Abstract
The role of epitope-specific regulatory CD4 T cells in modulating CD8 T-cell-mediated immunopathology during acute viral infection has not been well defined. In the murine model of respiratory syncytial virus (RSV) infection, CD8 T cells play an important role in both viral clearance and immunopathology. We have previously characterized two RSV epitope-specific CD4 T-cell responses with distinct phenotypic properties. One of them, the IAbM209-specific subset, constitutively expresses FoxP3 and modulates CD8 T-cell function in vitro. We show here that the IAbM209-specific CD4 T-cell response regulates CD8 T-cell function in vivo and is associated with diminished RSV-induced illness without affecting viral clearance at the site of infection. Achieving the optimal balance of regulatory and effector T-cell function is an important consideration for designing future vaccines.
A subset of CD4 T cells with regulatory function (Treg) has been shown to play an important role in modulating adaptive immune responses. Natural Tregs are characterized by the expression of FoxP3 and participate in reducing the activation of CD8 T-cell responses in peripheral lymphoid organs (11, 20, 35). This modulation can diminish the ability of adaptive immune responses to control systemic infections (4). However, the presence of natural regulatory CD4 T cells can have a beneficial effect on immune-mediated pathology, particularly at the site of infection. Tregs have been shown to limit pulmonary inflammation and lung injury induced by pneumocystis infection (29) and to modulate herpes simplex virus-induced inflammatory lesions of the eye (46). Natural Tregs also reduce the symptoms of West Nile virus infections in both humans and mice; Treg-deficient mice were more likely to develop lethal infection (25). Viral infection can also induce antigen-specific CD4 T cells that express FoxP3 (27), and their role in protective immunity and immunopathology needs more detailed investigation.
T lymphocytes are key components of adaptive immunity against respiratory syncytial virus (RSV) infection. Children with T-cell deficiencies have delayed virus clearance and are more susceptible to fatal RSV infection (10, 18). The absence of T cells infiltrating into lung is associated with fatal RSV infections in children without recognized underlying disease (49). In the murine model, CD8 T cells play a major role in RSV clearance, presumably through direct cytotoxicity to infected cells and the generation of immunocompetent molecules (2, 15, 43); depletion of CD8 T cells in mice results in delayed viral clearance (14). The CD8 T-cell response also induces immunopathology in primary infection of mice (15, 32, 48). Transferring high doses of CD8 T cells facilitates virus clearance but also causes hemorrhagic pneumonia and enhanced disease (6, 14). These studies demonstrate that while CD8 T cells are required for viral clearance, they are responsible for immunopathology. We have described the pattern of CD8 T-cell responses that occur in mice that are the F1 hybrid (H-2d/b) between BALB/c (H-2d) and C57BL/6 (H-2b) (39). These mice respond both to the well-characterized KdM282 epitope (24) and to a more recently described DbM187 epitope (38). Both CD8 T-cell responses are dominant in the parent strains but assume a hierarchy (KdM282 > DbM187) in the F1 hybrid (39). This model infection allows the analysis of factors that determine T-cell response hierarchy and provides multiple endpoints for the assessment of factors that modify or regulate CD8 T-cell responses.
We recently described epitope-specific CD4 T-cell responses distinguished by novel major histocompatibility complex (MHC) class II tetramers in RSV infection. The IAbM209-specific CD4 T cells have a high frequency of FoxP3 expression and suppress RSV-specific CD8 T-cell cytokine production in vitro (27). To investigate the regulatory role of IAbM209-specific CD4 T cells in vivo, we sought to determine how immunizing mice with the CD4 T-cell epitope peptide M209 would affect the RSV-specific CD8 T-cell response. We show here that the IAbM209-specific CD4 T cells have a regulatory effect on the dominant CD8 T-cell response to RSV infection, reducing both the magnitude and effector cytokine production in peripheral lymphoid organs while allowing effector functions at the site of infection to clear virus with normal kinetics. Viral clearance was thus achieved with less illness.
MATERIALS AND METHODS
Mice.
Pathogen-free CB6F1 female mice between the ages of 8 and 10 weeks were purchased from Jackson Laboratories (Bar Harbor, ME) and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, as described previously (16). CB6F1 mice are the F1 generation of a C57BL/6 × BALB/c mating and therefore express both H-2b and H-2d class I MHC proteins. They are able to present and respond to T-cell epitopes recognized by both parent strains (39). The NIH Animal Care and Use Committee approved all animal protocols in the present study. Experimental groups were age matched.
Immunization and Infection.
Detailed immunization and infection procedures were described previously (27). Mice were immunized with RSV M209-223 or M225-39 peptide at 100 μg/mouse intramuscularly. Peptides were conjugated with keyhole limpet hemocyanin (KLH) by using an Imject immunogen EDC kit (Thermo Fisher Scientific, Inc., Illinois). Control mice were immunized with KLH only. At day 28 after immunization, mice were boosted with a replication-defective recombinant adenovirus serotype 5 vector expressing M/M2 (rAd5-M/M2) that contains RSV M and M2 fusion genes (GenVec, Inc., Gaithersburg, MD), at 5 × 107 FFU/mouse via the quadriceps muscle, and then challenged with 107 PFU of live RSV intranasally 3 weeks after boosting. The challenge stock was derived from the A2 strain of RSV by sonicating HEp-2 cell monolayers as previously described (16).
Cell culture and flow cytometry reagents.
The cell culture medium was RPMI 1640 from HyClone (Logan, UT), supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 10 U of penicillin/ml, and 10 μg of streptomycin/ml. Fluorochrome-conjugated antibodies used to study cell phenotype and cytokine production with flow cytometry were anti-CD3-cyanine 7 (Cy7) allophycocyanin (APC), anti-IFN-γ-APC, and anti-IL-2-phycoerythrin (PE) that were obtained from BD Biosciences (San Jose, CA). Unconjugated antibodies to CD4, CD8, and CD62L were from Harlan (Indianapolis, IN) and were conjugated with Qdot605, Qdot705, and Alexa Fluor 688 from Invitrogen (Carlsbad, CA). Conjugates were validated by comparison with commercial conjugates. Anti-PD-1-fluorescein isothiocyanate and anti-CD127-Cy7-PE were purchased from eBioscience, Inc. (San Diego, CA). Violet fluorescent reactive dye and anti-CD19- and anti-CD16/32-Pacific Blue from Invitrogen were used as cell viability and lineage markers to exclude dead and non-T cells from analysis (33). Peptide OVA323-339 (ISQAVHAAHAEINEAGR) was purchased from AnaSpec (San Jose, CA). Peptide-MHC class I tetrameric complexes, Db-M187-195-APC and Kd-M282-90-PE, were purchased from Beckman Coulter (Fullerton, CA); the peptide-MHC class II tetrameric complexes, I-AbM209-223-APC and I-AbM226-39-PE, were prepared by NIH Tetramer Facility (Atlanta, GA). Staining protocols were described previously (27, 39).
T-cell cytokine expression and cytotoxic activity assays.
Using flow cytometry to assess T-cell cytokine expression intracellularly and cytotoxic activity were described previously (26, 27). Sample data of all flow cytometry studies were collected from at least 100,000 gated events and analyzed with FlowJo, version 8.8.5, of TreeStar (San Carlos, CA).
Viral load assessment.
Lung tissue was removed at indicated times after RSV infection and ethanol-dry ice frozen in 10% Eagle minimal essential medium (EMEM). After grinding and pelleting of tissue debris, serial dilutions of supernatant were inoculated onto 80% confluent HEp-2 cell monolayers in triplicate and overlaid with 0.75% methylcellulose in 10% EMEM. The cultures were incubated at 37°C for 4 days, fixed with 10% buffered formalin, and stained with hematoxylin and eosin. Plaques were counted and expressed as the log10 PFU/g of tissue. The limit of detection is 1.8 log10 PFU/g of tissue.
CD4 T-cell depletion.
Naive mice were treated daily with 500 μg of anti-CD4 antibody (clone GK1.5) or isotype control (clone DR5) i.p., both from Harlan (Indianapolis, IN), starting 2 days before RSV infection until the day of infection. Depletion of CD4 T cells was assessed by flow cytometry study.
RESULTS
The IAbM209-specific CD4 T-cell response is less frequent than the IAbM226-specific response.
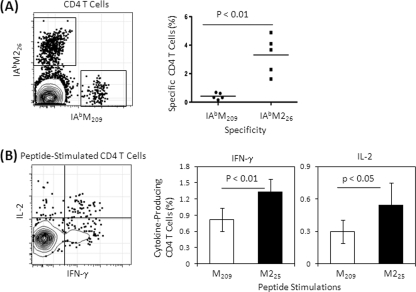
The frequencies of CD4 T-cell responses to RSV M and M2 proteins were quantitatively evaluated in lungs after primary RSV infection. IAbM226 tetramer-specific responses were 8-fold greater than those of IAbM209-specific responses (Fig. 1 A) and produced more gamma interferon (IFN-γ) and interleukin-2 (IL-2) after M225 peptide stimulation in vitro than peptide M209 stimulation (Fig. 1B). Therefore, we consider the IAbM209 a subdominant CD4 T-cell epitope relative to IAbM226 and have previously shown that the subdominant IAbM209 response preferentially differentiated into a FoxP3-expressing phenotype (27). The CD4 and CD8 T-cell epitopes of RSV M and M2 proteins, and related peptides used in this experiment, are listed in Table 1 .
FIG. 1.
CD4 T-cell responses to RSV M and M2. Lung lymphocytes were isolated at day 7 postinfection and stained with tetramers and phenotyping antibodies to identify specific CD4 T cells (A) or stimulated with MHC class II-restricted CD4 T-cell epitope-containing peptides (M209 or M225) in the presence of costimulators and brefeldin A for 5 h and then stained for lineage phenotype and cytokines intracellularly (B). Representative flow plots are gated on live CD4+ CD8− T cells. Frequencies of specific or cytokine-producing T cells refer to the total CD4 T-cell population. The cytokine-producing cell frequency is background subtracted, which is based on irrelevant OVA peptide stimulation. The data (mean ± the standard deviation [SD], n = 5 to 6/group) represent three independent experiments and are compared by using the Student t test.
TABLE 1.
Nomenclature of CB6F1 mouse CD4 and CD8 T-cell epitopes of RSV M and M2 proteins
| Epitope peptide or peptide-MHC tetramer | T-cell subseta |
|||
|---|---|---|---|---|
| Dominant |
Subdominant |
|||
| CD4 | CD8 | CD4 | CD8 | |
| Epitope peptide | M225-39 (M225) | M282-90 (M282) | M209-223 (M209) | M187-195 (M187) |
| Peptide-MHC tetramer | I-Ab-M226-39 (IAbM226) | H-2Kd-M282-90 (KdM282) | I-Ab-M209-223 (IAbM209) | H-2Db-M187-195 (DbM187) |
The abbreviation is given in parentheses.
IAbM209-specific CD4 T cells regulate the peripheral RSV-specific CD8 T-cell response against rAd5-M/M2.
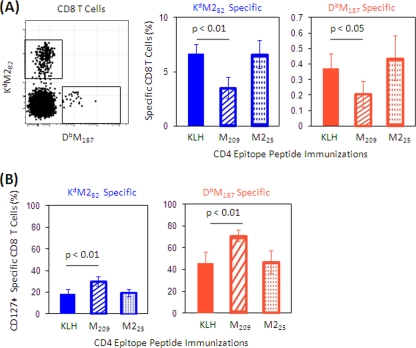
To explore the regulatory role of IAbM209-specific CD4 T cells on CD8 T-cell responses to RSV M and M2 and compare it to the effect of IAbM226-specific CD4 T cells, we immunized mice with KLH-conjugated M209 or M225, or KLH alone as a control. Immunization expanded the specific CD4 T-cell subsets as expected (see Fig. S1 in the supplemental material). After expanding the CD4 T-cell populations with peptide alone, mice were boosted with rAd5 expressing a fusion protein of RSV M and M2 to measure the impact of epitope-specific immunization on CD8 T-cell responses. After intramuscular administration of rAd5-M/M2, CD8 T cells responding to DbM187 and KdM282 epitopes were expanded. Interestingly, the expansion of CD8 T cells was diminished in mice primed with the CD4 epitope peptide M209 compared to the expansion in mice primed with KLH only (Fig. 2 A). This effect did not occur in mice primed with the M225 peptide. The modulatory effect in M209-immunized mice was associated with an increased frequency of CD127 expression on both Kd- and Db-restricted CD8 T cells (Fig. 2B). Few of the tetramer-specific CD8 T cells expressed CD62L in peripheral blood, while 2 to 3% of total CD8 T cells were CD62L+ (see Fig. S2 in the supplemental material). This suggests that M209 immunization suppressed CD8 T-cell expansion responding to vector-delivered immunization with M and M2 epitopes of CD8 T cells and resulted in more CD8 T cells retaining an effector memory phenotype rather than differentiating to terminal effectors.
FIG. 2.
Frequency and CD127 expression of RSV-specific CD8 cells in peripheral blood after administration of rAd5-M/M2. Mice were immunized with KLH or KLH-conjugated peptides M209 or M225 and then immunized with rAd5-M/M2 intramuscularly. (A) Peripheral blood samples were collected 10 days after rAd5-M/M2 immunization and stained with tetramers and phenotyping antibodies to assess the frequency of RSV tetramer-specific CD8 T cells. M209 peptide-immunized mice had a lower frequency of CD8 T-cell responses after rAd5-M/M2 than M25 peptide- or KLH-immunized mice. A representative plot is gated on CD4− CD8+ T cells. The percentages of specific CD8 T cells refer to total CD8 T-cell population. (B) We also assessed the frequency of CD127-expressing specific CD8 T cells in mice immunized with M209 or M25 peptides. The percentages refer to the specific CD8 T cells. The data (mean ± the SD, n = 5/group) are representative of three independent experiments and were compared by using the Student t test. Filled bars indicate the KLH-only-immunized control groups, hatched bars indicate M209-KLH-immunized mice, and dotted bars indicate M225-KLH-immunized mice.
IAbM209-specific CD4 T cells regulate spleen CD8 T cells and effector cytokine production responding to RSV infection.
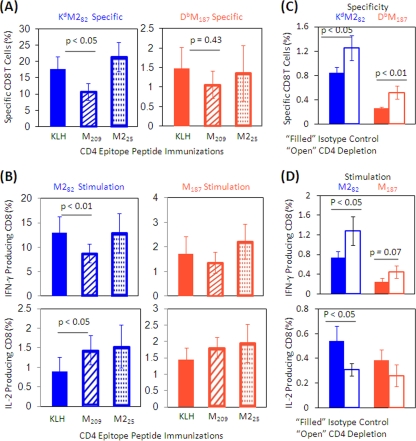
Next, we sought to determine whether the regulatory effect of the original priming with the M209 peptide was sustained during a subsequent virus infection. Immunized mice were challenged with live RSV, and CD8 T-cell responses to M and M2 were measured in the spleen. Peptide M209 immunization significantly decreased the frequency of CD8 T cells specific to the dominant KdM282 epitope compared to KLH immunization and tended to lower the frequency of CD8 T cells specific to the subdominant DbM187 epitope (Fig. 3 A). Peptide M209 immunization also decreased IFN-γ production of the KdM282-specific CD8 T cells and had a similar trend on DbM187-specific CD8 T cells (Fig. 3B). Conversely, IL-2 production in KdM282-specific CD8 T cells was increased. Peptide M225 immunization did not decrease the frequency of IFN-γ production in either tetramer-specific CD8 T-cell response and increased IL-2 production in KdM282-specific CD8 T cells. These data suggest that the IAbM209 response regulates RSV-specific CD8 T cells.
FIG. 3.
Frequency and cytokine production of RSV-specific CD8 T cells from the spleen after RSV challenge. Mice were immunized with KLH or KLH-conjugated peptides, followed by rAd5-M/M2 administration intramuscularly, and then challenged with live RSV intranasally. Spleen lymphocytes were isolated at 7 day postchallenge and stained with tetramers and phenotyping antibodies to identify specific CD8 T cells by flow cytometry (A) or stimulated with CD8 epitope-containing peptides in the presence of costimulators and brefeldin A for 5 h and then stained for lineage phenotype and cytokine expressions intracellularly (B). The frequencies of specific CD8 T cells or cytokine-producing cells refer to CD8 T-cell population. The cytokine-producing cell frequency is the background (ranging from 0.01 to 0.08%) subtracted based on irrelevant OVA peptide stimulation. The data (mean ± the SD, n = 5/group) are representative of three independent experiments and compared by using the Student t test. Filled bars indicate the KLH-only-immunized control groups, hatched bars indicate M209-KLH-immunized mice, and dotted bars indicate M225-KLH-immunized mice. We also assessed the CD8 T-cell responses in the spleen in unimmunized mice depleted of CD4 T cells just prior to RSV challenge to remove the impact of CD4 T-cell modulation on the CD8 T-cell responses. Naive mice were treated with anti-CD4 antibody (□) or isotype control (▪) and then challenged with RSV. Spleen lymphocytes were isolated at day 7 postchallenge. Frequencies of specific CD8 T cells (C) and cytokine-producing CD8 T cells (D) from CD4-depleted or control mice stimulated with epitope-containing peptides were assessed as described in panels A and B.
To prove the regulatory role of CD4 T cells, we depleted CD4 T cells from naive mice prior to primary RSV infection. The depletion of CD4 T cells by antibody treatment was effective, as demonstrated by flow cytometry study. There were 0.13% ± 0.07% and 0.19% ± 0.05% CD4 T cells in the lung and spleen T-cell populations, respectively, whereas the percentages were 47.9% ± 2.09% and 58.2% ± 0.86% in isotype controls. Depletion of CD4 T cells reversed the regulatory effect of Tregs. The frequency of both epitope-specific CD8 T cells and the IFN-γ production of KdM282-specific CD8 T cells were higher in CD4 T cell-depleted mice than they were in control mice; the IL-2 production of KdM282-specific CD8 T cells was decreased in the CD4 T-cell-depleted mice. Depletion of CD4 T cells had limited effect on subdominant DbM187-specific CD8 T-cell cytokine production (Fig. 3C and D).
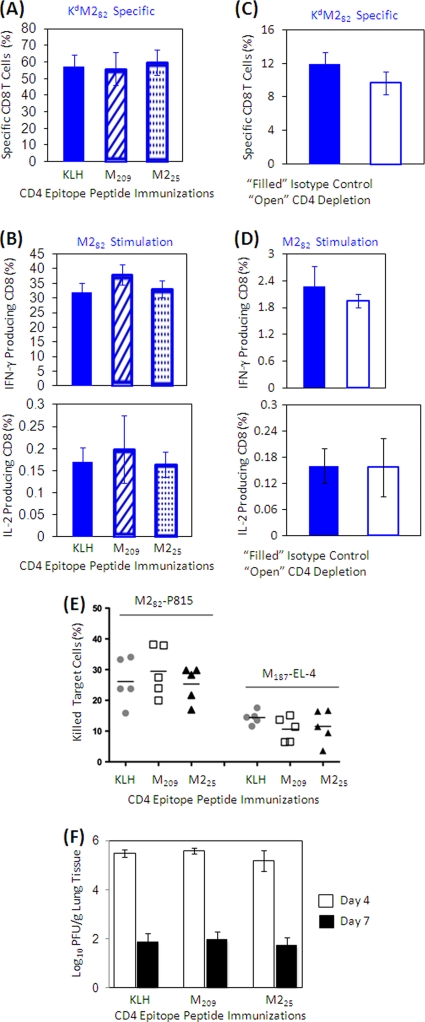
IAbM209-specific CD4 T cells have limited regulation on CD8 T-cell function at the site of infection.
In contrast to the diminished CD8 T-cell responses in peripheral blood and spleen, CD8 T-cell responses to M and M2 in the lung remained at the expected frequency and functional activity even in mice immunized with the M209 peptide. There was no significant difference in KdM282-specific CD8 T-cell frequencies (Fig. 4A) or cytokine production (Fig. 4B) in mice immunized with CD4 epitope peptides compared to KLH-immunized controls; neither were the DbM187-specific CD8 T-cell responses altered by peptide immunizations (data not shown). Neither CD8 T-cell response showed differences in memory phenotype (CD62L and CD127 expression) between immunization groups (data not shown). Depletion of CD4 T cells prior to RSV challenge did not significantly alter the frequency (Fig. 4C) or cytokine production (Fig. 4D) of the KdM282-specific CD8 T cells in the lung.
FIG. 4.
Frequency and cytokine production of specific CD8 cells from the lung after RSV challenge. Mice were immunized with KLH or KLH-conjugated peptides, followed by rAd5-M/M2 administration intramuscularly, and then challenged with RSV intranasally. Lung lymphocytes were isolated at day 7 postinfection and stained with tetramers and phenotyping antibodies to identify specific CD8 T cells by flow cytometry (A) or stimulated with CD8 epitope-containing peptides in the presence of costimulators and brefeldin A for 5 h and then stained for lineage and cytokine expression intracellularly (B). Frequencies of specific CD8 T cells or cytokine-producing cells refer to the total CD8 T-cell population. The cytokine-producing cell frequency is the background (ranging from 0.03 to 0.2%) subtracted based on irrelevant OVA peptide stimulation. The data (mean ± the SD, n = 5/group) are representative of three independent experiments. Filled bars indicate the KLH-only-immunized control groups, hatched bars indicate M209-KLH-immunized mice, and dotted bars indicate M225-KLH-immunized mice. We then assessed CD8 T-cell responses in the lungs in unimmunized mice depleted of CD4 T cells just prior to RSV challenge to remove the impact of CD4 T-cell modulation on the CD8 T-cell responses. Naive mice were treated with anti-CD4 antibody (□) or isotype control (▪) and then infected with RSV. Lung lymphocytes were isolated at day 7 postinfection. Frequencies of specific (C) or cytokine-producing (D) CD8 T cells from CD4-depleted or control mice stimulated with epitope-containing peptides were assessed as described in panels A and B. (E) Isolated lung lymphocytes were cultured with CD8 epitope peptide M282- and M187-loaded P815 and EL-4 cells, respectively, for 3 h. Dead P815 and EL-4 cells after culturing were assessed with flow cytometry by annexin V staining. The percentages of specific killings refer to total P815 and EL-4 cell populations, with nonspecific cell death subtracted based on irrelevant OVA peptide-loaded P815 and EL-4 cell cultures. The data (mean ± the SD, n = 5/group) are representative of three independent experiments. (F) Supernatant of ground lung tissue isolated at days 4 and 7 postinfection were plated on HEp-2 cell layer and cultured for 4 days. The virus titers are expressed as PFU per gram of lung tissue. The limit of detection is 1.8 log10 PFU/gram of tissue. The data (mean ± the SD, n = 5/group) are representative of two independent experiments. All data were statistically compared by using the Student t test.
Epitope-specific cytolytic functions of lung lymphocytes were also intact despite immunization with the M209 peptide, as measured by in vitro cytotoxicity assay (Fig. 4E). This is consistent with the finding that there was no significant difference in peak viral load or rate of viral clearance between immunization groups (Fig. 4F) and suggests there are factors present at the site of infection or inflammation that mitigate the regulatory effects of Tregs on effector function.
IAbM209-specific CD4 T-cell response reduces RSV-induced illness.
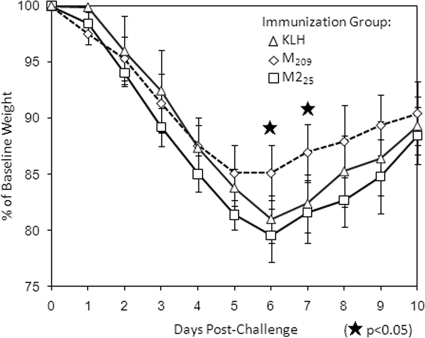
Mice lose weight during the acute phase of RSV infection, which correlates with the severity of illness (15). M209-immunized mice had significantly less peak weight loss than M225- or KLH-immunized mice and began recovering 1 day earlier (Fig. 5). This suggests that the IAbM209-specific CD4 T cells influenced the overall CD8 T-cell response to focus its effector functions at the site of virus infection in order to more efficiently clear virus and minimize immunopathology.
FIG. 5.
Weight loss after RSV challenge following immunization and boost. Mice were weighed daily after virus challenge (challenge day = day 0). Shown are the percentages of baseline weight. The data (mean ± the SD, n = 5/group) are representative of three independent experiments.
IAbM209-specific CD4 T cells express differentiated phenotype and cytokine profile in the spleen and lung.
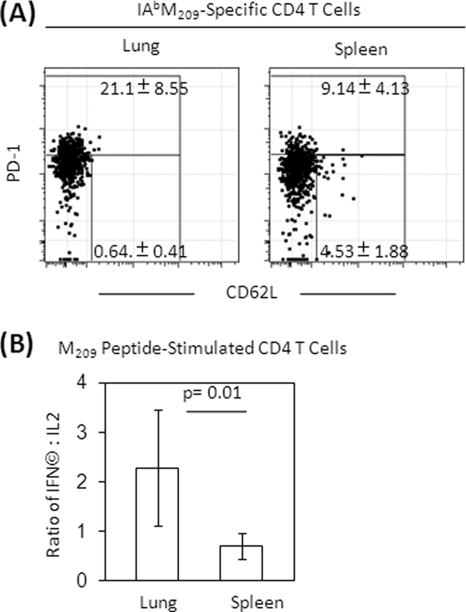
We next asked why the IAbM209-specific CD4 T cells had less influence on effector function at the site of infection. We found that more IAbM209-specific CD4 T cells isolated from the lung expressed PD-1 than those isolated from the spleen (P = 0.02, Fig. 6A). In contrast, more spleen IAbM209-specific CD4 T cells expressed CD62L than they did in the lung (P = 0.002), and most of the CD62L-expressing cells had low levels of PD-1 expression. Differences in PD-1 expression were not evident on RSV-specific CD8 T cells isolated from the lung and spleen (data not shown). Both the lung and spleen lymphocytes produced IFN-γ and IL-2 upon CD4 epitope peptide M209 stimulation. However, the ratios of IFN-γ- to IL-2-producing cells in the lung were significantly higher than the ratio of the cells in the spleen, indicating the majority of IAbM209-specific CD4 T cells from lung produced IFN-γ only, whereas the majority of specific CD4 T cells from spleen produced both IFN-γ and IL-2, with a bias toward IL-2 production (Fig. 6B). These data indicate that the IAbM209-specific CD4 population in lung is more activated and differentiated than in peripheral lymphoid organs and may therefore have less regulatory capacity.
FIG. 6.
Phenotype and cytokine expressions of CD4 and CD8 T cells in the spleen and lung. Mice were primed with KLH or KLH-conjugated peptides, followed by rAd5-M/M2 administration intramuscularly, and then challenged with RSV intranasally. (A) Lung and spleen lymphocytes were isolated at day 7 postinfection and stained with tetramers and phenotyping antibodies to identify specific CD8 T cells and their phenotypes by flow cytometry. Representative plots are gated on IAbM209-specific CD4 T cells. (B) Frequencies of PD-1 high or PD-1 low with CD62L expression cells refer to the gated population or stimulated with CD4 epitope peptide M209 in the presence of costimulators and brefeldin A for 5 h and then stained for lineage phenotype and intracellular cytokines. The ratio of CD4 T cells producing IFN-γ and IL-2 are the background subtracted based on irrelevant OVA peptide stimulation. The data (mean ± the SD, n = 5/group) are representative of three independent experiments and compared by using the Student t test.
DISCUSSION
Inflammation associated with viral infections is a consequence of the host attempting to eradicate the pathogen and control disease progression; immunopathology is the price paid to clear virus. Therefore, it is in the best interest of the host to rapidly identify and clear virus and then terminate the response expeditiously. We have shown that CD4+ CD25+ T cells regulate CD8 T-cell responses to RSV and reduce infection-induced illness (37) and identified two RSV-specific CD4 T-cell populations using novel peptide-MHC class II tetramers (27). Here, we find that IAbM226-specific CD4 T cells have a higher frequency and greater production of IFN-γ and IL-2 compared to IAbM209-specific CD4 T cells, a characteristic of effector T cells. Conversely, a high frequency of IAbM209-specific CD4 T cells express FoxP3 and exhibit a regulatory phenotype. We show that IAbM209-specific CD4 T cells expanded by M209 peptide immunization regulate the CD8 T-cell response to RSV in vivo. Mice with expanded IAbM209-specific CD4 T cells have fewer functional CD8 T cells and less effector cytokine production in peripheral lymphoid organs, with dominant CD8 T-cell responses being downregulated to a greater extent than subdominant responses. This regulatory effect can be reversed by CD4 T-cell depletion prior to RSV challenge. In addition to reduced magnitude of response and less IFN-γ production, IL-2 expression is increased in RSV-specific CD8 T cells from IAbM209-specific CD4 T-cell expanded mice, another sign of active regulation. In contrast, immunization with the M225 peptide results in a greater proportion of IFN-γ- and IL-2-producing CD8 T cells. This is consistent with our previous observation that the IAbM209-specific CD4 T cells preferentially expressed FoxP3 and appear to have a regulatory role on RSV-specific CD8 T-cell responses in vitro (27) and with reports that epitopes with weak T-cell-receptor (TCR) signaling properties or relatively low immunogenicity preferentially induced FoxP3-expressing CD4 T cells (23, 40).
Selective suppression of CD8 T-cell effector function by natural Tregs has been previously reported in Friend retrovirus infection and anti-CD3-activated responses. CD4+ CD25+ T cells were found to directly suppress IFN-γ production and effector CD8 T-cell expansion by inhibiting differentiation of CD8 T cells from memory to effector but had limited effect on IL-2 production (31, 36). In our study, the M209-immunized mice have fewer RSV-specific CD8 T cells in peripheral lymphoid organs, and those present have relatively high CD127 expression, which is a phenotypic marker associated with memory differentiation. This suggests that IAbM209-specific CD4 T cells may selectively inhibit CD8 T-cell effector differentiation and function resulting in a relatively higher memory/effector ratio in peripheral lymphoid organs. This may be advantageous in the context of vaccine-induced immunity.
Applying the RSV model in CB6F1 hybrid mice (expressing H-2d/b) allows us to evaluate the differential impact of the CD4 T-cell regulatory effect on dominant and subdominant CD8 T-cell responses. DbM187-specific CD8 T cells are downregulated to a lesser extent than the numerically dominant KdM282-specific CD8 T cells. This is consistent with our previous findings in Treg-depleted mice and in other model systems (17, 37) and suggests that CD4-mediated regulatory function targets selective features of effector cells that are associated with their property of dominance, thereby reducing epitope disparity.
While the dominant CD8 T cells and their effector function in peripheral blood and spleen are suppressed by the IAbM209-specific CD4 T-cell response, the CD8 T-cell effector response in lung is not significantly affected. Neither immunization nor CD4 depletion affects the dominant or subdominant CD8 T-cell frequency and function at the site of infection. In addition, peak viral load and kinetics of viral clearance are not affected by manipulation of the CD4 T-cell populations. Maintaining CD8 T-cell effector function at the site of infection while expanding the memory pool and regulating effector functions in peripheral lymphoid organs creates an advantage for the host by favoring viral clearance locally while establishing immunity against subsequent infections in peripheral lymphoid organs.
The limited suppression of CD8 T-cell functions in the lung may be due to reduced CD4 regulatory function. Alternatively, it could be that the site of infection is able to maintain CD8 T-cell effector function despite the regulatory influences. Our data suggest that the regulatory function may be impaired or minimized in lung based on the phenotype and cytokine profile. Expression of L-selectin molecule, CD62L, is a characteristic of T cells that have a memory phenotype and that still have the capacity to circulate and return to peripheral lymphoid organs. Few of the IAbM209-specific CD4 T cells express CD62L in the lung but ∼5% of them remain CD62L high in the spleen; the majority of lung IAbM209-specific CD4 T cells produce IFN-γ and very little IL-2, but they produce more IL-2 than IFN-γ in the spleen. Previous reports have shown CD62L+ Treg cells had more regulatory function than their CD62L− counterparts (5, 9, 19, 34), and IL-2 was required for Treg proliferation and functioning (13, 41, 47). Membrane protein programmed death-1 (PD-1) is an inhibitory receptor associated with downregulation of T-cell functions. We also find that a higher frequency of IAbM209-specific CD4 T cells express PD-1 in lung. Although the implication of elevated PD-1 is controversial, it was upregulated on exhausted virus-specific CD8 T cells (3, 8) and could negatively regulate regulatory T cells by controlling STAT-5 phosphorylation (12). Elevated PD-1-expressing CD4 T cells were reported to be defective in TCR-mediated proliferation (42), and ligation of PD-1 inhibited CD4 cytokine production and expansion (7). Although the elevated expression of PD-1 on Tregs in lungs suggests this cell population may be dysfunctional, it does not rule out the possibility of CD8 effectors in lung being more resistant to regulation, which is a question under active investigation in our laboratory. However, our data and interpretation are consistent with other observations showing the frequency of apoptotic or dysfunctional regulatory cells at the site of infection was greater than cells without regulatory function in tissue infected with Leishmania (44) or in pancreatic islets in a model of autoimmune diabetes (47). In addition, an inflammatory environment in the infected lung could diminish Treg function in other ways, such as activated CD8 T cells eliminating Tregs via FasL-mediated apoptosis (21), cytokines IL-6 and IL-1 downregulating FoxP3 expression (50), or viral RNA blocking Treg suppression through activation of RIG-I-like helicases (1). These possibilities are all consistent with the concept that it is in the best interest of the host to maintain effector functions locally at a site of inflammation or infection.
Regulation of dominant responses in peripheral lymphoid organs may reduce unnecessary inflammation induced by an overly robust CD8 T-cell effector activity that is associated with severity of illness (15, 32), and maintain a more favorable balance between protection and immunopathology. This may explain our observation that mice immunized with CD4 epitope peptide M209 experienced less illness after RSV challenge. Our previous studies also showed that depletion of natural Tregs resulted in increased effector CD8 T-cell frequency in peripheral lymphoid organs and enhanced infection-induced illness (37) and is consistent with the impact of Tregs on the response to herpes simplex virus at the site of infection (28). This would also explain why Tregs could diminish protection against a systemic infection such as Friend leukemia virus, while providing a benefit for a more localized infection.
Our study demonstrates that regulatory CD4 T cells can be induced by specific epitopes in viral pathogens, which is consistent with observations that regulatory and effector T cells are derived from the same naive precursor pool (30) and can differentiate from antigen-specific conventional naive precursors (22, 23, 45). The biological significance of antigen-induced Tregs relative to natural Tregs will require additional studies in more model systems. We show here that epitope-specific Tregs reduce the RSV-specific CD8 T-cell response and diminish CD8 T-cell effector function in peripheral lymphoid organs while effector function at the infection site is preserved. Therefore, effective viral clearance is achieved with less illness. Future studies on the relative value of antigen-induced Tregs in the control of virus replication and immunopathology may help guide vaccine design and refine the goals of immunization against viral diseases.
Supplementary Material
Acknowledgments
We thank the NIAID-sponsored tetramer core facility at Emory University (Atlanta, GA) for assisting in the construction of the class II tetramers and Mythreyi Shastri for her editorial work.
Footnotes
Published ahead of print on 4 August 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Anz, D., V. H. Koelzer, S. Moder, R. Thaler, T. Schwerd, K. Lahl, T. Sparwasser, R. Besch, H. Poeck, V. Hornung, G. Hartmann, S. Rothenfusser, C. Bourquin, and S. Endres. Immunostimulatory RNA blocks suppression by regulatory T cells. J. Immunol. 184:939-946. [DOI] [PubMed]
- 2.Aung, S., J. A. Rutigliano, and B. S. Graham. 2001. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J. Virol. 75:9918-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875-888. [DOI] [PubMed] [Google Scholar]
- 5.Biagi, E., I. Di Biaso, V. Leoni, G. Gaipa, V. Rossi, C. Bugarin, G. Renoldi, M. Parma, A. Balduzzi, P. Perseghin, and A. Biondi. 2007. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+ CD25+ GITR+ Foxp3+ CD62L+ functional regulatory T cells in patients with graft-versus-host disease. Transplantation 84:31-39. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, M. J., P. J. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemnitz, J. M., R. V. Parry, K. E. Nichols, C. H. June, and J. L. Riley. 2004. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T-cell stimulation, but only receptor ligation prevents T-cell activation. J. Immunol. 173:945-954. [DOI] [PubMed] [Google Scholar]
- 8.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Ermann, J., P. Hoffmann, M. Edinger, S. Dutt, F. G. Blankenberg, J. P. Higgins, R. S. Negrin, C. G. Fathman, and S. Strober. 2005. Only the CD62L+ subpopulation of CD4+ CD25+ regulatory T cells protects from lethal acute GVHD. Blood 105:2220-2226. [DOI] [PubMed] [Google Scholar]
- 10.Fishaut, M., D. Tubergen, and K. McIntosh. 1980. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J. Pediatr. 96:179-186. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot, J. D., J. P. Rasmussen, L. M. Williams, J. L. Dooley, A. G. Farr, and A. Y. Rudensky. 2005. Regulatory T-cell lineage specification by the forkhead transcription factor foxp3. Immunity 22:329-341. [DOI] [PubMed] [Google Scholar]
- 12.Franceschini, D., M. Paroli, V. Francavilla, M. Videtta, S. Morrone, G. Labbadia, A. Cerino, M. U. Mondelli, and V. Barnaba. 2009. PD-L1 negatively regulates CD4+ CD25+ Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Invest. 119:551-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtado, G. C., M. A. Curotto de Lafaille, N. Kutchukhidze, and J. J. Lafaille. 2002. Interleukin 2 signaling is required for CD4+ regulatory T-cell function. J. Exp. Med. 196:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham, B. S., L. A. Bunton, J. Rowland, P. F. Wright, and D. T. Karzon. 1991. Respiratory syncytial virus infection in anti-mu-treated mice. J. Virol. 65:4936-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 17.Haeryfar, S. M., R. J. DiPaolo, D. C. Tscharke, J. R. Bennink, and J. W. Yewdell. 2005. Regulatory T cells suppress CD8+ T-cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 174:3344-3351. [DOI] [PubMed] [Google Scholar]
- 18.Hall, C. B., K. R. Powell, N. E. MacDonald, C. L. Gala, M. E. Menegus, S. C. Suffin, and H. J. Cohen. 1986. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 315:77-81. [DOI] [PubMed] [Google Scholar]
- 19.Herbelin, A., J. M. Gombert, F. Lepault, J. F. Bach, and L. Chatenoud. 1998. Mature mainstream TCR alpha beta+ CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J. Immunol. 161:2620-2628. [PubMed] [Google Scholar]
- 20.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T-cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 21.Kilinc, M. O., R. B. Rowswell-Turner, T. Gu, L. P. Virtuoso, and N. K. Egilmez. 2009. Activated CD8+ T-effector/memory cells eliminate CD4+ CD25+ Foxp3+ T-suppressor cells from tumors via FasL mediated apoptosis. J. Immunol. 183:7656-7660. [DOI] [PubMed] [Google Scholar]
- 22.Knoechel, B., J. Lohr, E. Kahn, J. A. Bluestone, and A. K. Abbas. 2005. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 202:1375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M. C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T-cell populations by foreign antigen. Nat. Immunol. 6:1219-1227. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni, A. B., P. L. Collins, I. Bacik, J. W. Yewdell, J. R. Bennink, J. E. Crowe, Jr., and B. R. Murphy. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanteri, M. C., K. M. O'Brien, W. E. Purtha, M. J. Cameron, J. M. Lund, R. E. Owen, J. W. Heitman, B. Custer, D. F. Hirschkorn, L. H. Tobler, N. Kiely, H. E. Prince, L. C. Ndhlovu, D. F. Nixon, H. T. Kamel, D. J. Kelvin, M. P. Busch, A. Y. Rudensky, M. S. Diamond, and P. J. Norris. 2009. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J. Clin. Invest. 119:3266-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, J., and M. Roederer. 2007. Differential susceptibility of leukocyte subsets to cytotoxic T-cell killing: implications for HIV immunopathogenesis. Cytometry A 71:94-104. [DOI] [PubMed] [Google Scholar]
- 27.Liu, J., T. J. Ruckwardt, M. Chen, T. R. Johnson, and B. S. Graham. 2009. Characterization of respiratory syncytial virus M- and M2-specific CD4 T cells in a murine model. J. Virol. 83:4934-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lund, J. M., L. Hsing, T. T. Pham, and A. Y. Rudensky. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science 320:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinley, L., A. J. Logar, F. McAllister, M. Zheng, C. Steele, and J. K. Kolls. 2006. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J. Immunol. 177:6215-6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLachlan, J. B., D. M. Catron, J. J. Moon, and M. K. Jenkins. 2009. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 30:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolova, M., J. D. Lelievre, M. Carriere, A. Bensussan, and Y. Levy. 2009. Regulatory T cells modulate differentially the maturation and apoptosis of human CD8− T cell subsets. Blood 113:4556-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostler, T., W. Davidson, and S. Ehl. 2002. Virus clearance and immunopathology by CD8+ T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur. J. Immunol. 32:2117-2123. [DOI] [PubMed] [Google Scholar]
- 33.Perfetto, S. P., P. K. Chattopadhyay, L. Lamoreaux, R. Nguyen, D. Ambrozak, R. A. Koup, and M. Roederer. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313:199-208. [DOI] [PubMed] [Google Scholar]
- 34.Pop, S. M., C. P. Wong, D. A. Culton, S. H. Clarke, and R. Tisch. 2005. Single cell analysis shows decreasing FoxP3 and TGFβ1 coexpressing CD4+ CD25+ regulatory T cells during autoimmune diabetes. J. Exp. Med. 201:1333-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson, S. J., and K. J. Hasenkrug. 2006. The role of virus-induced regulatory T cells in immunopathology. Springer Semin. Immunopathol. 28:51-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson, S. J., R. J. Messer, A. B. Carmody, and K. J. Hasenkrug. 2006. In vitro suppression of CD8+ T-cell function by Friend virus-induced regulatory T cells. J. Immunol. 176:3342-3349. [DOI] [PubMed] [Google Scholar]
- 37.Ruckwardt, T. J., K. L. Bonaparte, M. C. Nason, and B. S. Graham. 2009. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 83:3019-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutigliano, J. A., M. T. Rock, A. K. Johnson, J. E. Crowe, Jr., and B. S. Graham. 2005. Identification of an H-2Db-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology 337:335-343. [DOI] [PubMed] [Google Scholar]
- 39.Rutigliano, J. A., T. J. Ruckwardt, J. E. Martin, and B. S. Graham. 2007. Relative dominance of epitope-specific CD8+ T-cell responses in an F1 hybrid mouse model of respiratory syncytial virus infection. Virology 362:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauer, S., L. Bruno, A. Hertweck, D. Finlay, M. Leleu, M. Spivakov, Z. A. Knight, B. S. Cobb, D. Cantrell, E. O'Connor, K. M. Shokat, A. G. Fisher, and M. Merkenschlager. 2008. T-cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U. S. A. 105:7797-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Setoguchi, R., S. Hori, T. Takahashi, and S. Sakaguchi. 2005. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 201:723-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimatani, K., Y. Nakashima, M. Hattori, Y. Hamazaki, and N. Minato. 2009. PD-1+ memory phenotype CD4+ T cells expressing C/EBPα underlie T-cell immunodepression in senescence and leukemia. Proc. Natl. Acad. Sci. U. S. A. 106:15807-15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srikiatkhachorn, A., and T. J. Braciale. 1997. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J. Virol. 71:678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suffia, I. J., S. K. Reckling, C. A. Piccirillo, R. S. Goldszmid, and Y. Belkaid. 2006. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J. Exp. Med. 203:777-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, C. M., J. A. Hall, R. B. Blank, N. Bouladoux, M. Oukka, J. R. Mora, and Y. Belkaid. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204:1775-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse. 2004. CD4+ CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123-4132. [DOI] [PubMed] [Google Scholar]
- 47.Tang, Q., J. Y. Adams, C. Penaranda, K. Melli, E. Piaggio, E. Sgouroudis, C. A. Piccirillo, B. L. Salomon, and J. A. Bluestone. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28:687-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang, Y. W., and B. S. Graham. 1997. T-cell source of type 1 cytokines determines illness patterns in respiratory syncytial virus-infected mice. J. Clin. Invest. 99:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, X. O., R. Nurieva, G. J. Martinez, H. S. Kang, Y. Chung, B. P. Pappu, B. Shah, S. H. Chang, K. S. Schluns, S. S. Watowich, X. H. Feng, A. M. Jetten, and C. Dong. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T-cell programs. Immunity 29:44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.