Abstract
Measles virus (MV) entry requires at least 2 viral proteins, the hemagglutinin (H) and fusion (F) proteins. We describe the rescue and characterization of a measles virus with a specific mutation in the stalk region of H (I98A) that is able to bind normally to cells but infects at a lower rate than the wild type due to a reduction in fusion triggering. The mutant H protein binds to F more avidly than the parent H protein does, and the corresponding virus is more sensitive to inhibition by fusion-inhibitory peptide. We show that after binding of MV to its receptor, H-F dissociation is required for productive infection.
Measles virus (MV) infection requires binding of the hemagglutinin (H) protein to its cognate receptors (9, 20, 21, 29, 41) while the fusion (F) protein triggers membrane lipid mixing and fusion. The H protein is a type II transmembrane homodimeric, disulfide-linked glycoprotein (33). The F protein is a type I membrane glycoprotein that exists as a homotrimeric complex. The protein is cleaved by furin in the trans-Golgi network into a metastable heterodimer with a membrane-spanning F1 domain and a membrane-distal F2 domain (16). Expressed alone, neither H nor F leads to membrane fusion, and therefore, both proteins are required and have to interact for productive infection of a target cell (46). There is evidence that these interactions start within the endoplasmic reticulum (34).
The H proteins of Paramyxoviridae family members have a globular head with a six-blade β-propellor structure that is responsible for receptor binding (4, 7, 13), a stalk region composed of alpha-helical coiled coils (18, 48) that anchors the complex to the plasma membrane, and a short cytoplasmic domain that can interact with the matrix (M) protein and modulate fusion (2). Given that the F protein does not interact with a receptor on the target cell but undergoes conformational changes to enable membrane fusion, it seems likely that the F protein must interact with the H protein that enables fusion (14, 19, 23, 24, 35, 47). The molecular interactions between the F and H proteins are being increasingly understood (6, 8, 24, 25, 30, 35, 42). Hummel and Bellini have described a mutation in the H glycoprotein where threonine replaced isoleucine 98, which led to loss of fusion in chronically infected cells, but the virus was not rescued (15). Corey and Iorio performed alanine-scanning mutagenesis to determine the role of specific, membrane-proximal residues in the stalk region of the H protein responsible for H-F interactions (6). Substitution of alanine for specific residues in this region altered cell-to-cell fusion and the strength of the H-F interaction in transient-transfection experiments (6). Replacement of isoleucine with alanine at position 98 reduced fusion but did not significantly alter hemadsorption, implying that binding of the mutant H protein to CD46 was not affected (6). More recently, Paal et al. showed that the H protein can tolerate significant additions to its alpha-helical coiled coils without loss of binding or fusion in transient-transfection assays (30). Although these studies confirm the importance of the interactions between the H protein stalk and the metastable F protein for enabling fusion after receptor binding, the exact steps leading to fusion are still unclear. Moreover, studies evaluating H-F interactions were performed with transient protein expression and not in the presence of the actual virus. This is potentially an important shortcoming since the M protein can modulate infection and fusion (1).
Rescue of hypofusogenic measles virus.
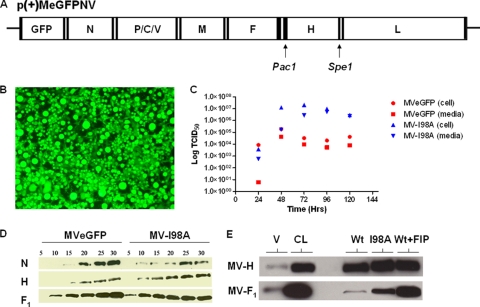
Site-directed mutagenesis at position 98 (I → A) (6) of MV H was performed on pCG-H (38), and the gene for H-I98A was cloned into the full-length plasmid of p(+)MeGFPNV (Fig. 1A). The recombinant virus, referred to as MV-I98A, was rescued as previously described (36). Cells infected with MV-I98A express high levels of green fluorescent protein (GFP) but exhibit minimal fusion (Fig. 1B). One-step growth curves for the virus show that it grows to titers similar to, if not higher than, those for the parental virus (Fig. 1C) (P = 0.012 for comparison of all groups, P = 0.06 for supernatants, and P = 0.04 for cell-associated virus). To further characterize the structural integrity of the recombinant virus, we performed Western blot analysis of purified virus preparations (Fig. 1D). The relative intensities of each protein band were quantitated using ImageJ in the linear range of the concentration/signal plot. The ratios of N/H were 1.0 and 1.1 for the wild-type and mutant viruses, respectively, and the ratios of H/F and N/F were 1.2 for both viruses. Therefore, the mutant virus is structurally similar to the parent.
FIG. 1.
Construction, rescue, and characterization of mutant measles virus. (A) Schematic representation of the measles virus genome. The mutant H gene was replaced in the full-length plasmid coding for the measles genome by use of PacI and SpeI restriction sites. (B) Rescue of the recombinant virus was documented by the presence of infection in Vero cells that expressed high levels of GFP without significant cell-to-cell fusion. (C) One-step growth curve of released and cell-associated virus for MVeGFP and MV-I98A after infection of Vero cells at a multiplicity of infection (MOI) of 1. (D) Western blot analysis of MVeGFP and MV-I98A by use of antibodies against measles N, H, and F proteins. The amount of purified virus loaded ranged from 5 × 103 to 30 × 103 50% tissue culture infectious doses (TCID50). (E) Coimmunoprecipitation of MV H-F complexes. Vero cell lysates (CL) collected after infection with MVeGFP or MV-I98A were pulled down with protein G agarose beads conjugated to mouse anti-MV-H IgG, and MV-F protein was then detected by Western blot analysis using rabbit anti-MV-F primary antibody. MVeGFP virions (V) were loaded at a TCID50 of 5 × 104. Wt, wild type.
Given that virus binding and fusion are so intimately related, we wanted to determine how the mutant or parent H and F proteins interact in the presence of the M protein, since the M protein is known to modulate fusion (2). Vero cells were infected with MVeGFP (with or without fusion-inhibitory peptide [FIP] added later) or MV-I98A. FIP is an oligopeptide similar to the N terminus of the F1 protein that may compete for binding sites on the plasma membranes of target cells and inhibits fusion (37). We performed coimmunoprecipitation studies using an anti-H antibody to pull down H and F complexes. The mutant H protein (H-I98A) coimmunoprecipitated F more efficiently (Fig. 1E), suggesting that H-I98A has a higher affinity for F, and binding or the dissociation kinetics between the mutant H protein and F are altered. The presence of FIP significantly increased the amount of F protein immunoprecipitated by MV-Edm H, suggesting that FIP might inhibit fusion by stabilizing the H-F complex and that dissociation of the two proteins is required for fusion to occur.
Syncytium formation after virus infection.
To evaluate the fusion properties of the proteins in the context of the virus, we infected Mel 624 cells with MVeGFP or MV-I98A and labeled target uninfected Mel 624 cells with cell tracker orange. After 24 h, infected and labeled target cells were mixed and allowed to attach. The following day, the cells were studied by confocal microscopy to determine the extent of fusion. Digital images were captured and analyzed for colocalization with ImageJ. As can be seen from Fig. 2, MVeGFP gave rise to significant cell-to-cell fusion, in contrast to MV-I98A (Spearman's ρ = 0.52 and −0.28, respectively [P = 0.04]; a positive correlation implies fusion and a negative correlation lack of fusion). Therefore, the hypofusogenic phenotype of H-I98A remains even in the presence of the matrix protein and the intact virus.
FIG. 2.
Membrane fusion after infection with wild-type or mutant viruses. Adherent Mel624 cells were infected with MVeGFP or MV-I98A and overlaid on target uninfected cells labeled with cell tracker orange. The presence/absence of fusion was detected as previously discussed and the Spearman's correlation coefficient calculated. The images are as follows: A and E, target cells labeled orange with the red filter only; B and F, phase contrast images; C and G, green filter showing infected cells expressing GFP; D and H, merged images showing colocalization (or lack) of signals. Fusion was apparent in MVeGFP-infected cells, as giant multicell syncytia were present (D) (Spearman's ρ = 0.52); however, these syncytia were absent from cells infected with MV-I98A (H), as no syncytia were observable (Spearman's ρ = −0.28).
Mutant virus binding and entry kinetics.
To determine whether the limiting step is binding or entry of the mutant virus, we incubated the parent or mutant virus with the suspension cell line KAS-6/1 (44) on ice for 10 min to allow the virus to bind but not enter cells. Subsequently, the cells were transferred to 37°C, and at specific time intervals, the cells were washed with acid buffer (pH = 3.0) for 1 min to eliminate any bound virus, and the medium was replaced. The fraction of cells infected was determined 48 h later by flow cytometry. FIP was added to the cells infected with MVeGFP but not the cells infected with MV-I98A. Infection with MV-I98A is slower, although with longer incubation times, the fraction of infected cells approaches that observed for the parent virus (Fig. 3A). In a similar experiment, cells were incubated with the viruses for 1 h on ice, followed by transfer to 37°C for variable time intervals. Entry of MVeGFP is significantly faster than that of MV-I98A, even though the binding kinetics are similar (Fig. 3B). Nonlinear least-squares fitting of the data for the two viruses represented in Fig. 3B also showed that the binding levels of the two viruses are similar, supporting the notion that binding efficiency is not the rate-limiting step. Therefore, the rate-limiting step is fusion.
FIG. 3.
Binding and entry kinetics of measles viruses. (A) KAS-6/1 cells were chilled and infected on ice at an MOI of 1.0 to allow virus binding but not entry. After incubation, the cells were transferred to 37°C for specified time intervals, at which point they were centrifuged and washed with acidic buffer (pH = 3.0) for 2 min to remove any bound virus that had not entered. Cells were then incubated for 48 h, at which point the fraction of GFP-positive cells was determined by flow cytometry. MVeGFP (red) entered cells at a much higher rate than MV-I98A (black), although, in time, both reached approximately the same level of infection. For panel B, the incubation on ice was for 1 h to ensure equilibration of the virus on binding sites without entry.
The sensitivities of the two viruses to inhibition by FIP were also determined. Vero cells were incubated with Opti-MEM containing the respective virus at 37°C. After specific time intervals, the cells were washed and incubated with fresh medium. FIP was added either immediately or after 6 h (delayed). MV-I98A infection was much more sensitive to the presence of FIP than MVeGFP (Fig. 4A). The viruses were incubated with chilled cells to allow binding for various time intervals, followed by washing and replacement with fresh medium with or without FIP (Fig. 4C to D). The impact of FIP on MVeGFP entry increased at 4°C, since the virus binds but cannot enter. In the absence of FIP, MV-I98A infection approached that of MVeGFP (compare Fig. 4A and D) although FIP reduced entry of the virus into cells (compare Fig. 4B and D). These results confirm that H-I98A binds to its receptor normally, and delayed infection is due to a reduction in the rate at which fusion of viral and cell membranes occur.
FIG. 4.
Specific virus sensitivity to fusion-inhibitory peptide. KAS-6/1 cells were chilled on ice and infected for specified time periods with either MVeGFP or MV-I98A at room temperature (A, B) or on ice (C, D). FIP was added immediately or at 6 h postinfection. The percentage of GFP-positive cells was determined by flow cytometry at 48 h postinfection. The addition of FIP to the medium reduced the percentages of GFP-positive cells in both viruses; however, FIP had a significantly greater effect on MV-I98A.
Conclusion.
Although several mutations in the stalk region of the measles virus H protein reduce fusion, none were tested in the context of a replicating virus (5, 6). Here, we describe the isolation of a fully infectious mutant virus that displays significantly reduced syncytium formation. Viruses with mutated F proteins have been produced in a low titer (3, 17, 22, 28, 39), but MV-I98A can be grown to titers similar to or higher than those for the parent MVeGFP. This virus provides a useful tool for elucidating the mechanisms of membrane fusion. The virus may provide a platform for gene therapy or vaccine applications (27) since the carboxyl terminus of H can be retargeted (10-12, 31, 32, 38, 43) without a reduction in the ability of H to trigger fusion.
Expression of F alone does not lead to fusion; binding of H to the receptor triggers the conformational changes in F. It has been suggested that after binding of H, F is released to allow fusion to occur (26, 45). Our results are most compatible with this hypothesis. By increasing the stability of the H-F complex, the I98A mutation reduces the probability of membrane fusion, hence explaining the slow kinetics of postbinding events (fusion). Our results also suggest that FIP may similarly stabilize the H-F complex and inhibit fusion. We cannot state that fusion does not occur; complete ablation of fusion will irreversibly prevent virus entry into cells.
This stalk region of the H glycoprotein has a rigid alpha-helical structure (19, 24, 40) that tolerates extensions without apparent loss of fusion triggering (30). The bulkier side chain of isoleucine imposes steric constrains on the proximity of the H-F contacts and modulates the strength of the protein-protein interactions. With an alanine substitution, this steric constraint is reduced and the corresponding H-F interaction stronger, explaining the higher stability of the complex and the reduced rates of fusion and infection. The almost inverse relationship between the levels of strength of H-F interactions and fusion, as reported by others (5, 6, 35) and us, highlights the dynamic nature of glycoprotein interactions within the measles viral envelope. Mutations that reduce the strength of H-F interactions are associated with a hyperfusogenic phenotype (35). In contrast, mutations in H or F that reduce the rate of fusion often exhibit a stronger H-F affinity. A mutant virus with a K97E substitution in the H protein that exhibits essentially no fusion was surreptitiously identified (L. K. Hallak, C. Hu, and S. J. Russell, unpublished observations). This suggests that charge-charge interactions can also (de)stabilize the H-F protein complexes and modulate fusion.
In summary, measles viruses with significant reduction in their fusogenic ability can be rescued. Receptor binding is normal, and reduced fusion is due to increased affinity between the H and F proteins. Modulation of these interactions by single-amino-acid substitutions profoundly alters the phenotype of this virus, including spread and production.
Acknowledgments
This work is supported by an early career development award to D.D. from the Mayo Clinic and Public Health Service grant CA-100634 from the National Cancer Institute to S.J.R.
We thank Roberto Cattaneo, Mayo Clinic, for critical review of the manuscript, Patricia Devaux for various reagents, and Justin Peters for assistance with quantitative image analysis.
Footnotes
Published ahead of print on 11 August 2010.
REFERENCES
- 1.Cathomen, T., B. Mrkic, D. Spehner, R. Drillien, R. Naef, J. Pavlovic, A. Aguzzi, M. A. Billeter, and R. Cattaneo. 1998. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 17:3899-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattaneo, R., and J. K. Rose. 1993. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J. Virol. 67:1493-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colf, L. A., Z. S. Juo, and K. C. Garcia. 2007. Structure of the measles virus hemagglutinin. Nat. Struct. Mol. Biol. 14:1227-1228. [DOI] [PubMed] [Google Scholar]
- 5.Corey, E. A., and R. M. Iorio. 2009. Measles virus attachment proteins with impaired ability to bind CD46 interact more efficiently with the homologous fusion protein. Virology 383:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corey, E. A., and R. M. Iorio. 2007. Mutations in the stalk of the measles virus hemagglutinin protein decrease fusion but do not interfere with virus-specific interaction with the homologous fusion protein. J. Virol. 81:9900-9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crennell, S., T. Takimoto, A. Portner, and G. Taylor. 2000. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 7:1068-1074. [DOI] [PubMed] [Google Scholar]
- 8.Deng, R., Z. Wang, A. M. Mirza, and R. M. Iorio. 1995. Localization of a domain on the paramyxovirus attachment protein required for the promotion of cellular fusion by its homologous fusion protein spike. Virology 209:457-469. [DOI] [PubMed] [Google Scholar]
- 9.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 10.Hallak, L. K., J. R. Merchan, C. M. Storgard, J. C. Loftus, and S. J. Russell. 2005. Targeted measles virus vector displaying echistatin infects endothelial cells via alpha(v)beta3 and leads to tumor regression. Cancer Res. 65:5292-5300. [DOI] [PubMed] [Google Scholar]
- 11.Hammond, A. L., R. K. Plemper, J. Zhang, U. Schneider, S. J. Russell, and R. Cattaneo. 2001. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J. Virol. 75:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa, K., T. Nakamura, M. Harvey, Y. Ikeda, A. Oberg, M. Figini, S. Canevari, L. C. Hartmann, and K. W. Peng. 2006. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin. Cancer Res. 12:6170-6178. [DOI] [PubMed] [Google Scholar]
- 13.Hashiguchi, T., M. Kajikawa, N. Maita, M. Takeda, K. Kuroki, K. Sasaki, D. Kohda, Y. Yanagi, and K. Maenaka. 2007. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. U. S. A. 104:19535-19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu, X. L., R. Ray, and R. W. Compans. 1992. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J. Virol. 66:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hummel, K. B., and W. J. Bellini. 1995. Localization of monoclonal antibody epitopes and functional domains in the hemagglutinin protein of measles virus. J. Virol. 69:1913-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb, R. A. 1993. Paramyxovirus fusion: a hypothesis for changes. Virology 197:1-11. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence, D. M., C. E. Patterson, T. L. Gales, J. L. D'Orazio, M. M. Vaughn, and G. F. Rall. 2000. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J. Virol. 74:1908-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence, M. C., N. A. Borg, V. A. Streltsov, P. A. Pilling, V. C. Epa, J. N. Varghese, J. L. McKimm-Breschkin, and P. M. Colman. 2004. Structure of the haemagglutinin-neuraminidase from human parainfluenza virus type III. J. Mol. Biol. 335:1343-1357. [DOI] [PubMed] [Google Scholar]
- 19.Lee, J. K., A. Prussia, T. Paal, L. K. White, J. P. Snyder, and R. K. Plemper. 2008. Functional interaction between paramyxovirus fusion and attachment proteins. J. Biol. Chem. 283:16561-16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard, V. H., P. L. Sinn, G. Hodge, T. Miest, P. Devaux, N. Oezguen, W. Braun, P. B. McCray, Jr., M. B. McChesney, and R. Cattaneo. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118:2448-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manchester, M., D. S. Eto, A. Valsamakis, P. B. Liton, R. Fernandez-Munoz, P. A. Rota, W. J. Bellini, D. N. Forthal, and M. B. Oldstone. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCullough, K. C. 1983. Characterization of a non-syncytiogenic autonomously replicating variant of measles virus. J. Gen. Virol. 64(3):749-754. [DOI] [PubMed] [Google Scholar]
- 23.McGinnes, L. W., and T. G. Morrison. 2006. Inhibition of receptor binding stabilizes Newcastle disease virus HN and F protein-containing complexes. J. Virol. 80:2894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melanson, V. R., and R. M. Iorio. 2006. Addition of N-glycans in the stalk of the Newcastle disease virus HN protein blocks its interaction with the F protein and prevents fusion. J. Virol. 80:623-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melanson, V. R., and R. M. Iorio. 2004. Amino acid substitutions in the F-specific domain in the stalk of the newcastle disease virus HN protein modulate fusion and interfere with its interaction with the F protein. J. Virol. 78:13053-13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison, T. G. 2003. Structure and function of a paramyxovirus fusion protein. Biochim. Biophys. Acta 1614:73-84. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura, T., and S. J. Russell. 2004. Oncolytic measles viruses for cancer therapy. Expert Opin. Biol. Ther. 4:1685-1692. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama, T., K. Komase, R. Uzuka, A. Hoshi, and T. Okafuji. 2001. Leucine at position 278 of the AIK-C measles virus vaccine strain fusion protein is responsible for reduced syncytium formation. J. Gen. Virol. 82:2143-2150. [DOI] [PubMed] [Google Scholar]
- 29.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paal, T., M. A. Brindley, C. St. Clair, A. Prussia, D. Gaus, S. A. Krumm, J. P. Snyder, and R. K. Plemper. 2009. Probing the spatial organization of measles virus fusion complexes. J. Virol. 83:10480-10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng, K. W., K. A. Donovan, U. Schneider, R. Cattaneo, J. A. Lust, and S. J. Russell. 2003. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood 101:2557-2562. [DOI] [PubMed] [Google Scholar]
- 32.Peng, K. W., P. D. Holler, B. A. Orr, D. M. Kranz, and S. J. Russell. 2004. Targeting virus entry and membrane fusion through specific peptide/MHC complexes using a high-affinity T-cell receptor. Gene Ther. 11:1234-1239. [DOI] [PubMed] [Google Scholar]
- 33.Plemper, R. K., A. L. Hammond, and R. Cattaneo. 2000. Characterization of a region of the measles virus hemagglutinin sufficient for its dimerization. J. Virol. 74:6485-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plemper, R. K., A. L. Hammond, and R. Cattaneo. 2001. Measles virus envelope glycoproteins hetero-oligomerize in the endoplasmic reticulum. J. Biol. Chem. 276:44239-44246. [DOI] [PubMed] [Google Scholar]
- 35.Plemper, R. K., A. L. Hammond, D. Gerlier, A. K. Fielding, and R. Cattaneo. 2002. Strength of envelope protein interaction modulates cytopathicity of measles virus. J. Virol. 76:5051-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson, C. D., and P. W. Choppin. 1983. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology 131:518-532. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, U., F. Bullough, S. Vongpunsawad, S. J. Russell, and R. Cattaneo. 2000. Recombinant measles viruses efficiently entering cells through targeted receptors. J. Virol. 74:9928-9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seligman, S. J., and F. Rapp. 1959. A variant of measles virus in which giant cell formation appears to be genetically determined. Virology 9:143-145. [DOI] [PubMed] [Google Scholar]
- 40.Stone-Hulslander, J., and T. G. Morrison. 1999. Mutational analysis of heptad repeats in the membrane-proximal region of Newcastle disease virus HN protein. J. Virol. 73:3630-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 42.Tsurudome, M., M. Kawano, T. Yuasa, N. Tabata, M. Nishio, H. Komada, and Y. Ito. 1995. Identification of regions on the hemagglutinin-neuraminidase protein of human parainfluenza virus type 2 important for promoting cell fusion. Virology 213:190-203. [DOI] [PubMed] [Google Scholar]
- 43.Ungerechts, G., C. Springfeld, M. E. Frenzke, J. Lampe, P. B. Johnston, W. B. Parker, E. J. Sorscher, and R. Cattaneo. 2007. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 67:10939-10947. [DOI] [PubMed] [Google Scholar]
- 44.Westendorf, J. J., G. J. Ahmann, P. R. Greipp, T. E. Witzig, J. A. Lust, and D. F. Jelinek. 1996. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia 10:866-876. [PubMed] [Google Scholar]
- 45.White, J. M., S. E. Delos, M. Brecher, and K. Schornberg. 2008. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 43:189-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild, T. F., E. Malvoisin, and R. Buckland. 1991. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J. Gen. Virol. 72(2):439-442. [DOI] [PubMed] [Google Scholar]
- 47.Yao, Q., X. Hu, and R. W. Compans. 1997. Association of the parainfluenza virus fusion and hemagglutinin-neuraminidase glycoproteins on cell surfaces. J. Virol. 71:650-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan, P., G. P. Leser, B. Demeler, R. A. Lamb, and T. S. Jardetzky. 2008. Domain architecture and oligomerization properties of the paramyxovirus PIV 5 hemagglutinin-neuraminidase (HN) protein. Virology 378:282-291. [DOI] [PMC free article] [PubMed] [Google Scholar]