Abstract
T-cell differentiation involves the early decision to commit to a particular pattern of response to an antigen. Here, we show that the leukocyte activation antigen CD69 limits differentiation into proinflammatory helper T cells (Th17 cells). Upon antigen stimulation in vitro, CD4+ T cells from CD69-deficient mice generate an expansion of Th17 cells and the induction of greater mRNA expression of interleukin 17 (IL-17), IL 23 receptor (IL-23R), and the nuclear receptor retinoic acid-related orphan receptor γt (RORγt). In vivo studies with CD69-deficient mice bearing OTII T-cell receptors (TCRs) specific for OVA peptide showed a high proportion of antigen-specific Th17 subpopulation in the draining lymph nodes, as well as in CD69-deficient mice immunized with type II collagen. Biochemical analysis demonstrated that the CD69 cytoplasmic tail associates with the Jak3/Stat5 signaling pathway, which regulates the transcription of RORγt and, consequently, differentiation toward the Th17 lineage. Functional experiments in Th17 cultures demonstrated that the selective inhibition of Jak3 activation enhanced the transcription of RORγt. Moreover, the addition of exogenous IL-2 restored Stat5 phosphorylation and inhibited the enhanced Th17 differentiation in CD69-deficient cells. These results support the early activation receptor CD69 as an intrinsic modulator of the T-cell differentiation program that conditions immune inflammatory processes.
Differentiation of T helper lymphocyte subsets is crucial for immune and inflammatory responses. In addition to the two classical T helper cell subsets (Th1 and Th2), a third T-lymphocyte subpopulation, designated Th17, characterized by the synthesis of interleukin 17A (IL-17A), IL-17F, and IL-22, has emerged as an independent differentiation pathway (9). Differentiation toward each Th subset is regulated by a variety of molecules, including cytokines and transcription factors. The key transcription factors that drive the differentiation of the Th1 and Th2 lineages are, respectively, T-bet and GATA-3, while the differentiation of Th17 cells is directed by retinoic acid-related orphan receptor γt (RORγt) (12). Th17 differentiation, moreover, is regulated by the balance of Stat3/Stat5 activation; Stat3 is necessary for Th17 differentiation, whereas the transcription factor Stat5 negatively regulates the development of these cells (1). The key cytokines involved in Th17 cell differentiation are transforming growth factor β (TGF-β) and IL-6 (3, 33). Another important cytokine for Th17 biology is IL-23. Although Th17 cells can arise in the absence of IL-23, the cytokine is required for their maintenance and survival (29, 33) and for their pathogenicity (20).
Dysregulated activation of T helper subpopulations is associated with immune pathogenesis (21). Th1 cells are clearly involved in autoimmune and inflammatory disorders mediated by the cellular immune response, and Th2 cells are involved in antibody-mediated allergic and inflammatory conditions (24). Recent evidence indicates that Th17 cells also exert a pathogenic effect in several autoimmune and hypersensitivity reactions. Indeed, a number of immune pathologies previously thought to be related to uncontrolled activation of Th1 or Th2 populations now appear to be related, at least in part, to Th17 cell differentiation. For example, involvement of Th17 lymphocytes has been reported in collagen-induced arthritis (CIA), experimental autoimmune encephalomyelitis (EAE), experimental autoimmune myocarditis (EAM), contact hypersensitivity (CHS), and airway hyperresponsiveness (AHR) (11, 13, 18, 22, 23, 30).
We previously found that CD69+ T cells are localized at sites of chronic inflammation and that these lymphocytes seem to be able to downregulate the inflammatory process. Although antigen-dependent T-cell activation and proliferation do not appear to be altered in CD69-deficient lymphocytes (16), CD69 knockout (CD69 KO) mice develop an exacerbated form of CIA characterized by diminished local synthesis of TGF-β (26). These and other studies (5) suggest that CD69 is a negative regulator of the immune response, in part through modulation of local levels of TGF-β. Here, we explore the role of CD69 in the differentiation of T helper lineages. Our results show that, while Th1 and Th2 differentiation remain unchanged in CD69-deficient mice, lymphocytes from these animals show an enhanced potential to differentiate toward Th17 cells both in vitro and in vivo. Biochemical and functional experiments demonstrated that the CD69 cytoplasmic tail associates with Jak3/Stat5, providing a mechanistic link between the regulatory effect of CD69 and Th17 differentiation. Our results support the conclusion that CD69 regulates immune and inflammatory responses by acting as a brake on the differentiation of Th17 effector cells.
MATERIALS AND METHODS
Mice.
CD69-deficient mice were generated as described previously (16) and backcrossed (for more than 12 generations) to the BALB/c and C57BL/6J genetic background. C57BL/6-Tg (TcraTcrb)425Cbn/J (called OTII) mice expressing a T-cell receptor (TCR) specific for peptide 323-339 of OVA in the context of I-Ab were purchased from Jackson Laboratory (stock number 004194). OTII mice were backcrossed with CD69-deficient mice in the C57BL/6 background. Sex-matched mice aged between 6 and 8 weeks were used for the in vitro experiments, and 10- to 12-week-old females, either littermates or age-matched offspring of these littermates, were used for the in vivo experiments. The mice were bred in homozygosity and kept under pathogen-free conditions at the Animal Unit of the School of Medicine, Universidad Autónoma de Madrid (UAM). Experimental procedures were approved by the Committee for Research Ethics of the Universidad Autónoma de Madrid and conducted under the supervision of the UAM Head of Animal Welfare and Health in accordance with Spanish and European guidelines.
CD4+ T-cell isolation and cell culture.
Naive CD4+ T cells were obtained from single-cell suspensions of the spleen and mesenteric lymph node (MS-LN). The cell suspensions were incubated with biotinylated antibodies against CD8, CD16, CD19, CD24, CD117, major histocompatibility complex (MHC) class II (I-Ab), CD11b, CD11c, and DX5 and subsequently with streptavidin microbeads (MACS; Miltenyi Biotec). CD4+ T cells were negatively selected in auto-MACS Pro Separator (Miltenyi Biotec) according to the manufacturer's instructions. The cells were then labeled with antibodies to CD4, CD25, and CD62L and analyzed by flow cytometry to confirm their naive status (data not shown). Naive CD4+ T cells (106 cells/ml) were cultured in the presence of irradiated antigen-presenting cells (APCs) (T-cell-depleted splenocytes) and OVA peptide 323-339 (OVA) (10 μg/ml) plus a cytokine or antibody combination appropriate for differentiation of the desired T-cell lineage: IL-2 (10 ng/ml), anti-gamma interferon (anti-IFN-γ) (4 μg/ml), and anti-IL-4 (4 μg/ml) for ThN (see below) differentiation; IL-2 (10 ng/ml), anti-IL-4 (4 μg/ml) and recombinant mouse IFN-γ (rmIFN-γ) (4 ng/ml) for Th1; IL-2 (10 ng/ml), anti-IFN-γ (4 μg/ml), and rmIL-4 (10 ng/ml) for Th2; and anti-IFN-γ (4 μg/ml), anti-IL-4 (4 μg/ml), rmIL-6 (10 ng/ml), rmIL-23 (10 ng/ml), and recombinant human TGF-β1 (rhTGF-β1) (5 ng/ml) for Th17. Where indicated, 3-day-differentiated Th17 T cells from OTII or CD69-deficient OTII (OTKO) mice were cultured in the presence of anti-IL-2 receptor (anti-IL-2R) antibody (clone PC61.5) and stained for phospho-Stat5 (p-Stat5) (Tyr694). No differences in viability were observed between OTII and OTKO ThN, Th1, Th2, or Th17 cells.
Intracellular staining and FACS.
Fully differentiated T helper cells were extensively washed and then restimulated for 4 h with 50 ng/ml phorbol myristate acetate (PMA) and 750 ng/ml ionomycin (P+I) plus brefeldin A (1 μg/ml). The cells were then fixed with 2% paraformaldehyde, permeabilized with 0.5% saponin, and stained with anti-IFN-γ, anti-IL-17, or anti-IL-2 antibody (BD Pharmingen) and analyzed by fluorescence-activated cell sorting (FACS).
The expression of other proteins was assessed in cells fixed in 2% formaldehyde and permeabilized with methanol. Antibodies to the following proteins were used: T-bet from Santa Cruz and phospho-Stat6 (Tyr641), phospho-Stat3 (Tyr705), and phospho-Stat5 (Tyr694) from Cell Signaling. Anti-mouse IgG1 and anti-rabbit antibodies were used as isotype controls.
Cytokine assays.
Naive CD4+ T cells were stimulated for 72 h with CD3 (10 μg/ml) plus CD28 (2 μg/ml); ThN, Th1, Th2, and Th17 differentiated cells were restimulated for 24 h with CD3 (10 μg/ml) plus CD28 (2 μg/ml). Supernatants were collected from the stimulated cells, and cytokine concentrations were measured with the FlowCytomix Mouse Th1/Th2 10plex kit (Bender MedSystems GmbH, Germany), followed by flow cytometry on a BD FACSCanto II cytometer (BD Biosciences). The following cytokines were measured: granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, IL-1α, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17, and tumor necrosis factor alpha (TNF-α). Data were analyzed with FlowCytomix Pro 2.2 software.
Real-time PCR.
Total RNA was extracted from cell lysates with the Tri-Reagent RNA isolation system (Sigma). cDNA was obtained with the ImProm-II Reverse Transcriptase Kit (Promega). Specific mRNA expression was analyzed by SYBR green real-time PCR (Roche Diagnostics) in a DNA LightCycler rapid thermal cycler (Roche). Primer sequences were as follows: GAPDH (glyceraldehyde-3-phosphate dehydrogenase) forward, 5′-GAA GGT GAA GGT CGG AGT C-3′, and reverse, 5′-GAA GAT GGT GAT GGG ATT TC-3′; RORγt forward, 5′-CCG CTG AGA GGG CTT CAC-3′, and reverse, 5′-TGC AGG AGT AGG CCA CAT TAC A-3′; IL-17A forward, 5′-CTC CAG AAG GCC CTC AGA CTA C-3′, and reverse, 3′-GGG TCT TCA TTG CGG TGG-5′; IL-17F forward, 5′-GAG GAT AAC ACT GTG AGA GTT GAC-3′, and reverse, 5′-GAG TTC ATG GTG CTG TCT TCC-3′; IL-23R forward, 5′-GCC AAG AAG ACC ATT CCC GA-3′, and reverse, 5′-TCA GTG CTA CAA TCT TCT TCA GAG GAC A-3′. The results for each gene were normalized to GADPH expression and measured in parallel in the same sample.
Immunoprecipitations, pulldown, and Western blotting.
Immunoprecipitations of endogenous proteins were performed with whole-cell lysates of human and mouse T-cell blasts. Cell extracts were prepared in lysis buffer containing Tris-buffered saline (TBS), 0.5% Nonidet P-40 (Boehringer Mannheim), protease inhibitor cocktail (Complete; Roche), and phosphatase inhibitor cocktail (PhosSTOP; Roche). Immunoprecipitations were performed with protein G-Sepharose beads (Biosciences) with the antibody TP1/33 to human CD69 or 2.2 to mouse CD69. Sepharose pellets were washed with the lysis buffer and resuspended in Laemmli buffer for Western blotting. Pulldown assays were performed with whole-cell lysates of human T-cell blasts incubated with biotinylated human CD69 peptide (MSSENCFVAENSSLHPESGQENDATSPHFSTRHEGSFQGSGSK) or human VCAM1 peptide (SGSGRKANMKGSYSLVEAQKSKV) as a control. The beads were washed and resuspended in Laemmli buffer for Western blotting.
Lysates of purified naive, ThN, Th1, Th2, or Th17 CD4+ T cells were prepared in RIPA buffer (20 mM Tris-HCl, pH 7.4, 37 mM NaCl, 2 mM EDTA, 1% NP-40, 10% glycerol, 0.5% sodium deoxycholate, 0.1% SDS) containing the protease inhibitor cocktail (Complete; Roche). Nuclear and cytosolic protein extracts were prepared with the ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem). Proteins (10 to 30 μg) were size separated on 8% SDS-polyacrylamide gels and transferred onto Trans-Blot nitrocellulose membranes (Bio-Rad). Primary antibodies for immunoblotting were as follows: anti-β-actin, anti-GATA-3, anti-Stat5, anti-Stat3, and anti-CD69 from Santa Cruz; anti-phospho-Stat3, anti-phospho-Stat5, anti-Stat1, and anti-Jak1, -Jak2, and -Jak3 (Jak isoform sampler kit) from Cell Signaling.
Immunization and ex vivo expansion of Th17 cells.
OTII and OTKO mice were immunized in both hind footpads with 20 μg OVA plus complete Freund's adjuvant (CFA) (1:1; Sigma) or curdlan (200 μg) as an adjuvant. Alternatively, wild-type (WT) and CD69 KO mice were injected subcutaneously in the hind footpads with chick type II collagen (CII) (Sigma Aldrich; 2 mg/ml) in CFA. After 4 days, single-cell suspensions were prepared from popliteal lymph nodes (LNs). Intracellular staining of activated cells for IFN-γ and IL-17 was as described above. Ex vivo expansion of Th17 cells was analyzed by incubating total popliteal LN cells with either 10 μg/ml OTII-specific peptide or 100 μg/ml CII for 48 h in 96-well plates (1 × 106 cells in 200 μl RPMI, 10% fetal bovine serum [FBS]). After incubation, cells were collected, washed, stimulated, with P+I, and stained for CD4, IFN-γ, and IL-17.
Statistical analysis.
P values were calculated with Student's t test, and P values of less than 0.05 were considered significant.
RESULTS
CD69-deficient CD4 T cells preferentially differentiate toward Th17.
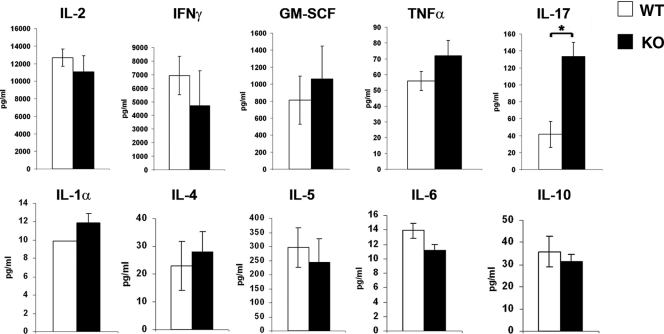
To assess whether CD69 affects the cytokine secretion pattern of T cells after activation, naive CD4+ T cells from lymph nodes and spleens of WT and CD69-deficient mice were stimulated for 72 h with anti-CD3/CD28 monoclonal antibodies (MAbs), and cytokine levels were measured in the culture supernatants. No significant differences in the secretion of IL-2, IFN-γ, GM-CSF, and TNF-α were detected between WT and CD69-deficient mice, and secretion of IL-1α, IL-4, IL-5, IL-6, and IL-10 was similarly unaffected (Fig. 1). In contrast, CD69 KO cells secreted significantly higher levels of IL-17 than lymphocytes from WT animals (Fig. 1).
FIG. 1.
CD69 deficiency increases IL-17 release from naive CD4+ T cells. Naive CD4+ T cells were isolated from mesenteric lymph nodes and spleens of WT and CD69 KO mice. The cells were cultured for 72 h with bound anti-CD3 and soluble anti-CD28, after which the indicated cytokines in the culture supernatants were determined by Flow Cytomix cytokine array. The bars represent means ± standard deviations (SD) of triplicate measurements of three independent experiments (means ± SD of six mice per genotype). *, P < 0.01 (Student's t test).
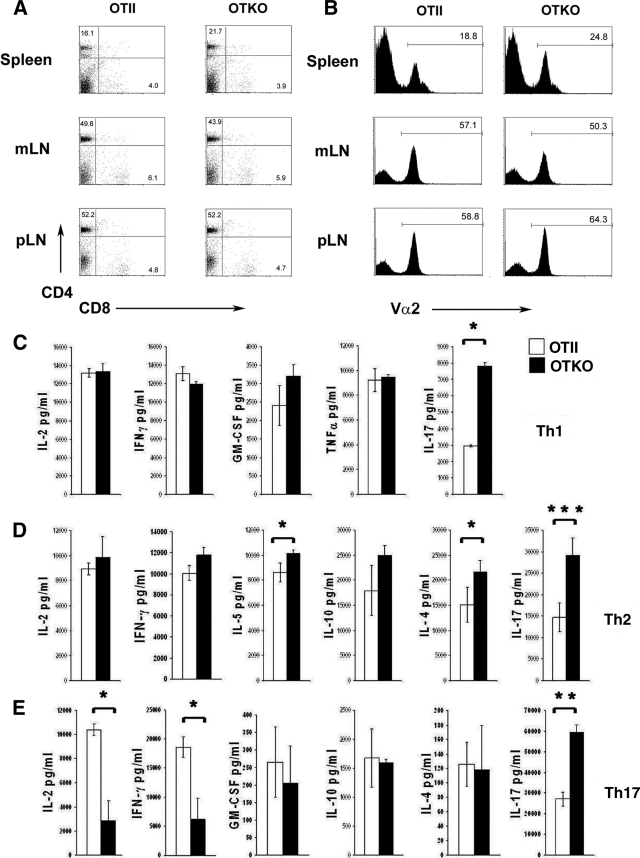
We next determined whether CD69 might play a role in the lineage commitment of CD4+ T helper cells. For this, we generated an antigen-specific model of CD69 deficiency by crossing CD69-deficient mice with transgenic mice expressing a TCR specific for peptide 323-339 of OVA in the context of I-Ab (OTII mice), hereafter referred to as OTKO mice. Phenotypic analysis of spleen and MS-LN and peripheral lymph node (p-LN) CD4+ and CD8+ T cells revealed no significant differences between OTII and OTKO mice (Fig. 2 A). TCR (Vα2 chain) membrane expression levels on transgenic T cells from these organs were also unaltered, which guarantees that the potential changes observed in T-cell activation and differentiation are not due to differences in TCR-mediated signaling (Fig. 2B). When naive CD4+ T cells from OTII or OTKO mice were differentiated in vitro under Th1, Th2, or Th17 polarizing conditions with OVA peptide-loaded APCs, no significant major differences were found in the secretion of Th1-specific cytokines, and a mild increase of IL-4 and IL-5 Th2 cytokines was seen in OTKO cells (Fig. 2C and D). Interestingly, IL-17 secretion was significantly higher in OTKO cells under all polarizing conditions tested (Fig. 2C to E). Also, we found low levels of IL-2 in OTKO cells cultured under Th17-polarized conditions. In addition, IFN-γ was also decreased in OTKO Th17 cells (Fig. 2E).
FIG. 2.
CD69 deficiency increases IL-17 release from CD4+ T cells differentiated under different lineage conditions. The T-lymphocyte population distribution in OTII mice was not altered by CD69 deficiency. Total cell suspensions from spleen, mesenteric LNs (mLN), or pLN were prepared from OTII and OTKO mice; stained with MAbs to CD4, CD8, and Vα2 (α chain type in OTII TCR); and analyzed by flow cytometry. (A) Dot plots showing CD4 and CD8 expression. The numbers indicate the percentages of CD4- and CD8-positive cells. (B) Histograms showing Vα2 chain expression. The percentages of Vα2-positive cells are indicated. The data are from one representative experiment out of three independent experiments. (C, D, and E) Naive CD4+ T cells obtained as for Fig. 1 were cultured for 3 days in the presence of OVA peptide under differentiation conditions for the Th1 (C), Th2 (D), or Th17 (E) subpopulation (see Materials and Methods). The cells were then washed and stimulated for 24 h with anti-CD3 and anti-CD28, and the cytokines in the culture supernatant were determined by Flow Cytomix cytokine array. The bars represent means ± SD of three independent experiments (means ± SD of three mice per genotype). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test).
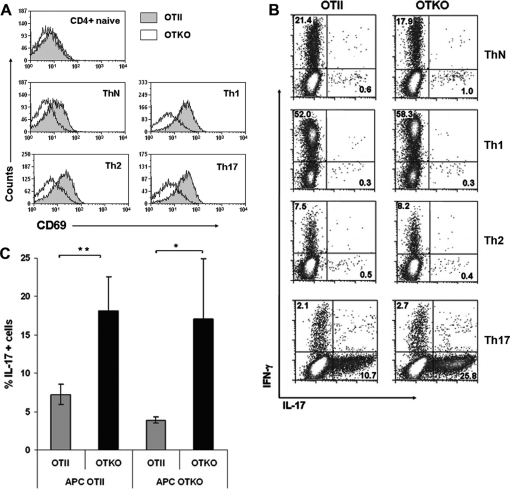
We then assessed the role of CD69 in determining the numbers of Th17 cells produced under different differentiation conditions. CD69 was absent from the membranes of naive CD4+ T cells but was similarly upregulated after the differentiation of T cells toward Th1, Th2, or Th17 lineages, as well as after culture with blocking antibodies under neutral conditions (ThN) (Fig. 3A). Likewise, the proportions of IFN-γ+ cells were similar under the different conditions tested and, moreover, did not vary between OTII and OTKO cells (Fig. 3B). In contrast, under Th17 differentiation conditions, the proportion of IL-17+ cells in OTKO cultures was statistically significantly higher than in OTII cultures (Fig. 3B and C). In these experiments, APCs from OTII and OTKO mice were equally able to induce strong Th17 differentiation of OTKO CD4+ T cells (Fig. 3C), indicating that the expression of CD69 by APCs is irrelevant for Th17 differentiation and supporting the idea that the enhanced Th17 differentiation observed is a cell-autonomous property of CD69-deficient T lymphocytes.
FIG. 3.
Enhancement of Th17 development in OTKO cells is T-cell intrinsic. (A) Flow cytometry of CD69 surface expression in naive and lineage-differentiated CD4+ T cells from OTII or OTKO mice. Anti-CD69 antibody was used to detect CD69 on freshly isolated (naive) cells; cells polarized toward the Th1, Th2, or Th17 lineage; and cells differentiated under neutral conditions (ThN). (B) Flow cytometry of intracellular IFN-γ and IL-17 expression. Effector T cells differentiated as in panel C were restimulated (4 h) with PMA and ionomycin in the presence of brefeldin A. The cells were permeabilized and stained with anti-IFN-γ-allophycocyanin and anti-IL-17-phycoerythrin antibodies. The numbers in each panel indicate the percentages of IFN-γ+ cells (top left) and IL-17+ cells (bottom right). (C) Enhanced Th17 differentiation of CD69-deficient cells is independent of CD69 on APCs. Naive CD4+ T cells from OTII or OTKO mice were cultured under Th17 differentiation conditions in the presence of APCs derived from OTII or OTKO spleens, and the percentage of IL-17+ cells was determined after restimulation as described in the legend to Fig. 2. The bars represent means ± SD of two independent experiments (means ± SD of three mice per genotype). *, P < 0.05; **, P < 0.01 (Student's t test).
CD69 KO mice show enhanced in vivo differentiation of antigen-specific Th17 cells after antigen challenge.
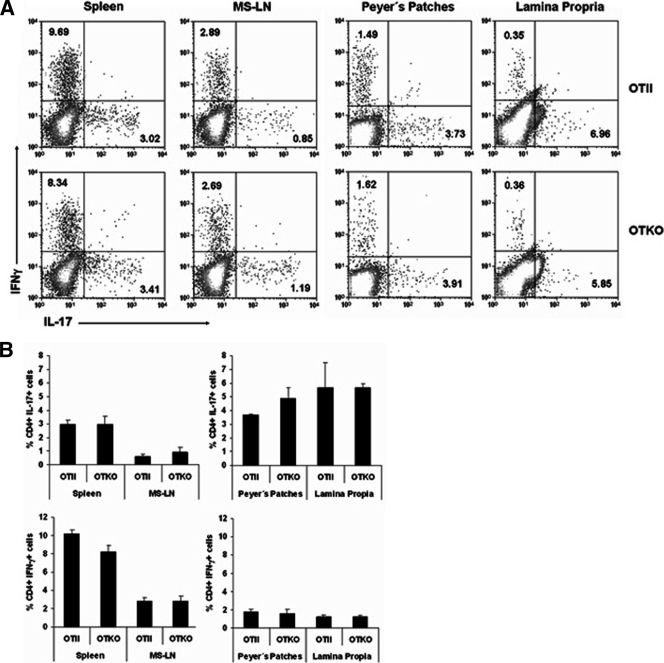
The key Th17 transcription factor RORγt is constitutively expressed by double-positive thymocytes and by T cells from lamina propria (LP), the location of most Th17 cells in naive mice (4, 12). To study the role of CD69 in the distribution of Th17 cells, we stimulated single-cell suspensions from spleen, MS-LNs, Peyer's patches (PP), and intestinal LP with P+I (4 h) and determined the percentages of IL-17- and IFN-γ-producing cells. For all tissues analyzed, the percentages of IL-17+ and IFN-γ+ cells did not vary between OTII and OTKO mice (Fig. 4A and B).
FIG. 4.
Normal distribution of IL-17+ and IFN-γ+ populations in OTII and OTKO mice in homeostasis. (A) Percentages of IL-17+ and IFN-γ+ T cells in the CD4+ gated population from the spleens, MS-LNs, Peyer's patches, and lamina propria of untreated OTII and OTKO mice. (B) Bar charts showing quantification of data from two independent experiments (means and SD of five mice per genotype). No significant differences were found between OTII and OTKO mice (Student's t test).
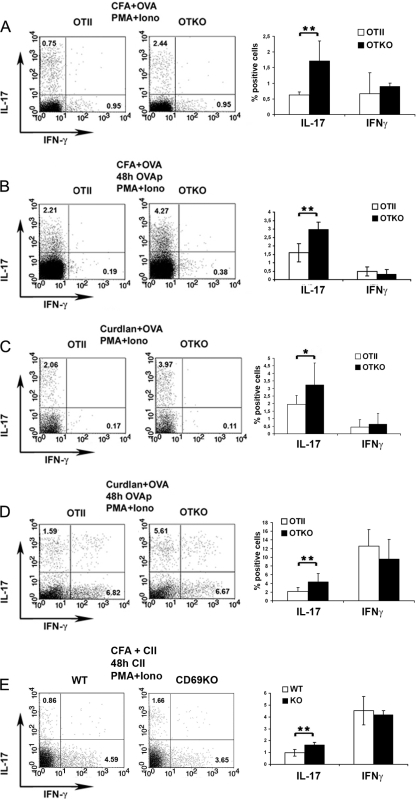
To study the influence of CD69 on the in vivo Th17 response to specific antigens, we immunized OTII and OTKO mice with OVA. The adjuvant used for immunization was either CFA, which contains Toll-like receptor (TLR) agonist and is a highly immunogenic inducer of Th1 and Th17 responses, or curdlan, a β-glucan that binds the C-type lectin Dectin-1 and preferentially induces Th17 responses (17). After 4 days, draining lymph node (DLN) cells were recovered and stimulated ex vivo for 6 h with P+I, and the proportions of IL-17+ and IFN-γ+ cells were determined (Fig. 5A and C). Alternatively, DLN cells were cultured in the presence of OTII-specific peptide for 48 h and then stimulated with P+I, and the proportions of IL-17+ and IFN-γ+ cells were determined (Fig. 5B and D). The percentage of IL-17+ cells was significantly higher in DLN cultures derived from OVA-plus-CFA-immunized OTKO mice than in equivalent cultures from OTII mice (Fig. 5A and B). Similar differences in IL-17+ populations were observed between OTKO and OTII cultures derived from mice sensitized with OVA plus curdlan (Fig. 5C and D). In contrast, the percentage of IFN-γ+ cells did not vary significantly under any experimental conditions. Moreover, the immunization of CD69-deficient mice with CII in CFA, following the protocol to induce arthritis in mice (26), resulted in a 2-fold increase in the IL-17+ population in draining lymph nodes (Fig. 5E), confirming that the exacerbated arthritis response observed in CD69 KO mice correlated with enhanced Th17. Thus, CD69-deficient mice were able to develop stronger in vivo Th17 responses to specific antigens, reinforcing the conclusion that CD69 negatively regulates Th17 cell differentiation.
FIG. 5.
OVA-induced differentiation of Th17 cells after antigen challenge is enhanced in CD69-deficient mice. Shown are percentages of IL-17+ and IFN-γ+ T cells in the CD4+ gated population of immunized mice. (A to D) OTII and OTKO mice were immunized in both hind footpads with OVA, with either CFA (A and B) or curdlan (C and D) as the adjuvant. (E) WT and CD69 KO mice were immunized with CII and CFA. Four days after immunization, cell suspensions were prepared from popliteal LNs. The cells were immediately activated with P+I (A and C) or had been previously stimulated for 48 h with OVA peptide (B and D) or collagen type II (E). After stimulation, the cells were stained for CD4, intracellular IFN-γ, and IL-17. The dot plots represent IL-17 and IFN-γ in CD4+ cells. The bar charts show quantification of data from 5 animals per genotype (means and SD) from 3 different experiments. *, P < 0.05; **, P < 0.01 (Student's t test).
The CD69 cytoplasmic tail interacts with Jak3 and Stat5.
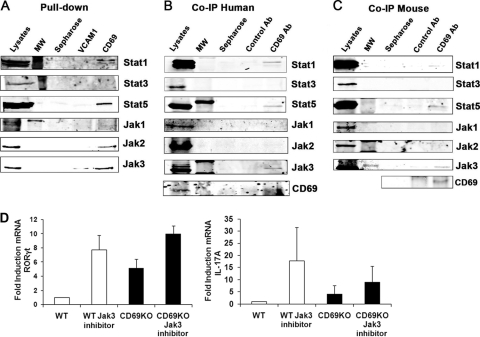
To investigate the mechanism by which CD69 limits Th17 differentiation, we performed a proteomic screen with biotinylated peptides from the cytoplasmic tail of human CD69 and identified Stat1 as an interacting protein (data not shown). Pulldown assays with a peptide containing the human CD69 cytoplasmic tail showed its molecular association with Stat1, Stat5, Jak2, and Jak3 (Fig. 6A). To ascertain whether endogenous CD69 binds to these signaling molecules, immunoprecipitation assays were performed with human (Fig. 6B) and mouse (Fig. 6C) CD69 from activated T-cell blast lysates. Whereas CD69 coprecipitated Stat1, Stat5, and Jak3, signals for Stat3, Jak1, or Jak2 were very weak or absent both in the human and in the mouse samples (Fig. 6B and C).
FIG. 6.
Analysis of CD69 interaction with Jak and Stat proteins. (A) Whole-cell lysates of human T-cell blasts were pulled down with biotinylated polypeptide containing the cytoplasmic tail of human CD69 or human VCAM1 as a control. The precipitates were analyzed by Western blotting with antibodies to the indicated Jak and Stat proteins. (B and C) Lysates of human and mouse activated T-cell blasts were prepared, and endogenous CD69 was immunoprecipitated (IP) with the antibody TP1/33 to human CD69 or 2.2 to mouse CD69. Associated endogenous proteins were detected by immunoblotting with the indicated antibodies. Immunoprecipitation specificity was controlled with an irrelevant isotype-matched antibody. The data are from one representative experiment out of three independent experiments with pooled cells from two different donors (A and B) or three mice per genotype (C). (D) CD69 regulates RORγt expression via Jak3. CD4+ T cells from WT or CD69-deficient mice were cultured for 72 h under Th17-skewed conditions and treated with a chemical Jak3 inhibitor for the last 16 hours. The mRNA expression levels of mouse RORγt and IL-17A were measured by quantitative RT-PCR.
To assess the role and the specificity of CD69 association with the Jak3/Stat5 pathway in the regulation of Th17 differentiation, we examined the effect of interfering with Stat5 signaling with chemical inhibitors of Jak3 and how these interactions affected the expression of Th17-related genes. Naive CD4+ T cells from OTII/OTKO mice were cultured under Th17-skewed conditions, and Jak3 inhibitor was added (Fig. 6D). Quantitative reverse transcription (qRT)-PCR revealed that selective inhibition of Jak3 enhanced the transcription of RORγt, IL-17A, and IL-23R in mouse OTII Th17 cells (Fig. 6D and data not shown), demonstrating that in CD69-expressing cells, Jak3 is essential for the regulation of Th17 differentiation under these conditions.
Expression of essential Th17 differentiation transcription factors is enhanced in CD69-deficient polarized CD4 T cells.
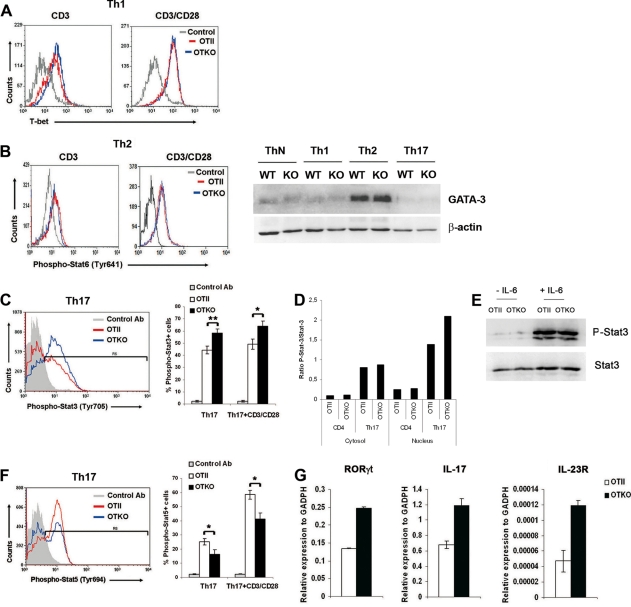
To further address the mechanism by which CD69 regulates Th17 differentiation transcription factors, we assessed the expression of the various transcription factors critical for the development of each effector T-cell lineage. Flow cytometry analysis of Th1-differentiated cells revealed no differences in the expression of T-bet between OTII and OTKO lymphocytes (Fig. 7A). Likewise, in Th2-differentiated cells, the levels of phospho-Stat6 and GATA3 were unaffected by CD69 deficiency (Fig. 7B). In contrast, the percentage of phospho-Stat3+ cells, a transcription factor which regulates cytokine-mediated generation of proinflammatory Th17 cells (10, 35), was higher in OTKO polarized T cells (Fig. 7C). Moreover, Western blot analysis revealed enhanced translocation of phospho-Stat3 to the nucleus in OTKO Th17 cells (Fig. 7D), whereas signaling through the IL-6 receptor in naïve CD4+ CD69-deficient T cells was not affected (Fig. 7E).
FIG. 7.
Analysis of transcription factors and cytokines implicated in the differentiation of T cells. (A and B) CD4+ T cells from OTII and OTKO mice were cultured for 3 days under Th1 or Th2 differentiation conditions and restimulated with anti-CD3 or CD3 plus anti-CD28 for 24 h, and the levels of intracellular T-bet (A) and phospho-Stat6 (Tyr641) (B) were measured by FACS. The Western blot shows the expression of the Th2 transcription factor GATA-3 in nuclear extracts from in vitro-differentiated ThN, Th1, Th2, and Th17 cells. (C and F) Three-day-differentiated Th17 T cells from OTII or OTKO mice were fixed, permeabilized, antibody stained for phospho-Stat3 (Tyr705) (C) or phospho-Stat5 (Tyr694) (F), and analyzed by FACS. The bar charts show the percentages of phospho-Stat3+ and phospho-Stat5+ cells in Th17 cell populations in one representative experiment out of three. Ab, staining of OTKO cells with an irrelevant polyclonal antibody. The bars represent means ± SD of three mice per genotype from three independent experiments; *, P < 0.01; **, P < 0.001 (Student's t test). (D) Semiquantitative Western blot analysis of the ratio of phospho-Stat3 (Tyr705) to Stat3 content in nuclear and cytosolic extracts from naive CD4+ T cells and 3-day-differentiated Th17 T cells from OTII or OTKO mice. (E) Naïve CD4+ T cells were stimulated or not with IL-6 for 10 min; then, phospho-Stat3 and Stat3 levels were determined by immunoblotting. The data are representative of two independent experiments with similar results. (G) RT-PCR analysis of the transcription factor RORγt, IL-17, and IL-23R in Th17 cells differentiated in vitro as for panel C. The data are representative of two independent experiments performed with six mice per genotype (the bars show means ± SD).
Recent studies have established that Stat5 is a critical negative regulator of Th17 differentiation (15, 28), and analysis of Stat5 phosphorylation revealed that the activation of this transcription factor was impaired in OTKO Th17 cells compared with OTII Th17 cells (Fig. 7F). Accordingly, mRNA levels of RORγt, another key Th17 transcription factor (12), were higher in CD69-deficient Th17 cells (Fig. 7G). These results were further supported by the enhanced expression of IL-17 and the IL-23R genes in OTKO Th17 cells (Fig. 7G). Thus, the absence of CD69 favors the differentiation of Th17 cells by enhancing the expression of transcription factors involved in their differentiation without affecting the levels of factors important for Th1 or Th2 development.
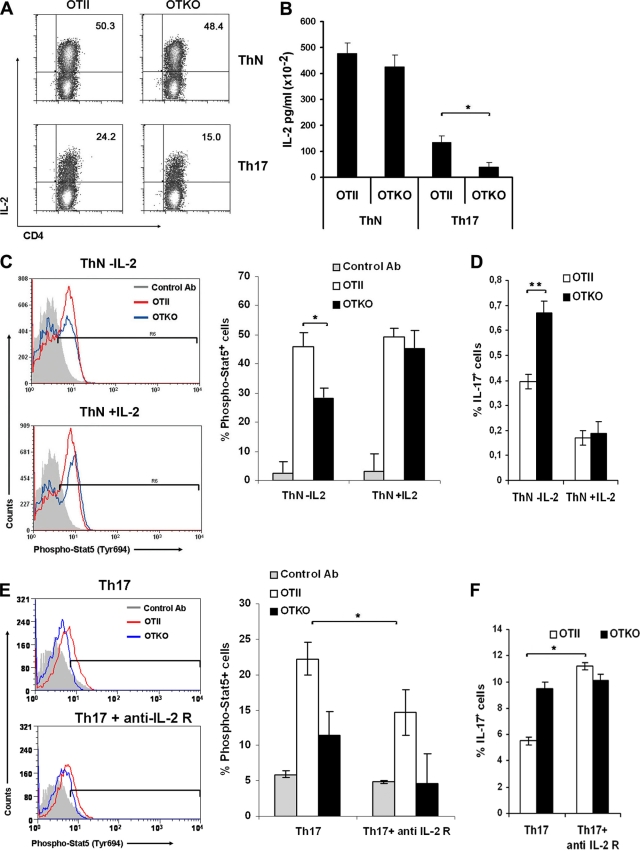
Exogenous IL-2 restores the Stat5 activation pathway in CD69-deficient cells.
IL-2 has been shown to negatively regulate Th17 differentiation via Stat5 signaling in mice (15). Although Th17-driven cultures lacked exogenous IL-2, the cytokine was produced in low concentrations by the cells (Fig. 2E). To avoid the differences between IL-2 secretion by Th17 OTII and OTKO cells in culture and to further address the role of CD69 in Stat5 phosphorylation, we stimulated the cells for 3 days under neutral conditions (ThN) in which the levels of IL-2 secreted by OTII and OTKO were comparable (Fig. 8A and B). ThN cells from OTII or OTKO mice were stimulated for 24 h with CD3 and CD28, and the levels of Stat5 phosphorylation were measured after intracellular staining by FACS (Fig. 8C). We found that, even though the levels of IL-2 were comparable in OTII and OTKO ThN cultures, the percentage of phospho-Stat5+ cells was lower in OTKO cells (Fig. 8C), indicating that in these cells, Stat5 activation is partially inhibited by the lack of CD69. In contrast, in the presence of exogenous IL-2, there were no significant differences in the phosphorylation of Stat5 between OTII and OTKO ThN cells (Fig. 8C), which indicates that the presence of this cytokine in the culture restores activation of the pathway. Consequently, in the presence of exogenous IL-2, the enhanced differentiation of OTKO cells toward Th17 is inhibited to the levels of OTII cells (Fig. 8D). To examine the effect of exogenous IL-2 directly in Th17 differentiation, we performed differentiation cultures in the presence of blocking antibodies to anti-IL-2 receptor (CD25) to block IL-2 signaling (Fig. 8E). The addition of anti-IL-2R antibodies partially inhibited Stat5 phosphorylation (Fig. 8E) and increased the percentage of Th17 differentiation in Th17 OTII cells (Fig. 8F), whereas these parameters were not affected in Th17 OTKO cultures. These data indicate that in the absence of CD69 Stat5 signaling is inhibited independently of exogenous IL-2, and therefore, Th17 regulation is not affected by this pathway, whereas the blockade of IL-2 signaling in CD69-expressing cells stimulates differentiation toward Th17 (Fig. 8F).
FIG. 8.
Exogenous IL-2 restores the Stat5 activation pathway in CD69-deficient cells. (A) CD4+ T cells from OTII and OTKO mice were cultured for 3 days under ThN or Th17 differentiation conditions, and the percentages of IL-2+ cells in the CD4+ gated population were measured by FACS. The number in each panel indicates the percentage of IL-2+ cells. The dot plots are representative of three independent experiments with similar results. (B) Levels of IL-2 determined in ThN or Th17 culture supernatant by Flow Cytomix cytokine array. (C) Three-day-cultured cells from OTII or OTKO mice under ThN conditions with or without exogenous rmIL-2 were fixed, permeabilized, antibody stained for phospho-Stat5 (Tyr694), and analyzed by FACS. (D) Percentages of IL-17+ cells in the CD4+ gated OTII and OTKO cells from cultures performed as for panel C. (E) Three-day-differentiated Th17 T cells from OTII or OTKO mice with or without anti-IL-2R antibody were stained for phospho-Stat5 (Tyr694). (C and E) The bar charts show the percentages of phospho-Stat5+ cells in ThN (C) or Th17 (E) cell populations. (F) Percentages of IL-17+ cells in the CD4+ gated OTII and OTKO cells from cultures performed as for panel E. The bars represent means ± SD of three independent experiments (means ± SD of three mice per genotype). *, P < 0.05; **, P < 0.001 (Student's t test).
DISCUSSION
In this study, we have established that CD69 negatively regulates the in vitro and in vivo differentiation of T lymphocytes toward the Th17 lineage. The increased potential of CD69-deficient T cells to secrete IL-17 in vitro is not restricted to Th17-polarizing conditions, since both IL-17 secretion and the proportion of IL-17+ cells were also enhanced under different culture conditions. These observations indicate that the loss of CD69 causes an increase in the capacity of naïve T cells to secrete IL-17 after TCR activation that is independent of the cytokine milieu. Notably, in the antigen-specific differentiation system of OTII transgenic mice, the effect observed in CD69 KO mice occurs independently of whether the APCs used are CD69 deficient, indicating that it is an intrinsic T-cell effect. Moreover, the enhanced secretion of IL-17 also occurs in the absence of APCs after activation with anti-CD3 plus anti-CD28 MAbs. In summary, all these data strongly indicate that the loss of CD69 has an effect on Th17 differentiation that is T-lymphocyte intrinsic and independent of differential interaction with costimulatory molecules or putative CD69 ligands present in APCs. Consistently, the absence of CD69 enhanced the expression of RORγt and Stat3 transcription factors, as well as Th17-associated molecules, like IL-23R. In contrast, typical Th1 or Th2 transcription factors, like T-bet for Th1 or Stat6 and GATA-3 for Th2 (27), were almost unaltered in CD69-deficient cells, indicating that, unlike that of Th17, Th1 and Th2 differentiation were mostly unaffected. Although Th1 cytokines were unaltered, the observation of mild enhancement of the Th2 cytokines IL-4 and IL-5 in cultures of T cells from CD69 KO mice, together with the effect of CD69-mediated suppression of IL-17, could be linked by the expression of transcription factor c-Maf, which has been involved in both Th2 and Th17 differentiation (2). In this regard, it has been recently proposed that Th17 polarization would result from a default response in CD4 T-cell differentiation, actively modified to give Th1 and Treg (19). Our data suggest that early induction of CD69 in T cells would facilitate the inhibition of the “natural tendency” to generate Th17, allowing other outcomes in the differentiation process. These findings provide the first demonstration of the role of CD69 in the modulation of Th17 cells and establish it as a key molecule in the regulation of T-cell differentiation (Fig. 9).
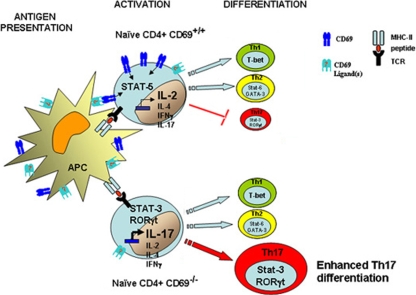
FIG. 9.
Scheme depicting the regulatory role of CD69 in Th17 differentiation. The antigen-specific signal induces activation of T cells through the TCR and induces CD69 expression on the membrane, which activates Stat5, a negative regulator of Th17 differentiation. In the absence of CD69, Stat3 activation and RORγt transcription, and therefore differentiation toward the Th17 lineage, are enhanced in naïve T cells.
The results presented here identify a previously unknown function of CD69 as a regulator of T-lymphocyte differentiation toward the Th17 lineage. The activation molecule CD69 is widely expressed at sites of inflammation (7, 14). Our previous work in the model of collagen-induced arthritis demonstrated that CD69 deficiency exacerbates inflammation in the joints of immunized mice (25, 26). We previously suggested that CD69 might prevent unchecked immune activation by regulating T-effector differentiation (25). Our present results show that CD69 acts as a brake on Th17 cell differentiation and demonstrate that the cytoplasmic tail of CD69 physically associates with Jak3/Stat5, establishing a link between CD69 and Stat5 phosphorylation and subsequent Th17 inhibition. Moreover, the inactivation of the Stat5 pathway with chemical inhibitors of Jak3, mimicking the situation in CD69-deficient cells, enhanced the transcription of RORγt and therefore differentiation toward the Th17 lineage. In accord with this, it has been demonstrated that IL-2 signaling via Stat5 negatively regulates the differentiation of Th17 cells (15, 28, 32). Consistently, the absence of CD69 partially abrogates the phosphorylation of Stat5 in vitro, whereas activation of the pathway can be restored by adding exogenous IL-2, indicating that signaling through CD69, together with IL-2, is an important factor in the control of Th17 differentiation. Although other cytokines, like IL-2 or IFN-γ, are inhibited in Th17-polarized cultures, this could be due to the lack of CD69 signaling, since it has been reported that the cross-linking of CD69 induced a prolonged elevation of intracellular Ca2+ and enhancement of IL-2 and IFN-γ gene expression (31).
Taken together, our work supports the role of CD69 as an early controller of Th17 differentiation by a new mechanism in which the early induction of the molecule following antigen activation modifies the fate of the differentiation of the T cells and prevents differentiation toward Th17. However, we do not propose CD69 as the unique inhibitory molecule implicated in these processes, since of course, activated CD69-expressing cells are able to differentiate toward Th17 cells. Additionally, as the ligand(s) is still unknown, its cellular expression could control the function of CD69 as a brake in vivo for Th17 differentiation. CD69 could play a role in the regulation of Treg function and somehow affect the balance of Th17 responses in vivo. In this regard, the role of CD69+ cells in the regulation of T-cell proliferation through TGF-β has recently been described (8). To experimentally address this possibility requires a detailed future study. Though much further work on the regulation and function of CD69 is needed, our study has revealed an important regulatory role for this molecule in T-cell differentiation, has linked its expression to Th17 differentiation for the first time, and has increased our knowledge of the origin and progression of important chronic immune inflammatory diseases. Finally, this work places CD69 in the category of other established negative regulators, such as CTLA4, LAG3, or PD1 (6, 34), and explains the persistent expression of this molecule, not only in inflammatory foci, but also in T-cell-regulatory environments.
Acknowledgments
This work was supported by grant SAF2008-02719 from the Spanish Ministry of Science and Innovation to P.M.; grant PI06/0937 from ISCIII-MSC to M.G.; and grants SAF2008-02635 from the Spanish Ministry of Science and Innovation, RECAVA [RD06/0014-0030] from the Instituto de Salud Carlos III, MEICA from the Genoma España Foundation, and INSINET 01592006 from Comunidad de Madrid to F.S.-M. P.M. is an investigator for the Spanish Ministry of Science and Innovation Ramón y Cajal Program (RYC-2006). The CNIC is supported by the Ministry of Science and Innovation and the Pro CNIC Foundation.
We thank R. R. Lobb, R. González-Amaro, D. Sancho, and S. Bartlett for comments and critical reading of the manuscript.
P.M. designed and performed research experiments, analyzed the data, and wrote the manuscript; M.G. designed and performed research experiments and analyzed the data; A.L., A.C.-A., M.R.-H., and M.A.U. performed research experiments; M.Y.-M. designed and performed proteomic analysis; and F.S.-M. supervised the research and wrote the paper.
We declare no competing financial interests.
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Adamson, A. S., K. Collins, A. Laurence, and J. J. O'Shea. 2009. The Current STATus of lymphocyte signaling: new roles for old players. Curr. Opin. Immunol. 21:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauquet, A. T., H. Jin, A. M. Paterson, M. Mitsdoerffer, I. C. Ho, A. H. Sharpe, and V. K. Kuchroo. 2009. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 4.Denning, T. L., Y. C. Wang, S. R. Patel, I. R. Williams, and B. Pulendran. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8:1086-1094. [DOI] [PubMed] [Google Scholar]
- 5.Esplugues, E., D. Sancho, J. Vega-Ramos, C. Martinez, U. Syrbe, A. Hamann, P. Engel, F. Sanchez-Madrid, and P. Lauzurica. 2003. Enhanced antitumor immunity in mice deficient in CD69. J. Exp. Med. 197:1093-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fife, B. T., and J. A. Bluestone. 2008. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 224:166-182. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Monzón, C., R. Moreno-Otero, J. M. Pajares, A. Garcia-Sanchez, M. Lopez-Botet, M. O. de Landazuri, and F. Sanchez-Madrid. 1990. Expression of a novel activation antigen on intrahepatic CD8+ T lymphocytes in viral chronic active hepatitis. Gastroenterology 98:1029-1035. [DOI] [PubMed] [Google Scholar]
- 8.Han, Y., Q. Guo, M. Zhang, Z. Chen, and X. Cao. 2009. CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J. Immunol. 182:111-120. [DOI] [PubMed] [Google Scholar]
- 9.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 10.Harris, T. J., J. F. Grosso, H. R. Yen, H. Xin, M. Kortylewski, E. Albesiano, E. L. Hipkiss, D. Getnet, M. V. Goldberg, C. H. Maris, F. Housseau, H. Yu, D. M. Pardoll, and C. G. Drake. 2007. Cutting edge: an in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179:4313-4317. [DOI] [PubMed] [Google Scholar]
- 11.He, D., L. Wu, H. K. Kim, H. Li, C. A. Elmets, and H. Xu. 2006. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 177:6852-6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov, I. I., B. S. McKenzie, L. Zhou, C. E. Tadokoro, A. Lepelley, J. J. Lafaille, D. J. Cua, and D. R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121-1133. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama, Y., S. Nakae, T. Matsuki, A. Nambu, H. Ishigame, S. Kakuta, K. Sudo, and Y. Iwakura. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566-573. [DOI] [PubMed] [Google Scholar]
- 14.Laffón, A., R. Garcia-Vicuna, A. Humbria, A. A. Postigo, A. L. Corbi, M. O. de Landazuri, and F. Sanchez-Madrid. 1991. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J. Clin. Invest. 88:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurence, A., C. M. Tato, T. S. Davidson, Y. Kanno, Z. Chen, Z. Yao, R. B. Blank, F. Meylan, R. Siegel, L. Hennighausen, E. M. Shevach, and J. O'Shea. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26:371-381. [DOI] [PubMed] [Google Scholar]
- 16.Lauzurica, P., D. Sancho, M. Torres, B. Albella, M. Marazuela, T. Merino, J. A. Bueren, A. C. Martinez, and F. Sanchez-Madrid. 2000. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood 95:2312-2320. [PubMed] [Google Scholar]
- 17.LeibundGut-Landmann, S., O. Gross, M. J. Robinson, F. Osorio, E. C. Slack, S. V. Tsoni, E. Schweighoffer, V. Tybulewicz, G. D. Brown, J. Ruland, and C. Reis e Sousa. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630-638. [DOI] [PubMed] [Google Scholar]
- 18.Liang, S. C., A. J. Long, F. Bennett, M. J. Whitters, R. Karim, M. Collins, S. J. Goldman, K. Dunussi-Joannopoulos, C. M. Williams, J. F. Wright, and L. A. Fouser. 2007. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179:7791-7799. [DOI] [PubMed] [Google Scholar]
- 19.Lohr, J., B. Knoechel, D. Caretto, and A. K. Abbas. 2009. Balance of Th1 and Th17 effector and peripheral regulatory T cells. Microbes Infect. 11:589-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeachy, M. J., and D. J. Cua. 2007. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin. Immunol. 19:372-376. [DOI] [PubMed] [Google Scholar]
- 21.Miossec, P., T. Korn, and V. K. Kuchroo. 2009. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361:888-898. [DOI] [PubMed] [Google Scholar]
- 22.Nakae, S., A. Nambu, K. Sudo, and Y. Iwakura. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173-6177. [DOI] [PubMed] [Google Scholar]
- 23.Rangachari, M., N. Mauermann, R. R. Marty, S. Dirnhofer, M. O. Kurrer, V. Komnenovic, J. M. Penninger, and U. Eriksson. 2006. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 203:2009-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiner, S. L. 2007. Development in motion: helper T cells at work. Cell 129:33-36. [DOI] [PubMed] [Google Scholar]
- 25.Sancho, D., M. Gomez, and F. Sanchez-Madrid. 2005. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 26:136-140. [DOI] [PubMed] [Google Scholar]
- 26.Sancho, D., M. Gomez, F. Viedma, E. Esplugues, M. Gordon-Alonso, M. A. Garcia-Lopez, H. de la Fuente, A. C. Martinez, P. Lauzurica, and F. Sanchez-Madrid. 2003. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J. Clin. Invest. 112:872-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinman, L. 2007. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13:139-145. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger, B. 2007. Good for Goose, but not for Gander: IL-2 interferes with Th17 differentiation. Immunity 26:278-279. [DOI] [PubMed] [Google Scholar]
- 29.Stritesky, G. L., N. Yeh, and M. H. Kaplan. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 181:5948-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki, S., F. Kokubu, M. Kawaguchi, T. Homma, M. Odaka, S. Watanabe, K. Ieki, S. Matsukura, M. Kurokawa, H. Takeuchi, Y. Sasaki, S. K. Huang, M. Adachi, and H. Ota. 2007. Expression of interleukin-17F in a mouse model of allergic asthma. Int. Arch. Allergy Immunol. 143(Suppl. 1):89-94. [DOI] [PubMed] [Google Scholar]
- 31.Testi, R., J. H. Phillips, and L. L. Lanier. 1989. T cell activation via Leu-23 (CD69). J. Immunol. 143:1123-1128. [PubMed] [Google Scholar]
- 32.Veldhoen, M., K. Hirota, J. Christensen, A. O'Garra, and B. Stockinger. 2009. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179-189. [DOI] [PubMed] [Google Scholar]
- 34.Wherry, E. J., S. J. Ha, S. M. Kaech, W. N. Haining, S. Sarkar, V. Kalia, S. Subramaniam, J. N. Blattman, D. L. Barber, and R. Ahmed. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670-684. [DOI] [PubMed] [Google Scholar]
- 35.Yang, X. O., A. D. Panopoulos, R. Nurieva, S. H. Chang, D. Wang, S. S. Watowich, and C. Dong. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282:9358-9363. [DOI] [PubMed] [Google Scholar]