Abstract
Dendritic cells represent a specialized class of professional antigen-presenting cells that are responsible for priming and maintaining antigen-specific effector cell responses and regulating immune activation by cytokine secretion. In HIV-1 infection, myeloid dendritic cells are highly dysfunctional, but mechanisms contributing to their functional alterations are not well defined. Here, we show that soluble molecules of the nonclassical major histocompatibility complex class Ib (MHC-Ib) antigen HLA-G are highly upregulated in the plasma during progressive HIV-1 infection, while levels of membrane-bound HLA-G surface expression on dendritic cells, monocytes, and T cells only slightly differ among HIV-1 progressors, HIV-1 elite controllers, and HIV-1-negative persons. These elevated levels of soluble HLA-G in progressive HIV-1 infection likely result from increased secretion of intracellularly stored HLA-G molecules in monocytes and dendritic cells and contribute to a functional disarray of dendritic cells by inhibiting their antigen-presenting properties, while simultaneously enhancing their secretion of proinflammatory cytokines. Interestingly, we observed that these immunoregulatory effects of soluble HLA-G were mainly mediated by interactions with the myelomonocytic HLA class I receptor leukocyte immunoglobulin-like receptor B2 (LILRB2; ILT4), while binding of soluble HLA-G to its alternative high-affinity receptor, LILRB1 (ILT2), appeared to be less relevant for its immunomodulatory functions on dendritic cells. Overall, these results demonstrate a critical role for soluble HLA-G in modulating the functional characteristics of professional antigen-presenting cells in progressive HIV-1 infection and suggest that soluble HLA-G might represent a possible target for immunotherapeutic interventions in HIV-1-infected persons.
The hallmark of HIV-1-associated immune deficiency is a progressive decline of T-cell immunity; however, HIV-1 infection also involves dysfunction of multiple other components of the innate and adaptive immune systems, including B cells (25, 28), NK cells (22), and NK T (NKT) cells (30). Perhaps most importantly, HIV-1 infection leads to functional deficiencies of myeloid dendritic cells (mDC) (2, 8, 10), which as professional antigen-presenting cells have critical roles in priming and maintaining adaptive and innate effector cell responses and in regulating immune activation (4). In progressive HIV-1 infection, myeloid dendritic cells show an activated phenotype, with upregulation of costimulatory molecules and maturation markers (2, 6), but their functional antigen-presenting properties are poor (7), which may be responsible for the dysfunctional properties of antigen-specific T- and B-cell responses during HIV-1 infection. In addition, mDC in progressive HIV-1 infection seem to secrete higher levels of proinflammatory cytokines (2) and by this mechanism may contribute to generalized activation and exhaustion of the immune system, two events that play important roles in the pathogenesis of HIV-1 infection (9). The molecular pathways that contribute to dendritic cell dysfunction in HIV-1 infection, however, are unclear, but their understanding holds promise for a targeted manipulation of dendritic cells for immunotherapeutic interventions.
HLA-G represents a nonclassical major histocompatibility complex class Ib (MHC-Ib) antigen, which, in comparison to classical HLA class I molecules, has limited functions for antigen presentation and restriction of T-cell immune responses but important immunoregulatory properties during various infectious, inflammatory, and malignant diseases (5). Unlike expression of classical HLA class I molecules, expression of HLA-G is mostly limited to fetal trophoblastic tissues (15), but ectopic expression of HLA-G on T cells (11), monocytes, and dendritic cells (3) has been documented in a variety of pathological conditions, including HIV-1 infection (16, 19). Moreover, it is well recognized that alternative splicing of HLA-G can lead to soluble isoforms which cause systemic immunoregulatory effects in the absence of localized tissue expression. The highest-affinity receptors for HLA-G include leukocyte immunoglobulin-like receptor B1 (LILRB1; ILT2) and LILRB2 (ILT4), two members of the LILR family, as well as the NK cell receptor KIR2DL4. By interacting with such receptors, HLA-G can induce a variety of immunomodulatory effects, including inhibition of antigen-specific T-cell (17) and NK cell responses (27). How HLA-G changes the functional profile of dendritic cells during chronic viral diseases such as HIV-1 infection remains unknown.
In the present study, we analyzed immunomodulatory effects of HLA-G in individuals with different rates of HIV-1 disease progression. Our studies show that soluble HLA-G in the plasma, but not membrane-bound HLA-G expression on leukocytes, is strikingly upregulated during progressive HIV-1 infection. This soluble HLA-G critically contributes to the functional deficiencies of myeloid dendritic cells by interacting with ILT4 (LILRB2), while interactions with its other high-affinity receptor, ILT2, seem to be less relevant. Overall, these data show that binding interactions between ILT4 and soluble HLA-G play a key role in mediating dendritic cell dysfunction in progressive HIV-1 infection and might represent a possible target for immunotherapeutic interventions in HIV-1 infection.
MATERIALS AND METHODS
Study subjects.
HIV-1-infected individuals, as well as a reference cohort of HIV-1-seronegative persons, were recruited at the Massachusetts General Hospital. The clinical and demographical characteristics of the study subjects are summarized in Table 1. HIV-1 patients were not taking antiretroviral therapy at the time of study participation. All subjects gave written informed consent to participate, and the study was approved by the Massachusetts General Hospital Institutional Review Board.
TABLE 1.
Clinical and demographic characteristics of the patient population
| Cohort (n) | Median age (yr) (range) | Male/female ratio | Median viral load (HIV RNA copies/ml) (range) | Median CD4 cells/μl (range) | No. of patients witha: |
||
|---|---|---|---|---|---|---|---|
| HCV coinfection | HBV coinfection | Prior HAART exposure | |||||
| Progressors (12) | 38.5 (30-51) | 11:1 | 28,428 (8,104-449,000) | 412.5 (195-1,000) | 0 | 0 | 2d |
| Controllers (10) | 50 (35-62) | 8:2 | <74 | 876 (432-1,134) | 5b | 2c | 0 |
| HIV negatives (13) | 24 (23-38) | 9:4 | NDe | 0 | 0 | 0 | |
HCV, hepatitis C virus; HBV, hepatitis B virus; HAART, highly active antiretroviral therapy.
Patients had positive HCV serum antibodies but undetectable HCV viral replication.
Patients had detectable HBV core and HBV surface antibodies but were negative for HBV envelope antigen or HBV DNA.
One patient exposed 6 years prior to study participation, one patient 16 years prior.
ND, not determined.
Flow cytometric analysis of HLA-G expression.
Peripheral blood mononuclear cells (PBMC) were incubated with monoclonal antibodies against CD4, CD3, CD8, CD14, HLA-DR, CD11c, and a cocktail of lineage antibodies (CD3, CD14, CD16, CD19, CD20, and CD56) to identify populations of T cells, monocytes, and myeloid dendritic cells. For HLA-G surface assessments, cells were additionally stained with a phycoerythrin (PE)-labeled HLA-G-specific antibody (clone MEM-G/9; no cross-reactivity to alternative HLA class I molecules [24]) for 20 min at room temperature. Cells were subsequently fixed in phosphate-buffered saline (PBS) containing 2% paraformaldehyde. For intracellular detection of HLA-G, cells were stained with surface antibodies, fixed, and permeabilized using a commercial kit (Caltag), followed by intracellular staining with HLA-G-specific antibodies (24). Cells were acquired on an LSRII (BD Biosciences) instrument. Compensation was performed with microbeads stained separately with individual antibodies used in the test samples.
Detection of soluble HLA-G.
Soluble HLA-G1/G5 was detected by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (US Biologicals) according to the manufacturer's instructions. To selectively remove HLA-G from plasma, samples were incubated with biotinylated HLA-G antibodies (clone 4H84) for 1 h at room temperature, followed by immunomagnetic depletion of HLA-G using antibiotin magnetic beads according to the manufacturer's protocol (Invitrogen).
Preparation and maturation of MDDC.
Freshly isolated PBMC were washed several times in RPMI medium to remove platelets and incubated for 60 min at 37°C to adhere monocytes. Adherent monocytes were propagated in the presence of 50 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; CellGenix) for 5 days, which maintains expression of the HLA-G receptors ILT2 and ILT4 (29). On day 5, immature myelomonocytic cells were harvested using Hanks-based cell disassociation buffer (Invitrogen) and incubated with either full plasma or HLA-G-depleted plasma from HIV-1 patients or negative controls at 37°C for 30 min. In some experiments, monocyte-derived dendritic cells (MDDC) were incubated with plasma from HIV-1-negative persons supplemented with recombinant HLA-G1/G5 molecules (US Biologicals; 5 μg/ml). After two washes, MDDC were incubated at 37°C for 3 h before maturation in the presence of a previously described cytokine cocktail (14) containing interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), prostaglandin E2 (PGE2), and IL-6 for MDDC maturation.
Dendritic cell functional assays.
Matured MDDC were subjected to flow cytometric analysis of the surface expression of dendritic cell maturation markers (CD80, CD86, HLA-DR, CD83, and HLA-ABC, all antibodies from BD Biosciences). Alternatively, matured MDDC were washed aggressively and mixed with carboxyfluorescein succinimidyl ester (CFSE)-labeled allogeneic T cells from identical HIV-1-negative donors; the proportion of proliferating T cells was afterwards assessed on day 6 using standard flow cytometry assays. For the assessment of cytokine secretion in MDDC, matured cells were stimulated with Toll-like receptor (TLR) ligands (CL097; 5 μg/ml) for 20 h in the presence of brefeldin A. Subsequently, cells were fixed and permeabilized using a commercial kit (Caltag), and intracellular cytokine staining was performed using antibodies against IL-6, IL-12p70, and TNF-α before processing for flow cytometric acquisition.
siRNA-mediated gene silencing.
Small interfering RNA (siRNA) pools specific for the ILT4 message (LILRB2 ON-TARGETplus SMARTpool; Dharmacon Technologies) or the ILT2 message (LILRB1 ON-TARGETplus SMARTpool; Dharmacon) were used at a concentration of 1 nmol/million cells. MDDC (106) were suspended in 300 μl Optimem in the presence of siRNA and transferred to a 4-mm electroporation cuvette (Bio-Rad Laboratories). Cells were left on ice for 5 min and then electroporated (900-V, 0.75-ms square wave; Bio-Rad; Genepulser Xcell).
Western blots.
Equal amounts of plasma from study patients were subjected to SDS-PAGE (Tris-glycine 4 to 20% gels; Invitrogen), electroblotted, and incubated with HLA-G antibodies (clone 4H84) in buffer (5% milk-PBS), followed by visualization with horseradish peroxidase (HRP)-labeled secondary antibodies and enhanced chemiluminescence (ECL) detection reactions (Amersham Biosciences) according to standard protocols.
Immunoprecipitation experiments.
Plasma samples from study patients were incubated with cell lysates from HIV-1 progressors or recombinant ILT2/ILT4 proteins (R&D Systems) as controls for 1 h at room temperature. Afterwards, HLA-G and associated proteins were isolated using biotinylated HLA-G antibodies (clone 4H84), followed by immunomagnetic enrichment with antibiotin magnetic beads. Eluted proteins was denatured in SDS sample buffer at 96°C for 6 min and subsequently subjected to SDS-PAGE using Tris-glycine 4 to 20% gels (Invitrogen). Samples were then electroblotted and hybridized with ILT2 or ILT4 antibodies (clone m402 or 42D1, respectively), and bands were visualized using HRP-labeled secondary antibodies and ECL detection reactions (Amersham Biosciences).
Statistics.
Data from different study cohorts were expressed using box-and-whisker plots, indicating the minimum, maximum, and 25th, 50th, and 75th percentiles. Statistical comparisons were made using Mann-Whitney U tests or Wilcoxon rank sum tests, as appropriate.
RESULTS
Upregulation of soluble HLA-G expression in progressive HIV-1 infection.
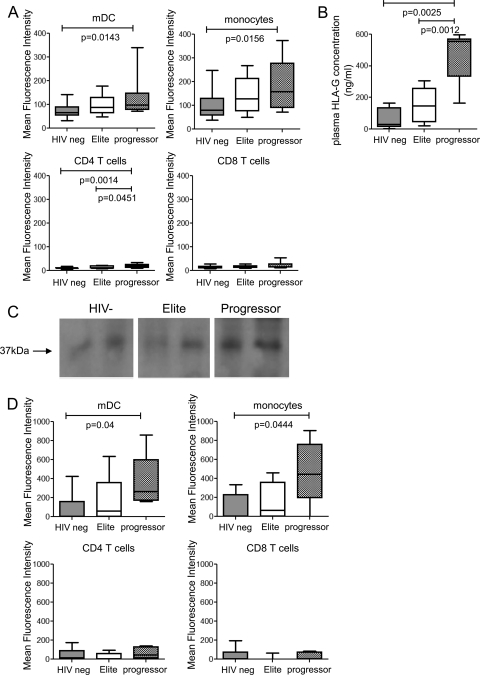
To analyze immunomodulatory effects of HLA-G in HIV-1 infection, we initially assessed the surface expression of HLA-G on T cells, monocytes, and dendritic cells in patients with progressive infection or treatment-naive elite control of HIV-1 infection, as well as in a background population of HIV-1-negative persons. The clinical and demographic characteristics of the study patients are summarized in Table 1. For these experiments, PBMC from the different study cohorts were analyzed by flow cytometric assays using an HLA-G-specific antibody recognizing β2-microglobulin-associated HLA-G isoforms. As shown in Fig. 1A, membrane-bound HLA-G surface expression on monocytes and dendritic cells was 1.5- to 2-fold higher in progressors than in HIV-1-negative persons (P < 0.02), while corresponding differences between elite controllers and HIV-1-negative persons were not statistically significant. Levels of HLA-G surface expression on T cells were low in all three study cohorts.
FIG. 1.
Upregulation of soluble plasma HLA-G during progressive HIV-1 infection. (A) Surface expression of HLA-G on CD14+ monocytes and CD11c+ HLA-DR+ lin− mDC and T cells in HIV-1 controllers (n = 10), HIV-1 progressors (n = 10), and HIV-1-negative study persons (n = 13). (B) Concentrations of soluble HLA-G in the plasma from the three patient cohorts (n = 9, 7, and 5, respectively). (C) Detection of soluble HLA-G in plasma by Western blotting. Equal amounts of plasma from two representative individuals from each of the indicated study cohorts were analyzed. (D) Intracellular levels of HLA-G in monocytes, dendritic cells, and T cells from the three study groups.
In contrast, we found that soluble HLA-G, as measured by ELISAs, was 3- to 5-fold more strongly expressed in the plasma of patients with progressive HIV-1 infection than in either HIV-1-negative persons or HIV-1 elite controllers; moreover, expression of soluble HLA-G also tended to be higher in plasma of elite controllers than in HIV-1-negative individuals, although this difference was not significant (Fig. 1B). To further characterize soluble HLA-G from these study patients, equal amounts of plasma from the study patients were analyzed using Western blots. These experiments revealed higher-intensity signals of the expected 37-kDA bands corresponding to soluble HLA-G isoforms (23) in samples from progressors (Fig. 1C), thus confirming our previous findings.
Since the elevated levels of soluble HLA-G in the plasma of HIV-1 progressors may result from the secretion of intracellularly stored HLA-G molecules by monocytes, dendritic cells, or T cells, we assessed intracellular contents of HLA-G in these cell populations by flow cytometry (Fig. 1D). In persons with progressive HIV-1 infection, intracellular HLA-G levels in monocytes and dendritic cells were 3- to 5-fold higher than in HIV-1 elite controllers and HIV-1-negative persons. Intracellular HLA-G expression in monocytes and dendritic cells also tended to be higher in elite controllers than in HIV-1-negative persons, while no systematic changes in intracellular HLA-G content between T cells from the three different study cohorts were found. Overall, these experiments suggest that soluble HLA-G is strongly upregulated in progressive HIV-1 infection, most likely as a result of enhanced secretion of intracellularly stored HLA-G from monocytes and dendritic cells.
Soluble HLA-G inhibits dendritic cell function in progressive HIV-1 infection.
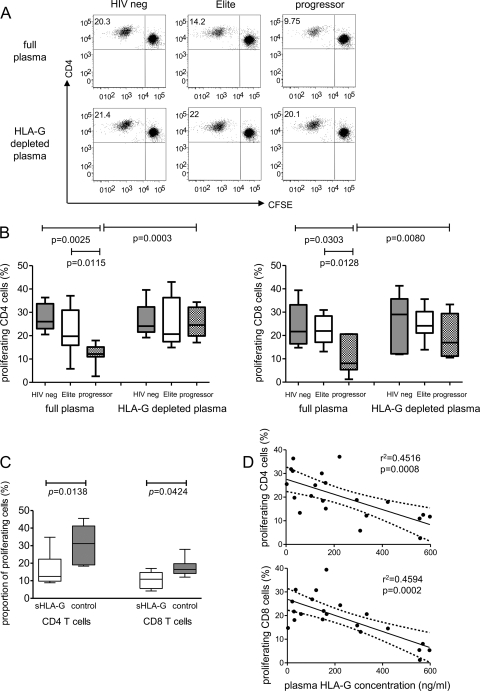
To analyze functional consequences of higher levels of circulating soluble HLA-G in HIV-1 progressors, we focused on the impact of soluble HLA-G on dendritic cell function. For this purpose, monocyte-derived dendritic cells (MDDC) were generated from HIV-1-negative donors and matured in the presence of plasma from elite controllers, progressors, and HIV-1-negative persons. After two washes, cells were mixed with allogeneic CFSE-labeled T cells, and allogeneic T-cell proliferation was monitored after 6 days in culture using flow cytometry. As indicated in Fig. 2A and B, we observed significantly weaker allostimulatory properties of MDDC matured in the presence of plasma from HIV-1 progressors than in corresponding experiments in which plasma from controllers or HIV-1-negative persons was used. There was no difference between the proportions of proliferating allogeneic T cells in experiments using MDDC matured in the presence of plasma from HIV-1 controllers or HIV-1-negative individuals. To investigate if these inhibitory effects of plasma from HIV-1 progressors are related to soluble HLA-G, we selectively removed soluble HLA-G from the plasma by antibody-mediated immunomagnetic depletion, which reduced soluble HLA-G concentrations below the threshold of detection by Western blotting (data not shown). This elimination of soluble HLA-G from the plasma of HIV-1 progressors resulted in significant increases in the allostimulatory properties of MDDC, while the removal of HLA-G from plasma of controllers or HIV-1-negative persons had no substantial effect on the allostimulatory function of MDDC (Fig. 2B). In addition, we also noted that MDDC matured in the presence of plasma from HIV-1-negative persons supplemented with recombinant HLA-G1/G5 had significantly reduced antigen-presenting properties in comparison to those of MDDC in control experiments without addition of exogenous recombinant HLA-G (Fig. 2C). Finally, we observed that proliferative activities of allogeneic CD4 and CD8 T cells were inversely correlated to the concentrations of HLA-G in the corresponding plasma samples tested by ELISA (Fig. 2D). Overall, these data suggest that large amounts of soluble HLA-G in the plasma of HIV-1 progressors can significantly suppress the antigen-presenting properties of MDDC.
FIG. 2.
Soluble HLA-G inhibits antigen-presenting properties of dendritic cells. MDDC generated from HIV-1-negative persons were mixed with CFSE-labeled allogeneic T cells from HIV-1-negative donors in mixed-lymphocyte reactions. (A and B) Proportions of allogeneic T cells proliferating after exposure to MDDC matured in the presence of full or HLA-G-depleted plasma from HIV-1 progressors, controllers, or HIV-1-negative persons. (A) Data from one representative experiment; (B) cumulative data from HIV-1-negative donors (n = 5), elite controllers (n = 9), and progressors (n = 7). (C) Allostimulatory properties of MDDC matured in the presence of plasma from HIV-1-negative persons supplemented with recombinant HLA-G1/G5 (sHLA-G) or without addition of exogenous HLA-G molecules (control experiments). (D) Correlation between plasma HLA-G concentrations and corresponding proportions of proliferating allogeneic CD4 and CD8 T cells. Data from all patients (n = 21) were included.
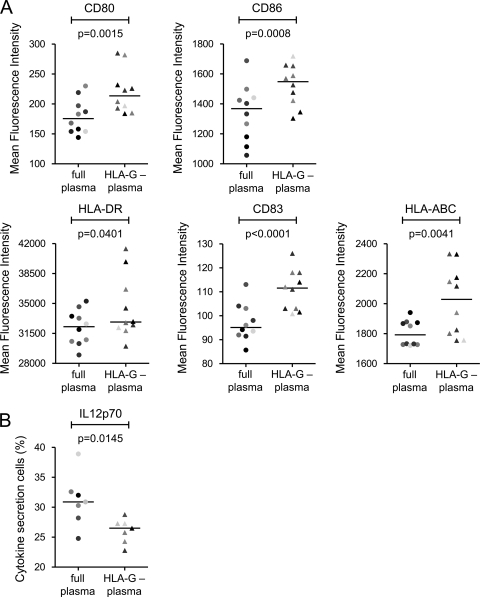
We next tested whether soluble HLA-G in the plasma of HIV-1 progressors also affects the maturation and cytokine secretion profile of MDDC. For these investigations, MDDC were matured in the presence of plasma from progressors and the expression of maturation markers (CD83, HLA-DR, and HLA-A/B/C) and costimulatory molecules (CD80 and CD86) and the production of cytokines (IL-6, TNF-α, and IL-12p70) were subsequently monitored by flow cytometry. These experiments showed that MDDC exposed to full plasma from progressors had significantly lower levels of expression of maturation markers (Fig. 3A) and secreted increased amounts of IL-12p70 compared to MDDC treated with identical plasma preparations after selective removal of HLA-G (Fig. 3B). Production of other cytokines was not affected by depletion of HLA-G from plasma of HIV-1 progressors. Overall, these studies show that soluble HLA-G in the plasma of HIV-1 progressors can impair the maturation of myeloid dendritic cells and enhance their secretion of the proinflammatory cytokine IL-12p70.
FIG. 3.
Soluble HLA-G leads to functional disarray of dendritic cells in progressive HIV-1 infection. (A) Surface expression of costimulatory molecules and dendritic cell maturation markers of MDDC exposed to full or HLA-G-depleted plasma from HIV-1 progressors. (B) IL-12p70 secretion of MDDC following stimulation with TLR ligands in the presence of full or HLA-G-depleted plasma from HIV-1 progressors.
Inhibitory effects of soluble HLA-G are mediated by interactions with ILT4 on dendritic cells.
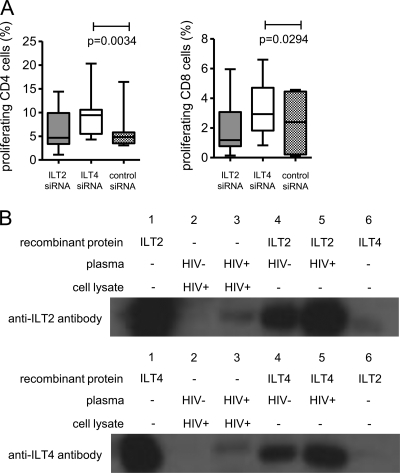
The physiologic high-affinity receptors for HLA-G include the myelomonocytic HLA class I receptors ILT2 (LILRB1) and ILT4 (LILRB2), which both belong to the leukocyte immunoglobulin-like receptor (LILR) family of molecules and are strongly expressed on dendritic cells. To test whether soluble HLA-G mediates its immunomodulatory effects on MDDC through binding to ILT2 or ILT4, MDDC constitutively expressing both ILT2 and ILT4 were incubated with plasma from HIV-1 progressors in the presence of siRNA-mediated silencing of ILT2/ILT4 gene expression or nontargeting control siRNA. siRNA was equally effective in reducing surface expression of ILT2 and ILT4 in comparison to untreated cells (data not shown). Functional properties of these MDDC were then tested using mixed-lymphocyte reactions. Interestingly, we found that, in comparison to control experiments, knockout of ILT2 did not significantly change the impact of plasma from progressors on the ability of MDDC to expand allogeneic T cells; however, following knockout of ILT4, allostimulatory activities of MDDC were significantly enhanced (Fig. 4A). These observations were surprising given that prior studies in a cell-free system using surface plasmon resonance technology showed higher affinities for the binding of recombinant HLA-G to ILT2 than to ILT4 (30). To further investigate this, we performed coimmunoprecipitation experiments in which cell lysates from HIV-1 progressors were mixed with plasma and precipitated with an HLA-G antibody. Coprecipitation of the isolated HLA-G-associated protein complexes with an ILT2- or an ILT4-specific antibody resulted in positive detection signals in both cases, indicating that soluble HLA-G from HIV-1 progressors can physically interact with physiological ILT4 and ILT2 molecules derived from primary dendritic cells (Fig. 4B). Thus, these data indicate that soluble HLA-G from HIV-1 progressors can bind to both ILT2 and ILT4, although biological effects of soluble HLA-G on MDDC are mediated by interactions with ILT4.
FIG. 4.
Immunomodulatory properties of soluble HLA-G are mediated by interactions with ILT4 but not with ILT2. (A) Proportions of proliferating allogeneic CD4 and CD8 T cells stimulated with MDDC (n = 7) after exposure to plasma from HIV-1 progressors in the presence of manipulation with the indicated siRNA. (B) Soluble HLA-G from plasma of HIV-1 progressors can interact with both ILT2 and ILT4. Plasma from HIV-1 progressors or HIV-1-negative persons was incubated with dendritic cell lysates from HIV-1 progressors (lanes 2 and 3) or recombinant ILT2 or ILT4 (lanes 4 and 5). Subsequently, HLA-G and associated proteins were immunomagnetically isolated and hybridized with ILT2 (top) or ILT4 (bottom) antibodies. See text for a detailed explanation of the experimental setup.
DISCUSSION
Myeloid dendritic cells are the most effective antigen-presenting cells and play critical roles in the generation and maintenance of antigen-specific T- and B-cell responses, regulation of immune activation, and orchestration of immune defense mechanisms through cross talk with various effector cell populations. A number of recent studies have shown that, during progressive HIV-1 infection, these cells are functionally defective, and it is very likely that dysfunction of dendritic cells is a critical factor underlying the defective functional characteristics of effector immune cells in progressive HIV-1 infection. However, the mechanisms that are responsible for the altered functional properties of dendritic cells in progressive HIV-1 infection are insufficiently understood. In this study, we show that soluble and, to a lesser extent, membrane-bound HLA-G is upregulated in persons with progressive HIV-1 infection and contributes to a functional disarray of mDC that includes impaired antigen-presenting properties and increased secretion of the proinflammatory cytokine IL-12p70. These changes were induced by interactions between HLA-G and the myelomonocytic MHC-I receptor ILT4, while ILT2, an alternative high-affinity HLA-G receptor expressed on myelomonocytic cells, did not appear to be involved in HLA-G-mediated functional alterations of mDC. Overall, these results indicate that immunoregulatory networks between soluble HLA-G and ILT4 on mDC play a causal role in the dysfunctional profile of mDC in progressive HIV-1 infection and suggest that a targeted manipulation of HLA-G-mediated immunoregulatory effects may lead to beneficial clinical consequences in vivo.
The immunomodulatory properties of HLA-G are generally executed via interactions with its three high-affinity receptors, ILT2, ILT4, and KIR2DL4. Of these receptors, ILT2 and KIR2DL4 are expressed on T cells and NK cells, and interactions of HLA-G with either of these two receptors can modulate the cytotoxic and proliferative properties of these cells (3). Our data indicate that, in addition to directly affecting these effector cells, soluble HLA-G from HIV-1-infected persons can modulate the functional characteristics of dendritic cells. Notably, professional antigen-presenting cells such as monocytes and dendritic cells express both ILT2 and ILT4, but how individual interactions between these receptors and soluble HLA-G influence the functional profile of mDC remained unclear in prior work. In a cell-free system using surface plasmon resonance technology, previous studies have shown that recombinant HLA-G can bind with higher affinity to ILT2 than to ILT4 (31), and prior data suggested that functional effects of HLA-G on decidual leukocytes are indeed mediated by interactions with ILT2 (3). Yet, although we observed that soluble HLA-G from the plasma of HIV-1-infected persons can physically bind to both ILT4 and ILT2 in immunoprecipitation assays, we found that immunomodulatory effects of HLA-G on antigen-presenting properties of mDC were mediated by interactions with ILT4, while selective knockout of ILT2 did not affect any of the investigated biological effects of HLA-G on mDC. The definitive reason for this finding remains to be determined, but it is possible that soluble HLA-G might be incapable of effectively engaging the functional signal transduction cascade of ILT2 in MDDC. Moreover a specific clustering of ILT4 or ILT2 on the surfaces of dendritic cells could induce preferential binding of HLA-G to ILT4 and prevent effective engagement of ILT2. Indeed, prior observations showed that soluble HLA-G tetramers preferentially bind to ILT4 and not to ILT2 on dendritic cells and monocytes (1).
In prior studies, dendritic cell dysfunction in HIV-1 infection has been related to Vpr-induced inhibition of maturation (26), direct infection of dendritic cells by HIV (32), and functional defects induced by gp120 (10). By showing that elevated levels of soluble HLA-G in progressive HIV-1 infection can decrease the antigen-presenting properties of dendritic cells while enhancing their abilities to secrete proinflammatory cytokines, we identify an important additional mechanism that modulates dendritic cell properties during progressive HIV-1 infection. Importantly, we observed that HLA-G increased the ability of dendritic cells to secrete IL-12p70, a proinflammatory cytokine with potent ability to activate effector cells of the immune system. In this way, soluble HLA-G may be able to significantly enhance bystander cell activation and contribute to a proinflammatory cytokine milieu, which plays important roles in HIV-1 immunopathogenesis (12). Moreover, it is noteworthy that soluble HLA-G levels tended to be elevated in the plasma of HIV-1 elite controllers, although to a substantially lesser extent than in HIV-1 progressors; this circulating HLA-G might contribute to the abnormally high immune activation levels that have been documented in this specific subset of patients despite undetectable levels of viral replication (13). However, it is important to recognize that the functional effects of soluble HLA-G were analyzed using soluble monocyte-derived dendritic cells and not primary dendritic cells, and although MDDC imitate many of the biological characteristics of mDC (18), we cannot exclude the possibility that soluble HLA-G may induce additional or alternative functional alternations in vivo.
In order to analyze the source of elevated soluble HLA-G levels in persons with progressive HIV-1 infection, we assessed intracellular HLA-G contents in dendritic cells and monocytes, which can be secreted into the plasma. Given the significant upregulation of intracellular HLA-G in monocytes and dendritic cells from progressors, the main cellular source of the soluble plasma HLA-G appeared to be these myelomonocytic cells themselves. Notably, we were unable to detect significant amounts of intracellular HLA-G in T cells regardless of the study cohort, despite the fact that ectopic expression of HLA-G on the surfaces of T cells in HIV-1 infection has been reported before (19, 20). Overall, these data suggest an autocrine regulatory feedback loop in which soluble HLA-G serves as a systemic immunomodulatory agent that is secreted by professional antigen-presenting cells and simultaneously plays a key role in regulating the functional characteristics of these cells. It is, however, important to mention that HLA-G can also be synthesized in alternative tissues such as the thymus (21) and the bone marrow (23), and it remains to be determined to what extent production of soluble HLA-G in these compartments may contribute to the elevation of circulating HLA-G levels during progressive HIV-1 infection. Moreover, the genetic and epigenetic mechanisms responsible for increased HLA-G transcription during progressive HIV-1 infection also need to be analyzed in future studies.
In conclusion, we here show that progressive HIV-1 infection is associated with high levels of circulating soluble HLA-G levels, which at least in part stem from monocytes and dendritic cells and regulate the functional properties of these cells by autocrine secretion. These investigations add novel insight into immunomodulatory mechanisms in progressive HIV-1 infection and might be important for developing immunotherapeutic approaches for HIV-1 infection.
Acknowledgments
This study was supported by the U.S. National Institutes of Health (to X.G.Y., AI078799 and AI074415). X.G.Y. and M.L. are both recipients of the Doris Duke Clinical Scientist Development Award. The recruitment of study patients was supported by the William and Melinda Gates Foundation and the Mark and Lisa Schwartz Foundation.
We thank Richard Apps and Mary Carrington (both NCI/NIH) and Des Jones and Rachel Allen (both University of London, United Kingdom) for helpful discussions of the manuscript.
Footnotes
Published ahead of print on 11 August 2010.
REFERENCES
- 1.Allan, D. S., M. Colonna, L. L. Lanier, T. D. Churakova, J. S. Abrams, S. A. Ellis, A. J. McMichael, and V. M. Braud. 1999. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J. Exp. Med. 189:1149-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, M., M. Cordero, J. Almeida, and A. Orfao. 2005. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS 19:261-271. [PubMed] [Google Scholar]
- 3.Apps, R., L. Gardner, and A. Moffett. 2008. A critical look at HLA-G. Trends Immunol. 29:313-321. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Carosella, E. D., B. Favier, N. Rouas-Freiss, P. Moreau, and J. Lemaoult. 2008. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 111:4862-4870. [DOI] [PubMed] [Google Scholar]
- 6.Dillon, S. M., K. B. Robertson, S. C. Pan, S. Mawhinney, A. L. Meditz, J. M. Folkvord, E. Connick, M. D. McCarter, and C. C. Wilson. 2008. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J. Acquir. Immune Defic. Syndr. 48:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 8.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 9.El-Far, M., R. Halwani, E. Said, L. Trautmann, M. Doroudchi, L. Janbazian, S. Fonseca, J. van Grevenynghe, B. Yassine-Diab, R. P. Sekaly, and E. K. Haddad. 2008. T-cell exhaustion in HIV infection. Curr. HIV/AIDS Rep. 5:13-19. [DOI] [PubMed] [Google Scholar]
- 10.Fantuzzi, L., C. Purificato, K. Donato, F. Belardelli, and S. Gessani. 2004. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J. Virol. 78:9763-9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feger, U., E. Tolosa, Y. H. Huang, A. Waschbisch, T. Biedermann, A. Melms, and H. Wiendl. 2007. HLA-G expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood 110:568-577. [DOI] [PubMed] [Google Scholar]
- 12.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 13.Hunt, P. W., J. Brenchley, E. Sinclair, J. M. McCune, M. Roland, K. Page-Shafer, P. Hsue, B. Emu, M. Krone, H. Lampiris, D. Douek, J. N. Martin, and S. G. Deeks. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavanagh, D. G., D. E. Kaufmann, S. Sunderji, N. Frahm, S. Le Gall, D. Boczkowski, E. S. Rosenberg, D. R. Stone, M. N. Johnston, B. S. Wagner, M. T. Zaman, C. Brander, E. Gilboa, B. D. Walker, and N. Bhardwaj. 2006. Expansion of HIV-specific CD4+ and CD8+ T cells by dendritic cells transfected with mRNA encoding cytoplasm- or lysosome-targeted Nef. Blood 107:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovats, S., E. K. Main, C. Librach, M. Stubblebine, S. J. Fisher, and R. DeMars. 1990. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248:220-223. [DOI] [PubMed] [Google Scholar]
- 16.Lajoie, J., M. Massinga Loembe, J. Poudrier, F. Guédou, J. Pépin, A. C. Labbé, M. Alary, and M. Roger. 2010. Blood soluble human leukocyte antigen G levels are associated with human immunodeficiency virus type 1 infection in Beninese commercial sex workers. Hum. Immunol. 71:182-185. [DOI] [PubMed] [Google Scholar]
- 17.Le Gal, F. A., B. Riteau, C. Sedlik, I. Khalil-Daher, C. Menier, J. Dausset, J. G. Guillet, E. D. Carosella, and N. Rouas-Freiss. 1999. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 11:1351-1356. [DOI] [PubMed] [Google Scholar]
- 18.Leon, B., and C. Ardavin. 2008. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol. Cell Biol. 86:320-324. [DOI] [PubMed] [Google Scholar]
- 19.Lozano, J. M., R. Gonzalez, J. M. Kindelan, N. Rouas-Freiss, R. Caballos, J. Dausset, E. D. Carosella, and J. Pena. 2002. Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS 16:347-351. [DOI] [PubMed] [Google Scholar]
- 20.Lozano, J. M., R. Gonzalez, J. Luque, M. Frias, A. Rivero, and J. Pena. 2009. CD8+HLA-G+ regulatory T cells are expanded in HIV-1-infected patients. Viral Immunol. 22:463-465. [DOI] [PubMed] [Google Scholar]
- 21.Mallet, V., A. Blaschitz, L. Crisa, C. Schmitt, S. Fournel, A. King, Y. W. Loke, G. Dohr, and P. Le Bouteiller. 1999. HLA-G in the human thymus: a subpopulation of medullary epithelial but not CD83+ dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int. Immunol. 11:889-898. [DOI] [PubMed] [Google Scholar]
- 22.Mantegani, P., G. Tambussi, L. Galli, C. T. Din, A. Lazzarin, and C. Fortis. 2010. Perturbation of the natural killer cell compartment during primary human immunodeficiency virus 1 infection primarily involving the CD56(bright) subset. Immunology 129:220-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menier, C., M. Rabreau, J. C. Challier, M. Le Discorde, E. D. Carosella, and N. Rouas-Freiss. 2004. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood 104:3153-3160. [DOI] [PubMed] [Google Scholar]
- 24.Menier, C., B. Saez, V. Horejsi, S. Martinozzi, I. Krawice-Radanne, S. Bruel, C. Le Danff, M. Reboul, I. Hilgert, M. Rabreau, M. L. Larrad, M. Pla, E. D. Carosella, and N. Rouas-Freiss. 2003. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Hum. Immunol. 64:315-326. [DOI] [PubMed] [Google Scholar]
- 25.Moir, S., and A. S. Fauci. 2008. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J. Allergy Clin. Immunol. 122:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muthumani, K., D. S. Hwang, A. Y. Choo, S. Mayilvahanan, N. S. Dayes, K. P. Thieu, and D. B. Weiner. 2005. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int. Immunol. 17:103-116. [DOI] [PubMed] [Google Scholar]
- 27.Park, G. M., S. Lee, B. Park, E. Kim, J. Shin, K. Cho, and K. Ahn. 2004. Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem. Biophys. Res. Commun. 313:606-611. [DOI] [PubMed] [Google Scholar]
- 28.Peruchon, S., N. Chaoul, C. Burelout, B. Delache, P. Brochard, P. Laurent, F. Cognasse, S. Prevot, O. Garraud, R. Le Grand, and Y. Richard. 2009. Tissue-specific B-cell dysfunction and generalized memory B-cell loss during acute SIV infection. PLoS One 4:e5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ristich, V., S. Liang, W. Zhang, J. Wu, and A. Horuzsko. 2005. Tolerization of dendritic cells by HLA-G. Eur. J. Immunol. 35:1133-1142. [DOI] [PubMed] [Google Scholar]
- 30.Sandberg, J. K., N. M. Fast, E. H. Palacios, G. Fennelly, J. Dobroszycki, P. Palumbo, A. Wiznia, R. M. Grant, N. Bhardwaj, M. G. Rosenberg, and D. F. Nixon. 2002. Selective loss of innate CD4+ Vα24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 76:7528-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiroishi, M., K. Kuroki, T. Ose, L. Rasubala, I. Shiratori, H. Arase, K. Tsumoto, I. Kumagai, D. Kohda, and K. Maenaka. 2006. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J. Biol. Chem. 281:10439-10447. [DOI] [PubMed] [Google Scholar]
- 32.Steinman, R. M., A. Granelli-Piperno, M. Pope, C. Trumpfheller, R. Gnatius, G. Arrode, P. Racz, and K. Tenner-Racz. 2003. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top. Microbiol. Immunol. 276:1-30. [DOI] [PubMed] [Google Scholar]