Abstract
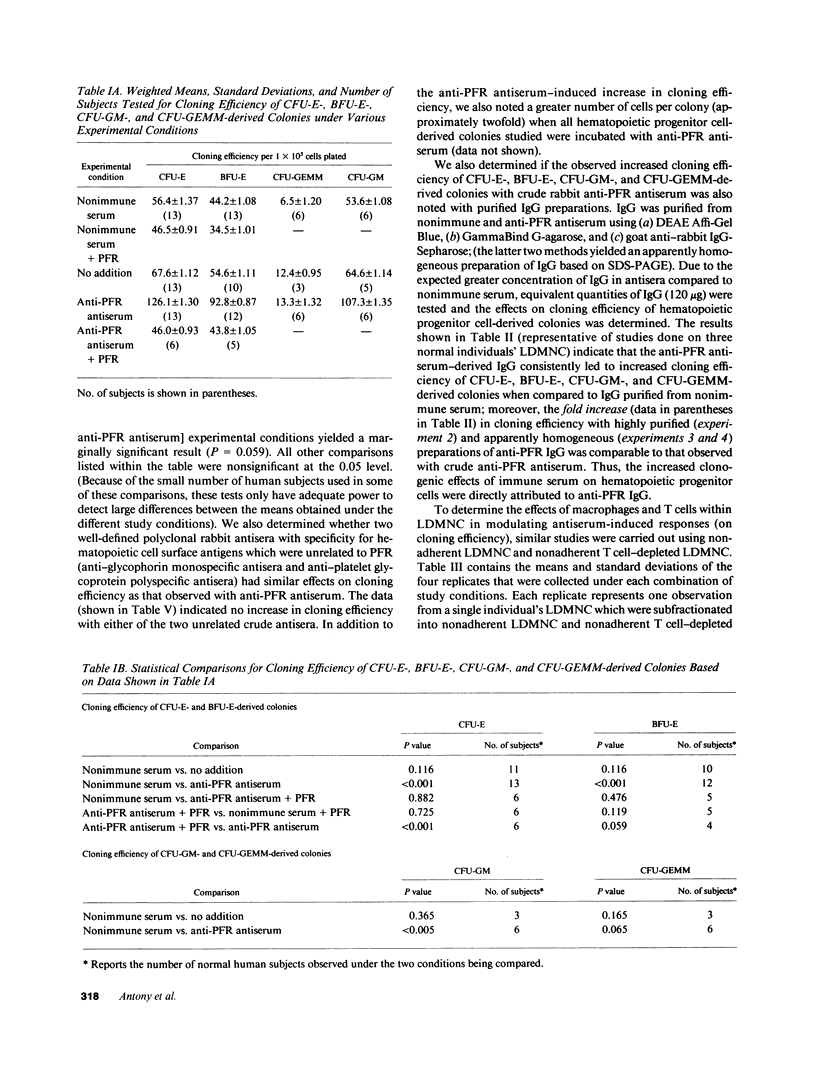
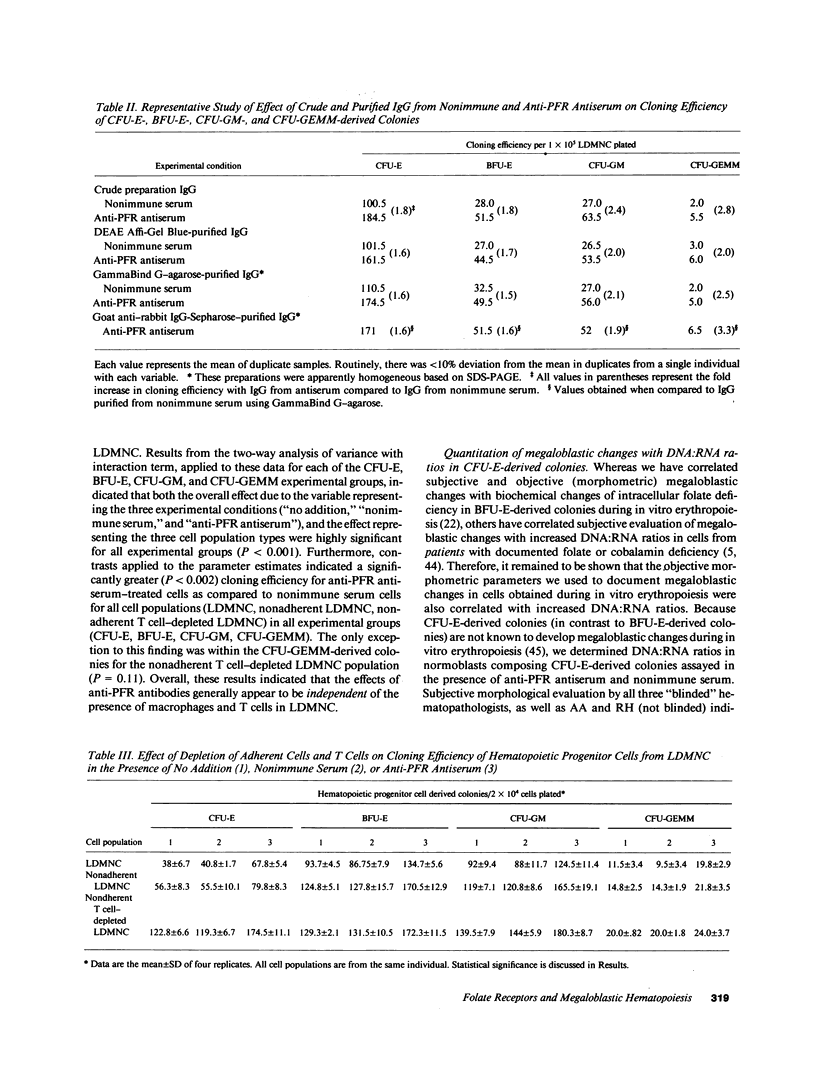
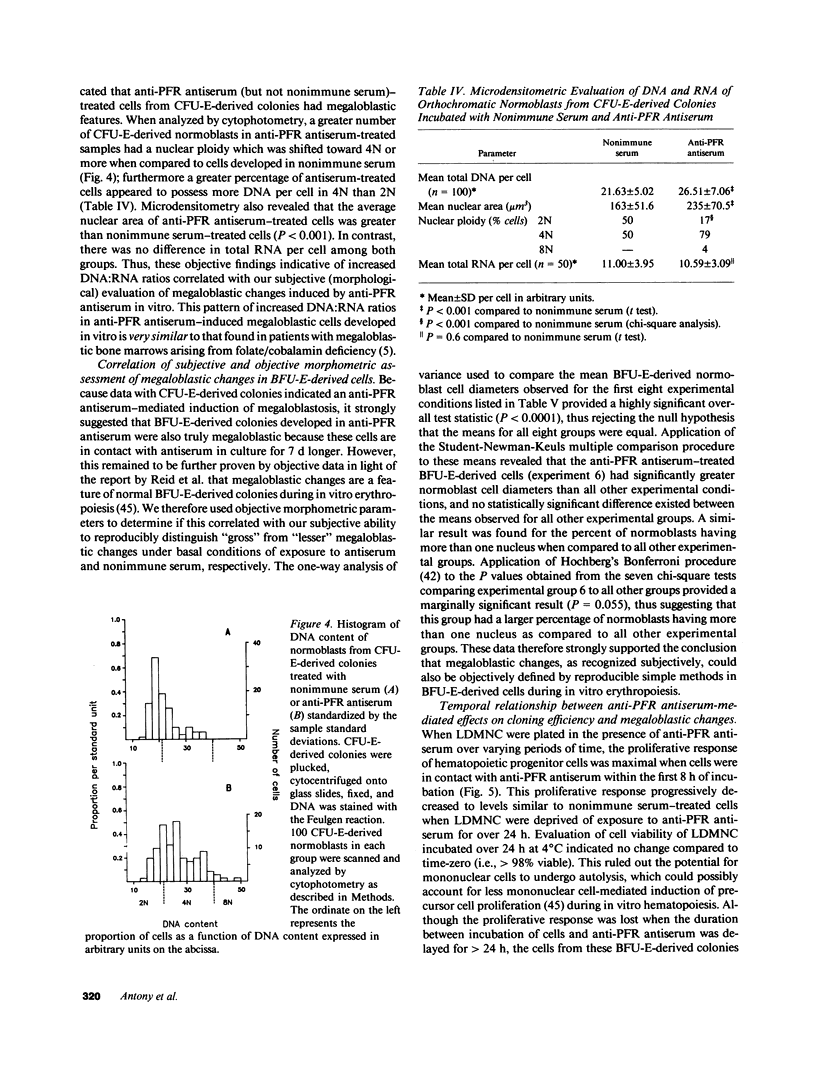
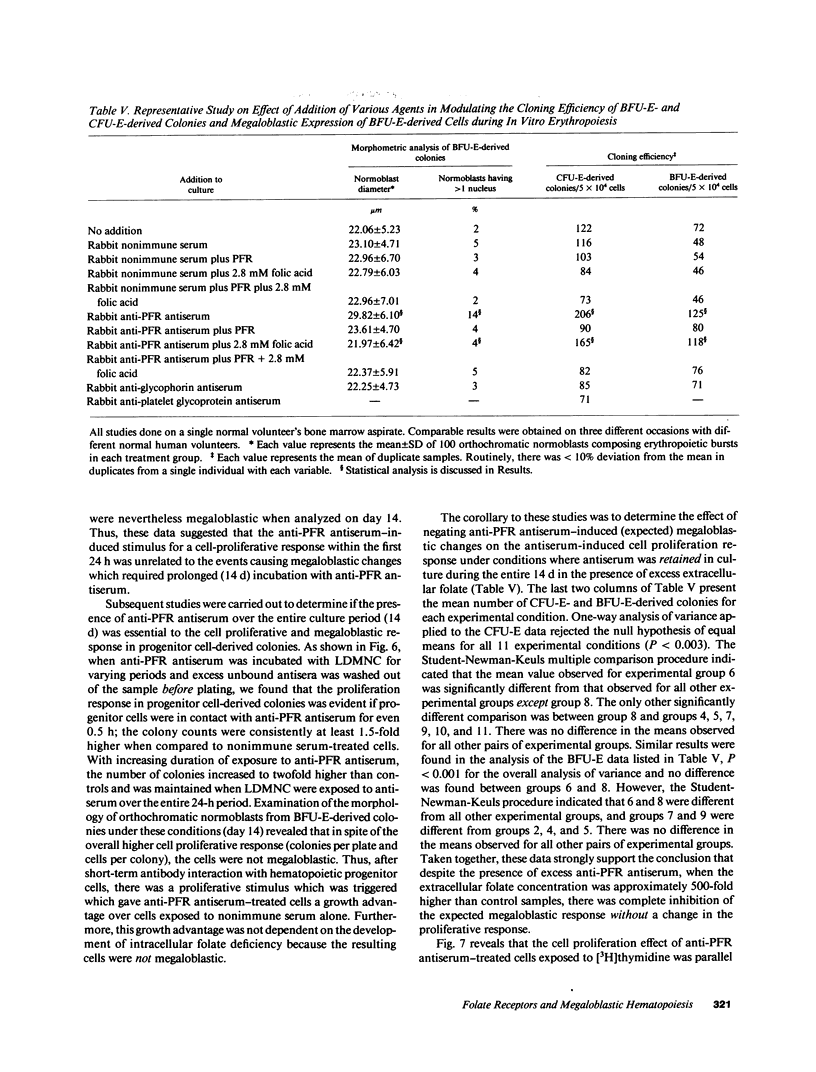
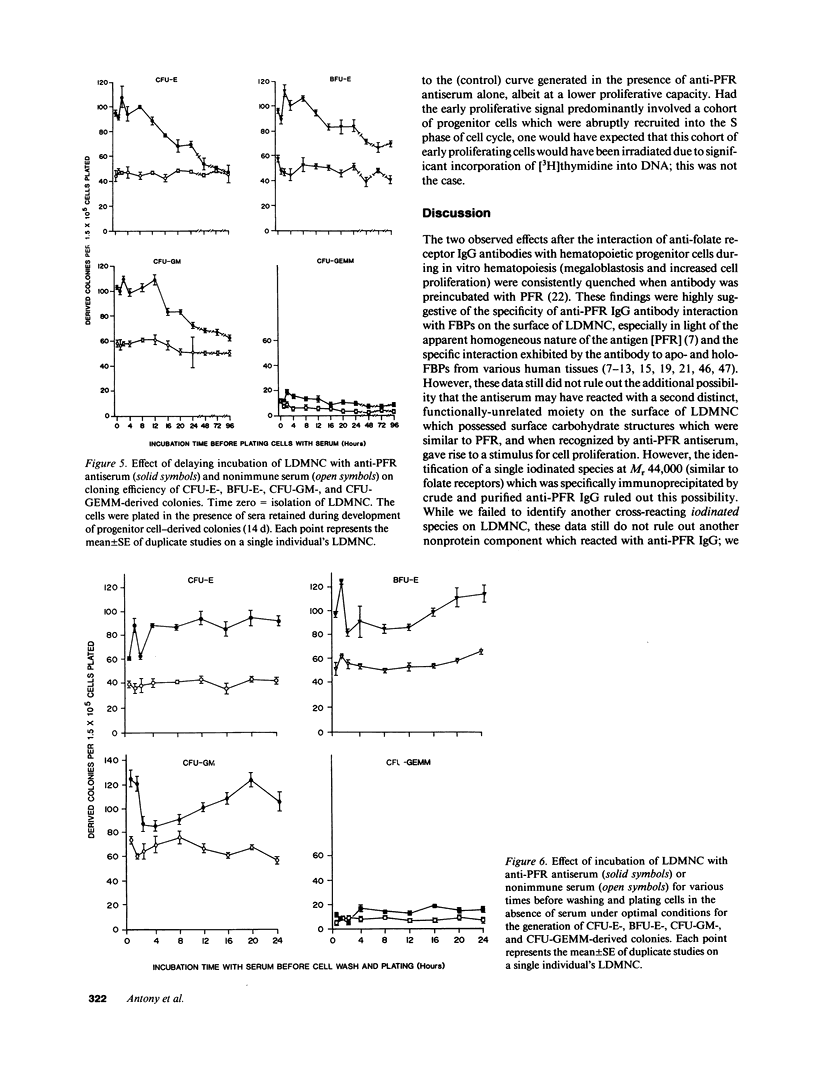
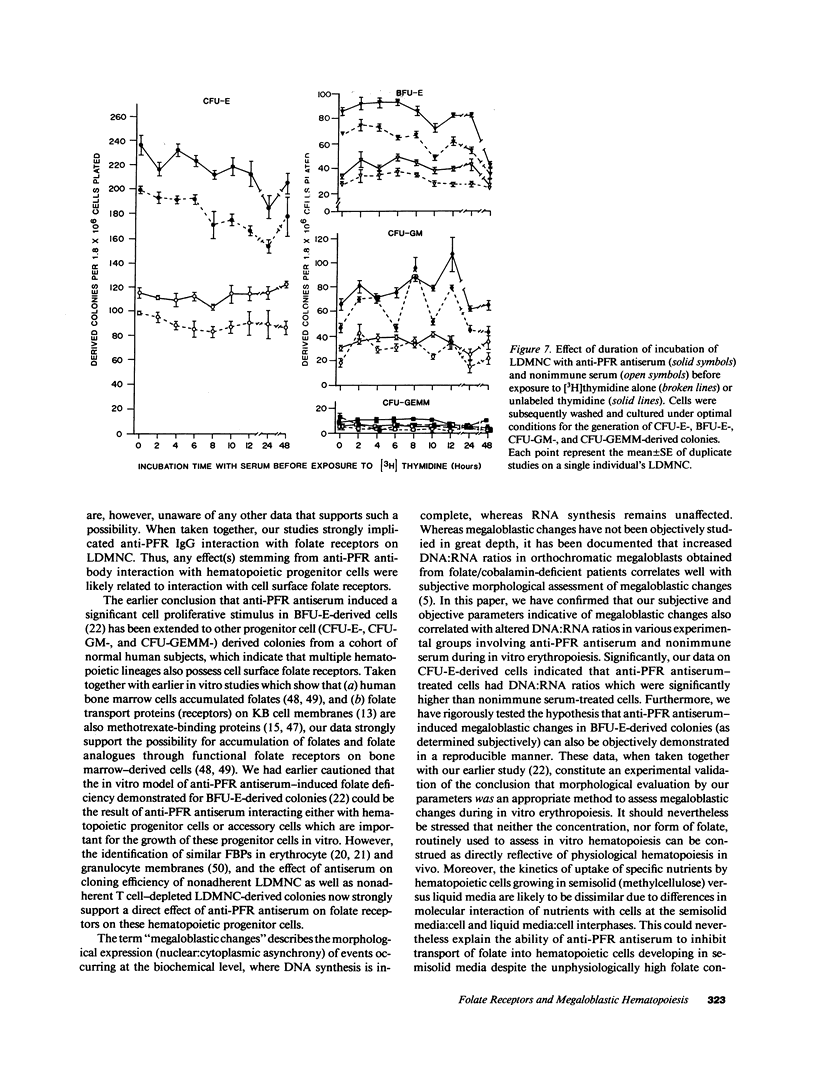
We tested the hypothesis that anti-placental folate receptor (PFR) antiserum-mediated effects on hematopoietic progenitor cells in vitro of increased cell proliferation and megaloblastic morphology were independent responses. We determined that (a) purified IgG from anti-PFR antiserum reacted with purified apo- and holo-PFR and specifically immunoprecipitated a single (44-kD) iodinated moiety on cell surfaces of low density mononuclear cells (LDMNC); (b) when retained in culture during in vitro hematopoiesis, anti-PFR IgG (in contrast to PFR-neutralized anti-PFR IgG and nonimmune IgG) consistently led to increased cloning efficiency of colony forming unit-erythroid (CFU-E), burst forming unit-E (BFU-E), CFU-granulocyte macrophage (CFU-GM), and CFU-GEM megakaryocyte (CFU-GEMM), and objectively defined megaloblastic changes in orthochromatic normoblasts from CFU-E- and BFU-E-derived colonies; (c) when anti-PFR antiserum was removed after initial (less than 1 h) incubation with LDMNC, a cell proliferation response was induced, but megaloblastic changes were not evident. (d) Conversely, delay at 4 degrees C for 24 h before long-term plating with antiserum resulted in megaloblastosis without increased cell proliferation; (e) however, 500-fold molar excess extracellular folate concentrations completely abrogated the expected anti-PFR antiserum-induced megaloblastic changes, without altering cell proliferative responses. Thus, although cell proliferative and megaloblastic changes are induced after short-term and prolonged interaction of antibody with folate receptors on hematopoietic progenitors, respectively, they are independent effects.
Full text
PDF


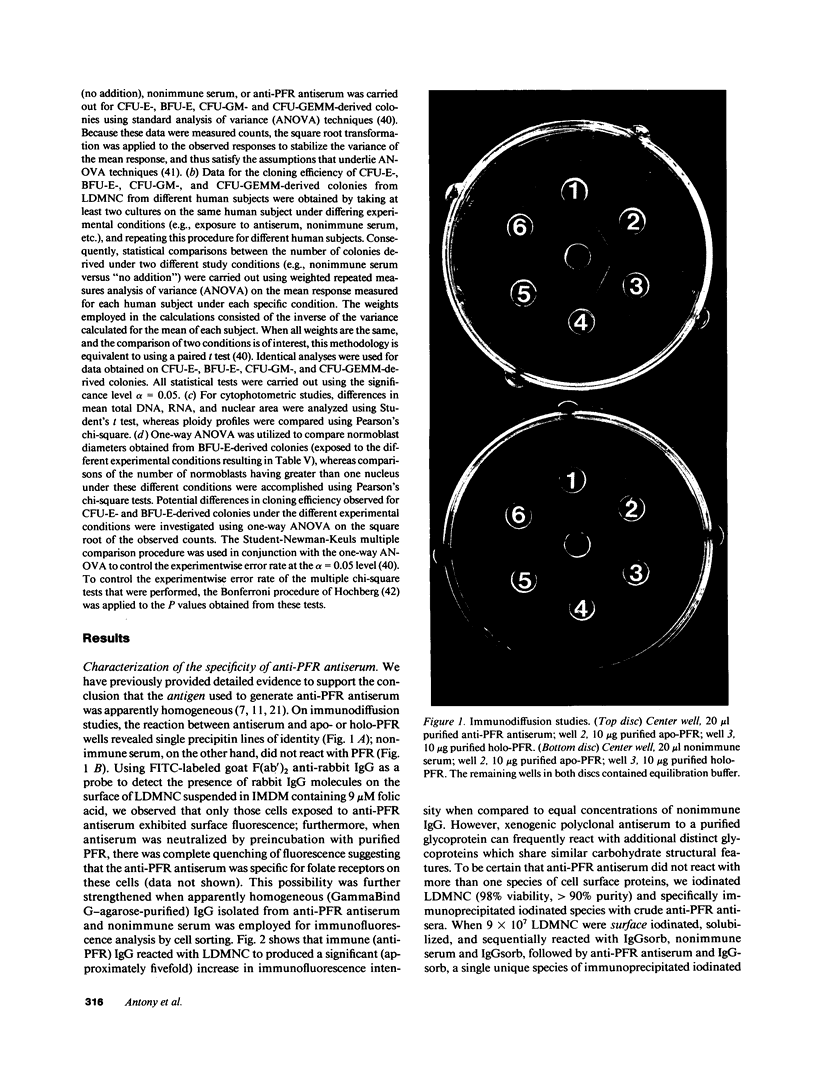
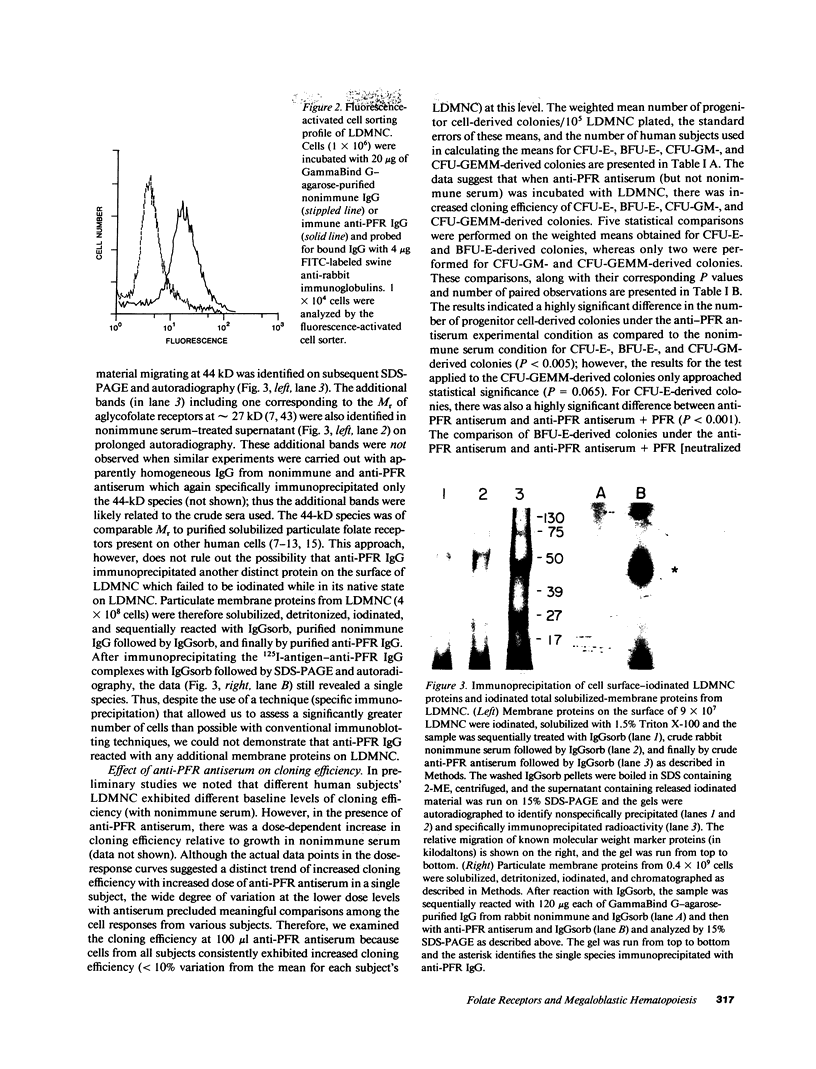


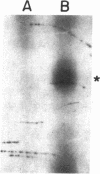
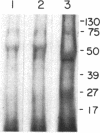
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Björck L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J Biol Chem. 1986 Aug 5;261(22):10240–10247. [PubMed] [Google Scholar]
- Antony A. C., Bruno E., Briddell R. A., Brandt J. E., Verma R. S., Hoffman R. Effect of perturbation of specific folate receptors during in vitro erythropoiesis. J Clin Invest. 1987 Dec;80(6):1618–1623. doi: 10.1172/JCI113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Krishnan S. R., Kincade R. S., Verma R. S. Folate (pteroylglutamate) uptake in human red blood cells, erythroid precursors and KB cells at high extracellular folate concentrations. Evidence against a role for specific folate-binding and transport proteins. Biochem J. 1989 Jun 1;260(2):401–411. doi: 10.1042/bj2600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Kincade R. S., Verma R. S., Krishnan S. R. Identification of high affinity folate binding proteins in human erythrocyte membranes. J Clin Invest. 1987 Sep;80(3):711–723. doi: 10.1172/JCI113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C., Utley C. S., Marcell P. D., Kolhouse J. F. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J Biol Chem. 1982 Sep 10;257(17):10081–10089. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Antony A. C., Verma R. S. Hydrophobic erythrocyte folate binding proteins are converted to hydrophilic forms by trypsin in vitro. Biochim Biophys Acta. 1989 Feb 13;979(1):62–68. doi: 10.1016/0005-2736(89)90523-3. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Verma R. S., Kincade R. S. Development of a specific radioimmunoassay for the placental folate receptor and related high-affinity folate binding proteins in human tissues. Anal Biochem. 1987 Apr;162(1):224–235. doi: 10.1016/0003-2697(87)90031-5. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E. Relationship of cell-cycle expression of Ia-like antigenic determinants on normal and leukemia human granulocyte-macrophage progenitor cells to regulation in vitro by acidic isoferritins. J Clin Invest. 1982 Mar;69(3):632–642. doi: 10.1172/JCI110490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch J. C., Elwood P. C., Portillo R. M., Macey M. G., Kolhouse J. F. Role of the membrane-associated folate binding protein (folate receptor) in methotrexate transport by human KB cells. Arch Biochem Biophys. 1989 Nov 1;274(2):327–337. doi: 10.1016/0003-9861(89)90446-3. [DOI] [PubMed] [Google Scholar]
- Elwood P. C. Molecular cloning and characterization of the human folate-binding protein cDNA from placenta and malignant tissue culture (KB) cells. J Biol Chem. 1989 Sep 5;264(25):14893–14901. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Herbert V. Megaloblastic anemias. Lab Invest. 1985 Jan;52(1):3–19. [PubMed] [Google Scholar]
- Hoffbrand V., Tripp E., Catovsky D., Das K. C. Transport of methotrexate into normal haemopoietic cells and into leukaemic cells and its effects on DNA synthesis. Br J Haematol. 1973 Oct;25(4):497–511. doi: 10.1111/j.1365-2141.1973.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Kamen B. A., Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5983–5987. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Johnson C. A., Wang M. T., Anderson R. G. Regulation of the cytoplasmic accumulation of 5-methyltetrahydrofolate in MA104 cells is independent of folate receptor regulation. J Clin Invest. 1989 Nov;84(5):1379–1386. doi: 10.1172/JCI114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Wang M. T., Streckfuss A. J., Peryea X., Anderson R. G. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988 Sep 25;263(27):13602–13609. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Kolhouse J. F. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem. 1986 Nov 25;261(33):15625–15631. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Najfeld V., Finley A., Waxman S., Kolhouse J. F. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988 May;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. A., Portillo R. M., Elwood P. C., Antony A. C., Kolhouse J. F. The influence of extracellular folate concentration on methotrexate uptake by human KB cells. Partial characterization of a membrane-associated methotrexate binding protein. J Biol Chem. 1986 Jan 5;261(1):44–49. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacey S. W., Sanders J. M., Rothberg K. G., Anderson R. G., Kamen B. A. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J Clin Invest. 1989 Aug;84(2):715–720. doi: 10.1172/JCI114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Low M. G. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Lu L., Briddell R. A., Graham C. D., Brandt J. E., Bruno E., Hoffman R. Effect of recombinant and purified human haematopoietic growth factors on in vitro colony formation by enriched populations of human megakaryocyte progenitor cells. Br J Haematol. 1988 Oct;70(2):149–156. doi: 10.1111/j.1365-2141.1988.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E., Meyers P. A., Moore M. A., Thaler H. T. Association of cell cycle expression of Ia-like antigenic determinations on normal human multipotential (CFU-GEMM) and erythroid (BFU-E) progenitor cells with regulation in vitro by acidic isoferritins. Blood. 1983 Feb;61(2):250–256. [PubMed] [Google Scholar]
- Luhrs C. A., Slomiany B. L. A human membrane-associated folate binding protein is anchored by a glycosyl-phosphatidylinositol tail. J Biol Chem. 1989 Dec 25;264(36):21446–21449. [PubMed] [Google Scholar]
- Marchesi S. L., Chasis J. A. Isolation of human platelet glycoproteins. Biochim Biophys Acta. 1979 Aug 23;555(3):442–459. doi: 10.1016/0005-2736(79)90398-5. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur E. M., Hoffman R., Chasis J., Marchesi S., Bruno E. Immunofluorescent identification of human megakaryocyte colonies using an antiplatelet glycoprotein antiserum. Blood. 1981 Feb;57(2):277–286. [PubMed] [Google Scholar]
- McHugh M., Cheng Y. C. Demonstration of a high affinity folate binder in human cell membranes and its characterization in cultured human KB cells. J Biol Chem. 1979 Nov 25;254(22):11312–11318. [PubMed] [Google Scholar]
- Mikel U. V., Fishbein W. N., Bahr G. F. Some practical considerations in quantitative absorbance microspectrophotometry. Preparation techniques in DNA cytophotometry. Anal Quant Cytol Histol. 1985 Jun;7(2):107–118. [PubMed] [Google Scholar]
- Parmley R. T., Ogawa M., Spicer S. S., Bank H. L., Wright N. J. Human marrow erythropoiesis in culture: III. Ultrastructural and cytochemical studies of cellular interactions. Exp Hematol. 1978 Jan;6(1):78–90. [PubMed] [Google Scholar]
- RITTER C., DI STEFANO H. S., FARAH A. A method for the cytophotometric estimation of ribonucleic acid. J Histochem Cytochem. 1961 Jan;9:97–102. doi: 10.1177/9.1.97. [DOI] [PubMed] [Google Scholar]
- Reid C. D., Baptista L. C., Deacon R., Chanarin I. Megaloblastic change is a feature of colonies derived from an early erythroid progenitor (BFU-E) stimulated by monocytes in culture. Br J Haematol. 1981 Dec;49(4):551–561. doi: 10.1111/j.1365-2141.1981.tb07263.x. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K. M., Larrick J. W., Fulcher S. A., Burck K. B., Pacely J., Davis J. C., Ring D. B. Selective inhibition of the growth of human erythroid bursts by monoclonal antibodies against transferrin or the transferrin receptor. Blood. 1986 Jun;67(6):1631–1638. [PubMed] [Google Scholar]
- Srour E. F., Leemhuis T., Jenski L., Redmond R., Jansen J. Cytolytic activity of human natural killer cell subpopulations isolated by four-color immunofluorescence flow cytometric cell sorting. Cytometry. 1990;11(3):442–446. doi: 10.1002/cyto.990110316. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S., Schreiber C. Determination of folate by use of radioactive folate and binding proteins. Methods Enzymol. 1980;66:468–483. doi: 10.1016/0076-6879(80)66490-8. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N., Chalmers D. G., Cooper E. H. Disturbed proliferation of erythropoietic cells in pernicious anemia. Nature. 1967 Jul 8;215(5097):189–191. doi: 10.1038/215189a0. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N. RNA content of megaloblasts in pernicious anaemia. Scand J Haematol. 1970;7(5):398–400. doi: 10.1111/j.1600-0609.1970.tb01920.x. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Bocchini V., Becker M., Givol D. A general method for the specific isolation of peptides containing modified residues, using insoluble antibody columns. Biochemistry. 1971 Jul 20;10(15):2828–2834. doi: 10.1021/bi00791a004. [DOI] [PubMed] [Google Scholar]
- da Costa M., Iqbal M. P. The transport and accumulation of methotrexate in human erythrocytes. Cancer. 1981 Dec 1;48(11):2427–2432. doi: 10.1002/1097-0142(19811201)48:11<2427::aid-cncr2820481115>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]