EGF and ErbB2 activation of transcription by the serum response element (SRE). This effect is also detected in response to H-Ras(G12V) or B-Raf(V600E) oncogenes and is accompanied by a reduction in phosphorylated extracellular signal-regulated kinase (ERK) levels, p90RSK levels, and SRE-dependent transcription. Furthermore, VRK2 knockdown has the opposite effect, increasing the transcriptional response to stimulation with EGF and leading to increased levels of ERK phosphorylation. The molecular mechanism lies between MAPK/ERK kinase (MEK) and ERK, since MEK remains phosphorylated while ERK phosphorylation is blocked by VRK2A. This inhibition of the ERK signaling pathway is a consequence of a direct protein-protein interaction between VRK2A, MEK, and kinase suppressor of Ras 1 (KSR1). Identification of new correlations in human cancer can lead to a better understanding of the biology of individual tumors. ErbB2 and VRK2 protein levels were inversely correlated in 136 cases of human breast carcinoma. In ErbB2+ tumors, there is a significant reduction in the VRK2 level, suggesting a role for VRK2A in ErbB2-MAPK signaling. Thus, VRK2 downregulation in carcinomas permits signal transmission through the MEK-ERK pathway without affecting AKT signaling, causing a signal imbalance among pathways that contributes to the phenotype of breast cancer.
Members of the ErbB receptor-tyrosine kinase family are indicators of poor prognosis in several types of cancer, including breast cancer, colorectal cancer, head and neck squamous cell carcinoma (HNSCC), and non-small cell lung carcinoma (NSCLC) (18). Vaccinia-related kinases (VRK) are a novel family of Ser-Thr kinases composed of three members (26), two of which are catalytically active (36). VRK2A and its shorter, spliced isoform, VRK2B, are located, respectively, anchored to membranes of the endoplasmic reticulum and mitochondria or in soluble form in the cytoplasm and within the nucleus (4, 5). VRK2A, the more abundant isoform, modulates the stress response to hypoxia (6) and cytokines, such as interleukin 1β (IL-1β) (7). This signal modulation is independent of VRK2A kinase activity but is dependent on protein interactions with JIP1, which assembles mitogen-activated protein kinase (MAPK) complexes, functioning as a scaffold protein for the c-Jun N-terminal kinase (JNK) pathway. VRK2A blocks signal transmission mediated by the assembly of JIP1-MAPK complexes, reducing JNK phosphorylation and AP1-dependent transcription (6, 7). On the other hand, the nuclear VRK2B isoform has been proposed to functionally replace VRK1 in adenocarcinoma tumor cell lines (4). VRK1 is expressed at high levels in proliferating tumor cell lines and during cellular expansion in murine embryonic hematopoietic development (49). VRK1 expression correlates with several proliferation markers in human head and neck squamous cell carcinomas (42). VRK1 knockdown causes a block in cell cycle progression in tumor cells (50) and fibroblasts (46). Indeed, VRK1 is an early response gene expressed in parallel with MYC and FOS genes and required for cyclin D1 expression (21) and entry into the G1 phase of the cell cycle (46). Nuclear VRK1 phosphorylates several transcription factors, such as p53 (29, 50), c-Jun (43), ATF2 (44), and CREB (21), as well as Baf, during nuclear envelope assembly (17, 37). VRK1 and p53 form an autoregulatory loop (48) that is disrupted in tumors with p53 mutations, such as lung cancer (47). In estrogen receptor-positive (ER+) breast cancers, tumors with poor prognosis and a shorter time to relapse were associated with higher VRK1 expression (30). All of these data together suggest that VRK proteins might be good candidates to be deregulated in tumors.
The specificity of any biological effect in response to stimulation initiated in ErbB receptors (33), such as epidermal growth factor receptor (EGFR) or ErbB2, can be determined by interacting proteins that may alter the distribution of the signal among alternative response pathways (14, 45). Thus, the signal transmitted by Ras can be channeled mainly between MAPK and phosphatidylinositol 3-kinase (PI3K)-Akt (9, 54). However, how signal distribution is modulated among different pathways is not known, suggesting that additional elements can intervene, either by protein-protein interactions or by interactions with additional pathways, among which the MAPK pathway is the best known (28). This interconnectivity may result in functional differences that can help to explain differential responses to identical stimulation in either normal or tumor cells. The study of pathway interactions and their components has received relatively limited attention; however, it will be essential to identify specific effects in the biological systems in which they are likely to play important roles. The identification of new regulatory components can also lead to the discovery of novel molecular therapeutic targets.
Breast cancer-specific molecular markers help determine the prognosis, outcome, and treatment to be followed in these patients (15). Tumors positive for ER and/or progesterone receptor (PR) have a better prognosis, since they can be managed with antihormonal therapies. A third marker is ErbB2 (HER2/neu), a member of the EGF receptor family, which is usually detected in ER− cases, which represent approximately one-fourth of cases (27). Nevertheless, ErbB2 is the target of novel therapies based on the use of either specific antibodies or small tyrosine kinase inhibitors of the receptor (18).
In this report, we characterize how VRK2A modulates the ErbB-MAPK signaling pathway by a downstream interaction of the kinase with the kinase suppressor of Ras 1 (KSR1) scaffold protein. Analysis of the expression of VRK2 proteins in human breast carcinomas showed that ErbB2 receptor and VRK2A levels were inversely correlated, suggesting a facilitation of MAPK signaling. We conclude that VRK2A downregulation causes an imbalance in signaling pathways and thus contributes to the phenotype of breast carcinomas.
MATERIALS AND METHODS
Plasmids.
The following plasmids were used: pCEFL-HA-VRK1 (50); plasmids for VRK2A (pCEFL-HA-VRK2A), VRK2B (pCEFL-HA-VRK2B), and the inactive kinase VRK2(K169E) (pCEFL-GST-VRK2A) and the control pCEFL-GST (4); pCDNA3-ErbB2 (53); pCEFL-HA-MEK, pCEFL-HA-ERK, and pCEFL-H-RasG12V (from Piero Crespo, IBBTEC-CSIC, Santander, Spain); pG12B-RafV600E (from M. Soengas, University of Michigan); pCMV-Flag-KSR1 (20); pA3Cyclin D1(−1720)-Luc (1); pFC-MEK1 [constitutively active MEK1(S218/222E, Δ32-51)]; pSRE-Luc reporter (Stratagene); and pRL-tk control (Promega Biotech).
Cell lines, transfections, and immunoblotting.
MDA-MB-231 and MCF7 breast cancer cells (19) and HeLa and HEK-293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum. Transfections were performed using the JetPEI reagent (Polyplus, Illkirch, France) or Lipofectamine 2000 (Invitrogen). The total amount of DNA in transfections was kept constant by completion with the corresponding empty vector. The methods for cell lysates and the conditions for immunoprecipitations, pulldown, and Western blotting have been reported previously (4, 6, 7).
Transcriptional assays and quantitative reverse transcription (qRT)-PCR.
For assays of transcriptional activity using a reporter plasmid, cells were transfected with the reporter plasmids pSRE-Luc (1 μg), pCDNA3-ErbB2 (1.5 μg), pCEFL-H-RasG12V (0.2 μg), pG12B-RafV600E (0.2 μg), and pFC-MEK1(0.05 μg) and the indicated amounts of the specific kinase constructs. Cell lysates were prepared 48 h after transfection in luciferase lysis buffer (Promega). Luciferase activity was determined with a Dual-Luciferase reporter reagent from Promega (4, 6, 7). In some experiments, cells were stimulated 4 h before lysis with 10 nM EGF (Strathmann Biotec) for the indicated times.
For qRT-PCR, cells were washed in ice-cold phosphate-buffered saline (PBS). Total RNA was extracted using the RNAeasy extraction kit from Qiagen and analyzed as previously reported (46). The following forward (F) and reverse (R) primers were used: human c-myc (Myc-F, 5′-CCAGCAGCCTCCCGCGACGATG-3′; Myc-R, 5′-GAGGGGTCGATGCACTCTGAGG-3′) and human cyclin D1 (CycD1-F, 5′-CTTCCTCTCCAAAATGCCAG-3′; CycD1-R, 5′-AGAGATGGAAGGGGGAAAGA-3′), with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) amplification used as an internal control (GAPDH-F, 5′-GGTCTTACTCCTTGGAGGCCATGT-3′; GAPDH-R, 5′-ACCTAACTACATGGTTTACATGTT-3′).
Antibodies and reagents.
The antibodies used were VRK2 polyclonal antibody (4); ErbB2 (44E7); p90 ribosomal S6 kinase (RSK) (32D7), p90RSK (Ser 380), phosphorylated AKT (p-AKT) (Ser473), and p-MEK1/2 (S217/S221) from Cell Signaling; antibodies for extracellular signal-regulated kinase 2 (ERK2) (sc-154), ERK1/2 (Y204) (sc-7383), MAPK/ERK kinase 1 (MEK-1) (C-18 polyclonal or 9G3 monoclonal), B-Raf (sc-55522), glutathione S-transferase (GST) (sc-138), AKT (H-136), cyclin D1 (M-20), Ksr-1 (sc-9317), and c-myc (sc-764), all from Santa Cruz; anti-hemagglutinin (HA) from Covance; actin (AC-15) and anti-Flag (M5) from Sigma; and EGF (Strathmann Biotec).
VRK2, KSR, or JIP1 knockdown by siRNA interference.
The targeted sequence for VRK2 (GenBank NM_006296) was 5′-GCAAGGUUCUGGAUGAUAUUU-3′ (duplex siVRK2-06) (Dharmacon). The targeted JIP1sequence (GenBank NM_005456) was 5′-CTGGAGGAGTTTGAGGATGAA-3′ (small interfering RNA [siRNA] JIP1 number 2; Qiagen) (25, 26). Human Ksr-1 siRNA contains a pool of 3 target-specific 20- to 25-nucleotide (nt) siRNAs (Santa Cruz; sc-35762). The siControl nontargeting siRNA pool (Dharmacon) was used as a negative control. Transfection of siRNA duplexes in HeLa cells at a final concentration of 160 mM was carried out using Lipofectamine 2000 reagent (Invitrogen). Cells were processed for immunoblotting 4 days after transfection. In assays of VRK2 depletion, cells were transfected with the reporter plasmid 72 h after siRNA transfection and EGF stimulated 24 h after retransfection.
Immunofluorescence and confocal microscopy.
HeLa cells were grown on uncoated glass coverslips placed in 60-mm plates. The cells were prepared as previously reported (4). Fluorescence images were captured with a Zeiss LSM 510 confocal microscope.
Immunohistochemistry in tissue sections.
Biopsy specimens from human breast cancer were provided by the National Tumor Bank at CIC-Hospital Universitario de Salamanca. The biopsy specimens were prepared as previously reported (47), and sections were counterstained with hematoxylin. VRK2 was detected with a rabbit polyclonal antibody (4). For analysis of VRK2 and ErbB2, consecutive sections were used. ErbB2 immunostaining was scored from 1 to 3 for both the intensity of the staining and the number of ErbB2-positive cells. The final score was obtained by multiplying these two parameters. Briefly, the frequencies were compared either by the chi-square method or by Fisher's exact test (SPSS version 18; SPSS, Chicago, IL), and differences with a P value of <0.05 were considered statistically significant (42, 47).
RESULTS
Overexpression or knockdown of VRK2A inversely regulates SRE transcription induced by EGF.
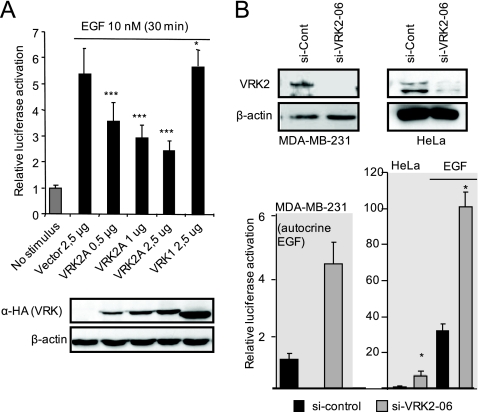
Because VRK2A downregulates JNK signaling in response to IL-1β and hypoxia (6, 7), it was hypothesized that the VRK2 protein might also interfere with transcriptional response signals to growth factor receptors mediated by MAPK. To address whether VRK2A could affect signaling induced upon EGF stimulation, we determined the effect of increasing levels of VRK2A protein on the serum response element (SRE) transcriptional response induced by EGF in MCF7 cells (Fig. 1A ) and HeLa cells (see Fig. S1A in the supplemental material). EGF, whose receptor is ErbB1, stimulated SRE-dependent transcription in MCF7 and HeLa cells, and this transcriptional response was inhibited by VRK2A in a dose-dependent manner, but the closely related member VRK1, or VRK2B, did not affect this response. We also tested if overexpression of VRK2A could prevent the activation of the cyclin D1 gene promoter in response to EGF. VRK2A prevented activation of this promoter, as expected, while VRK1, which is required for cyclin D1 expression (46), had a strong stimulatory effect (see Fig. S1B in the supplemental material).
FIG. 1.
VRK2A protein modulates EGF activation of SRE-dependent transcription. (A) Effect of VRK2A overexpression. MCF7 cells were transfected with 1 μg of the SRE-luciferase reporter, as well as plasmids, for overexpression of VRK2A or VRK1. The immunoblot shows the correct expression of the corresponding VRK proteins detected with an antibody to the HA epitope. The error bars indicate standard deviations. ***, P < 0.001; *, P < 0.05. (B) (Top) Knockdown of the VRK2 intracellular protein level with siVRK2-06 in MDA-MB-231 breast cancer cells and HeLa cells. MDA-MB-231 cells express only VRK2A, and HeLa cells express both VRK2 isoforms. Mean values with standard deviations for three experiments are shown. *, P < 0.05. (Bottom) Effect of VRK2 knockdown on the activation of SRE-dependent transcription induced by EGF in MDA-MB-231 and HeLa cells. MDA-MB-231 cells have autocrine stimulation by EGF. MCF7 cells express both isoforms of VRK2, which are not affected by the specific siVRK2-06, while its RNA is effectively reduced (see Fig. S2B in the supplemental material). In the HeLa cell line, siVRK2-06 was very effective at reducing VRK2A protein. The reduction of VRK2 by siVRK2-06 was approximately 63% (see Fig. S2A in the supplemental material).
From the previous result, it was predicted that lowering the levels of endogenous VRK2 protein would make cells more sensitive to EGF. To test this hypothesis, VRK2 protein was knocked down in MDA-MB-231 and HeLa cells (Fig. 1B, top; see Fig. S2A in the supplemental material) with a specific siRNA (6, 7), which effectively reduced the VRK2 protein levels in both cell lines between 80 and 90%. In MCF7 cells, the RNA knockdown was very effective, but the protein was extremely stable (see Fig. S2B in the supplemental material). MDA-MB-231 cells, which have autocrine stimulation by EGF (31), and HeLa cells were stimulated with EGF. In both, there was an increase in the response to EGF stimulation when VRK2 was eliminated (Fig. 1B, bottom). These results confirm the inverse correlation between VRK2 levels and the transcriptional response to EGF.
VRK2A downregulates the transactivation of transcription induced by ErbB2, H-Ras(G12V), B-Raf(V600E), and active MEK.
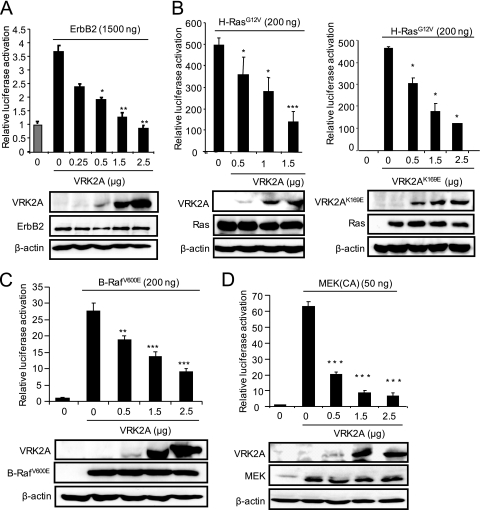
The above observations suggest that expression of VRK2A protein might have an effect on growth factor response signaling mediated by the Ras-Raf-MEK-Erk pathway, among which ErbB2 is the most relevant for breast and other carcinomas. ErbB2 is active by itself and has no known ligand (18, 52). Initially, a dose-response assay to ErbB2 was performed in HEK293T cells with the expected increase in SRE-dependent transcription (see Fig. S3A in the supplemental material). A 1.5-μg amount of ErbB2 was selected to determine the effect of the VRK2A protein on the transcriptional response to ErbB2 in MCF7 cells. As the VRK2A protein level increased, there was a significant reduction, in a dose-dependent manner, of the transcriptional response induced by ErbB2 in MCF7 cells (Fig. 2 A) and HEK293T cells (see Fig. S3B in the supplemental material). These data indicated that ErbB2 was able to activate SRE-dependent gene transcription and that its response was inhibited by VRK2A.
FIG. 2.
VRK2A inhibits the transcriptional response to overexpression of oncogenic ErbB2, H-Ras(G12V), B-Raf(V600E), and MEK1 in MCF7 cells. (A) Effect of VRK2A on the response to ErbB2. Breast carcinoma MCF7 cells were transfected with a fixed amount of ErbB2 and increasing amounts of pCEFL-HA-VRK2A. At the bottom are immunoblots showing the expression of ErbB2 and VRK2A. The experiments to select the amount of ErbB2 to be used and to determine the effects of increasing amounts of VRK2 in HEK293T cells are shown in Fig. S3A and B in the supplemental material. The error bars indicate standard deviations. (B) Effect of VRK2A (left) or VRK2A(K169E) (right) on the SRE transcriptional response to H-Ras(G12V). MCF7 cells were transfected with a fixed amount of H-Ras(G12V) and increasing amounts of pCEFL-HA-VRK2A or pCEFL-HA-VRK2AK169E. At the bottom is shown the expression of VRK2A proteins by immunoblotting with an anti-HA antibody. The experiments to select the amount of H-Ras(G12V) to be used and to determine the effect of increasing amounts of VRK2 in HEK293T cells are shown in Fig. S3C and D in the supplemental material. (C) Effect of VRK2A on the response to B-Raf(V600E). MCF7 cells were transfected with a fixed amount of B-Raf(V600E) and increasing amounts of pCEFL-HA-VRK2A, as indicated. At the bottom is shown an immunoblot determining the expression of B-Raf(V600E) and VRK2A. (D) Effect of VRK2A overexpression on constitutively active MEK (CA) activation of the SRE. Plasmid pFC-MEK1, expressing constitutively active MEK1(S218/222E, Δ32-51), was transfected in the presence of different amounts of VRK2A. The control (0) had the maximum amount of empty vector. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The signal transduction pathway activating SRE in response to EGF, or ErbB2, is mediated by the Ras oncogene (40) and the B-Raf Ser-Thr kinase (24), both frequently mutated in many types of human cancer (34, 38), and thus cells become independent of EGF/ErbB signaling (8). Therefore, we tested whether the VRK2A protein was acting downstream of the Ras/Raf proteins, since they are in intracellular membranes (16). First, a dose-response assay for H-Ras(G12V) was performed (see Fig. S3C in the supplemental material), and a dose of 200 ng was selected to determine the effect of VRK2A in HEK293T (see Fig. S3C and D in the supplemental material) and MCF7 (Fig. 2B) cells. VRK2A inhibited H-Ras(G12V) activation of SRE-dependent transcription in a dose-dependent manner (Fig. 2B). Since the Ras oncogene was the stronger inducer of transcription, it was used to determine if the effect of VRK2A was due either to its kinase activity or to a protein interaction. Overexpression of kinase-dead VRK2A(K169E) (4) was equally effective in inhibiting the SRE-dependent transcription induced by H-Ras(G12V) (Fig. 2B, right), suggesting that the observed effect was a consequence of a VRK2A protein interaction with a component of the MAPK pathway.
A similar approach was followed using the B-Raf(V600E) mutant and a constitutively active MEK1(S218/222E, Δ32-51) (plasmid pFC-MEK1), two downstream components of Ras in the MAPK signaling pathway. Activation of transcription by B-Raf(V600E) (Fig. 2C) or MEK1(S218/222E, Δ32-51) (Fig. 2D) was also blocked by VRK2A in a dose-dependent manner. Therefore, it can be concluded that VRK2A acts downstream of Ras, B-Raf, and MEK1 in the ErbB signal transduction pathway.
VRK2A blocks the phosphorylation of ERK in response to ErbB2 and H-Ras(G12V)..
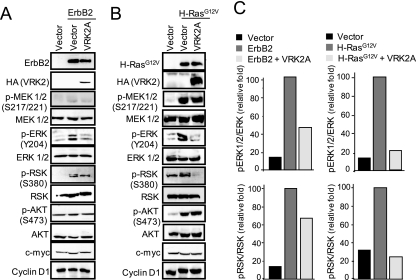
The signal initiated in the EGF receptor (ErbB1) is partly transmitted by phosphorylation of ERK, which activates SRE-dependent transcription. To gain insight into how VRK2A can regulate ERK signaling, we determined whether overexpression of ErbB2 or H-Ras(G12V) affected the phosphorylation of ERK, the last kinase in the MAPK complex. Both ErbB2 and H-Ras(G12V) induced increased levels of p-MEK and p-ERK in MCF7 cells (Fig. 3 A and B). However, when VRK2A was overexpressed, there was a reduction in the level of p-ERK, but not of p-MEK, in response to either ErbB2 or H-Ras(G12V), suggesting it might be acting between MEK and ERK (Fig. 3A and B). In these experiments, AKT activation by phosphorylation was not affected by VRK2A overexpression, since there were no changes in its phosphorylation level (Fig. 3A and B). These data indicated that VRK2A acts downstream of MEK and does not affect PI3K-AKT signaling, one of the most relevant pathways activated by the ErbB2 receptor. In addition, the phosphorylation of RSK, an ERK cytoplasmic substrate, was also increased by ErbB2 and H-Ras(G12V) and was reduced in the presence of overexpressed VRK2A (Fig. 3A and B). The reduction in p-ERK and p-RSK was approximately 50%, similar to the efficiency of VRK2A transfection (Fig. 3C). Other proteins, such as cyclin D1 and c-myc, were not affected by ErbB2, H-Ras(G12V), or VRK2A overexpression. The effects of several proteins of the VRK family (VRK1, VRK2A, and VRK2B) on ERK activation were also tested; VRK2A was the only one able to inhibit ERK phosphorylation (see Fig. S4 in the supplemental material). These results indicate that VRK2A interferes with signal transmission between MEK and ERK.
FIG. 3.
VRK2A inhibits ErbB2 and H-Ras(G12V) activation of ERK phosphorylation without affecting MEK or AKT phosphorylation. MCF7 cells were transfected with empty vector (control) or with plasmids expressing ErbB2 (A) or H-Ras(G12V) (B), and the effect of VRK2A overexpression on the activation at different levels of several pathways, such as the MAPK and AKT routes, that respond to EGF was determined. Proteins were detected in immunoblots with the corresponding antibodies (see Materials and Methods). (C) Changes in relative p-ERK and p-RSK levels induced by ErbB2 (A) and H-Ras(G12V) (B).
Knockdown of VRK2A leads to increased levels of phospho-ERK.
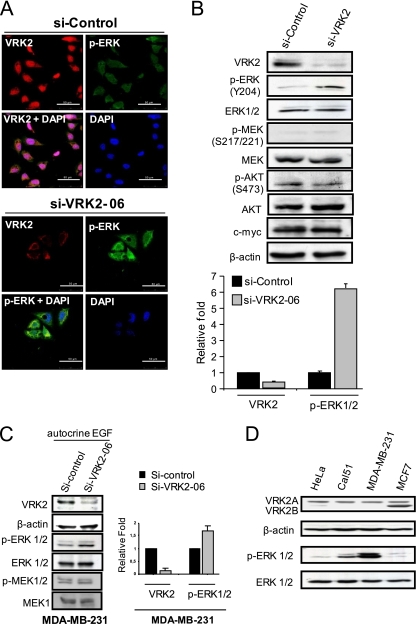
In order to further analyze the effect of VRK2 on the ERK1/2 pathway, HeLa cells were used for knockdown of endogenous VRK2. The effect of VRK2 depletion on ERK phosphorylation was studied by immunofluorescence assays in cells grown in the presence of serum. In cells transfected with siControl, there was a significant level of VRK2 protein and low levels of p-ERK (Fig. 4 A, top); on the other hand, in cells transfected with siVRK2-06, the level of VRK2 dropped, and there was an increase in p-ERK (Fig. 4A, bottom). Next, the levels of different proteins were determined by Western blotting under these two conditions. VRK2 knockdown resulted in accumulation of p-ERK but had no effect on either p-MEK or p-AKT (Fig. 4B). The changes in VRK2A and p-ERK were quantified, and the reduction in VRK2 was accompanied by a 6-fold increase in p-ERK (Fig. 4B). These results are consistent with the effect on transcription levels observed in these cells (Fig. 1B) and with VRK2A overexpression results (Fig. 3). The effect of siVRK2 on p-ERK was also determined in MDA-MB-231 cells, in which the reduction in VRK2A was accompanied by increased p-ERK without affecting MEK phosphorylation; in this cell line, the increase in p-ERK was smaller due to its high basal level of p-ERK (Fig. 4C). The levels of VRK2 and p-ERK in different cell lines are shown in Fig. 4D; in MDA-MB-231 cells, there was a very high basal level of p-ERK, consistent with its autocrine secretion of EGF and its Ras and B-Raf mutations (19).
FIG. 4.
VRK2 knockdown in HeLa cells leads to increased ERK phosphorylation. HeLa cells were transfected with either siControl or siVRK2-06, and the level of intracellular VRK2 protein, as well as of p-ERK, were determined. (A) Knockdown by siVRK2-06 induces a reduction in VRK2 (red) with respect to the siControl that is accompanied by increased levels of p-ERK (green) detected by immunofluorescence. Scale bars, 50 μm. (B) Expression levels of different signaling proteins in HeLa cells treated with siControl or siVRK2-06. The specific siVRK2-06 caused an increase in the level of p-ERK, but not of p-Akt or p-MEK. The error bars indicate standard deviations. Quantification of ERK1/2 activation, detected as p-ERK in VRK2A-depleted cells. The data presented are the means of three independent experiments. (C) Knockdown of VRK2A in MDA-MB-231 cells. siVRK2-06 effectively reduces VRK2 levels and is accompanied by an increase in p-ERK (left) that was quantified (right). (D) The basal level of VRK2 and p-ERK in different cells lines is shown.
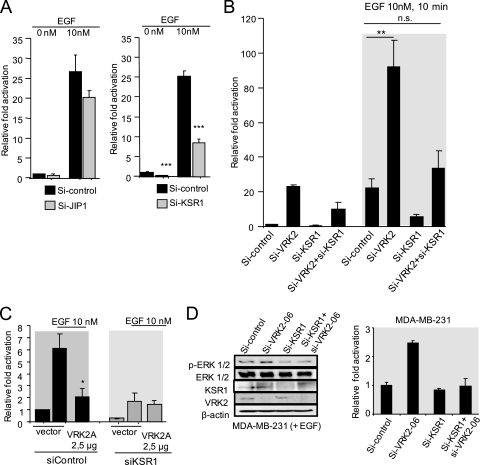
Effect of knockdown of JIP1 and KSR1 on the transcriptional response to EGF.
Transmission by MAPK protein complexes requires their assembly on scaffold proteins (13). Two of them, KSR1 and JIP1, are likely to be implicated in the SRE transcriptional response to EGF. Therefore, it was tested if knockdown of each of them affected the activation of transcription induced by EGF. Both siKSR1 (10) and siJIP1 (7) were very effective in knocking down their respective protein levels. The SRE transcriptional response to EGF was very sensitive to the knockdown of KSR1, resulting in its loss, but was only slightly affected by the knockdown of JIP1 (Fig. 5A ). HeLa cells can respond very well to EGF; thus, it was also determined if knockdown of VRK2, KSR1, or both affected the transcriptional response to EGF. VRK2 knockdown by itself significantly increased the transcriptional response to EGF, a response that is lost in combination with siKSR1 (Fig. 5B). The knockdown of KSR1 showed that this protein is necessary for the EGF response, and the VRK2A inhibitory effect was detected only in the presence of KSR1 (Fig. 5C). These results suggested that the EGF response is at least partially mediated by the KSR1 scaffold protein. Also, in MDA-MB231 cells in the presence of EGF, knockdown of VRK2 resulted in an increase in phospho-Erk levels, which was also lost by knockdown of KSR1 in combination with VRK2 knockdown (Fig. 5D). These data point to contributions by both proteins to signal transmission and rule out the possibility that the effect of VRK2 is mediated by an alternative mechanism independent of KSR1.
FIG. 5.
(A) Effects of knocking down scaffold proteins, JIP1 (left) and KSR1 (right), on the SRE-dependent transcriptional response to EGF in HeLa cells. ***, P < 0.001. The error bars indicate standard deviations. (B) Effects of siVRK2 and siKSR1 on the SRE-dependent transcriptional response to EGF in HeLa cells. **, P < 0.005; n.s., not significant. (C) Effect of VRK2A in response to EGF after knockdown of KSR1 in MCF7 cells. *, P < 0.01. (D) Effects of KSR1 and VRK2 knockdown on phospho-Erk levels in MDA-BB-231 cells in the presence of EGF. The effect on the activation of the SRE promoter is also shown (right). The change in luciferase activity resulting from activation of the SRE-luc reporter coincides with the result for phospho-ERK (left).
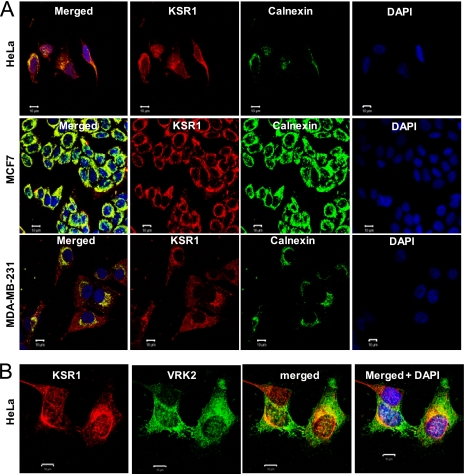
Colocalization of KSR1 with calnexin and VRK2.
Mechanistically, the effect of VRK2 on the ERK pathway may be a consequence of a direct interaction between VRK2 and KSR1, or one of the kinases in the complex, as has been described for JNK-JIP1 regulation mediated by VRK2A (6, 7). Initially, the potential colocalization of endogenous KSR1 and calnexin in three cell lines was determined, and in all of them, the KSR1 pool was larger than the calnexin pool (Fig. 6 A), which is where VRK2A was localized (4); the fraction of KSR1 colocalizing with calnexin varied depending on the cell line used (Fig. 6A). In MCF7 cells, almost all KSR1 colocalized with calnexin, and thus, a reduction in VRK2 can explain the large increase in p-ERK observed in this cell line. In the case of MDA-MB-231 cells, the overlapping signal represents a small fraction. In these cells, there is much more KSR1, and most of it is dispersed in the cytosol; this distribution explains why eliminating VRK2 had a smaller effect on the level of p-ERK (Fig. 4C), which already had a very high basal level (Fig. 4D). The colocalization of KSR1 with calnexin indicates that KSR1, even if it is a subpopulation, might also colocalize with VRK2 in the endoplasmic reticulum. This VRK2-KSR1 colocalization was confirmed by immunofluorescence (Fig. 6B).
FIG. 6.
(A) Colocalization of KSR1 with calnexin, an endoplasmic reticulum marker, in three cell lines. Bars, 10 μm. (B) Colocalization of VRK2 and KSR1 in HeLa cells by confocal microscopy. Bars, 10 μm.
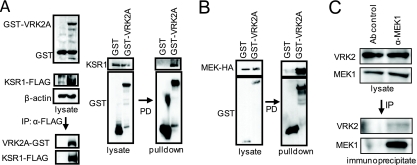
Direct interaction of VRK2 with MEK and KSR1.
Next, we determined if these potential protein interactions could be detected. First, a direct KSR1-VRK2A interaction could be detected by immunoprecipitation of transfected KSR1, which was clearly bound to VRK2A (Fig. 7 A, left), and also in a reciprocal experiment by pulldown of VRK2, which binds to endogenous KSR1 (Fig. 7A, right). However, since several proteins are anchored on the KSR1 scaffold, the inhibition of ERK phosphorylation might also implicate additional interactions with any of the MAP kinases, Raf, MEK, or ERK. Therefore, we also determined if B-Raf(V600E), MEK-HA, or ERK could also directly interact with VRK2A in pulldown assays. Only MEK stably interacted with VRK2A (Fig. 7B), and no interaction was detected with either Raf or ERK (see Fig. S5A and B in the supplemental material). The interaction between endogenous VRK2 and MEK1 was also determined and detected by immunoprecipitation (Fig. 7C), suggesting that it is likely to be a direct interaction. These data indicated that in the signaling complex assembled on the KSR1 scaffold protein, signal transmission can be blocked by the incorporation of VRK2A in the complex, since MEK1 can form a complex with both proteins but additional proteins can also participate in this complex, forming a signalosome (unpublished observations).
FIG. 7.
(A) Interaction between VRK2A and the KSR1 scaffold. 293T cells were transfected with plasmid pCMV-Flag-KSR1 or pCMV-Flag (control) and pCEFL-GST-VRK2A or pCEFL-GST, the cell extract was immunoprecipitated (IP) with an anti-Flag antibody, and the proteins present in the immunoprecipitate were detected with antibodies for the epitopes. For the pulldown (PD) assay, cells were transfected with pCEFL-GST-VRK2A or pCEFL-GST, and endogenous KSR1 was detected with a specific antibody. (B) Interaction of VRK2A with MEK. HEK293T cells were transfected with plasmid pCEFL-GST VRK2A or pCEFL-GST as a control, in combination with plasmid pCEFL-HA-MEK. The lysates were used for pulldown, and proteins (right) were detected by immunoblotting. (C) Direct interaction between endogenous MEK1 and VRK2 proteins in MCF7 cells. Endogenous MEK1 was immunoprecipitated with 9G3 monoclonal antibody (Ab), and the endogenous VRK2 protein present in the immunoprecipitate was detected by Western blotting.
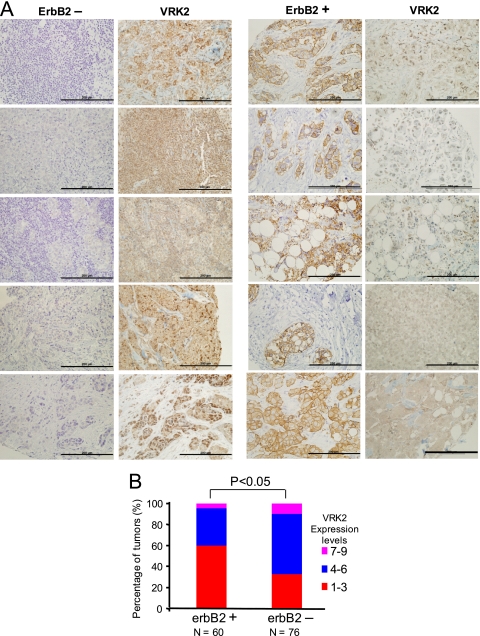
Inverse correlation between VRK2 and ErbB2 expression levels in breast carcinomas.
The above-mentioned results showing the effect of VRK2A on MAPK signaling suggested that in those tumors in which the MAPK pathway is activated, VRK2 is likely to be downregulated to reduce the block in signal transmission. Therefore, we determined if there was any correlation between VRK2 and the ErbB2 receptor protein levels, using breast cancer as a model. These two proteins were determined by immunohistochemistry in 136 cases of human breast carcinomas. Interestingly, an inverse correlation was found between VRK2 expression and ErbB2 protein levels (Fig. 8 A and B). VRK2 was mostly located in endoplasmic reticulum membranes, as expected (4), independent of the ErbB2 status (see Fig. S6 in the supplemental material).
FIG. 8.
ErbB2 and VRK2 proteins in human breast carcinomas detected by immunohistochemistry. (A) Detection of ErbB2 and VRK2 in five cases representing the two groups identified. Bars, 200 μm. (B) Distribution of cases in different groups depending on the level of VRK2 protein within ErbB2-positive and -negative groups. The score was determined by multiplying the scores from each tumor, representing the number of positive cells, and the intensity of the signal. Immunohistochemistry methods and statistics used the chi-square method, as previously reported (42, 47).
The correlation between ER or PR and VRK2 was also determined in these carcinomas. Both receptors were positively correlated with VRK2 expression, and thus, tumors positive for ER or PR also had higher levels of VRK2 than those negative for the receptors (see Fig. S7 in the supplemental material), which is consistent with the inverse correlation between VRK2 and ErbB2 detected in this work.
DISCUSSION
Signaling by the ErbB family of receptors is partly transmitted by the RAF-MEK-ERK pathway, activating transcription that promotes survival and expansion of the cell population. Modulation of MAPK signaling by interaction of its canonical components with other proteins is an emerging theme in signal transduction, highlighting the importance of signaling pathway cross talk and its contribution to signal specificity (45). Thus, the incorporation of annexin A6 in H-Ras-containing complexes inhibits EGFR and Ras signaling in breast cancer cells (12). Also, thyroid receptor β1 blocks the response to EGF, IGF1, and transforming growth factor β (TGF-β) functioning as a suppressor of tumor invasion (32). The VRK2A protein, by interacting with the JIP1 scaffold protein, blocks JNK activation in response to interleukin 1β or hypoxia (6, 7).
Blocking of EGF or its receptor (ErbB) at the membrane level by monoclonal antibodies or small-molecule tyrosine kinase inhibitors blocks any pathway downstream of the ligand-receptor complex. In contrast, interference with the signal at a downstream level can alter the balance among the different pathways responding to that signal, thus generating functional heterogeneity that may affect proliferation, quiescence, mobility, survival, or apoptosis. Physiologically, this modulation can be achieved through protein-protein interactions with any downstream component, altering the signal distribution among alternative pathways. In this regard, homodimerization of the scaffold KSR1 and heterodimerization of MEK1 and MEK2 have been shown to mediate ERK activation and inhibition, respectively (11). Mechanistically this effect may be achieved by alternative mechanisms that are currently under investigation, such as competition between VRK2A and ERK for interaction with KSR1, recruitment of a phosphatase by VRK2A, or interference with activated MEK1. Furthermore, KSR1 is inhibited by Nm23-H1 (41), and recently, it was found that the activation of the KSR1-B-Raf complex (39) can also be inhibited by direct interaction between c-Raf and B-Raf(V600E) (23). The incorporation of VRK2 in signaling complexes assembled on KSR1, and its regulation, is a process that will need to be further characterized, since KSR1 also interacts with members of the 14-3-3 protein family, which modulates signal transduction (20), and these alternative complexes can modify the balance among different signals and biological effects. The subcellular location of KSR1 in the cytoplasm is partly dispersed and partly colocalized with endogenous calnexin, an endoplasmic reticulum marker, but the proportions vary depending on the cell line (Fig. 6A). KSR1 is mostly cytoplasmic and has no membrane-spanning sequence or hydrophobic region (35, 51). Thus, its association with the endoplasmic reticulum must occur through interactions with proteins anchored to the endoplasmic reticulum, as is the case with VRK2A (4), or with common interacting proteins that function as a bridge between them.
In ErbB2+ breast tumors, we have detected a significant reduction in VRK2 levels, but not lack of expression, which functionally may alter the balance among EGF- or ErbB2-dependent pathways. VRK2 downregulation resulted in increased levels of p-ERK-, p-RSK-, and SRE-dependent transcriptional response, while overexpression of VRK2 had the opposite effect. This is a consequence of blocking the MAPK signaling pathway at the level of its last component; thus, not all EGF response routes are likely to be affected, as is the case with the AKT-mediated response. Hence, the low level of VRK2 in ErbB2+ tumors suggests that the proliferative signal is stronger, and thus, the likelihood of a more aggressive tumor is increased. The identification of VRK2A as a downstream modulator of ErbB2 can have wider implications than those related to breast carcinoma. The alteration of MAPK signaling by mutation of some of its components is common to many carcinomas. Thus, a specific B-Raf(V600E) mutation is commonly detected in melanomas (38), and Ras mutations are detected in many types of carcinomas (8). In these cancers, the prediction is that the level of VRK2A will be downregulated in tumors with any of these mutations compared with those that do not have alterations in MAPK signaling. This is consistent with the loss of heterozygosity detected in the VRK2 locus in the lung (3) and adrenocortical carcinomas (25).
In this work, we have shown that VRK2A, a cytoplasmic kinase, is able to reduce the signal from ErbB2 or EGF. Thus, in those tumors, like breast carcinomas, that have amplified ErbB2, its signal could be potentiated if there was a reduction in the levels of VRK2A, which functions as an inhibitor. The effect of removing an inhibitor of the MAPK route is consistent with the relative importance of this signaling pathway in carcinomas. Thus, in colon carcinomas treated with cetuximab, if there is an activating Ras mutation, the effect of the antibody is reduced, pointing to the importance of the route (2, 22). The use of targeted therapies directed at kinases (40) will require the characterization of the mutational status of signaling molecules downstream in the pathway, such as Ras or B-Raf, as well as that of modulators of the signal, such as VRK2, particularly in the subgroup with high ErbB2 and VRK2 levels. However, at this time, it is not known if this VRK2 downregulation will identify a subpopulation of ErbB2+ tumors or if therapeutic decisions will be affected by this change. Better selection of patients based on signaling criteria might contribute to achieving better clinical responses to current inhibitors, either antibodies or small molecules. Thus, identification of new regulators opens up an area for potential intervention in those tumors that, because of downstream mutations, such as in Ras or Raf, can make patients less responsive to current therapies directed at the EGF/ErbB2 signaling pathway.
Supplementary Material
Acknowledgments
This work was funded by grants from the Ministerio de Educación y Ciencia (SAF2007-60242, SAF2010-14935, and CSD2007-0017), Junta de Castilla y León (Consejería de Educación, CSI14A08; Consejería de Sanidad, GR-15), Fundación Sandra Ibarra to P.A.L. and grants BFU2007-66100 and SAF2010-20203 from the Ministerio de Educación y Ciencia to J.L. I.F.F. has a JAE-CSIC predoctoral fellowship.
Footnotes
Published ahead of print on 2 August 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. [DOI] [PubMed] [Google Scholar]
- 2.Amado, R. G., M. Wolf, M. Peeters, E. Van Cutsem, S. Siena, D. J. Freeman, T. Juan, R. Sikorski, S. Suggs, R. Radinsky, S. D. Patterson, and D. D. Chang. 2008. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26:1626-1634. [DOI] [PubMed] [Google Scholar]
- 3.Benachenhou, N., S. Guiral, I. Gorska-Flipot, D. Labuda, and D. Sinnett. 1998. High resolution deletion mapping reveals frequent allelic losses at the DNA mismatch repair loci hMLH1 and hMSH3 in non-small cell lung cancer. Int. J. Cancer 77:173-180. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, S., L. Klimcakova, F. M. Vega, and P. A. Lazo. 2006. The subcellular localization of vaccinia-related kinase-2 (VRK2) isoforms determines their different effect on p53 stability in tumour cell lines. FEBS J. 273:2487-2504. [DOI] [PubMed] [Google Scholar]
- 5.Blanco, S., and P. A. Lazo. 2009. Vaccinia-related kinase-2. UCSD-Nature Molecule Page doi: 10.1038/mp.a000905.01. [DOI]
- 6.Blanco, S., C. Santos, and P. A. Lazo. 2007. Vaccinia-related kinase 2 modulates the stress response to hypoxia mediated by TAK1. Mol. Cell. Biol. 27:7273-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco, S., M. Sanz-Garcia, C. R. Santos, and P. A. Lazo. 2008. Modulation of interleukin-1 transcriptional response by the interaction between VRK2 and the JIP1 scaffold protein. PLoS One 3:e1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos, J. L. 1989. ras oncogenes in human cancer: a review. Cancer Res. 49:4682-4689. [PubMed] [Google Scholar]
- 9.Burgess, A. W. 2008. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors 26:263-274. [DOI] [PubMed] [Google Scholar]
- 10.Casar, B., I. Arozarena, V. Sanz-Moreno, A. Pinto, L. Agudo-Ibanez, R. Marais, R. E. Lewis, M. T. Berciano, and P. Crespo. 2009. Ras subcellular localization defines extracellular signal-regulated kinase 1 and 2 substrate specificity through distinct utilization of scaffold proteins. Mol. Cell. Biol. 29:1338-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, C., R. E. Lewis, and M. A. White. 2008. IMP modulates KSR1-dependent multivalent complex formation to specify ERK1/2 pathway activation and response thresholds. J. Biol. Chem. 283:12789-12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Muga, S. V., P. Timpson, L. Cubells, R. Evans, T. E. Hayes, C. Rentero, A. Hegemann, M. Reverter, J. Leschner, A. Pol, F. Tebar, R. J. Daly, C. Enrich, and T. Grewal. 2009. Annexin A6 inhibits Ras signalling in breast cancer cells. Oncogene 28:363-377. [DOI] [PubMed] [Google Scholar]
- 13.Dhanasekaran, D. N., K. Kashef, C. M. Lee, H. Xu, and E. P. Reddy. 2007. Scaffold proteins of MAP-kinase modules. Oncogene 26:3185-3202. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon, A. S., S. Hagan, O. Rath, and W. Kolch. 2007. MAP kinase signalling pathways in cancer. Oncogene 26:3279-3290. [DOI] [PubMed] [Google Scholar]
- 15.Di Cosimo, S., and J. Baselga. 2008. Targeted therapies in breast cancer: where are we now? Eur. J. Cancer 44:2781-2790. [DOI] [PubMed] [Google Scholar]
- 16.Fehrenbacher, N., D. Bar-Sagi, and M. Philips. 2009. Ras/MAPK signaling from endomembranes. Mol. Oncol. 3:297-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorjanacz, M., E. P. Klerkx, V. Galy, R. Santarella, C. Lopez-Iglesias, P. Askjaer, and I. W. Mattaj. 2008. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J. 26:132-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes, N. E., and H. A. Lane. 2005. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 5:341-354. [DOI] [PubMed] [Google Scholar]
- 19.Ikediobi, O. N., H. Davies, G. Bignell, S. Edkins, C. Stevens, S. O'Meara, T. Santarius, T. Avis, S. Barthorpe, L. Brackenbury, G. Buck, A. Butler, J. Clements, J. Cole, E. Dicks, S. Forbes, K. Gray, K. Halliday, R. Harrison, K. Hills, J. Hinton, C. Hunter, A. Jenkinson, D. Jones, V. Kosmidou, R. Lugg, A. Menzies, T. Mironenko, A. Parker, J. Perry, K. Raine, D. Richardson, R. Shepherd, A. Small, R. Smith, H. Solomon, P. Stephens, J. Teague, C. Tofts, J. Varian, T. Webb, S. West, S. Widaa, A. Yates, W. Reinhold, J. N. Weinstein, M. R. Stratton, P. A. Futreal, and R. Wooster. 2006. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 5:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jagemann, L. R., L. G. Perez-Rivas, E. J. Ruiz, J. A. Ranea, F. Sanchez-Jimenez, A. R. Nebreda, E. Alba, and J. Lozano. 2008. The functional interaction of 14-3-3 proteins with the ERK1/2 scaffold KSR1 occurs in an isoform-specific manner. J. Biol. Chem. 283:17450-17462. [DOI] [PubMed] [Google Scholar]
- 21.Kang, T. H., D. Y. Park, W. Kim, and K. T. Kim. 2008. VRK1 phosphorylates CREB and mediates CCND1 expression. J. Cell Sci. 121:3035-3041. [DOI] [PubMed] [Google Scholar]
- 22.Karapetis, C. S., S. Khambata-Ford, D. J. Jonker, C. J. O'Callaghan, D. Tu, N. C. Tebbutt, R. J. Simes, H. Chalchal, J. D. Shapiro, S. Robitaille, T. J. Price, L. Shepherd, H. J. Au, C. Langer, M. J. Moore, and J. R. Zalcberg. 2008. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359:1757-1765. [DOI] [PubMed] [Google Scholar]
- 23.Karreth, F. A., G. M. DeNicola, S. P. Winter, and D. A. Tuveson. 2009. C-Raf inhibits MAPK activation and transformation by B-Raf(V600E). Mol. Cell 36:477-486. [DOI] [PubMed] [Google Scholar]
- 24.Kizaka-Kondoh, S., K. Sato, K. Tamura, H. Nojima, and H. Okayama. 1992. Raf-1 protein kinase is an integral component of the oncogenic signal cascade shared by epidermal growth factor and platelet-derived growth factor. Mol. Cell. Biol. 12:5078-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjellman, M., L. Roshani, B. T. Teh, O. P. Kallioniemi, A. Hoog, S. Gray, L. O. Farnebo, M. Holst, M. Backdahl, and C. Larsson. 1999. Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. J. Clin. Endocrinol. Metab. 84:730-735. [DOI] [PubMed] [Google Scholar]
- 26.Klerkx, E. P., P. A. Lazo, and P. Askjaer. 2009. Emerging biological functions of the Vaccinia-Related Kinase (VRK) family. Histol. Histopathol. 24:749-759. [DOI] [PubMed] [Google Scholar]
- 27.Klijn, J. G., M. P. Look, H. Portengen, J. Alexieva-Figusch, W. L. van Putten, and J. A. Foekens. 1994. The prognostic value of epidermal growth factor receptor (EGF-R) in primary breast cancer: results of a 10 year follow-up study. Breast Cancer Res. Treat. 29:73-83. [DOI] [PubMed] [Google Scholar]
- 28.Kolch, W. 2005. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6:827-837. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Borges, S., and P. A. Lazo. 2000. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene 19:3656-3664. [DOI] [PubMed] [Google Scholar]
- 30.Martin, K. J., D. R. Patrick, M. J. Bissell, and M. V. Fournier. 2008. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS One 3:e2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Carpio, P. A., C. Mur, P. Rosel, and M. A. Navarro. 1999. Constitutive and regulated secretion of epidermal growth factor and transforming growth factor-beta1 in MDA-MB-231 breast cancer cell line in 11-day cultures. Cell Signal. 11:753-757. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Iglesias, O., S. Garcia-Silva, S. P. Tenbaum, J. Regadera, F. Larcher, J. M. Paramio, B. Vennstrom, and A. Aranda. 2009. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 69:501-509. [DOI] [PubMed] [Google Scholar]
- 33.McKay, M. M., and D. K. Morrison. 2007. Integrating signals from RTKs to ERK/MAPK. Oncogene 26:3113-3121. [DOI] [PubMed] [Google Scholar]
- 34.Michaloglou, C., L. C. Vredeveld, M. S. Soengas, C. Denoyelle, T. Kuilman, C. M. van der Horst, D. M. Majoor, J. W. Shay, W. J. Mooi, and D. S. Peeper. 2005. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436:720-724. [DOI] [PubMed] [Google Scholar]
- 35.Muller, J., S. Ory, T. Copeland, H. Piwnica-Worms, and D. K. Morrison. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983-993. [DOI] [PubMed] [Google Scholar]
- 36.Nichols, R. J., and P. Traktman. 2004. Characterization of three paralogous members of the mammalian vaccinia related kinase family. J. Biol. Chem. 279:7934-7946. [DOI] [PubMed] [Google Scholar]
- 37.Nichols, R. J., M. S. Wiebe, and P. Traktman. 2006. The vaccinia-related kinases phosphorylate the N′ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol. Biol. Cell 17:2451-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patton, E. E., H. R. Widlund, J. L. Kutok, K. R. Kopani, J. F. Amatruda, R. D. Murphey, S. Berghmans, E. A. Mayhall, D. Traver, C. D. Fletcher, J. C. Aster, S. R. Granter, A. T. Look, C. Lee, D. E. Fisher, and L. I. Zon. 2005. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15:249-254. [DOI] [PubMed] [Google Scholar]
- 39.Rajakulendran, T., M. Sahmi, M. Lefrancois, F. Sicheri, and M. Therrien. 2009. A dimerization-dependent mechanism drives RAF catalytic activation. Nature 461:542-545. [DOI] [PubMed] [Google Scholar]
- 40.Roberts, P. J., and C. J. Der. 2007. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291-3310. [DOI] [PubMed] [Google Scholar]
- 41.Salerno, M., D. Palmieri, A. Bouadis, D. Halverson, and P. S. Steeg. 2005. Nm23-H1 metastasis suppressor expression level influences the binding properties, stability, and function of the kinase suppressor of Ras1 (KSR1) Erk scaffold in breast carcinoma cells. Mol. Cell. Biol. 25:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos, C. R., M. Rodriguez-Pinilla, F. M. Vega, J. L. Rodriguez-Peralto, S. Blanco, A. Sevilla, A. Valbuena, T. Hernandez, A. J. van Wijnen, F. Li, E. de Alava, M. Sanchez-Cespedes, and P. A. Lazo. 2006. VRK1 signaling pathway in the context of the proliferation phenotype in head and neck squamous cell carcinoma. Mol. Cancer Res. 4:177-185. [DOI] [PubMed] [Google Scholar]
- 43.Sevilla, A., C. R. Santos, R. Barcia, F. M. Vega, and P. A. Lazo. 2004. c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK). Oncogene 23:8950-8958. [DOI] [PubMed] [Google Scholar]
- 44.Sevilla, A., C. R. Santos, F. M. Vega, and P. A. Lazo. 2004. Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J. Biol. Chem. 279:27458-27465. [DOI] [PubMed] [Google Scholar]
- 45.Shin, S. Y., O. Rath, S. M. Choo, F. Fee, B. McFerran, W. Kolch, and K. H. Cho. 2009. Positive- and negative-feedback regulations coordinate the dynamic behavior of the Ras-Raf-MEK-ERK signal transduction pathway. J. Cell Sci. 122:425-435. [DOI] [PubMed] [Google Scholar]
- 46.Valbuena, A., I. Lopez-Sanchez, and P. A. Lazo. 2008. Human VRK1 is an early response gene and its loss causes a block in cell cycle progression. PLoS One 3:e1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valbuena, A., A. Suarez-Gauthier, F. Lopez-Rios, A. Lopez-Encuentra, S. Blanco, P. L. Fernandez, M. Sanchez-Cespedes, and P. A. Lazo. 2007. Alteration of the VRK1-p53 autoregulatory loop in human lung carcinomas. Lung Cancer 58:303-309. [DOI] [PubMed] [Google Scholar]
- 48.Valbuena, A., F. M. Vega, S. Blanco, and P. A. Lazo. 2006. p53 downregulates its activating vaccinia-related kinase 1, forming a new autoregulatory loop. Mol. Cell. Biol. 26:4782-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vega, F. M., P. Gonzalo, M. L. Gaspar, and P. A. Lazo. 2003. Expression of the VRK (vaccinia-related kinase) gene family of p53 regulators in murine hematopoietic development. FEBS Lett. 544:176-180. [DOI] [PubMed] [Google Scholar]
- 50.Vega, F. M., A. Sevilla, and P. A. Lazo. 2004. p53 stabilization and accumulation induced by human vaccinia-related kinase 1. Mol. Cell. Biol. 24:10366-10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing, H., K. Kornfeld, and A. J. Muslin. 1997. The protein kinase KSR interacts with 14-3-3 protein and Raf. Curr. Biol. 7:294-300. [DOI] [PubMed] [Google Scholar]
- 52.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]
- 53.Yuste, L., J. C. Montero, A. Esparis-Ogando, and A. Pandiella. 2005. Activation of ErbB2 by overexpression or by transmembrane neuregulin results in differential signaling and sensitivity to herceptin. Cancer Res. 65:6801-6810. [DOI] [PubMed] [Google Scholar]
- 54.Zandi, R., A. B. Larsen, P. Andersen, M. T. Stockhausen, and H. S. Poulsen. 2007. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell. Signal. 19:2013-2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.