Abstract
Infection of quiescent cells by human cytomegalovirus (HCMV) elicits severe cell cycle deregulation, resulting in a G1/S arrest, which can be partly attributed to the inactivation of the anaphase-promoting complex (APC). As we previously reported, the premature phosphorylation of its coactivator Cdh1 and/or the dissociation of the core complex can account for the inactivation. We have expanded on these results and further delineated the key components required for disabling the APC during HCMV infection. The viral protein kinase UL97 was hypothesized to phosphorylate Cdh1, and consistent with this, phosphatase assays utilizing a virus with a UL97 deletion mutation (ΔUL97 virus) indicated that Cdh1 is hypophosphorylated at early times in the infection. Mass spectrometry analysis demonstrated that UL97 can phosphorylate Cdh1 in vitro, and the majority of the sites identified correlated with previously characterized cyclin-dependent kinase (Cdk) consensus sites. Analysis of the APC core complex during ΔUL97 virus infection showed APC dissociation occurring at the same time as during infection with wild-type virus, suggesting that the UL97-mediated phosphorylation of Cdh1 is not required for this to occur. Further investigation of the APC subunits showed a proteasome-dependent loss of the APC5 and APC4 subunits that was temporally associated with the disassembly of the APC. Immediate early viral gene expression was not sufficient for the degradation of APC4 and APC5, indicating that a viral early gene product(s), possibly in association with a de novo-synthesized cellular protein(s), is involved.
Human cytomegalovirus (HCMV), a highly prevalent β-herpesvirus, can cause serious birth defects and disease in immunocompromised individuals, and it may be associated with cancer and cardiovascular disease (53). Viral gene expression is temporally regulated and is dependent on many cellular factors for a productive infection. Immediate early (IE) genes are expressed by 2 h postinfection (p.i.) and transactivate the early genes required for viral DNA replication. The expression of the late genes, which encode proteins involved in virion maturation and egress, is dependent on viral DNA replication.
The virus has adopted different strategies for altering the cellular environment to make it more conducive to productive infection, including the stimulation of host cell DNA replication pathways, cell cycle deregulation and arrest, immune evasion, and inhibition of apoptosis (53). Although HCMV encodes its own DNA polymerase, it is dependent on other cellular resources for DNA replication. Infection of quiescent cells induces passage toward S phase such that the host cell is stimulated to generate proteins and DNA precursors necessary for genome replication; however, entry into S phase and cellular DNA replication are subsequently blocked and the cell arrests in G1/S (1, 10, 11, 14, 30, 45). Cellular resources are thereby presumably free to be efficiently utilized for viral replication. Cell cycle arrest by HCMV is achieved in part through the misregulation of several cell cycle proteins, including the phosphorylation and accumulation of the Rb family pocket proteins, upregulation of cyclins E and B and their associated kinase activities, inhibition of cyclin A expression, stabilization of p53, and accumulation of Cdc6 and geminin, which inhibits licensing of the cellular origins of DNA replication (8, 17, 30, 49, 54, 65). Some of these cell cycle defects can be attributed to a deregulation of the anaphase-promoting complex (APC) (8, 72, 79, 80), an E3 ubiquitin ligase that is responsible for the timely degradation of cell cycle proteins and mitotic cyclins to promote cycle progression from mitosis through G1 to S phase (58, 74). As the APC also appears to be a common target among other viruses, including the chicken anemia virus, adenoviruses, and poxviruses (23, 36, 52, 70), understanding the mechanisms leading to its inactivation during viral infection has been of great interest.
As we have previously reported, multiple mechanisms may be involved in disabling the APC during HCMV infection (72), which is not surprising given the complexity of its structure and regulation (for a review, see references 58 and 74). The APC is a large multisubunit complex consisting of at least 11 conserved core subunits, as well as other species-specific subunits. In metazoans, the APC2 and APC11 subunits form the catalytic core, and along with APC10, provide the platform for binding the E2 ubiquitin-conjugating enzyme. Each of the APC3, APC8, APC6, and APC7 subunits contain multiple copies of the tetratricopeptide repeat (TPR) motif and together make up the TPR subcomplex, which provides a platform of protein interaction surfaces for binding the coactivators (i.e., Cdh1 and Cdc20) and various substrates. These two subcomplexes are bridged by the large scaffolding subunit APC1, with the TPR subcomplex tethered to APC1 through APC4 and APC5. The binding between APC1, APC4, APC5, and APC8 is also interdependent, such that the loss of one subunit decreases the association of the other three (71).
The APC is activated by either of its coactivators, Cdh1 or Cdc20, which also function in recruiting specific substrates to the APC during different phases of the cell cycle. The phosphorylation of several APC subunits at the onset of mitosis, including APC1 and the TPR subunits, by cyclin B/cyclin-dependent kinase 1 (Cdk1) and Plk1 allows the binding of Cdc20 and subsequent activation of the APC (APCCdc20) (19, 37), whereas the binding and activation of the complex by Cdh1 is inhibited through its phosphorylation by cyclin B/Cdk1 (9, 29, 38, 83). As cells pass the spindle assembly checkpoint, APCCdc20 ubiquitinates securin (to allow for sister chromatid separation) and cyclin B for degradation by the proteasome (42, 67). The subsequent inactivation of Cdk1 and activation of mitotic phosphatases during late anaphase relieves the inhibitory phosphorylation on Cdh1, presumably by Cdc14 (6, 38, 44), which then allows Cdh1 to bind and activate the APC (APCCdh1). APCCdh1 ubiquitinates Cdc20 and mitotic cyclins for degradation to facilitate mitotic exit and maintains their low levels, along with S-phase regulators (e.g., Cdc6, geminin, etc.), during G1 (16, 50, 59, 63). The inactivation of APCCdh1 as cells enter S phase may be mediated in part through the phosphorylation of Cdh1 by cyclin A/Cdk2 (46) and Cdh1 binding to the inhibitor Emi1 (25). The inactivation of Cdh1 by phosphorylation has been shown in all organisms studied thus far (e.g., yeast, Drosophila, plants, mammals, etc.), and mutants mimicking constitutively phosphorylated Cdh1 on Cdk consensus sites can neither bind nor activate the APC in vivo or in vitro (9, 29, 38, 69, 83).
During HCMV infection of fibroblasts in G0/G1, however, Cdh1 becomes prematurely phosphorylated in a Cdk-independent manner and no longer associates with the APC (72). This dissociation does not appear to be due to an overexpression of Emi1 (79). Cdc20 also can no longer associate with the APC (79), suggesting a defect in the APC core. We have further shown that the APC core complex disassembles during the infection, with the TPR subunits (i.e., APC3, APC7, and APC8) and APC10 localizing to the cytosol, while APC1 remains nuclear (72). Interestingly, both the phosphorylation of Cdh1 and the dissociation of the APC occur at similar times during HCMV infection. Although either of these mechanisms could render the APC inactive, it was unclear whether these processes are linked or represent independent (or redundant) pathways. The causative factor(s) in mediating these events and the question of whether such a factor(s) was of cellular or viral origin also remained unresolved.
On the basis of the results of several recent studies (26, 32, 62), the viral protein kinase UL97 emerged as a likely candidate for involvement in the phosphorylation of Cdh1. Conserved among herpesviruses, UL97 functions in viral genome replication (7, 32, 81) and in nuclear egress of viral capsids (21, 39, 48). UL97 is present in the tegument of the virus particle (76) and is also expressed de novo with early kinetics (i.e., detectable by 5 h p.i. by Western blot assay), with increased expression at later times of the infection (51, 76, 77). UL97 is a serine/threonine (S/T) protein kinase (22), and recent studies have further characterized it as a Cdkl mimic, with predicted structural similarity to Cdk2 (64) and common substrates. UL97 has been shown to phosphorylate in vitro nuclear lamin A/C (21), the carboxyl-terminal domain of RNA polymerase II (5), the translation elongation factor 1δ (EF1δ) (33), and Rb (26, 62) on sites targeted by Cdks, and there is considerable evidence that UL97 phosphorylates lamin A/C, EF1δ, and Rb on these sites in infected cells as well (21, 26, 33, 62). Given that cyclin A/Cdk2 and cyclin B/Cdk1 complexes normally phosphorylate Cdh1, thus preventing its association with the APC, we hypothesized that UL97 phosphorylates Cdh1 during HCMV infection.
In the present study, we provide further mechanistic details of the events and players involved in inactivating the APC during HCMV infection. Evidence that UL97 is the viral factor mediating the phosphorylation of Cdh1 was obtained. However, APC disassembly still occurred at similar times in ΔUL97 and wild-type virus infections, indicating that UL97-mediated phosphorylation of Cdh1 is not required for this event. The inactivation of the APC core complex is further attributed to the loss of the APC5 and APC4 subunits early during the infection. The degradation of these subunits is proteasome dependent and requires de novo synthesis of viral early or cellular proteins. While the primary mechanism of inactivation appears to be the dissociation of the complex and the targeted loss of APC5 and APC4, phosphorylation of Cdh1 may provide a small kinetic advantage and backup mechanism for disabling the APC.
MATERIALS AND METHODS
Cells and virus.
Human foreskin fibroblasts (HFFs), obtained from the University of California, San Diego, Medical Center, were cultured in minimum essential medium with Earle's salts supplemented with 10% heat-inactivated fetal bovine serum, 1.5 μg/ml amphotericin B, 2 mM l-glutamine, 200 U/ml penicillin, and 200 μg/ml streptomycin. All cell culture media were from Gibco-BRL. Cells were kept in incubators maintained at 37°C and 7% CO2. The Towne and AD169 strains of HCMV were obtained from the American Type Culture Collection and propagated as previously described (68). The UL97 deletion virus RCΔ97.08 (ΔUL97 virus) was a generous gift from Mark Prichard (University of Alabama, Birmingham) and has been described previously (61).
Infections and drug treatments.
Experiments were done under G0 synchronization conditions unless otherwise noted. Cells were trypsinized 3 days after the monolayer reached confluence and either infected with virus at the indicated multiplicity of infection (MOI) or mock infected with tissue culture supernatants as described previously (65). The cells were then replated at a lower density to induce cell cycle progression. For the confluent culture infections, virus or mock tissue culture supernatants were added to the monolayer 3 days after it reached confluence. Infections with the ΔUL97 virus were done at an MOI of 3 with AD169 as the wild-type control. UV-inactivated virus was prepared by subjecting virus or mock tissue culture supernatants to UV irradiation via a Stratalinker UV cross-linker (Stratagene). Sodium pyruvate was added to a final concentration of 5 mM immediately after irradiation to scavenge free radicals prior to infection at the equivalent of an MOI of 2. The presence of tegument proteins and absence of viral immediate early or early gene expression were confirmed by Western blot analysis.
For proteasome inhibition assays, mock- or virus-infected HFFs (MOI of 2) were treated with dimethyl sulfoxide (DMSO; vehicle control), 2.5 μM MG132 (Calbiochem), or 100 nM salinosporamide A (Sal A; gift from Bradley Moore, Scripps Institute of Oceanography, University of California, San Diego) at the times indicated and harvested at the end of treatment. PYR-41 (10 μM; Calbiochem) was used to inhibit E1 activity at the times indicated below. Similarly, 20 μM actinomycin D (ActD; Calbiochem) or 100 μg/ml cycloheximide (CHX; Sigma) was added at the time of infection or as indicated below for viral gene expression assays. For the inhibitor release experiments, cell cultures were rinsed twice in phosphate-buffered saline (PBS) and then cultured in fresh medium.
Western blot analysis.
Cells were lysed in Laemmli reducing sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol) supplemented with a protease inhibitor cocktail (Roche) and phosphatase inhibitors (50 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mM sodium orthovanadate), sonicated, and boiled. Equal amounts of lysate (i.e., by cell number) were run on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels unless otherwise stated. Following electrophoresis, proteins were transferred to nitrocellulose (Schleicher & Schuell), and Western blot analyses were performed using appropriate antibodies. The SuperSignal West Pico chemiluminescent substrate (Pierce/Thermo Scientific) was used to visualize the proteins according to the manufacturer's instructions.
Phosphatase assay.
Cell lysates were prepared in buffer A (50 mM Tris-HCl, pH 7.5, 10 mM KCl, 1 mM MgCl2, 10% glycerol, 300 mM NaCl, 0.1% NP-40, protease inhibitor cocktail) or buffer B (buffer A plus the phosphatase inhibitors 50 mM sodium fluoride, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate) as previously described (72), with protein concentrations determined by Bradford assay (Bio-Rad). Lysates were incubated with 1× λ protein phosphatase (λPP) buffer (New England Biolabs), 2 mM MnCl2, and λPP (New England Biolabs) at 5 U/μg protein for 30 min at 30°C. The reactions were terminated with the addition of 2× Laemmli reducing sample buffer. Samples were then boiled and analyzed by Western blot assay.
Immunoprecipitation.
Cells were harvested and lysed directly in extraction buffer (20 mM Tris-HCl, pH 8, 150 mM NaCl, 5 mM MgCl2, 0.2% NP-40, 10% glycerol supplemented with 1× protease inhibitor cocktail, 50 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mM ATP, and 1 mM dithiothreitol [DTT]) using an end-over-end rotator at 4°C. Lysates were clarified by centrifugation at 16,000 × g. For APC3 coimmunoprecipitation (co-IP) assays, lysates were incubated with protein G Plus-agarose beads (Santa Cruz Biotechnology) coupled with an anti-APC3 monoclonal antibody (AF3.1; Santa Cruz Biotechnology) or with beads coupled with mouse IgG (Jackson ImmunoResearch) as a negative control. Beads were washed with TBS-T, pH 8 (Tris-buffered saline with 0.01% Tween 20 supplemented with a protease inhibitor cocktail, phosphatase inhibitors, and 1 mM ATP), and proteins were eluted in Laemmli reducing sample buffer by boiling for 5 min. All IP steps were performed at 4°C. Pre- and post-IP and post-IgG samples were also collected and boiled in reducing sample buffer. Samples were analyzed by Western blot assay, with same-cell-number equivalents being loaded for the pre- and post-IP lanes, while the IP and IgG lanes were loaded with 5 times more.
Kinase assays and mass spectrometry (MS) analyses.
Human Cdh1 cDNA was cloned into the glutathione S-transferase (GST) fusion vector pGEX-6P1 (gift from Karen Oegema, University of California, San Diego) using the EcoRI and NotI sites. This positioned the PreScission protease site in between GST and Cdh1. Protein expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) in Escherichia coli BL21(DE3) cells, and GST-Cdh1 was affinity purified from cell lysates using glutathione-Sepharose 4B beads (GE Healthcare). The GST-Cdh1-bound beads were washed in TBSE (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA) supplemented with 1 M NaCl, 5 mM DTT, 10 mM ATP, and 0.2% Triton X-100, followed by washes in TBSE containing 2 mM DTT and, subsequently, by TBSE. The washed beads were then incubated with PreScission protease (GE Healthcare) in TBSE at 4°C overnight to cleave off Cdh1 from GST. The released Cdh1 was eluted and dialyzed into HEPES buffer (20 mM HEPES, pH 8, 100 mM NaCl, 0.5 mM EDTA, 2 mM DTT). Aliquots were stored at −80°C until use.
The in vitro kinase assays were performed as previously described (21, 26) except with 1 μg Cdh1, 20 ng GST-UL97, and 50 μM ATP supplemented with 2.5 μCi of [γ-32P]ATP (Easytides, 6,000 Ci/mmol; Perkin Elmer) in kinase reaction buffer (50 mM Tris-HCl, pH 8.5, 10 mM MgCl2, 2 mM DTT, 5 mM β-glycerophosphate). As a control, maribavir (1 μM in DMSO) or 1% (vol/vol) DMSO (carrier control) was added to inhibit UL97 phosphorylation activity. The reactions were initiated with the addition of ATP, incubated at 37° for 30 min, and terminated in 4× SDS-PAGE sample buffer with heating at 85°C for 10 min. Samples were resolved by SDS-PAGE, stained with colloidal Coomassie blue G-250, and dried onto filter paper. 32P incorporation was imaged on a phosphor screen and a Bio-Rad 2-dimensional scanner.
For analysis of phosphorylation sites by MS, kinase reactions were performed with 1 μg Cdh1 alone or together with 250 ng GST-UL97 in kinase reaction buffer with 300 μM unlabeled ATP and incubation at 37°C for 75 min. The reactions were stopped by the addition of 4× SDS-PAGE sample buffer, and the mixtures heated at 85°C for 5 min. Samples were resolved by SDS-PAGE and stained with colloidal Coomassie blue G-250, and bands corresponding to Cdh1 were excised with a clean razor blade and submitted to the Taplin Biological Mass Spectrometry Facility (Harvard Medical School) for analysis. Briefly, gel pieces were reduced with DTT, alkylated with iodoacetamide, and subjected to a modified in-gel trypsin digestion procedure (66). Peptides were extracted, washed in a solution containing 50% acetonitrile and 1% formic acid, and dried in a speed-vac. Samples were reconstituted in HPLC solvent and subjected to liquid chromatography-tandem MS (LC-MS-MS) analysis. Peptide sequences were analyzed by Sequest (15) with the included modification of 79.9663 mass units to serine, threonine, and tyrosine to identify phosphopeptides. The results were manually inspected to ensure confidence.
Quantitative RT-PCR.
Total RNA from mock- or HCMV-infected cells was isolated using a NucleoSpin RNA II kit (Machery-Nagel). The eluates were subjected to a second DNase treatment using Turbo DNA-free DNase (Ambion) to ensure complete DNA removal. The RNA concentrations were determined by UV spectrophotometry. Quantitative reverse transcriptase PCR (qRT-PCR) was performed with an ABI Prism 7000 sequence detection system (Applied Biosystems) using a TaqMan one-step RT-PCR master mix reagents kit (Applied Biosystems) with 50 ng RNA, oligonucleotide primers and TaqMan dually labeled (5′,6-carboxyfluorescein and 3′ black hole quencher-1) probes (Integrated DNA Technologies). Standard curves were generated using dilutions of RNA isolated from uninfected cells harvested at 24 h p.i. Values were normalized to the expression of glucose-6-phosphate dehydrogenase (G6PD) as a control for input RNA. The primers and probes used were as follows: geminin forward (5′-GCCTTCTGCATCTGGATCTCTT-3′), geminin reverse (5′-CGATGTTTCCTTTTGGACAAGC-3′), and geminin probe (5′-TGGAAGAGAAAATGAGCTGTCCGCAG-3′); APC4 forward (5′-ATTCTCGTCCTTGGAGGAAGCTCT-3′), APC4 reverse (5′-TTCTGGCCATCCGAGTTACTTCAG-3′), and APC4 probe (5′-AATTGCTCGAGTCACAGGGATTGCTGGT-3′); APC5 forward (5′-GTGCCATGTTCTTAGTGGCCAAGT-3′), APC5 reverse (5′-GATGCGCTCTTTGCAGTCAACCTT-3′), and APC5 probe (5′-AAGAAAGCAGAAGCTCTGGAGGCTGCCA-3′); and G6PD forward (5′-TCTACCGCATCGACCACTACC-3′), G6PD reverse (5′-GCGATGTTGTCCCGGTTC-3′), and G6PD probe (5′-ATGGTGCTGAGATTTGCCAACAGGA-3′).
Antibodies.
Antibodies to HCMV proteins IE1 72/IE2 86 (CH16.0), UL57, UL44, UL83, and UL99 were from Virusys. Other antibodies used were as follows: Cdh1 (Ab-2; Calbiochem); geminin (Santa Cruz Biotechnology); Cdc6 (Ab-1 and DCS-180) and cyclin B1 from LabVision/Thermo Scientific, securin (Zymed; Invitrogen); APC3 (AF3.1; Santa Cruz Biotechnology); APC4 (A301-176A), APC5 (A301-026A), and APC6 (A301-165A) from Bethyl Laboratories; APC7 (poly6113) and APC8 (poly6114) from Biolegend; APC1 (gift from Jose Teodoro, McGill University); actin (AC-15; Sigma); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (6c5; Fitzgerald).
RESULTS
UL97 and phosphorylation of Cdh1 during HCMV infection.
We previously showed that Cdh1 becomes phosphorylated during HCMV infection beginning 8 to 12 h p.i. and that this phosphorylation still occurs in the presence of the Cdk inhibitor roscovitine (72). Based on recent studies characterizing the HCMV early protein kinase UL97 as a Cdk mimic that is involved in the hyperphosphorylation of Rb during the infection (26, 62), it emerged as the leading viral candidate involved in the phosphorylation of Cdh1.
The UL97 deletion virus RCΔ97.08 (ΔUL97 virus) was utilized in testing this hypothesis. The ΔUL97 virus has over 70% of the UL97 open reading frame deleted (including subdomains homologous to those of other protein kinases) and replaced with the selectable genetic markers gpt and lacZ (61). Previous reports have indicated a replication impairment of the virus in primary fibroblasts, with 10- to 1,000-fold lower titers (26, 31, 32, 39, 61). This may be attributed in part to reduced viral DNA synthesis, abnormal aggregation and intracellular localization of virion proteins, and/or impaired nuclear egress, although the extent of each defect appears to depend on the culture conditions (3, 32, 39, 60, 62, 81). To assess viral replication and determine whether cell cycle defects still occur with the ΔUL97 virus in our system, the levels of expression of viral genes and cell cycle regulators were monitored by Western blot assay and qRT-PCR and compared to their levels in the wild-type parental virus AD169 during an infection time course. To help mitigate induced protein expression due to normal cell cycle progression, infections were done in G0-synchronized cells maintained at confluence through the course of the infection. As shown by the results in Fig. 1 A, no difference between IE protein expression levels in the two viruses was observed, while the levels of the early proteins UL44 and UL57 were similar or perhaps slightly lower in the ΔUL97 virus-infected samples than in the wild type-infected samples at 48 h p.i., as observed in previous studies (32, 39, 40). The cell cycle proteins geminin, Cdh1, cyclin B, Cdc6, and securin are APC substrates that are normally targeted for degradation in G1, and in general, these proteins remained at low or undetectable levels through the time course in the mock-infected cells, as expected. Increased protein expression of the different APC substrates was observed in the ΔUL97-infected cells compared to the levels in mock-infected cells, albeit with a slight delay compared to the time of expression in AD169 virus. The RNA levels of the APC substrates tested remained comparable between samples from mock- and virus-infected cells, suggesting a stabilization of the protein. The results for geminin are shown as a representative example in Fig. 1B. Similar results were also obtained with infections done with G0-synchronized cells that were replated at a lower density to induce cell cycle progression (data not shown). These data suggested that the accumulation of the APC substrates still occurred in cells infected with the ΔUL97 virus.
FIG. 1.
APC substrates accumulate during ΔUL97 virus infection. HFFs were mock-infected (M) or virus-infected with ΔUL97 virus (Δ) or AD169 (V) at an MOI of 3. (A) Cells were harvested over an infection time course and analyzed by Western blot assay for the expression of HCMV genes and cell cycle regulators. Actin is shown as a loading control. (B) Total RNA isolated from cells harvested over an infection time course was analyzed for geminin and G6PD expression by qRT-PCR. Geminin values were normalized to the expression of G6PD as a control for input RNA. Values are expressed as the fold induction over the level at 0 h p.i. (C) Cell samples were harvested at 16 h p.i., treated with or without (−) lambda protein phosphatase (λpp), and analyzed by Western blot assay. Equivalent cell numbers were loaded for each sample, except for lane 7 (†), which contained one-fifth the amount. Results for short and long exposures of the Cdh1 blot are shown. IE2 86 was a positive control for the phosphatase assay, while actin served as a negative and loading control. Lane numbers are shown below.
Although the increase in Cdh1 expression was not as great in the ΔUL97 virus infection as in the wild-type infection, it is interesting to note that the migration pattern remained comparable to that observed in the mock-infected samples through the time course, while Cdh1 in the AD169-infected cells migrated slightly more slowly (Fig. 1A). In our previous study, we showed that the decrease in the mobility of Cdh1 in cells infected with the wild-type Towne strain of HCMV was due to phosphorylation (72). To determine whether phosphorylation accounted for the change in the mobility of Cdh1 in the wild-type AD169-infected cells, phosphatase assays were performed using lysates from HFFs infected with ΔUL97, AD169, or mock infection supernatants that were harvested at 16 h p.i. and treated with lambda protein phosphatase (λPP) (Fig. 1C). The phosphatase treatment shifted the migration pattern of Cdh1 from the AD169-infected cells (Fig. 1C, lane 5) to a faster-migrating form (lane 6) comparable to that seen in the uninfected cells (lanes 1 and 2). A longer exposure of the Cdh1 blot is shown to better exemplify the migration shift in the untreated AD169 sample with one-fifth of the sample loaded (lane 7). No change in the migration of the Cdh1 bands was observed after phosphatase treatment in the ΔUL97 virus infection samples (lanes 3 and 4), suggesting that Cdh1 is hypophosphorylated. IE2 86 is further shown as a positive control for the phosphatase assay, while IE1 72 and actin are negative and loading controls.
In vitro kinase assays were done to investigate whether Cdh1 can be a direct substrate of UL97. GST-Cdh1 was expressed in E. coli cells and affinity purified from bacterial lysates using glutathione-Sepharose 4B beads, which were subsequently treated with PreScission protease to release untagged Cdh1. Purified Cdh1 and highly purified GST-UL97 (21, 26) were incubated together with 32P-labeled ATP. As shown in Fig. 2 A, 32P incorporation was readily observed in samples containing both Cdh1 and GST-UL97 but not when either protein was incubated alone with labeled ATP. However, the 32P signal was markedly reduced upon the addition of maribavir, a highly specific inhibitor of UL97 kinase activity (7), indicating that UL97 can directly phosphorylate Cdh1.
FIG. 2.
UL97 phosphorylates Cdh1 at multiple sites in vitro. (A) In vitro kinase assays using purified Cdh1 and GST-UL97 were performed with [γ-32P]ATP in the presence or absence of maribavir (MBV). 32P incorporation was imaged on a phosphor screen, and the Coomassie-stained gel of the samples is shown below as a loading control. (B) The phosphopeptides identified by MS are listed with the phosphorylated amino acid shown by boldface with a lowercase p, and the corresponding position numbers are indicated. Cdk consensus sites and those conforming to the UL97 preferential sequence (P+5) are indicated.
Samples from reaction mixtures in which Cdh1 was phosphorylated by GST-UL97 in the presence of unlabeled ATP were analyzed by mass spectrometry for the sites of phosphorylation to determine whether UL97 also utilizes the same sites on Cdh1 as Cdks. Interestingly, all of the previously characterized conserved Cdk consensus sites (i.e., S/T-P-X-K/R) on Cdh1 from mammalian cells (38) were positively identified, with the exception of S146 (Fig. 2B). Phosphorylation of these sites has been shown to inhibit the binding of Cdh1 to the APC both in vitro and in vivo (38). Eight additional phosphorylation sites were detected, of which S58, S131, S172, and S286 conform to the P+5 site (i.e., S-X-X-X-X-K/R) preference previously described for UL97 (4). The majority of the sites identified fall within the N-terminal region comprised of amino acids 1 to 173 that contains all of the previously identified Cdk consensus sites, while S286, T300, and S374 are within the C-terminal WD40 repeats. It is possible that additional phosphorylation sites were exposed due to differences in Cdh1 expressed and phosphorylated in vitro and in vivo. The results with the UL97-null mutant taken together with these in vitro results are consistent with UL97 mediating at least some of the phosphorylation of Cdh1 in infected cells.
APC dissociation during HCMV infection does not require UL97-mediated phosphorylation of Cdh1.
The accumulation of the APC substrates in the ΔUL97 virus-infected cells suggested that inactivation of the APC was still occurring. Since virus-mediated phosphorylation of Cdh1 was not observed during ΔUL97 virus infection, we next determined whether Cdh1 was still capable of binding to the APC and whether other APC subunits remained associated. APC3 co-IP assays using an antibody to the APC3 subunit were performed with lysates from mock-, ΔUL97 virus-, or AD169-infected HFFs at 8, 16, and 24 h p.i. It should be noted that proteins in the IP lanes appeared to migrate more slowly, a phenomenon we have often observed. As shown in Fig. 3, the APC subunits APC7, APC8, and APC1, as well as Cdh1, remained in complex with APC3 in the mock- and virus-infected samples at 8 h p.i. (lanes 2, 5, and 8). By 16 h p.i., however, the association of Cdh1 and APC1 with APC3 was severely diminished in the ΔUL97 virus infection sample (lane 14), as in the wild-type virus infection (lane 17), and their association was no longer detected at 24 h p.i. (lanes 23 and 26). These results suggest that the dissociation of the APC occurs independently from the phosphorylation of Cdh1. Since APC3, APC7, and APC2 can bind Cdh1, all three subunits may be necessary to provide proper conformation and support for the association. Disparate localization and/or modification of the APC subunits themselves may also account for the decreased binding of Cdh1 in the ΔUL97 virus-infected cells.
FIG. 3.
The APC dissociates with similar kinetics during ΔUL97 virus infection and wild-type virus infection. HFFs infected with ΔUL97 virus (Δ) or AD169 (V) at an MOI of 3 or mock-infected (M) were harvested at the times indicated. The APC was immunoprecipitated using an anti-APC3 antibody, and coimmunoprecipitated proteins were analyzed by Western blot assay with antibodies to APC3, APC7, APC8, APC1, and Cdh1. GAPDH is shown as a negative and loading control. Lane numbers are shown below. Pre, input lysate; IP, APC3 IP; PIP, post-IP.
Destabilization of APC4 and APC5 protein expression during HCMV infection.
To further delineate the mechanism governing the dissociation of the APC, we reexamined the structure of the core complex. As shown above and previously reported (72), separation of the TPR subcomplex (i.e., APC3, APC7, and APC8) from APC1 was observed in cells infected with either wild-type or ΔUL97 virus. Previous structural studies of the APC have suggested that the TPR subcomplex (via APC8) is associated to APC1 through APC4 and APC5 (71). Moreover, the association between APC1, APC4, APC5, and APC8 is interconnected such that each subunit is necessary for the others to bind effectively (71). Thus, APC4 and APC5 might also be targets in the disrupted association between the TPR subunits and APC1 during the infection.
Although all APC subunits previously tested exhibited stable protein expression through the infection time course (72, 79), the expression of APC4, APC5, and APC6 had not been confirmed due to the lack of specific antibodies. We have now examined APC4, APC5, and APC6 expression during HCMV infection. Surprisingly, the levels of both APC4 and APC5 were substantially reduced in the HCMV Towne-infected samples by 12 h p.i. and remained at or below the limits of detection through the end of the time course, while the levels were unchanged in the mock-infected cells (Fig. 4 A). In contrast, the APC6 and APC3 levels remained relatively similar in the comparison between the mock- and virus-infected samples (Fig. 4A). APC4 and APC5 subunit expression was further examined in a more detailed time course analysis using the ΔUL97 and AD169 viruses. As shown by the results in Fig. 4B, decreased abundance of APC4 and APC5 was also observed (beginning at 6 to 8 h p.i.) in both virus infections, similar to the Towne infection.
FIG. 4.
Decreased APC4 and APC5 protein expression is observed during HCMV infection. (A) Levels of APC4, APC5, APC6, and APC3 protein expression were assessed by Western blot assay through an HCMV Towne (V) infection time course. GAPDH is shown as a loading control. (B) APC subunit expression was further examined in cells infected with ΔUL97 virus (Δ) and AD169 (V) by Western blot. Actin is shown as a loading control. (C) Total RNA was isolated from mock- or HCMV Towne-infected cells and analyzed for APC4, APC5, and G6PD expression by qRT-PCR. APC4 and APC5 values were normalized to the expression of G6PD as a control for input RNA. Values are expressed as the fold induction over the level at 0 h p.i.
To differentiate between a defect at the protein or RNA level, total RNA from mock- or Towne-infected cells was isolated over an infection time course and analyzed by qRT-PCR with primers and probes to APC4 and APC5. Values were normalized to those of G6PD, which served as an internal control for RNA input, and expressed as the fold induction from 0 h p.i. As shown in Fig. 4C, no meaningful differences (less than 2-fold) in either APC5 or APC4 transcript levels in mock- and virus-infected samples were observed through the time course. In fact, if anything, the levels were slightly higher in virus-infected cells at 16 h p.i. These data indicate that the decrease in APC4 and APC5 is likely due to a destabilization of the proteins or a translational defect.
Proteasome inhibitors were used to address whether APC4 and APC5 are targeted for degradation during the infection. HFFs infected with Towne virus at an MOI of 2 or mock infected were treated with either the reversible proteasome inhibitor MG132 (2.5 μM) or the irreversible inhibitor Sal A (100 nM) from 6 to 12 h p.i. Cells were harvested at the end of treatment (12 h p.i.), or the drug was washed out and the cells cultured in fresh medium for an additional 12 h before being harvested at 24 h p.i. The results in Fig. 5 A (lanes 3 to 8) show that the addition of either proteasome inhibitor from 6 to 12 h p.i. prevented the loss of APC4 and APC5 protein expression. Decreased expression of both proteins was observed upon washout of the MG132-treated cells between 12 and 24 h p.i. (lanes 11 and 12), while the expression levels remained stable in the Sal A-treated cells (lanes 13 and 14), strongly suggesting the loss of expression to be proteasome dependent. APC8 expression remained unchanged with the various treatments, indicating that this targeted degradation is specific to the APC4 and APC5 subunits. p53 expression was monitored as a control for proteasome inhibition and drug washout. Increased levels of p53 were seen in the mock-infected cells during inhibitor treatment (lanes 5 and 7 compared to lane 3), and there was a return to basal levels after drug washout in the MG132-treated cells (lane 11), while elevated levels remained in the Sal A-treated cells (lane 13). As we recently reported (73), treatment with the proteasome inhibitors also had no effect on IE gene expression but did result in lower levels of the early gene product UL44.
FIG. 5.
APC4 and APC5 are degraded by the ubiquitin-proteasome pathway during HCMV infection. (A) HFFs infected with HCMV Towne (V) (MOI of 2) or mock-infected (M) were treated with proteasome inhibitor MG132 (2.5 μM, reversible) or Sal A (100 nM, irreversible) at 6 h p.i. Cells were harvested at 12 h p.i. or placed in fresh medium without drug and harvested at 24 h p.i. Samples were processed by Western blotting for protein expression. Lane numbers are shown below. (B) Mock-infected (M) or Towne-infected (V) cells were treated with Sal A (100 nM) or the E1 inhibitor PYR-41 (10 μM) at 6 h p.i. and harvested at 18 h p.i., and protein expression analyzed by Western blot assay.
To determine whether proteasome degradation of the subunits is ubiquitin dependent, mock- or Towne-infected HFFs were treated with the E1 (i.e., ubiquitin-activating enzyme) inhibitor PYR-41 or Sal A from 6 to 18 h p.i. and harvested at 18 h p.i. As shown by the results in Fig. 5B, PYR-41 was also effective in preventing the loss of both APC4 and APC5 compared to the results for the untreated samples. These results further support that APC4 and APC5 are degraded through the ubiquitin-proteasome pathway.
APC dissociation during HCMV infection is blocked with proteasome inhibitors.
If the loss of APC4 and APC5 is associated with the disassembly of the APC during the infection, then preventing the degradation of the subunits may maintain the integrity of the complex. APC3 co-IP studies were done with lysates from mock- or Towne-infected HFFs that were treated with or without Sal A from 6 to 14 h p.i. Cells were harvested at 14 h p.i., and coimmunoprecipitated proteins were analyzed by Western blot assay. As shown by the results in Fig. 6, APC3 and APC8 were pulled down equally in all the APC3 IP samples but not with the immunoglobulin (IgG) negative control (lane 2). The levels of coimmunoprecipitated APC4, APC5, APC1, and Cdh1 remained comparable in the uninfected samples with or without inhibitor treatment (lanes 4 and 7). In contrast, treatment of the infected cells with Sal A appeared to have inhibited the dissociation of the complex, as the levels of APC4, APC5, APC1, and Cdh1 that coimmunoprecipitated with APC3 were greater than with no drug treatment (lane 13 compared to lane 10). GAPDH is further shown as a negative control. Together, these results support that the disassembly of the APC and loss of APC4 and APC5 are proteasome dependent.
FIG. 6.
APC dissociation is prevented with proteasome inhibitors. Mock-infected (M) and Towne-infected (V) HFFs (MOI of 2) were treated with or without Sal A (100 nM) from 6 to 14 h p.i. and harvested at 14 h p.i. APC3 co-IP assays were performed as previously described with coimmunoprecipitated proteins analyzed by Western blot assay. GAPDH is shown as a negative and loading control. Lane numbers are shown below. Pre, input lysate; IgG, IgG IP; PIgG, post-IgG; IP, APC3 IP; PIP, post-IP.
Viral early gene expression is necessary to mediate the degradation of APC4 and APC5.
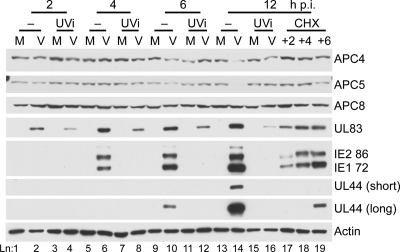
Given the time at which APC4 and APC5 protein are lost during the infection, the potential viral protein(s) involved are likely expressed at IE or early times of the infection or brought in with the viral tegument. To establish whether input viral-tegument proteins alone are sufficient, HFFs were infected with Towne or UV-inactivated (UVi) virus and harvested over an infection time course with protein expression assayed by Western blotting. As shown by the results in Fig. 7, both APC4 and APC5 expression remained stable in the UVi virus infection through 12 h p.i., whereas a marked decrease in APC5 and, to a lesser extent APC4, was observed in the Towne-infected cells at 12 h p.i. (lane 14). APC8 expression was also assayed as a negative control, and no change was observed. As a control for viral entry, samples were probed for the tegument protein UL83. Although there were lower levels of UL83 protein expression in the UVi virus-infected cells at all time points, APC4 and APC5 expression was not greatly affected in the Towne-infected cells at 2 or 4 h p.i. (lanes 2 and 6). A slight decrease in APC4 and APC5 levels was detected at 6 h p.i. (lane 10), but a marked difference was only observed at 12 h p.i. in the Towne-infected cells (lane 14), when both IE and early (UL44) proteins were more highly expressed, suggesting that viral input tegument proteins themselves were insufficient to induce the expression loss. The decrease in the amount of APC4 and APC5 appeared to be proportional to the IE and early protein expression.
FIG. 7.
Viral tegument and IE protein expression alone are insufficient to cause the loss of APC4 and APC5 protein expression. HFFs were treated with mock-infected (M), virus-infected (V), or UV-inactivated (UVi) tissue culture supernatants and harvested at 2, 4, 6, and 12 h p.i. To assess the requirement for IE expression, HFFs infected with HCMV Towne (MOI of 2) were treated with 100 μg/ml CHX from 2 to 12 h p.i. (+2), 4 to 12 h p.i. (+4), or 6 to 12 h p.i. (+6) to inhibit synthesis of early proteins. Samples were analyzed by Western blot assay. Short and long exposures of the UL44 blot are shown, and lane numbers are indicated below.
CHX was also used to inhibit protein synthesis, so that the requirement for IE expression could be examined. CHX was added to Towne-infected HFFs at 2, 4, or 6 h p.i. to allow for increasing IE expression, and cells were harvested at 12 h p.i. Compared to the expression in mock-infected cells at 12 h p.i. (Fig. 7, lane 15), APC subunit expression was not significantly altered with the addition of CHX at 2 h p.i. (lane 17) or 4 h p.i. (lane 18), whereas a modest decrease in APC5 was observed with the treatment at 6 to 12 h p.i. (lane 19), which also allowed for low levels of UL44 expression. The amount of UL83 present in the virus-infected cells treated with CHX beginning at 2 h p.i. (lane 17) was more comparable to that seen in the UVi virus-infected cells, suggesting that the increased UL83 levels observed at 6 and 12 h p.i. in the virus-infected cells (lanes 6 and 10) may be due to some newly synthesized protein rather than input protein. Collectively, these results indicate that de novo expression of viral gene products or their effect(s) on cellular gene expression may be required for the degradation of APC4 and APC5.
A combination of CHX and ActD treatments were then used to further define the involvement of viral early gene expression in mediating the loss of APC4 and APC5 (Fig. 8). Untreated mock- or Towne-infected HFFs were harvested at 6, 18, and 24 h p.i. (Fig. 8, lanes 1 to 6). No significant differences in APC4 and APC5 expression were seen in the uninfected samples, whereas decreased expression was observed in the infected samples after 6 h p.i. (lanes 4 and 6), as expected. These results were used as controls to evaluate the effectiveness of the inhibitors in preventing the loss of APC4 and APC5. First, cells were treated with CHX at the beginning of the infection from 0 to 6 h p.i. and then harvested (lane 7) or washed and released into fresh medium and harvested at 18 and 24 h p.i. (lanes 8 and 9). APC4 or APC5 expression at 6 h p.i. in the virus-infected sample treated with CHX (lane 7) remained comparable to the expression in the untreated sample (lane 2). After the inhibitor was removed and viral gene expression was allowed to recover in the absence of drug, a decrease in both APC4 and APC5 expression was observed at 18 and 24 h p.i. (lanes 8 and 9), albeit not to the extent of the decrease in the untreated cells (lanes 4 and 6), probably due to the delay in viral gene expression. These results provide further support for the idea that the input tegument and IE proteins themselves are not sufficient for the degradation of APC4 and APC5. Alternatively, the CHX-treated cells (from 0 to 6 h p.i.) were released from CHX at 6 h p.i., placed in medium containing ActD to prevent early gene transcription, and harvested at 18 and 24 h p.i. (lanes 10 and 11). No additional decreases in APC4 and APC5 protein levels were seen with the ActD treatment, as the levels remained comparable to those at 6 h p.i. (lane 7) and even to those in the uninfected cells at 18 and 24 h p.i. (lanes 3 and 5). Accordingly, IE expression was comparable to that in the untreated samples but UL44 expression was not detected (lanes 10 and 11 compared to lanes 8 and 9). To further assess the contribution of viral genes expressed by 6 h p.i., infected cells were treated with ActD at 6 h p.i. and harvested at 18 and 24 h p.i. (lanes 12 and 13). This allowed for continued translation of viral transcripts already present by 6 h p.i. A similar decrease in APC4 and APC5 levels (lanes 12 and 13) compared to the levels in the CHX-treated cells that were released in the absence of ActD (lanes 8 and 9) was observed, but the loss of APC4 and APC5 was still less than that seen in the untreated viral samples (lanes 4 and 6). Taken together, these results suggest that the transcription of the viral (or cellular) gene(s) likely involved in the degradation of APC4 and APC5 begins by 6 h p.i. and requires the expression of viral immediate early proteins.
FIG. 8.
Viral early gene expression is required to mediate the degradation of APC4 and APC5. Mock-infected (M) or Towne-infected (V) HFFs (MOI of 2) were treated with CHX (100 μg/ml) from 0 to 6 h p.i. and harvested at 6 h p.i. or washed and released into fresh medium or ActD (20 μM) and harvested at 18 and 24 h p.i. ActD (20 μM) was added at 6 h p.i., and cells were harvested at 18 and 24 h p.i. Lysates were analyzed by Western blot assay for APC4, APC5, IE, and UL44 expression. Actin is shown as a loading control. Lane numbers are shown below.
DISCUSSION
We have further delineated the mechanisms by which the APC is disabled during HCMV infection and determined that the phosphorylation of Cdh1 and the dissociation of the APC core complex are independent events despite occurring at similar times postinfection. These results are consistent with UL97-mediated phosphorylation of Cdh1 early during the infection. Based on the results of the phosphatase assays and gel migration analysis, it appears that Cdh1 becomes phosphorylated beginning at 8 to 10 h p.i. during wild-type HCMV infection but is seemingly unphosphorylated through at least 24 h p.i. in ΔUL97 virus-infected cells. We cannot exclude the possibility, however, that there is a low level of phosphorylation on Cdh1 that is below the limit of detection in these experiments. The results from in vitro kinase assays and MS analysis are consistent with Cdh1 being a direct substrate of UL97. Positive identification of eight of the nine Cdk consensus sites was not surprising given the functional similarity and common substrates shared between UL97 and Cdks (5, 21, 26, 33, 62). Interestingly, the previously characterized Cdk consensus sites, as well as the majority of the non-Cdk sites identified in this study, reside within the N-terminal 180 amino acids of Cdh1, which appears to be the major regulatory domain of the protein as it also contains the nuclear localization signal (amino acids 151 to 178) (84), as well as two putative RXXL D-boxes (RRLLRQIVI and RRTLTPASS) that are required for mediating the autoubiquitination and degradation of Cdh1 by APCCdh1 during G1 (43). Other phosphorylation sites have also been identified by MS in yeast (20) and mammalian cells (13, 18, 28), but it is unclear if these phosphorylation sites also affect Cdh1 function.
Since in mammalian cells, phosphorylation of Cdh1 inhibits its binding and the activation of the APC (9, 29, 38, 83), UL97-mediated phosphorylation of Cdh1 could potentially have a similar effect, and this may account for the small delay in the accumulation of APC substrates in cells infected with the ΔUL97 mutant relative to the time of accumulation in cells infected with wild-type virus. The question of whether the UL97-mediated phosphorylation of Cdh1 is sufficient to block its association and the activation of the APC still needs to be addressed. Although there may be discrepancies in the number of sites phosphorylated on Cdh1 with expression in vitro or in vivo, the numerous potential UL97 phosphorylation sites strongly suggest Cdh1 to be sufficiently modified to interfere with binding to the APC. Nevertheless, Cdh1 still dissociated from APC3 during ΔUL97 virus infection. This result suggested that either the UL97-mediated phosphorylation of Cdh1 is insufficient to inhibit binding to the APC or the modification of the APC itself no longer facilitated binding to Cdh1. The latter possibility is supported by the data showing that the dissociation of APC1 occurred in both the ΔUL97 and wild-type virus infections. Cdh1 has been shown to bind to APC3, APC7, and APC2 (78), all of which may be necessary to facilitate the stable association of Cdh1. Thus, the dissociation of the APC core complex and subsequent relocation of the TPR subunits to the cytosol would account for the subsequent dissociation of Cdh1 in ΔUL97 virus-infected cells. Phosphorylation of Cdh1 by UL97 may, however, provide a small kinetic advantage in disabling the APC earlier during the infection. The results from this study (Fig. 3) and our previous work (72) indicate that complete dissociation of Cdh1 occurs prior to that of APC1 from APC3. It is also possible that the phosphorylation of Cdh1 serves another purpose in facilitating viral replication other than mediating the inactivation of the APC.
We have further shown that the disassembly of the APC is associated with the proteasome-mediated degradation of APC4 and APC5 beginning at 6 to 8 h p.i. This was an interesting result given that all other subunits previously examined showed comparable, if not elevated, expression levels compared to their levels in mock-infected cells (72, 79). Moreover, this degradation appears to be ubiquitin mediated, as treatment with the E1 (ubiquitin-activating enzyme) inhibitor also prevented the loss of APC4 and APC5. This suggests that a cellular ubiquitin ligase may be involved in ubiquitinating the subunits to target their degradation by the proteasome, as there are no known HCMV-encoded ubiquitin ligases. A viral early protein may be necessary to mediate this interaction.
The addition of proteasome inhibitors prevented further loss of the subunits, as well as APC dissociation. The effect of the inhibitors could be 2-fold. Proteasome inhibition could prevent the degradation of the subunits directly or result in decreased expression of the viral early proteins (or cellular proteins) that may be involved in targeting the APC subunits. It is unclear whether APC5 and APC4 are individually targeted or if the loss of APC4 is the result of losing APC5 and vice versa. The binding of APC8, APC4, APC5, and APC1 is interdependent, such that the loss of any one subunit greatly decreases the binding of the other three (71). While the loss of either APC5 or APC4 alone may not sufficiently dissociate the complex, the concerted loss of both subunits seems to ensure the complete detachment of the APC3/TPR subcomplex from APC1 and, thereby, free the catalytic core containing APC2 and APC11 as well. This mechanism would account for the observed dissociation of the TPR subcomplex from APC1 during the infection. However, we cannot completely discount the possibility that partial dissociation of the complex may precede and/or be necessary for the targeted degradation of APC4 and APC5.
A model of HCMV-mediated inactivation of the APC is proposed in Fig. 9. Based on the experimental conditions of these studies and our previously published data (72), the critical time window for the virus-mediated inactivation of the APC appears to be 6 to 8 h p.i. At this time, there is sufficient viral gene expression to mediate the proteasome-dependent degradation of APC5 and APC4, dissociation of the complex, and accumulation of substrates. Meanwhile, Cdh1 is also phosphorylated and dissociates from the complex. While the phosphorylation of Cdh1 does not seem to be required for disabling the APC in fibroblasts, it may be important in other cell types infected by HCMV.
FIG. 9.
Inactivation of the APC during HCMV infection. (A) Schematic diagram of the APCCdh1. The essential mammalian APC core subunits are shown and numbered accordingly. (B) Model illustrating the mechanisms by which the APC is disabled during HCMV infection. (I) APC5 and APC4 are targeted for degradation by the proteasome and the APC dissociates, with the TPR subunits and APC10 localizing to the cytosol while APC1 remains nuclear. (II) Cdh1 is phosphorylated, mediated by UL97, and no longer associates with the complex.
Whether APC inactivation is essential for viral replication remains unresolved. Disabling of the APC by the specific targeting of individual subunits appears to be a common strategy adopted by different viruses to arrest the cell cycle. Adenovirus E4orf4 targets PP2A to APC6 to inactivate the complex through dephosphorylation (36), chicken anemia virus has been shown to target APC1 through its apoptin protein (23, 70), and a recent report suggests that the PACR protein encoded by poxviruses (e.g., parapoxviruses, molluscipoxviruses, and crocodile and squirrel poxvirus) acts as an APC11 mimic to inhibit APC activity (52). APC inactivation may also be required to allow HCMV replication proteins to be stably expressed. Interestingly, five of the six core proteins (i.e., UL54, UL44, UL57, UL70, and UL105) and all the accessory proteins required for oriLyt-dependent viral DNA replication in a transient assay (i.e., UL37 exon 1, IRS1/TRS1, UL122, UL84, and UL112-113) (56, 57) contain the consensus D-box (RXXLXXXXN/D/E) that is recognized by APCCdh1.
The ramifications of APC inactivation may also contribute to the clinical complications caused by the infection. While numerous studies have characterized the role of the APC in regulating the cell cycle, several recent studies have also highlighted its importance in regulating neuronal physiology, differentiation, and tumor suppression. Congenital HCMV infection may lead to birth defects (i.e., mental retardation, hearing loss, and vision loss), which may be present at birth or may not develop until a later age. Impaired brain development is likely linked to HCMV infection and subsequent deregulation of neuronal cells. HCMV readily infects neuronal precursor cells, as well as differentiated neurons and astroglia (12, 47, 55). It is interesting to speculate whether the neuronal defects caused by the infection may be associated with the inactivation of the APC given that APCCdh1 is involved in regulating axon growth and patterning, synapse development, neuronal survival, and neural stem cell differentiation (2, 34, 35, 41, 75, 82). A recent study characterizing a constitutively phosphorylated phosphomimic of Cdh1 in neurons showed decreased APCCdh1 function and a defect in axon growth regulation of primary cerebellar granule neurons (27). Another study has also shown the importance of APCCdh1 activity in preventing glycolysis-associated oxidative stress and cell death in neurons (24). Pfkfb3, which mediates the downstream activation of glycolysis, is maintained at low levels through APCCdh1-mediated degradation in neurons. The upregulation of Pfkfb3 in neurons, either through the inhibition of Cdh1 or overexpression of Pfkfb3, led to the activation of glycolysis, along with decreased oxidation of glucose, oxidative stress, and apoptotic death (24). These studies suggest that inactivation of the APC may cause deleterious effects on neurons or neuronal development. Thus, understanding the mechanisms involved in the inactivation of the APC will provide further insight into not only the molecular pathology of the infection but also its clinical manifestations.
Acknowledgments
We are grateful to Mark Prichard for the UL97 deletion virus RCΔ97.08, Bradley Moore for the sample of Sal A, Jose Teodoro and Michael Green for the sample of APC1 antibody, Karen Oegama for the pGEX6P-1 vector, and Ross Tomaino of the Taplin Mass Spectroscopy Facility for his expert assistance in analysis of phosphorylation sites.
This work was supported by NIH grants CA73490, CA34729, and AI083991 to D.H.S and AI26077 to D.M.C, as well as an NIH Ruth L. Kirschstein NRSA postdoctoral fellowship F32 AI075766 to J.P.K.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Albrecht, T., M. P. Fons, I. Boldogh, S. AbuBakar, C. Z. Deng, and D. Millinoff. 1991. Metabolic and cellular effects of human cytomegalovirus infection. Transplant. Proc. 23:48-55. [PubMed] [Google Scholar]
- 2.Almeida, A., J. P. Bolanos, and S. Moreno. 2005. Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J. Neurosci. 25:8115-8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzeh, M., A. Honigman, A. Taraboulos, A. Rouvinski, and D. Wolf. 2006. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology 354:69-79. [DOI] [PubMed] [Google Scholar]
- 4.Baek, M.-C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. Importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 5.Baek, M.-C., P. M. Krosky, A. Pearson, and D. M. Coen. 2004. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virology 324:184-193. [DOI] [PubMed] [Google Scholar]
- 6.Bembenek, J., and H. Yu. 2001. Regulation of the anaphase-promoting complex by the dual specificity phosphatase human Cdc14a. J. Biol. Chem. 276:48237-48242. [DOI] [PubMed] [Google Scholar]
- 7.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas, N., V. Sanchez, and D. H. Spector. 2003. Human cytomegalovirus infection leads to accumulation of geminin and inhibition of the licensing of cellular DNA replication. J. Virol. 77:2369-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco, M. A., A. Sánchez-Díaz, J. M. de Prada, and S. Moreno. 2000. APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J. 19:3945-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldogh, I., S. AbuBakar, C. Z. Deng, and T. Albrecht. 1991. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J. Virol. 65:1568-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:156-160. [DOI] [PubMed] [Google Scholar]
- 12.Cheeran, M., S. Hu, H. Ni, W. Sheng, J. Palmquist, P. Peterson, and J. Lokensgard. 2005. Neural precursor cell susceptibility to human cytomegalovirus diverges along glial or neuronal differentiation pathways. J. Neurosci. Res. 82:839-850. [DOI] [PubMed] [Google Scholar]
- 13.Dephoure, N., C. Zhou, J. Villén, S. A. Beausoleil, C. E. Bakalarski, S. J. Elledge, and S. P. Gygi. 2008. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 105:10762-10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng, J., A. McCormack, and J. Yates. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 16.Fang, G., H. Yu, and M. W. Kirschner. 1998. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2:163-171. [DOI] [PubMed] [Google Scholar]
- 17.Fortunato, E. A., and D. H. Spector. 1998. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 72:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauci, S., A. O. Helbig, M. Slijper, J. Krijgsveld, A. J. Heck, and S. Mohammed. 2009. Lys-N and trypsin cover complementary parts of the phosphoproteome in a refined SCX-based approach. Anal. Chem. 81:4493-4501. [DOI] [PubMed] [Google Scholar]
- 19.Golan, A., Y. Yudkovsky, and A. Hershko. 2002. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1-cyclin B and Plk. J. Biol. Chem. 277:15552-15557. [DOI] [PubMed] [Google Scholar]
- 20.Hall, M. C., E. N. Warren, and C. H. Borchers. 2004. Multi-kinase phosphorylation of the APC/C activator Cdh1 revealed by mass spectrometry. Cell Cycle 3:1278-1284. [DOI] [PubMed] [Google Scholar]
- 21.Hamirally, S., J. Kamil, Y. Ndassa-Colday, A. Lin, W. Jahng, M. Baek, S. Noton, L. Silva, M. Simpson-Holley, D. Knipe, D. Golan, J. Marto, and D. Coen. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He, Z., Y. S. He, Y. Kim, L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilman, D. W., J. G. Teodoro, and M. R. Green. 2006. Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies. J. Virol. 80:7535-7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrero-Mendez, A., A. Almeida, E. Fernández, C. Maestre, S. Moncada, and J. P. Bolaños. 2009. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 11:747-752. [DOI] [PubMed] [Google Scholar]
- 25.Hsu, J. Y., J. D. R. Reimann, C. S. Sørensen, J. Lukas, and P. K. Jackson. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4:358-366. [DOI] [PubMed] [Google Scholar]
- 26.Hume, A. J., J. S. Finkel, J. P. Kamil, D. M. Coen, M. R. Culbertson, and R. F. Kalejta. 2008. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science 320:797-799. [DOI] [PubMed] [Google Scholar]
- 27.Huynh, M. A., J. Stegmüller, N. Litterman, and A. Bonni. 2009. Regulation of Cdh1-APC function in axon growth by Cdh1 phosphorylation. J. Neurosci. 29:4322-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imami, K., N. Sugiyama, Y. Kyono, M. Tomita, and Y. Ishihama. 2008. Automated phosphoproteome analysis for cultured cancer cells by two-dimensional nanoLC-MS using a calcined titania/C18 biphasic column. Anal. Sci. 24:161-166. [DOI] [PubMed] [Google Scholar]
- 29.Jaspersen, S. L., J. F. Charles, and D. O. Morgan. 1999. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and phosphatase Cdc14. Curr. Biol. 9:227-236. [DOI] [PubMed] [Google Scholar]
- 30.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamil, J. P., and D. M. Coen. 2007. Human cytomegalovirus protein kinase UL97 forms a complex with the tegument phosphoprotein pp65. J. Virol. 81:10659-10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamil, J. P., A. J. Hume, I. Jurak, K. Münger, R. F. Kalejta, and D. M. Coen. 2009. Human papillomavirus 16 E7 inactivator of retinoblastoma family proteins complements human cytomegalovirus lacking UL97 protein kinase. Proc. Natl. Acad. Sci. U. S. A. 106:16823-16828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1delta. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, A. H., and A. Bonni. 2007. Thinking within the D box: initial identification of Cdh1-APC substrates in the nervous system. Mol. Cell Neurosci. 34:281-287. [DOI] [PubMed] [Google Scholar]
- 35.Konishi, Y., J. Stegmuller, T. Matsuda, S. Bonni, and A. Bonni. 2004. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303:1026-1030. [DOI] [PubMed] [Google Scholar]
- 36.Kornitzer, D., R. Sharf, and T. Kleinberger. 2001. Adenovirus E4orf4 protein induces PP2A-dependent growth arrest in Saccharomyces cerevisiae and interacts with the anaphase-promoting complex/cyclosome. J. Cell Biol. 154:331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraft, C., F. Herzog, C. Gieffers, K. Mechtler, A. Hagting, J. Pines, and J.-M. Peters. 2003. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22:6598-6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer, E. R., N. Scheuringer, A. V. Podtelejnikov, M. Mann, and J. M. Peters. 2000. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11:1555-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krosky, P. M., M.-C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krosky, P. M., M.-C. Baek, W. J. Jahng, I. Barrera, R. J. Harvey, K. K. Biron, D. M. Coen, and P. B. Sethna. 2003. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J. Virol. 77:7720-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasorella, A., J. Stegmüller, D. Guardavaccaro, G. Liu, M. S. Carro, G. Rothschild, L. De La Torre-Ubieta, M. Pagano, A. Bonni, and A. Iavarone. 2006. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 442:471-474. [DOI] [PubMed] [Google Scholar]
- 42.Lim, H. H., P. Y. Goh, and U. Surana. 1998. Cdc20 is essential for the cyclosome-mediated proteolysis of both Pds1 and Clb2 during M phase in budding yeast. Curr. Biol. 8:231-234. [DOI] [PubMed] [Google Scholar]
- 43.Listovsky, T., Y. S. Oren, Y. Yudkovsky, H. M. Mahbubani, A. M. Weiss, M. Lebendiker, and M. Brandeis. 2004. Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 23:1619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Listovsky, T., A. Zor, A. Laronne, and M. Brandeis. 2000. Cdk1 is essential for mammalian cyclosome/APC regulation. Exp. Cell Res. 255:184-191. [DOI] [PubMed] [Google Scholar]
- 45.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukas, C., C. S. Sørensen, E. Kramer, E. Santoni-Rugiu, C. Lindeneg, J. M. Peters, J. Bartek, and J. Lukas. 1999. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401:815-818. [DOI] [PubMed] [Google Scholar]
- 47.Luo, M. H., P. H. Schwartz, and E. A. Fortunato. 2008. Neonatal neural progenitor cells and their neuronal and glial cell derivatives are fully permissive for human cytomegalovirus infection. J. Virol. 82:9994-10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marschall, M., A. Marzi, P. aus den Siepen, R. Jochmann, M. Kalmer, S. Auerochs, P. Lischka, M. Leis, and T. Stamminger. 2005. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J. Biol. Chem. 280:33357-33367. [DOI] [PubMed] [Google Scholar]
- 49.McElroy, A. K., R. S. Dwarakanath, and D. H. Spector. 2000. Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130. J. Virol. 74:4192-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGarry, T. J., and M. W. Kirschner. 1998. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93:1043-1053. [DOI] [PubMed] [Google Scholar]
- 51.Michel, D., I. Pavic, A. Zimmermann, E. Haupt, K. Wunderlich, M. Heuschmid, and T. Mertens. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mo, M., S. Fleming, and A. Mercer. 2009. Cell cycle deregulation by a poxvirus partial mimic of anaphase-promoting complex subunit 11. Proc. Natl. Acad. Sci. U. S. A. 106:19527-19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mocarski, E. S., T. Shenk, and R. F. Pass. 2007. Cytomegaloviruses, p. 2701-2772. In D. M. Knipe and P. M. Howley (ed.), Fields Virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 54.Muganda, P., O. Mendoza, J. Hernandez, and Q. Qian. 1994. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J. Virol. 68:8028-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odeberg, J., N. Wolmer, S. Falci, M. Westgren, A. Seiger, and C. Söderberg-Nauclér. 2006. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J. Virol. 80:8929-8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pari, G. S., M. A. Kacica, and D. G. Anders. 1993. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67:2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters, J.-M. 2006. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7:644-656. [DOI] [PubMed] [Google Scholar]
- 59.Petersen, B. O., C. Wagener, F. Marinoni, E. R. Kramer, M. Melixetian, E. L. Denchi, C. Gieffers, C. Matteucci, J. Peters, and K. Helin. 2000. Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev. 14:2330-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prichard, M. N., W. J. Britt, S. L. Daily, C. B. Hartline, and E. R. Kern. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 79:15494-15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prichard, M. N., E. Sztul, S. L. Daily, A. L. Perry, S. L. Frederick, R. B. Gill, C. B. Hartline, D. N. Streblow, S. M. Varnum, R. D. Smith, and E. R. Kern. 2008. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 82:5054-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prinz, S., E. S. Hwang, R. Visintin, and A. Amon. 1998. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr. Biol. 8:750-760. [DOI] [PubMed] [Google Scholar]
- 64.Romaker, D., V. Schregel, K. Maurer, S. Auerochs, A. Marzi, H. Sticht, and M. Marschall. 2006. Analysis of the structure-activity relationship of four herpesviral UL97 subfamily protein kinases reveals partial but not full functional conservation. J. Med. Chem. 49:7044-7053. [DOI] [PubMed] [Google Scholar]
- 65.Salvant, B. S., E. A. Fortunato, and D. H. Spector. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 67.Shirayama, M., A. Toth, M. Galova, and K. Nasmyth. 1999. APC(Cdc20) promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature 402:203-207. [DOI] [PubMed] [Google Scholar]
- 68.Tamashiro, J. C., L. J. Hock, and D. H. Spector. 1982. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J. Virol. 42:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tarayre, S., J. M. Vinardell, A. Cebolla, A. Kondorosi, and E. Kondorosi. 2004. Two classes of the CDh1-type activators of the anaphase-promoting complex in plants: novel functional domains and distinct regulation. Plant Cell 16:422-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teodoro, J. G., D. W. Heilman, A. E. Parker, and M. R. Green. 2004. The viral protein apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53. Genes Dev. 18:1952-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thornton, B. R., T. M. Ng, M. E. Matyskiela, C. W. Carroll, D. O. Morgan, and D. P. Toczyski. 2006. An architectural map of the anaphase-promoting complex. Genes Dev. 20:449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran, K., J. A. Mahr, J. Choi, J. G. Teodoro, M. R. Green, and D. H. Spector. 2008. Accumulation of substrates of the anaphase-promoting complex (APC) during human cytomegalovirus infection is associated with the phosphorylation of Cdh1 and the dissociation and relocalization of the APC subunits. J. Virol. 82:529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran, K., J. A. Mahr, and D. H. Spector. 2010. Proteasome subunits relocalize during human cytomegalovirus infection, and proteasome activity is necessary for efficient viral gene transcription. J. Virol. 84:3079-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Leuken, R., L. Clijsters, and R. Wolthuis. 2008. To cell cycle, swing the APC/C. Biochim. Biophys. Acta 1786:49-59. [DOI] [PubMed] [Google Scholar]
- 75.van Roessel, P., D. A. Elliott, I. M. Robinson, A. Prokop, and A. H. Brand. 2004. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell 119:707-718. [DOI] [PubMed] [Google Scholar]
- 76.van Zeijl, M., J. Fairhurst, E. Z. Baum, L. Sun, and T. R. Jones. 1997. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology 231:72-80. [DOI] [PubMed] [Google Scholar]
- 77.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vodermaier, H. C., C. Gieffers, S. Maurer-Stroh, F. Eisenhaber, and J. M. Peters. 2003. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13:1459-1468. [DOI] [PubMed] [Google Scholar]
- 79.Wiebusch, L., M. Bach, R. Uecker, and C. Hagemeier. 2005. Human cytomegalovirus inactivates the G0/G1-APC/C ubiquitin ligase by Cdh1 dissociation. Cell Cycle 4:1435-1439. [DOI] [PubMed] [Google Scholar]
- 80.Wiebusch, L., R. Uecker, and C. Hagemeier. 2003. Human cytomegalovirus prevents replication licensing by inhibiting MCM loading onto chromatin. EMBO Rep. 4:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. U. S. A. 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao, W., W. Qian, C. Zhu, L. Gui, J. Qiu, and C. Zhang. 2010. Cdh1-APC is involved in the differentiation of neural stem cells into neurons. Neuroreport 21:39-44. [DOI] [PubMed] [Google Scholar]
- 83.Zachariae, W., M. Schwab, K. Nasmyth, and W. Seufert. 1998. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282:1721-1724. [DOI] [PubMed] [Google Scholar]
- 84.Zhou, Y., Y.-P. Ching, A. C. S. Chun, and D.-Y. Jin. 2003. Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J. Biol. Chem. 278:12530-12536. [DOI] [PubMed] [Google Scholar]