Abstract
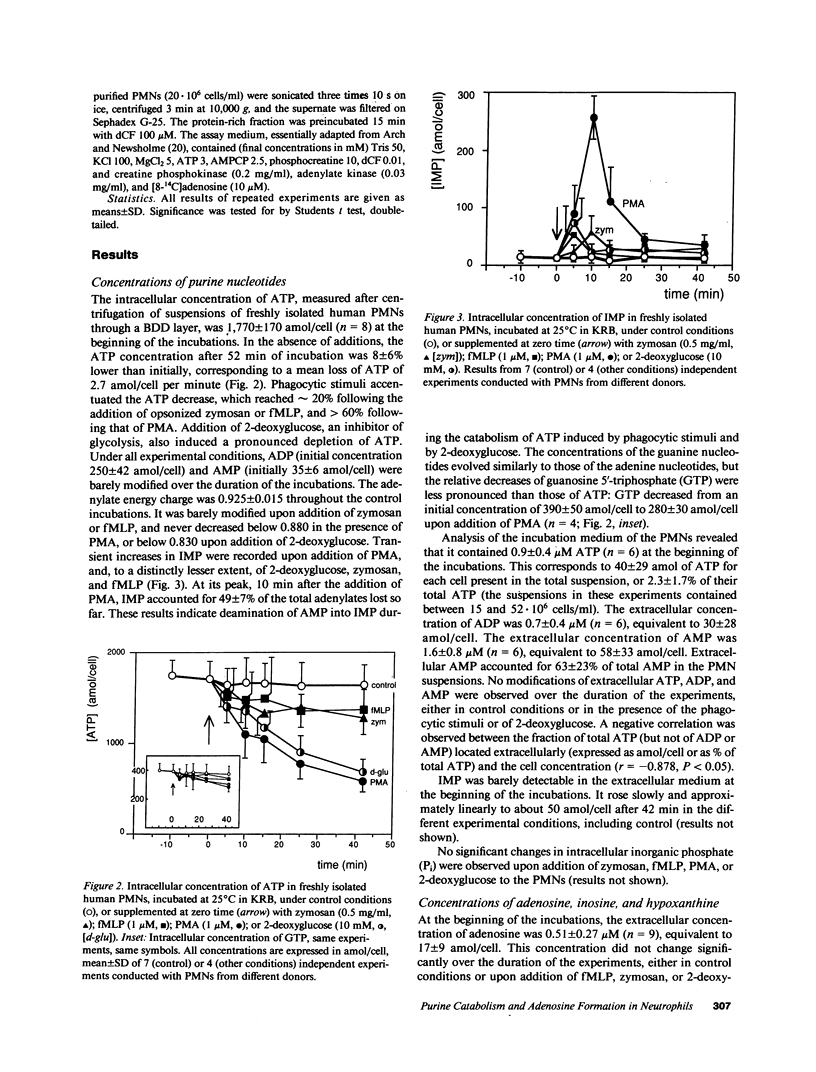
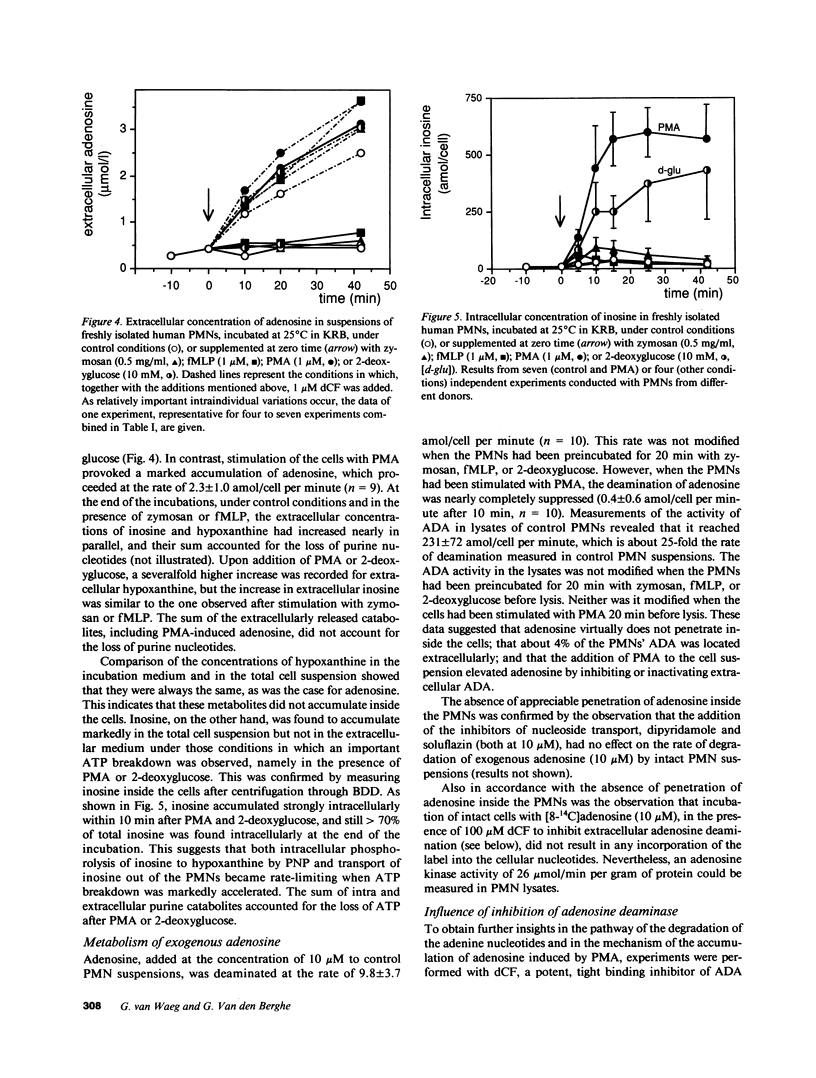
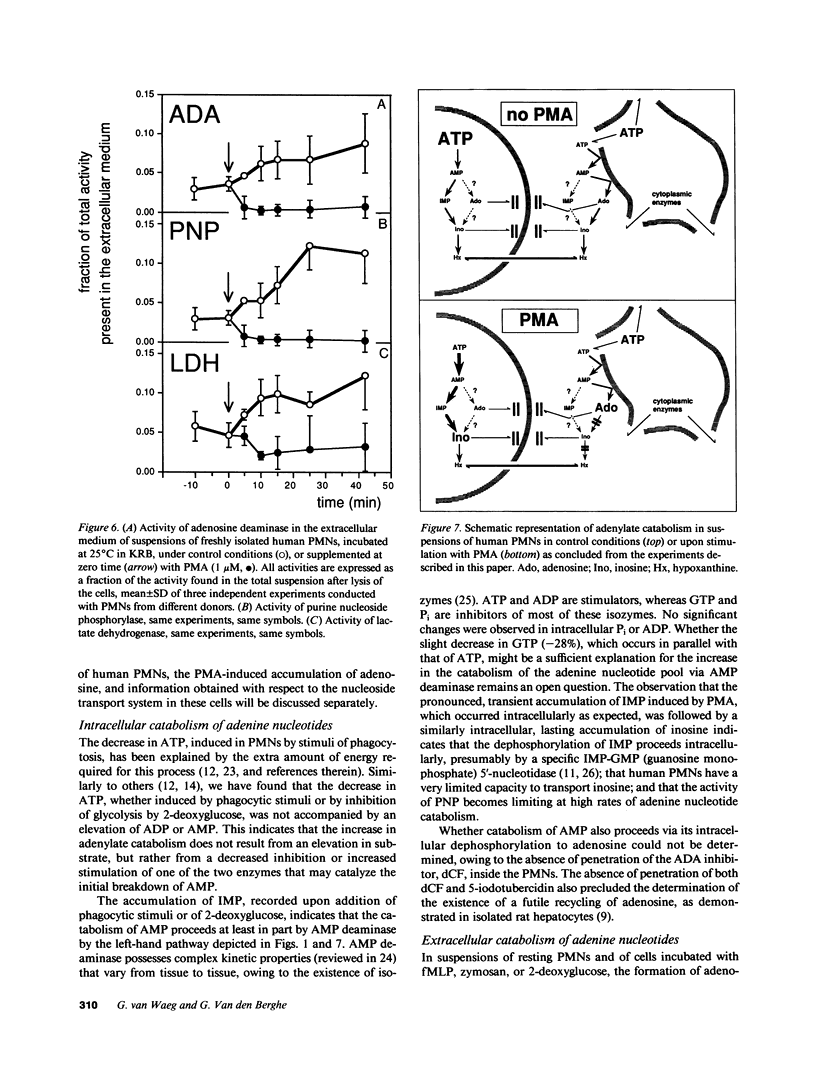
Since physiological concentrations (0.1-1 microM) of adenosine influence the functions of human polymorphonuclear neutrophils (PMNs), we investigated the metabolism of adenosine in suspensions of stimulated and unstimulated PMNs. Stimulation with phorbol myristate acetate (PMA, 1 microM), but not by zymosan (0.5 mg/ml) or N-formyl-methionyl-leucyl-phenylalanine (fMLP, 1 microM), provoked an accumulation of endogenous adenosine at a rate of 2.3 +/- 1.0 amol/cell per minute. A similar accumulation was observed with both unstimulated and stimulated PMNs after the addition of deoxycoformycin (dCF, 1-100 microM), an inhibitor of adenosine deaminase. Exogenous adenosine (10 microM) was deaminated at a rate of 9.8 +/- 3.7 amol/cell per minute in control or zymosan or fMLP-stimulated PMN suspensions. This deamination was nearly completely suppressed when the PMNs had been stimulated with PMA. In contrast, the activity of adenosine deaminase in PMN lysates (231 +/- 72 amol/cell per minute) was not modified by PMA stimulation. alpha, beta-Methyleneadenosine 5'-diphosphate (AMPCP, 2.5 mM), an inhibitor of membranous ecto-5'-nucleotidase, profoundly inhibited endogenous adenosine accumulation under all conditions. PMA stimulation also provoked an inactivation of extracellular adenosine deaminase, purine nucleoside phosphorylase, and lactate dehydrogenase in PMN suspensions. We concluded that PMNs, even when not stimulated, continuously produce adenosine by dephosphorylation of extracellularly released adenylates; and that stimulation of PMNs by PMA causes adenosine accumulation owing to the inactivation of adenosine deaminase released by broken cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. 5'-Nucleotidase activities in human erythrocytes. Identification of a purine 5'-nucleotidase stimulated by ATP and glycerate 2,3-bisphosphate. Biochem J. 1988 Mar 15;250(3):687–696. doi: 10.1042/bj2500687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Pathways of adenine nucleotide catabolism in erythrocytes. J Clin Invest. 1986 Mar;77(3):824–830. doi: 10.1172/JCI112379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G. Mechanism of adenosine triphosphate catabolism induced by deoxyadenosine and by nucleoside analogues in adenosine deaminase-inhibited human erythrocytes. Cancer Res. 1989 Sep 15;49(18):4983–4989. [PubMed] [Google Scholar]
- Bontemps F., Vincent M. F., Van den Bergh F., van Waeg G., Van den Berghe G. Stimulation by glycerate 2,3-bisphosphate: a common property of cytosolic IMP-GMP 5'-nucleotidase in rat and human tissues. Biochim Biophys Acta. 1989 Jul 27;997(1-2):131–134. doi: 10.1016/0167-4838(89)90144-1. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982 Sep;70(3):550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. 5'-Nucleotidase from smooth muscle of small intestine and from brain. Inhibition of nucleotides. Biochemistry. 1975 Jun 3;14(11):2362–2366. doi: 10.1021/bi00682a014. [DOI] [PubMed] [Google Scholar]
- Burnham D. N., Tyagi S. R., Uhlinger D. J., Lambeth J. D. Diacylglycerol generation and phosphoinositide turnover in human neutrophils: effects of particulate versus soluble stimuli. Arch Biochem Biophys. 1989 Feb 15;269(1):345–353. doi: 10.1016/0003-9861(89)90116-1. [DOI] [PubMed] [Google Scholar]
- Cornell N. W. Rapid fractionation of cell suspensions with the use of brominated hydrocarbons. Anal Biochem. 1980 Mar 1;102(2):326–331. doi: 10.1016/0003-2697(80)90162-1. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Rosenstein E. D., Korchak H. M., Weissmann G., Hirschhorn R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem J. 1988 Jun 15;252(3):709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Harlan J., DeChatelet L. R., Iverson D. B., McCall C. E. Magnesium-dependent adenosine triphosphatase as a marker enzyme for the plasma membrane of human polymorphonuclear leukocytes. Infect Immun. 1977 Feb;15(2):436–443. doi: 10.1128/iai.15.2.436-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. F., Brox L., Zombor G., Hunting D., Lomax C. A. Specificity of adenosine deaminase inhibitors. Biochem Pharmacol. 1977 Nov 1;26(21):1967–1972. doi: 10.1016/0006-2952(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Hammond J. R., Paterson A. R., Clanachan A. S. Species differences in nucleoside transport. A study of uridine transport and nitrobenzylthioinosine binding by mammalian erythrocytes. Biochem J. 1982 Oct 15;208(1):83–88. doi: 10.1042/bj2080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H. Purine accumulation in human fat cell suspensions. Evidence that human adipocytes release inosine and hypoxanthine rather than adenosine. J Biol Chem. 1988 Jun 25;263(18):8803–8809. [PubMed] [Google Scholar]
- Lane T. A., Lamkin G. E. Defective energy metabolism in stored granulocytes. Transfusion. 1982 Sep-Oct;22(5):368–373. doi: 10.1046/j.1537-2995.1982.22583017460.x. [DOI] [PubMed] [Google Scholar]
- McGarrity S. T., Stephenson A. H., Webster R. O. Regulation of human neutrophil functions by adenine nucleotides. J Immunol. 1989 Mar 15;142(6):1986–1994. [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A. Adenosine production inside rat polymorphonuclear leucocytes. Biochem J. 1981 Nov 15;200(2):399–403. doi: 10.1042/bj2000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Watanabe T. Isozymes of rat AMP deaminase. Biochim Biophys Acta. 1975 Oct 22;403(2):530–537. doi: 10.1016/0005-2744(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Rose F. R., Hirschhorn R., Weissmann G., Cronstein B. N. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988 Mar 1;167(3):1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Schrier D. J., Imre K. M. The effects of adenosine agonists on human neutrophil function. J Immunol. 1986 Nov 15;137(10):3284–3289. [PubMed] [Google Scholar]
- Sedgwick J. B., Vrtis R. F., Gourley M. F., Busse W. W. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J Allergy Clin Immunol. 1988 May;81(5 Pt 1):876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- Skubitz K. M., Wickham N. W., Hammerschmidt D. E. Endogenous and exogenous adenosine inhibit granulocyte aggregation without altering the associated rise in intracellular calcium concentration. Blood. 1988 Jul;72(1):29–33. [PubMed] [Google Scholar]
- Smith G. P., Peters T. J. Subcellular localization and properties of adenosine diphosphatase activity in human polymorphonuclear leukocytes. Biochim Biophys Acta. 1981 Mar 18;673(3):234–242. [PubMed] [Google Scholar]
- Taube R. A., Berlin R. D. Membrane transport of nucleosides in rabbit polymorphonuclear leukocytes. Biochim Biophys Acta. 1972 Jan 17;255(1):6–18. doi: 10.1016/0005-2736(72)90003-x. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Roberts M. F. 31 P NMR spectroscopy of phorbol-myristate-acetate stimulated polymorphonuclear human leukocytes. FEBS Lett. 1981 Jun 29;129(1):105–108. doi: 10.1016/0014-5793(81)80766-1. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Extracellular metabolites in suspensions of isolated hepatocytes. Biochem J. 1987 Dec 1;248(2):517–521. doi: 10.1042/bj2480517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. Metabolism of hypoxanthine in isolated rat hepatocytes. Biochem J. 1984 Aug 15;222(1):145–155. doi: 10.1042/bj2220145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., McCulloch K. K., Phan S. H., Powell J., Johnson K. J. Platelet enhancement of O2-. responses in stimulated human neutrophils. Identification of platelet factor as adenine nucleotide. Lab Invest. 1988 Jan;58(1):37–47. [PubMed] [Google Scholar]
- White J. G., Estensen R. D. Selective labilization of specific granules in polymorphonuclear leukocytes by phorbol myristate acetate. Am J Pathol. 1974 Apr;75(1):45–60. [PMC free article] [PubMed] [Google Scholar]



