Abstract
In order to better understand the broad applicability of adenovirus (Ad) as a vector for human vaccine studies, we compared four adenovirus (Ad) vectors from families C (Ad human serotype 5 [HAdV-5; here referred to as AdHu5]), D (HAdV-26; here referred to as AdHu26), and E (simian serotypes SAdV-23 and SAdV-24; here referred to as chimpanzee serotypes 6 and 7 [AdC6 and AdC7, respectively]) of the Adenoviridae. Seroprevalence rates and titers of neutralizing antibodies to the two human-origin Ads were found to be higher than those reported previously, especially in countries of sub-Saharan Africa. Conversely, prevalence rates and titers to AdC6 and AdC7 were markedly lower. Healthy human adults from the United States had readily detectable circulating T cells recognizing Ad viruses, the levels of which in some individuals were unexpectedly high in response to AdHu26. The magnitude of T-cell responses to AdHu5 correlated with those to AdHu26, suggesting T-cell recognition of conserved epitopes. In mice, all of the different Ad vectors induced CD8+ T-cell responses that were comparable in their magnitudes and cytokine production profiles. Prime-boost regimens comparing different combinations of Ad vectors failed to indicate that the sequential use of Ad vectors from distinct families resulted in higher immune responses than the use of serologically distinct Ad vectors from the same family. Moreover, the transgene product-specific antibody responses induced by the AdHu26 and AdC vectors were markedly lower than those induced by the AdHu5 vector. AdHu26 vectors and, to a lesser extent, AdC vectors induced more potent Ad-neutralizing antibody responses. These results suggest that the potential of AdHu26 as a vaccine vector may suffer from limitations similar to those found for vectors based on other prevalent human Ads.
Due to their ability to induce potent transgene product-specific B- and T-cell responses, replication-defective adenovirus (Ad) vectors are being explored for use as carriers of vaccines for a variety of pathogens, including human immunodeficiency virus type 1 (HIV-1) (7), Plasmodium falciparum (9), and Mycobacterium tuberculosis (20). Initial enthusiasm for the use of Ad vectors based on Ad human serotype 5 (AdHu5) was dampened by the finding that preexisting antibodies to this virus, which are found in ∼40% of humans residing in the United States and up to 90% of humans residing in some African countries (28), can reduce transgene product-specific immune responses (16) by reducing vector uptake (19). Enthusiasm further decreased after the phase IIb STEP trial, in which an AdHu5 vector was tested for induction of protection in cohorts at high risk for HIV-1 infection. The vector failed to show efficacy in reducing acquisition rates or lowering viral loads in individuals who became infected and instead appeared to increase susceptibility to infection in humans with preexisting neutralizing antibodies to the vaccine carrier (4). As a result of these setbacks, the use of Ad vectors based on other less common serotypes of human Ads (1) or Ads isolated from different species, such as chimpanzees (21, 25), bovines (24), and canines (31), to circumvent preexisting neutralizing antibodies is being explored. Of these, vectors based on adenovirus family D (AdHu26) were shown to have a low seroprevalence in some countries (1) and are now viewed as promising carriers for Ad vector-based gene transfer.
A number of studies showed that AdHu26 vectors are highly immunogenic in nonhuman primates (NHPs), where they induced potent transgene product-specific CD8+ T-cell responses (13) that, when they were combined in a prime-boost regimen with an AdHu5 vector expressing gag of simian immunodeficiency virus (SIV), achieved a sustained reduction in viral loads upon SIV challenge of vaccinated animals (14). Intriguingly, AdHu26 vectors have been shown to induce a CD8+ T-cell response in NHPs that is qualitatively superior to that induced by AdHu5 vectors. AdHu26-induced CD8+ T cells showed a broader response, recognizing more epitopes within the transgene product, and had a more polyfunctional response, in that vector-induced individual CD8+ T cells produced multiple factors rather than predominantly gamma interferon (IFN-γ) only (13). This suggests that AdHu26 may have fundamental differences in immunogenicity from other Ad vectors.
To elucidate this further, we developed a molecular clone of AdHu26 and a number of recombinant AdHu26 vectors from which E1 was deleted and used these to test human samples for the prevalence of AdHu26-neutralizing antibodies and responding CD4+ and CD8+ T cells. In addition, we conducted a series of studies with mice to determine if this species showed an immune response to a transgene product delivered by an AdHu26 vector markedly different from that induced by the same transgene product delivered by other Ad vectors. Our results showed that AdHu26, strictly speaking, is not a rare serotype, especially in African countries, where the seroprevalence rates of antibodies to AdHu26 are high. Similarly, most humans carry AdHu26-reactive T cells, which in some individuals are present at very high frequencies. In mice, AdHu26 induces potent CD8+ T-cell responses that are quantitatively and qualitatively similar to those induced by other Ad vectors. AdHu26 and chimpanzee-origin Ad (AdC) vectors stimulated only marginal transgene product-specific B-cell responses in comparison to those stimulated by AdHu5 vectors but induced more potent neutralizing antibodies to their capsid antigens.
MATERIALS AND METHODS
Serum samples.
Human serum or plasma samples were obtained from adult donors from Thailand (Bangkok and vicinity, n = 200) Cameroon (n = 26), Ivory Coast (n = 200), Nigeria (n = 193), South Africa (n = 40), Uganda (n = 59), and the United States (n = 100). The sera were stored at −20°C and heat inactivated at 56°C for 30 min prior to their testing. Repeated freeze-thawing was avoided.
PBMCs.
Peripheral blood mononuclear cells (PBMCs) were obtained by aphaeresis of sera from HIV- and hepatitis C virus-negative adult healthy donors by the University of Pennsylvania Center for AIDS Research Immunology Core, under the institutional guidelines required for the conduct of experiments with human samples.
Cells.
HEK 293 cells, used for propagation and titration of Ad vectors and for neutralization assays, were grown in Dulbecco's modified Eagle's (DME) medium supplemented with 10% fetal bovine serum (FBS), 1% glutamine, nonessential amino acids, and antibiotics at 37°C in a 5% CO2 humidified incubator. CHO cells stably transfected to express the coxsackievirus-adenovirus receptor (CAR) (CHO-CAR cells) or, as a control, the herpesvirus entry mediator (HVEM) (CHO-HVEM cells) and BHK-21 cells were grown in DME medium supplemented with 10% FBS, 1% glutamine, nonessential amino acids, 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, and antibiotics at 37°C in a 10% CO2 humidified incubator.
Ad vectors.
Construction of the molecular clone of AdHu26 and the recombinant vectors is described in the Results section. Construction of other molecular clones and vectors has been described previously (16, 21, 23, 25, 28). Ad vectors from which E1 was deleted expressing green fluorescent protein (GFP), Gag of HIV-1, or the rabies virus glycoprotein (rab.gp) under the control of the cytomegalovirus promoter were propagated on HEK 293 cells. Virus particles (vps) were determined by spectrophotometry, and the vectors were, in addition, titrated in 96-well plates or in Terasaki plates on confluent monolayers of HEK 293 cells to determine the multiplicity of infection (MOI). The vp-to-MOI ratios ranged from 50:1 to 200:1. The vectors used in this study had endotoxin levels below the level of detection and were free of replication-competent virus particles. It should be pointed out that the official nomenclature of the International Committee of Taxonomy of Viruses is as follows for the adenoviruses used for construction of the vectors described herein: HAdV-5 for AdHu5, HAdV-26 for AdHu26, SAdV-23 for AdC6, and SAdV-24 for AdC7.
Rabies virus.
Rabies virus of the Evelyn Rokitniki Abelseth (ERA) strain and the challenge virus standard 11 (CVS-11) strain were propagated on BHK-21 cells. ERA virus was purified by sucrose gradient centrifugation and inactivated by treatment with β-propionolactone (BPL). The protein concentration of inactivated rabies virus (ERA inactivated with BPL) was measured and adjusted to 0.1 mg/ml. The CVS-24 strain of rabies virus was propagated in the brains of newborn mice, and the lethal dose (LD) was determined by titration in adult ICR mice.
Infection studies and microscopy.
CHO-CAR or CHO-HVEM cells were infected with different amounts of Ad vectors expressing GFP. Cells either were incubated for 2 h with virus and then washed, prior to an additional incubation of 22 h or were incubated in virus-containing medium for 24 h. Cells were analyzed by fluorescent microscopy at a ×10 magnification using the same setup for all samples, or they were analyzed by flow cytometry.
Mice.
Six- to 8-week-old female BALB/c mice were purchased from ACE Animals Inc. (Boyertown, PA) and kept at the animal facility of The Wistar Institute (Philadelphia, PA). Five- to 7-week-old outbred female ICR mice were purchased from Charles River Laboratories (Boston, MA). All experiments with animals were performed in accordance with institutional regulations.
Immunization and challenge of mice.
Mice were immunized intramuscularly at 6 to 8 weeks of age with recombinant viruses diluted in sterile phosphate-buffered saline. Booster immunizations were applied 2 months after the mice were primed. For preexposure, mice were immunized with vectors at 1011 vps/mouse 2 to 3 weeks prior to vaccination, and the doses used for immunization varied. Four weeks after vaccination, mice were challenged with rabies virus strain CVS-24, given intramuscularly at 10 50% LDs (LD50s). After challenge, the mice were checked daily and survival was recorded for at least 3 weeks. Mice were euthanized once they developed complete hind leg paralysis, indicative of terminal rabies encephalitis.
Adenovirus neutralization assay.
The adenovirus neutralization assays were conducted either in flat-bottom 96-well plates or, if the amount of available serum was very limited, in Terasaki plates. A dose of GFP-expressing Ad vector that caused expression in 70 to 90% of the cells within 24 h was chosen. Vectors diluted to the appropriate concentration were preincubated for 60 min at room temperature with an equal volume of diluted serum. Dilutions of vectors and sera were made in DME medium supplemented as described above. The vector-serum mixture was then mixed with an equal volume of HEK 293 cells at 106 cells/ml (5 μl of cells for Terasaki plates, 50 μl of cells for 96-well plates) and that mixture was transferred into culture wells. The cells were incubated for 24 h at 37°C and then screened visually for green fluorescent cells under a fluorescent microscope. The titer was determined as the reciprocal serum dilution that caused an ∼50% reduction of fluorescence in comparison to that for the control wells infected with vector only. Samples were initially prescreened at a 1:10 dilution, and those that caused a reduction in the level of GFP expression were then rescreened using serial (log2) dilutions of sera.
Neutralizing antibodies to rabies virus.
Sera from individual mice were tested on BHK-21 cells for neutralization of the CVS-11 strain of rabies virus (29), which is antigenically closely related to the ERA strain, from which the vaccine antigen originated. Assays were standardized by inclusion of a reference serum sample from the World Health Organization (WHO). Data are expressed as international units (IU). The assay has a lower limit of detection of 0.2 IU.
Intracellular cytokine staining (ICS) and tetramer staining. (i) Human samples.
T-cell responses to replication-defective AdHu5 and AdHu26 vectors in PBMCs were measured as described previously (11). Cells were stained for surface phenotype (CD3, CD4, CD8, CD27, CD45RO, CD14, CD16), viability (live-dead amine reactive dye), and functionality (interleukin-2 [IL-2], tumor necrosis factor alpha [TNF-α], IFN-γ). All antibodies were titrated to determine the optimal staining. We analyzed cells on a modified LSR II flow cytometer (BD Immunocytometry Systems, San Jose, CA) and collected 200,000 to 1,000,000 events per sample. Data were analyzed using FlowJo (version 8.7) software (TreeStar, Ashland, OR). The data obtained after subtraction of the values for the background no-stimulation condition are reported.
(ii) Mouse samples.
ICS was performed on lymphocytes isolated from the blood or spleens of mice, as described previously (5). The antibodies used for ICS were peridinin chlorophyll protein (PerCP)-Cy5.5- or Alexa 700-labeled anti-CD8, phycoerythrin (PE)-Cy7-labeled anti-TNF-α, antigen-presenting cell (APC)-labeled anti-macrophage inflammatory protein-1α (MIP-1α), and PE-Gr-A-labeled anti-IFN-γ. For tetramer staining, cells were harvested from the indicated tissues; and red blood cells were lysed with ACK lysis buffer (Gibco, Carlsbad, CA) for 3 min on ice, washed 3 times with L-15 medium (Gibco), and stained with APC-labeled HIV-1 Gag tetramer (NIH Tetramer Core Facility, Emory University, Atlanta, GA), PerCP-Cy5.5-labeled anti-CD8, fluorescein isothiocyanate-labeled anti-CD3, Alexa Fluor 700-labeled anti-CD44, and an AmCyan (Live/Dead fixable aqua dead cell stain; Invitrogen, Carlsbad, CA)-labeled live cell stain. For flow cytometry, cells were acquired on a BD FACSCalibur fluorescence-activated cell sorter (FACS) apparatus (BD Bioscience, San Jose, CA) and analyzed using FlowJo software. Cells were gated onto lymphocytes, live CD3+ cells, and then CD8+ T cells with the different functions.
Statistical analysis.
Most results show the means ± standard deviations (SDs) or standard errors of the means (SEMs). Differences between two groups were analyzed by one-tailed Student's t tests. Correlation was determined by Spearman rank correlation. Correlation of T-cell responses between human subjects was determined by a multivariable analysis of variance. Data with P values of ≤0.05 are viewed as showing a statistically significant difference.
RESULTS
Construction and characterization of AdHu26.
AdHu26 (VR-224, strain BP-2) was purchased from the American Type Culture Collection (ATCC; Manassas, VA) (GenBank accession number EF153474). The virus was expanded on HEK 293 cells, and its 35,152-bp genome was isolated. A molecular clone of AdHu26 from which the E1 was deleted was generated, and open reading frame 6 (orf6) of the E4 genes was replaced with that of AdHu5. Briefly, a 3.4-kb EcoRI fragment which contains the 3′ inverted terminal repeat (ITR) was cloned after the ends were filled into the XbaI site of pNEB193, resulting in clone pAdHu26-R. orf6 of E4 of AdHu5 was amplified by PCR and cloned downstream of the 3′ ITR into the PmeI site of pAdH26-R, resulting in pAdHu26-Rorf6. The 5′ ITR was amplified by PCR using a 3′ primer with unique restriction enzyme sites for I-Ceu I and PI-SceI and ligated into the HindIII-XbaI sites of the pNEB193 vector, resulting in pAdHu26-L. The pAdHu26-L vector was digested with HindIII and XbaI, and the resulting ∼650-bp fragment was cloned into pAdHu26-Rorf6, which had also been digested with HindIII and XbaI, resulting in pAdHu26-LR containing both sides of the ITR and the unique cloning sites of I-Ceu I and PI-SceI. A 24-kb fragment was excised from the AdHu26 genome upon digestion with NdeI and was inserted into pAdHu26-LR to generate pAdHu26-NdeI. Finally, an 8-kb piece of BamHI-EcoRI was excised from the AdHu26 genome and cloned into the corresponding sites of pAdHu26-NdeI, resulting in the final construct of a molecular clone of pAdHu26 with the E1 deletion and a modified E4 orf6 (Fig. 1). The resulting molecular clone was tested by multiple restriction enzyme digestions, which showed the expected banding patterns. The parts of the genome encoding the hexon (the main target for virus-neutralizing antibody), fiber (the virus's attachment molecule), and both ITRs were sequenced using the molecular clone as the template; and the sequences were found to be identical to those described previously (A. J. Bett, D. R. Casimiro, J. W. Shiver, E. A. Emini, M. Chastain, and D. C. Kaslow, U.S. patent application 20,080,254,059). To generate recombinant Ad vectors, the transgenes were cloned into pShuttle (Clontech, Mountain View, CA). The expression cassette was excised from pShuttle using the rare cutters I-Ceu I and PI-SceI and cloned into the molecular viral clone cut with the same enzymes. Recombinant vectors in transformed bacteria were identified through diagnostic restriction enzyme digestion. For viral rescue, recombinant molecular clones were digested with PacI. The DNA was transfected onto semiconfluent monolayers of HEK 293 cells for viral rescue. Once viral plaques became visible, cells were harvested and virus was released by freeze-thawing. The virus was then expanded on HEK 293 cells, purified, titrated, and quality controlled.
FIG. 1.
Construction of the AdHu26 molecular clone. The graph shows the molecular clone of AdHu26 in which orf6 of E4 was replaced with that of AdHu5. Enzyme sites that were used to construct the clone are shown.
The AdHu26 vectors described in this article were tested for their expression of the transgene by Western blotting (gag) or indirect immunofluorescence assay (rabies virus glycoprotein). Expression was verified, and the level was found to be slightly below that achieved by AdHu5 vectors expressing the same transgene product and tested at the same doses (dosed according to the numbers of vps; data not shown). In addition, DNA was isolated from purified AdHu26 virions, and the genes encoding the hexon and fiber were sequenced. Both sequences were identical to those of the molecular clone.
Receptor binding activity of AdHu26.
Although previous studies have shown that adenoviruses of family D use CAR for cell attachment (22), a more recent publication indicated that AdHu26 bound to CD46 (1). To further address this controversy, we infected CHO-CAR cells and, as a control, CHO-HVEM cells with GFP-expressing AdHu5 (AdHu5GFP; a known CAR binder) and AdHu26GFP vectors. Infection with the AdHu5GFP and AdHu26GFP vectors resulted in the robust transduction and intense immunofluorescence of nearly all cells at a dose of ∼103 vps/cell. The CHO-HVEM cells used as controls showed lower levels of background fluorescence for AdHu5 and AdHu26 (Fig. 2 A). To ensure that AdHu26 was as effective as AdHu5 at entering cells upon binding to CAR, we incubated CHO-CAR cells and, as a control, CHO-HVEM cells with 103 or 104 vps of the AdHu26GFP or AdHu5GFP vector per cell. Some of the cells were washed 2 h later and then incubated for an additional 22 h to allow transgene product expression, while in other cultures, cells were incubated with the Ad vectors for 24 h. Cells were analyzed by flow cytometry for GFP expression. As shown in Fig. 2B, at the lower dose of the Ad vectors, the numbers of CHO-CAR cells expressing GFP were lower for AdHu26 during the 2- or 24-h incubation period, while at the higher dose, the levels of GFP expression achieved with either vector were comparable for AdHu5 and AdHu26. AdHu26 caused a more pronounced transduction of the CHO-HVEM cell line, which does not express CAR. To further confirm that AdHu26 does not bind to CD46, we tested the vectors for agglutination of red blood cells from rhesus macaques (rhRBCs), which are known to express CD46 (25). A vector originating from AdC1, which we had previously identified as a CD46 binder (25), readily agglutinated rhRBCs; however, this was not seen with the AdHu26 vectors, confirming that AdHu26 is not a CD46 binding virus (Fig. 2C). Taken together, these results suggest that AdHu26 binds to CAR, although potentially with a lower affinity than AdHu5 binding. In addition, the more pronounced transduction of CAR-negative cells at high vector doses and upon prolonged incubation may suggest that AdHu26 more readily enters cells upon binding to alternative receptors, such as integrins, than AdHu5 does.
FIG. 2.
Receptor binding specificity of AdHu26. CHO-CAR and CHO-HVEM cells were infected with 104 vps/cell of AdHu5GFP or AdHu26GFP. Cells were incubated for 24 h and evaluated microscopically (A). CHO-CAR or CHO-HVEM cells were incubated for 2 h with the Ad vectors expressing GFP, washed several times, and then incubated for an additional 22 h (2 h). Other cultures were incubated in the presence of AdGFP vectors for 24 h. Ad vectors were used at 103 or 104 vps per cell. Cells were trypsinized, stained with a live cell dye, and evaluated by flow cytometry. The histograms show the levels of GFP expression over the numbers of events (B). A 0.2% solution of red blood cells from a rhesus macaque was incubated with serial dilutions of AdC1, AdHu26, or saline without virus. The picture shows the original wells (C).
Prevalence of neutralizing antibodies to AdHu26 in humans.
AdHu26 was originally generated as a vaccine carrier for antigens of HIV-1 on the basis of the notion that the prevalence of neutralizing antibodies to this virus were found to be low in sub-Saharan Africa (1). To further determine prevalence rates in additional human cohorts, we tested sera from adult volunteers from the United States, Thailand, and several African countries for neutralizing antibodies to AdHu26 (Fig. 3 A). For comparison, some sera were also tested for their titers of neutralizing antibodies to AdHu5 and two chimpanzee-origin adenoviruses, i.e., AdC6 and AdC7. The prevalence rates of neutralizing antibodies to AdHu26 were relatively low in sera from Thailand and the United States, although they were higher than those reported previously (1). Prevalence rates were high in the sera from all of the African countries that we tested, and 60 to 80% of the serum samples from these countries neutralized AdHu26. Focusing on samples with titers of or above 1:40, which were shown in animal models to reduce transgene product-specific B- and T-cell responses to AdHu5 vector immunization (30), ∼10% of the serum samples from the United States and Thailand remained positive for antibodies to AdHu26, while 20 to 70% of the serum samples obtained from African cohorts were positive. In most African countries, the prevalence rates of neutralizing antibodies to AdHu26 were similar to or higher than those to AdHu5. In contrast, the percentages of samples positive for antibodies to the two AdC viruses were low in the United States and South Africa and only slightly higher in Uganda, confirming our previous results (28).
FIG. 3.
Prevalence and titers of neutralizing antibodies to AdHu26 in human sera. Human sera collected in the United States (n = 100), Thailand (n = 200), Ivory Coast (n = 200), Nigeria (n = 193), Cameroon (n = 26), Uganda (n = 59), and South Africa (n = 40) were tested for neutralizing antibodies to AdHu26; the same sets of sera from the United States, Uganda, and South Africa were also tested for neutralizing antibodies to AdHu5, AdC7, and AdC6. (A) Prevalence of positive sera. Black bars, percentage of serum samples that were positive at the lowest serum dilution of 1:10; gray bars, percentage of serum samples that were positive at dilutions at or above 1:40. (B) Average antibody titers of the positive sera.
The average titers of neutralizing antibodies to AdHu26 were slightly (∼2-fold) lower than the titers of antibodies to AdHu5 and, with the exception of samples from South Africa, ranged from 1:100 to 1:180 (Fig. 3B). Average titers to the AdCs were lower (≤1:50). In African samples positive for at least one serotype, there was no correlation in seropositivity between AdHu5 and AdHu26 (P = 0.17), AdHu5 and AdC6 (P = 0.32), and AdHu26 and AdC7 (P = 0.89) or AdC6 (P = 0.31). There was a weak correlation between sera that were positive for antibodies to AdHu5 and AdC7 (P = 0.046) and a more pronounced correlation between sera positive for antibodies to AdC6 and AdC7 (P = 0.007). Mouse sera induced to have antibodies to the different vectors were tested for the cross-reactivity of antibodies to the different serotypes. No such cross-reactivity was observed, indicating that this correlation was unlikely to reflect recognition of a conserved epitope. It is more likely that the correlation of sera positive for antibodies to both AdCs reflects their high prevalence in chimpanzees (28), the original hosts for these viruses. Contact of humans with chimpanzees may thus result in concomitant exposure to several AdCs.
Prevalence of AdHu26-specific T cells.
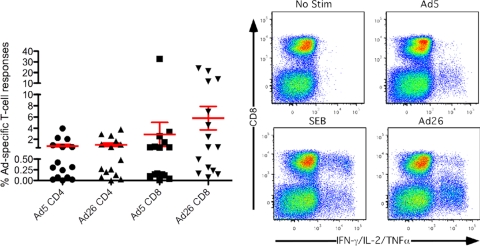
PBMCs from healthy human adults collected at the University of Pennsylvania Center for AIDS Research Immunology Core were tested as described previously (11) for T-cell responses to AdHu26 and AdHu5 after in vitro stimulation with Ad vectors. Cells were tested for production of cytokines (IL-2, TNF-α, and IFN-γ) in response to the antigen and for pertinent phenotypic markers. Most humans had readily detectable CD4+ and CD8+ T cells reactive to AdHu5, and most also carried T cells reactive to AdHu26 (Fig. 4), despite the low AdHu26 seroprevalence in the United States. Due to the likelihood for cross-serotype-reactive T cells to recognize conserved elements present within multiple Ad serotypes (10), it cannot be determined whether the AdHu26-specific T-cell responses that we detected were indeed originally primed by AdHu26. When we have previously compared human T-cell responses to AdHu5 with those to AdC7 and AdC6 (12), we found that the responses were universally detectable and exhibited similar magnitudes and functionalities. Likewise, there was no statistical difference in the magnitudes of the CD4+ and CD8+ T-cell responses to AdHu26 and AdHu5, although the average frequencies of CD8+ T cells reactive to the former were higher. There was also a correlation between the magnitudes of the AdHu26 and AdHu5 responses for a given subject (P < 0.01).
FIG. 4.
Human T-cell responses to AdHu5 and AdHu26. T-cell responses to AdHu5 and AdHu26 were measured by flow cytometry following whole-vector stimulation. (Left panel) Magnitudes of the CD4+ or CD8+ T-cell responses producing IL-2, IFN-γ, and/or TNF-α to AdHu5 and AdHu26 in 15 healthy subjects. Lines represent the average ± SEM. (Right panel) Representative flow plots of the T-cell response to no stimulation (top left) and staphylococcus endotoxin B (SEB) (bottom left) controls as well as AdHu5 (top right) and AdHu26 (bottom right).
Induction of transgene product-specific CD8+ T cells by AdHu26 vectors.
We tested an AdHu26 vector expressing a codon-optimized gene encoding HIV-1 gag for induction of transgene product-specific CD8+ T cells. In a dose titration experiment (Fig. 5 A and B), the AdHu26 vector induced, in a dose-dependent fashion, a potent gag-specific CD8+ T-cell response that could be detected by both tetramer staining and IFN-γ ICS. A 2nd experiment, in which different Ad vectors were compared (Fig. 5C), showed that at 1010 vps, AdHu26 induced gag-specific CD8+ T-cell responses that were comparable in magnitude to those induced by the AdHu5, AdC6, and AdC7 vectors also expressing gag. To test if prime-boost regimens based on Ad vectors from different families outperform those based on serologically distinct vectors from the same family, mice were primed with 108 vps of AdC6, AdC7 (both family E), AdHu5 (family C), or AdHu26 (family D) expressing gag of HIV-1. The frequencies of circulating gag-specific CD8+ T cells were tested 21 days later. Mice were boosted with the same dose of a heterologous vector 2 months after they were primed, and again, the frequencies of gag-specific CD8+ T cells in blood were tested 21 days later (Fig. 5D). After the priming, AdHu5 induced slightly higher responses than the other vectors. After booster immunization, the responses to the different regimens (AdC6/AdC7, AdC7/AdC6, AdHu5/AdHu26, AdHu26/AdHu5, AdC7/AdHu26, AdHu26/AdC6) were comparable. These results are in contrast to those described previously (13), which showed that priming with AdHu26 followed by an AdHu5 boost induced transgene product-specific CD8+ T-cell responses higher than those achieved by priming with AdHu5 followed by an AdHu26 boost. The discrepancy between these results could reflect differences in target antigens or mouse strains. We used vectors expressing gag of HIV-1 in BALB/c mice, while the previous study tested responses to gag of SIV in BALB/c mice. Differences could also relate to the time interval between priming and boosting and/or the vector dose; our study used a dose 10-fold lower than that used in the earlier study, and booster immunizations were given after a longer in-between resting period.
FIG. 5.
Transgene product-specific CD8+ T-cell responses to AdHu26 vector vaccination in mice. Groups of four BALB/c mice were immunized with varied doses of an AdHu26 vector expressing gag of HIV-1. Three weeks later the mice were bled and the frequencies of CD8+ cells and tetramer staining-positive CD8+ T cells from individual mice were determined. (A) Numbers of tetramer staining-positive (tet+) CD8+ cells/107 PBMCs. In addition, PBMCs from individual mice were stimulated for 5 h with the immunodominant epitope of Gag and then stained for CD8 and intracellular IFN-γ. (B) Frequencies of gag-specific IFN-γ-positive CD8+ cells as a proportion of all circulating CD8+ cells. (C) Comparison of CD8+ T-cell responses to gag using different Ad vectors for immunization. Mice were immunized with 1010 vps of vector; the frequencies of tetramer staining-positive CD8+ T cells were determined 21 days later. (D) Frequencies of gag-specific CD8+ T cells tested 21 days after they were primed with different Ad vectors at 108 vps or 21 days after booster immunization given 2 months after the cells were primed.
To assess if preexisting immunity to AdHu26 affected induction of an AdHu26 vector-induced transgene product-specific CD8+ T-cell response, groups of mice were vaccinated with 1011 vps of an AdHu26 vector expressing the rabies virus glycoprotein (AdHu26rab.gp). Mice were bled 2 weeks later, and neutralizing antibody titers to AdHu26 were tested and found to exceed 1:100 in most animals. Preexposed mice, together with naïve control mice, were then immunized with 5 × 109 vps of the AdHu26 gag vector. The gag-specific CD8+ T-cell responses tested 2 weeks later were strongly attenuated in animals with preexisting immunity to AdHu26, as shown by tetramer staining and ICS for IFN-γ, MIP-1α, and TNF-α (Fig. 6). The latter method also showed that preexisting immunity did not affect the cytokine production profiles of the gag-specific CD8+ T cells, which were dominated by cells producing IFN-γ alone or in combination with MIP-1α.
FIG. 6.
Preexposure to AdHu26 dampens the transgene product-specific CD8+ T-cell response to an AdHu26 vector. Mice were immunized with 1011 vps of an AdHu26 vector expressing the rabies virus glycoprotein. Two weeks later, sera were harvested and tested for neutralizing antibodies to AdHu26. All of the serum samples had titers in excess of 1:80. Mice were then vaccinated with 5 × 109 vps of an AdHu26gag vector (Ad26/Ad26gag), and naïve control mice were vaccinated with the AdHu26gag vector at the same time (−/Ad26rab). Two weeks later, the frequencies of gag-specific CD8+ T cells in blood were determined. A group of naïve mice (Naive) was tested in parallel. Preexposed animals developed significantly lower responses than non-pre-exposed animals (P = 0.0007). The graph on the left shows the numbers of tetramer staining-positive CD8+ cells/107 PBMCs. The same samples were tested for the production (+) or lack of production (−) of IFN-γ (I), MIP-1α (M), and TNF-α (T) in response to the epitope. The graph on the right shows the frequencies of CD8+ T cells with different cytokine profiles. The asterisk indicates a statistically significant difference in the responses of preexposed and non-pre-exposed animals.
Induction of transgene product-specific antibody responses by AdHu26 vectors.
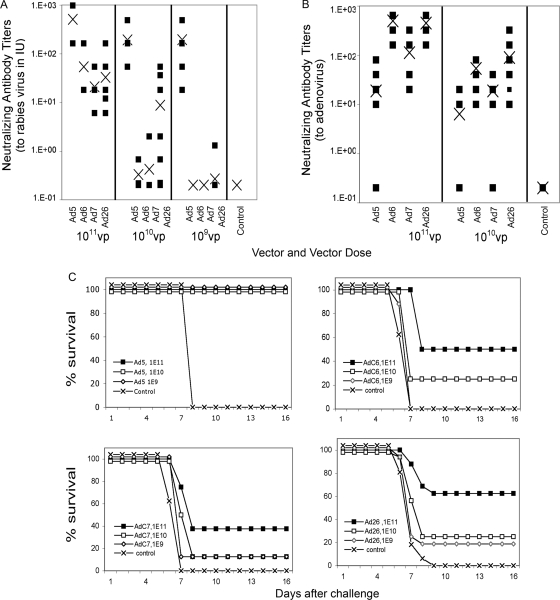
To assess induction of transgene product-specific antibody responses, groups of eight ICR mice were vaccinated intramuscularly with varied doses of AdHu26, AdHu5, AdC6, and AdC7 vectors expressing rab.gp. Sera from individual mice were tested 4 weeks later for neutralizing antibodies to rabies virus, and the titers, expressed in IU, were compared to those of a WHO standard serum sample. The titers were similar at both time points, and the results obtained for the week 4 bleeds are shown in Fig. 7 A. At all doses tested (109 to 1011 vps), the AdHu5rab.gp vector induced neutralizing antibody titers that were above those generally required for protection against challenge (≥0.5 IU) (Fig. 7A). In contrast, the AdHu26 and AdC vectors expressing rab.gp failed to induce detectable neutralizing antibodies at the 109-vp dose except in 1 of 16 mice in the AdHu26 group; several animals in all three groups failed to develop protective titers at the 1010-vp dose. All of the animals had titers of >0.5 IU after vaccination with 1011 vps of the AdC or AdHu vector, but the titers induced by the AdHu5 vector were significantly higher than those achieved by the other vectors. Upon challenge with rabies virus at 4 weeks postvaccination, all of the control mice died, while all of the AdHu5rab.gp-vaccinated mice survived regardless of the vaccine dose. Upon challenge, ∼40 to 60% of mice that received the highest vaccine dose of the AdC6, AdC7, or AdHu26 vector died; ∼80 to 90% of those vaccinated with the intermediate dose died; and 100% vaccinated with the lowest dose died (Fig. 7C).
FIG. 7.
Transgene product and capsid-specific antibody responses to AdHu26. To obtain neutralizing antibodies, groups of ICR mice were immunized with various doses of the AdHu5rab.gp (n = 8), AdC6rab.gp (n = 8), AdC7rab.gp (n = 8), or AdHu26rab.gp (n = 16) vector. They were bled 4 weeks later. (A) Titers of neutralizing antibodies to rabies virus were compared with those in a reference serum sample allows expression of the data in IU. Closed squares, results from individual mice, ×, averages. (B) Neutralizing antibody titers to the Ad vector used for immunization from individual mice (squares) and averages (×). (C) Protection against rabies virus challenge. The same groups of mice described for panel A were challenged with 10 LD50s of rabies virus at 4 weeks after vaccination. Survival was recorded over time. Animals were checked for a total of 3 weeks. All deaths occurred within 14 days after challenge.
Induction of adenovirus capsid-specific antibodies by AdHu26 vectors.
Replication-defective Ad vectors induce capsid-specific neutralizing antibodies. Nevertheless, depending on the dose, these antibody responses are, in general, modest and have thus allowed use of the same Ad vector repeatedly for booster immunizations, such as in the STEP trial. Serum samples from ICR mice immunized with 1011 and 1010 vps of the Adrab.gp vectors were used to test for neutralizing antibody titers to the Ad vector used for immunization (Fig. 7B). Only half of the mice immunized with the AdHu5rab.gp vector at either dose developed detectable neutralizing antibodies to AdHu5 within 4 weeks. In contrast, all of the mice immunized with AdHu26rab.gp and AdC6rab.gp developed robust titers of antibodies to the corresponding virus. At the 1011-vp vector dose, titers of antibodies to AdHu26 or AdC6 exceeded those to AdHu5 by ∼23- and 26-fold, respectively, while at the 1010-vp dose, the titers were 14- and 9-fold higher, respectively. The titers of neutralizing antibody to AdC7 were slightly higher than those to AdHu5, i.e., 6-fold higher at the 1011-vp dose and 3-fold higher at the 1010-vp dose, but well below those induced by AdHu26 or AdC6. This unexpected finding was repeated with Ad vectors expressing a different antigen to ensure that the results were not related to the transgene product or a specific vector batch. Again, AdHu26 induced more potent capsid-specific neutralizing antibody responses than AdHu5 (data not shown). We also conducted experiments with a different mouse strain (BALB/c mice), and again, AdHu26 induced exceptionally high titers of neutralizing antibodies to its capsid, confirming that this trait was not restricted to a particular mouse strain.
DISCUSSION
Ads from rare human or animal serotypes are being developed as vaccine carriers under the assumption that such vectors would outperform those based on common human serotypes, such as AdHu5 (1). In addition, Ads from different families may exhibit unique properties that could differentially affect the functionality of a transgene product-specific immune response to Ad vector-based vaccination. Ad vectors also differ in their tropisms, with most using CAR for cell entry, while others, such as those from family B (e.g., AdHu35 and AdC1), use the more ubiquitously expressed CD46 (6). Ad vectors also vary in their interactions with the innate immune system, with AdC vectors of family E typically inducing a more pronounced proinflammatory cytokine response than AdHu5 vectors (8).
AdHu26 was initially termed a rare serotype on the basis of its reported low seroprevalence rates in certain African countries (1). These results are in contrast to those of a more recent study, which showed neutralizing antibodies to AdHu26 in ∼65 to 88% of human samples from a number of other African countries (15). Our results confirm those of the latter study, as we also observed very high prevalence rates of AdHu26-neutralizing antibodies in human serum samples from Africa. Both of the studies show that average titers of antibodies to AdHu26 are lower than those to AdHu5, but nevertheless, in many individuals, they are above those anticipated to affect the performance of an AdHu26-based vaccine vector. The discrepancy between these results is noteworthy. The three different studies employed similar methods to assess neutralizing antibody responses, although the actual constructs used for the assays differed. In our studies, we used GFP as the reporter protein and titers were determined visually. The study that reported AdHu26 as a rare serotype used a luciferase readout (1), and the Merck study, whose results are in agreement with our results, used a chemiluminescence assay to assess the level of reduction of alkaline phosphatase expression (15). The sensitivities of these assays would be expected to differ; nevertheless, one would still expect concordant results, as all three methods are based on the same principle: measurement of antibody-mediated inhibition of cell transduction. We assume that the striking difference more likely reflects cohort variability. Both the Merck study and our study measured prevalence rates of antibodies to AdHu26 in Thailand; while Merck reported prevalence rates above 60%, in our study, less than 20% of serum samples were positive. Both sets of samples were collected in Bangkok, thus excluding regional variability, as could be envisioned between urban and rural settings, with samples from the former setting potentially showing higher Ad infection rates due to crowding. A sizable percentage of samples from the Merck study were obtained from individuals at high risk for HIV-1 infection (15), while in our study, random samples were received from King Chulalongkorn Memorial Hospital, which serves as a referral hospital for Bangkok and vicinity. We would thus assume that seroprevalence rates of antibodies to a given Ad serotype might vary markedly not only between geographic regions but also between cohorts. As was shown in the STEP trial, preexisting neutralizing antibodies to AdHu5 reduce the AdHu5 vaccine vectors' immunogenicity (17). AdHu5 vectors, for reasons that remain poorly understood, express higher levels of transgene product than other Ad vectors (1, 27), and one would thus assume that preexisting immunity to the latter vectors may have an even graver impact on their ability to induce transgene product-specific immune responses.
Preexisting T cells reactive to Ad may also reduce the efficacy of Ad vector-based vaccines, as has been shown in mice (5). T cells to Ads whose reactivities are commonly directed against conserved viral epitopes are highly prevalent in humans, and seropositivity for antibodies to a given strain does not predict either the presence or magnitude of Ad-specific CD4+ or CD8+ T cells (11). As we reported previously, the frequencies of T cells with reactivity to AdHu5 in human blood mirror those to unrelated Ads, such as AdCs (12). In the present study, we tested a set of human PBMCs for their responsiveness to AdHu26 and compared that to the responsiveness to AdHu5 using polyfunctional ICS as a readout (11). Unexpectedly, the frequencies of CD8+ T cells reactive to AdHu26 were extraordinarily high in some samples, exceeding 10% of all CD8+ T cells in 4 out of 15 subjects, while only 1 subject had AdHu5-specific CD8+ T-cell frequencies greater than 5%. The unexpectedly high frequencies of AdHu26-specific T cells in the blood of healthy human adults detected by ICS are in contrast to those detected in a previous study by enzyme-linked immunospot (ELISPOT) assay for IFN-γ (18), which reported the frequencies of AdHu26-reactive T cells to be comparable to those of AdHu5-reactive T cells. ELISPOT assays, which are commonly used to assess the immunogenicity of T-cell-inducing vaccines, require a lengthy incubation time, which may lead to the death of highly activated effector T cells (21).
The AdHu26 vector expressing HIV-1 gag induced potent CD8+ T-cell responses in mice that, at an intermediate vector dose, were comparable in magnitude to those observed upon immunization with the AdHu5 or AdC vector. As expected, preexisting immunity to AdHu26 strongly reduced the transgene product-specific CD8+ T-cell response to the AdHu26 vector. AdHu26 vectors stimulated a response dominated by CD8+ T cells producing IFN-γ with or without MIP-1α, unlike other Ad vectors that typically induce CD8+ T cells that produce IFN-γ and TNF-α (26). There was no marked difference in the magnitudes of the CD8+ T-cell responses using AdHu26 for priming or boosting from the magnitudes of the responses achieved with other regimens that used AdC vectors, indicating that prime-boost regimens that use Ad vectors derived from different families do not offer an advantage over prime-boost regimens that use serologically distinct Ad vectors from the same family.
The RV144 trial, which tested a vaccine regimen composed of multiple immunizations with a canary pox virus vector and a recombinant gp120 protein, reported only marginal induction of HIV-specific T cells or neutralizing antibodies, although most vaccine recipients developed HIV-1 binding antibodies. Nevertheless, the trial showed low-level efficacy against HIV-1 acquisition (3), unlike the STEP trial, which used a vaccine that focused solely on induction of T-cell responses (4). The albeit limited success of the RV144 trial, which awaits experimental confirmation, suggests that antibodies to the envelope of HIV-1, even if they fail to neutralize, may be crucial for protection against HIV-1 acquisition (2). We therefore tested an AdHu26 vector expressing the glycoprotein of rabies virus, which alone induces neutralizing and protective antibodies, for induction of a transgene product-specific antibody response. For comparison, we tested antibody responses induced by AdHu5 and AdC vectors carrying the same transgene cassette. As reported previously, the AdHu5rab.gp vector induced a robust neutralizing antibody response (30) that even at the lowest vector dose tested conferred complete protection against an otherwise fatal rabies virus infection. In comparison, the AdHu26 and the AdC vectors stimulated only low antibody responses, and even with the higher vector doses used, several of the animals failed to develop neutralizing antibodies to rabies virus. As we reported previously, while NHPs with preexisting Ad-neutralizing antibodies can still develop transgene product-specific T-cell responses to a high dose of the same type of Ad vector, they are no longer able to mount transgene product-specific antibodies (16), which suggests that induction of antibodies is more sensitive to a reduction in antigenic load. Considering that AdHu26 vectors induce only marginal titers of antibodies to an immunogenic protein such as the rabies virus glycoprotein, one would expect that in humans with preexisting antibodies to the virus, AdHu26 might not be the vector of choice for the induction of a transgene product-specific antibody response.
Our serological studies with mice revealed one additional curious finding: the AdHu26 vector and, to a lesser degree, the AdC vector, regardless of the transgene or the mouse strain tested, induced markedly higher titers of neutralizing antibodies to their own capsids than the AdHu5 vectors. This may reflect differences in the structure of the viral hexon, differences in tropism, or differences in the vectors' interaction with cells of the innate immune system. It remains an open question whether this finding is peculiar to mice or will also be observed in human recipients of AdHu26 or AdC vaccine vectors.
In conclusion, the prevalence rates of neutralizing antibodies to AdHu26 are high in humans from countries of sub-Saharan Africa and the frequencies of AdHu26-reactive CD8+ T cells are exceptionally high in some humans. Taken together, these results suggest that AdHu26 vectors may suffer from the limitations of preexisting immune responses similar to those encountered with AdHu5 vectors, especially in developing countries, where the need for novel vaccine strategies against many human pathogens is most dire.
Acknowledgments
This report was funded by grant U19, AI074078, from the NIH, NIAID.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal, G. P., A. Malaspina, and J. Flores. Future paths for HIV vaccine research: exploiting results from recent clinical trials and current scientific advances. Curr. Opin. Mol. Ther. 12:39-46. [PubMed]
- 4.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 6.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 7.Harro, C., X. Sun, J. E. Stek, R. Y. Leavitt, D. V. Mehrotra, F. Wang, A. J. Bett, D. R. Casimiro, J. W. Shiver, M. J. DiNubile, and E. Quirk. 2009. Safety and immunogenicity of the Merck adenovirus serotype 5 (MRKAd5) and MRKAd6 human immunodeficiency virus type 1 trigene vaccines alone and in combination in healthy adults. Clin. Vaccine Immunol. 16:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hensley, S. E., W. Giles-Davis, K. C. McCoy, W. Weninger, and H. C. Ertl. 2005. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J. Immunol. 175:6032-6041. [DOI] [PubMed] [Google Scholar]
- 9.Hill, A. V., A. Reyes-Sandoval, G. O'Hara, K. Ewer, A. Lawrie, A. Goodman, A. Nicosia, A. Folgori, S. Colloca, R. Cortese, S. C. Gilbert, and S. J. Draper. Prime-boost vectored malaria vaccines: progress and prospects. Hum. Vaccin. 6:78-83. [DOI] [PubMed]
- 10.Reference deleted.
- 11.Hutnick, N. A., D. G. Carnathan, S. A. Dubey, G. Makedonas, K. S. Cox, L. Kierstead, S. J. Ratcliffe, M. N. Robertson, D. R. Casimiro, H. C. Ertl, and M. R. Betts. 2009. Baseline Ad5 serostatus does not predict Ad5 HIV vaccine-induced expansion of adenovirus-specific CD4+ T cells. Nat. Med. 15:876-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutnick, N. A., D. Carnathana, K. Demers, G. Makedonas, H. C. J. Ertl, and M. R. Betts. 2010. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine 23:1932-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, J., B. A. Ewald, D. M. Lynch, M. Denholtz, P. Abbink, A. A. Lemckert, A. Carville, K. G. Mansfield, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mast, T. C., L. Kierstead, S. B. Gupta, A. A. Nikas, E. G. Kallas, V. Novitsky, B. Mbewe, P. Pitisuttithum, M. Schechter, E. Vardas, N. D. Wolfe, M. Aste-Amezaga, D. R. Casimiro, P. Coplan, W. L. Straus, and J. W. Shiver. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950-957. [DOI] [PubMed] [Google Scholar]
- 16.McCoy, K., N. Tatsis, B. Korioth-Schmitz, M. O. Lasaro, S. E. Hensley, S. W. Lin, Y. Li, W. Giles-Davis, A. Cun, D. Zhou, Z. Xiang, N. L. Letvin, and H. C. Ertl. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81:6594-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien, K. L., J. Liu, S. L. King, Y. H. Sun, J. E. Schmitz, M. A. Lifton, N. A. Hutnick, M. R. Betts, S. A. Dubey, J. Goudsmit, J. W. Shiver, M. N. Robertson, D. R. Casimiro, and D. H. Barouch. 2009. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichla-Gollon, S. L., S. W. Lin, S. E. Hensley, M. O. Lasaro, L. Herkenhoff-Haut, M. Drinker, N. Tatsis, G. P. Gao, J. M. Wilson, H. C. Ertl, and J. M. Bergelson. 2009. Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J. Virol. 83:5567-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radosevic, K., C. W. Wieland, A. Rodriguez, G. J. Weverling, R. Mintardjo, G. Gillissen, R. Vogels, Y. A. Skeiky, D. M. Hone, J. C. Sadoff, T. van der Poll, M. Havenga, and J. Goudsmit. 2007. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect. Immun. 75:4105-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes-Sandoval, A., J. C. Fitzgerald, R. Grant, S. Roy, Z. Q. Xiang, Y. Li, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2004. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J. Virol. 78:7392-7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy, S., G. Gao, Y. Lu, X. Zhou, M. Lock, R. Calcedo, and J. M. Wilson. 2004. Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum. Gene Ther. 15:519-530. [DOI] [PubMed] [Google Scholar]
- 24.Singh, N., A. Pandey, L. Jayashankar, and S. K. Mittal. 2008. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol. Ther. 16:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatsis, N., A. Blejer, M. O. Lasaro, S. E. Hensley, A. Cun, L. Tesema, Y. Li, G. P. Gao, Z. Q. Xiang, D. Zhou, J. M. Wilson, and H. C. Ertl. 2007. A CD46-binding chimpanzee adenovirus vector as a vaccine carrier. Mol. Ther. 15:608-617. [DOI] [PubMed] [Google Scholar]
- 26.Tatsis, N., J. C. Fitzgerald, A. Reyes-Sandoval, K. C. Harris-McCoy, S. E. Hensley, D. Zhou, S. W. Lin, A. Bian, Z. Q. Xiang, A. Iparraguirre, C. Lopez-Camacho, E. J. Wherry, and H. C. Ertl. 2007. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 110:1916-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang, Z., Y. Li, A. Cun, W. Yang, S. Ellenberg, W. M. Switzer, M. L. Kalish, and H. C. Ertl. 2006. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 12:1596-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang, Z. Q., S. Spitalnik, M. Tran, W. H. Wunner, J. Cheng, and H. C. Ertl. 1994. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology 199:132-140. [DOI] [PubMed] [Google Scholar]
- 30.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 31.Yuan, Z. G., X. M. Li, Y. S. Mahmmod, X. H. Wang, H. J. Xu, and X. X. Zhang. 2009. A single immunization with a recombinant canine adenovirus type 2 expressing the Seoul virus Gn glycoprotein confers protective immunity against Seoul virus in mice. Vaccine 27:5247-5251. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.