Abstract
The ebolavirus (EBOV) VP35 protein binds to double-stranded RNA (dsRNA), inhibits host alpha/beta interferon (IFN-α/β) production, and is an essential component of the viral polymerase complex. Structural studies of the VP35 C-terminal IFN inhibitory domain (IID) identified specific structural features, including a central basic patch and a hydrophobic pocket, that are important for dsRNA binding and IFN inhibition. Several other conserved basic residues bordering the central basic patch and a separate cluster of basic residues, called the first basic patch, were also identified. Functional analysis of alanine substitution mutants indicates that basic residues outside the central basic patch are not required for dsRNA binding or for IFN inhibition. However, minigenome assays, which assess viral RNA polymerase complex function, identified these other basic residues to be critical for viral RNA synthesis. Of these, a subset located within the first basic patch is important for VP35-nucleoprotein (NP) interaction, as evidenced by the inability of alanine substitution mutants to coimmunoprecipitate with NP. Therefore, first basic patch residues are likely critical for replication complex formation through interactions with NP. Coimmunoprecipitation studies further demonstrate that the VP35 IID is sufficient to interact with NP and that dsRNA can modulate VP35 IID interactions with NP. Other basic residue mutations that disrupt the VP35 polymerase cofactor function do not affect interaction with NP or with the amino terminus of the viral polymerase. Collectively, these results highlight the importance of conserved basic residues from the EBOV VP35 C-terminal IID and validate the VP35 IID as a potential therapeutic target.
Ebolaviruses (EBOVs) are enveloped, nonsegmented, negative-strand RNA viruses belonging to the family Filoviridae (34). Because filoviruses cause outbreaks of severe, often lethal hemorrhagic fever, they are of concern as potential bioweapons and as an emerging public health threat. Currently, the determinants of EBOV virulence are incompletely defined, but enhanced understanding of the biochemical and structural properties of EBOV proteins will facilitate development of prophylactic or therapeutic measures toward these viruses. One EBOV protein that functions as a virulence determinant is VP35. VP35 binds to double-stranded RNA (dsRNA), inhibits host innate immune responses, is a viral structural protein, and serves as a component of the viral RNA polymerase complex (1).
VP35 inhibits alpha/beta interferon (IFN-α/β) production, activation of the IFN-inducible protein kinase R (PKR) antiviral protein, and RNA silencing (3, 10, 12, 35). Among these functions, inhibition of IFN-α/β production clearly contributes to efficient virus replication in cell culture and in vivo (15-17, 33). Inhibition of IFN-α/β production primarily occurs through inhibition of retinoic acid-inducible gene I (RIG-I)-dependent signaling, which activates interferon regulatory factor 3 (IRF-3) and IRF-7, transcription factors that regulate IFN-α/β gene expression (2, 6, 7, 18, 32, 33). Recent studies demonstrated that VP35 dsRNA binding activity strongly correlates with IFN inhibition (6, 25, 33). However, VP35 likely interacts with and inhibits additional signaling molecules downstream of RIG-I (7, 25, 32). Altogether, these combined inhibitory activities likely contribute to the IFN suppression observed in cells expressing VP35 or infected with EBOV (11, 14, 17, 33). In addition to immune suppression, VP35 is an essential cofactor in the filoviral polymerase complex (28-30). The functional viral polymerase complex includes four EBOV proteins: nucleoprotein (NP), the VP35 and VP30 proteins, and the large protein (L), which is the RNA-dependent RNA polymerase (29, 30). In this complex, VP35 interacts with NP and L, and it is thought that VP35 bridges the catalytic subunit of the polymerase complex, L, to the NP-associated viral RNA (4, 8). Both VP35-NP and VP35-L interactions are therefore expected to be essential for viral RNA synthesis (4).
Recent structural studies of the carboxy-terminal dsRNA binding domain of VP35, referred to as the interferon inhibitory domain (IID), identified several structural features that are important for VP35 interaction with dsRNA and for inhibition of IFN-α/β production (21, 23, 25). Specifically, a central basic patch within the VP35 IID was demonstrated to make contacts with the phosphodiester backbone of dsRNA, and a hydrophobic pocket was found to form an end cap that recognizes the blunt ends of dsRNA (25). Mutation of either central basic patch residues or end-cap residues disrupted VP35-dsRNA interaction and impaired its ability to block signaling by RIG-I, a cellular protein which is likely to be the primary sensor of EBOV in most cell types (13). However, mutations impairing dsRNA binding and IFN-antagonist functions did not affect the VP35 polymerase cofactor function (25).
In addition to the central basic patch, structural studies identified a separate cluster of conserved basic residues, called the first basic patch, as well as additional conserved basic residues that border the central basic patch (23, 25). From these studies, it was not clear if these additional basic residues within the structurally defined VP35 IID region are important for VP35-mediated functions. In the present study, we demonstrate that basic residues outside the central basic patch are not critical for IFN inhibition but are important for viral RNA synthesis, as mutation of these basic residues abrogates viral RNA synthesis by the polymerase complex. Interestingly, residues located within the first basic patch are also important for VP35 interaction with NP. However, not all basic residues important for replication are required for NP binding, as several basic residue mutants that show diminished replication can bind to NP. These results support a model where VP35 bridges viral NP and L. Together, these results further support the role of EBOV VP35 as a critical, multifunctional virulence factor and a key component of the viral RNA replication complex.
MATERIALS AND METHODS
Antibodies.
Monoclonal antibodies against Zaire EBOV VP35 and against Zaire EBOV NP were generated in collaboration with the Mount Sinai Hybridoma Center and have been described previously (6, 27). A monoclonal anti-hemagglutinin (anti-HA) antibody was purchased from Sigma (St. Louis, MO). A monoclonal anti-maltose binding protein (anti-MBP) antibody was purchased from New England Biolabs.
Cell lines and viruses.
HEK293T cells and Vero cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. Sendai virus (SeV) strain Cantell was grown in 10-day-old embryonated chicken eggs for 2 days at 37°C.
Plasmids.
The Zaire EBOV VP35 expression plasmid pCAGGS-VP35 and an IFN-β promoter reporter plasmid were described previously (2, 3). VP35 point mutations were generated by standard PCR-based methods and cloned into the mammalian expression plasmid pCAGGS (31). Firefly luciferase was expressed from the pCAGGS plasmid in the minigenome assays (see below). The pRL-TK Renilla luciferase expression plasmid was purchased from Promega (Madison, WI). Sequences encoding Zaire EBOV L amino acids 1 to 505 (HA-L amino acids 1 to 505) were amplified from plasmid pTM1-L by PCR (33), cloned with an amino-terminal HA tag, and cloned into pCAGGS. The expression plasmid for Zaire EBOV NP, pcDNA3 EBOV NP, has been described previously (27).
MBP-fusion VP35 IID and VP35 IID protein expression and purification.
MBP-fusion VP35 IID proteins were expressed and purified as described previously (23, 24). Briefly, BL21(DE3) cells expressing MBP-fusion VP35 IID proteins were lysed using an EmulsiFlex-C5 homogenizer (Avestin) and clarified by centrifugation at 30,000 × g at 4°C for 30 min. The supernatant was purified by affinity and ion-exchange chromatography, prior to final purification by size-exclusion chromatography. For VP35 IID proteins, the fusion tags were removed prior to final purification by cleavage with tobacco etch virus (TEV) protease followed by size-exclusion chromatography. The purity of the protein samples was assessed by SDS-PAGE.
Isothermal titration calorimetry assays.
Quantitative analysis of VP35 IID protein binding interactions with dsRNA was performed on a microcalorimeter (VP-ITC; Microcal, North Hampton, MA) by isothermal titration calorimetry (ITC), using protein samples dialyzed against 500 ml of dialysis buffer [10 mM HEPES (pH 7.0), 150 mM NaCl, 1 mM MgCl2, 2 mM Tris(2-carboxyethyl)phosphine (TCEP)] for 12 h. The resulting raw microcalorimeter data were processed and analyzed to determine n (number of binding sites) and KD (binding constant) using ORIGIN software.
IFN-β-luciferase reporter assay.
HEK293T cells were transfected by using Lipofectamine 2000 (Invitrogen) with the indicated amounts of expression plasmid, an IFN-β-firefly luciferase reporter plasmid (400 ng), and a constitutively expressed Renilla luciferase reporter plasmid (pRLTK, 200 ng). To induce reporter gene expression, cells were infected with SeV at a multiplicity of infection of 10. Twenty-four hours posttransfection, the cell lysates were assayed with the dual luciferase reporter assay (Promega), and firefly luciferase activity was normalized to Renilla luciferase activity. The results are presented as percent induction of the positive control (SeV infected, empty vector transfected [no VP35]), the value for which was set equal to 100%.
EBOV transcription/replication assay.
The EBOV transcription/replication assay was based on a previously described system (30). HEK293T cells were cotransfected by the calcium phosphate precipitation method with phage T7-driven expression plasmids encoding the Zaire EBOV NP, L, and VP30 and VP35 proteins. Also transfected were a T7 RNA polymerase expression plasmid, a plasmid that expresses from a T7 promoter a Zaire EBOV minigenome which encodes a fused green fluorescent protein (GFP)-chloramphenicol acetyltransferase (CAT) reporter gene. This is flanked by the cis-acting sequences necessary for replication and transcription of the RNA by a reconstituted EBOV polymerase complex. Also transfected was a constitutively expressing luciferase expression plasmid that served as a transfection control. At 36 h posttransfection, cells were lysed with reporter lysis buffer (Promega) and both CAT and luciferase reporter activities were determined. CAT activity was normalized to luciferase activity. Minigenome reporter activation is presented as percent activity relative to that of the positive-control reaction (250 ng of wild-type [WT] VP35 plasmid), which was set equal to 100%. Error bars represent the standard deviation (SD) from at least three experiments.
Immunoprecipitations (IPs).
To immunoprecipitate full-length VP35 proteins, lysates from transfected cells were incubated with 1 μg of anti-VP35 monoclonal antibody overnight at 4°C, followed by 1 h incubation with protein G-Sepharose beads (Roche). The beads were washed five times with lysis buffer. After the beads were washed, they were resuspended in SDS-PAGE sample loading buffer, separated by 10% SDS-PAGE, and analyzed by Western blotting, as indicated.
MBP-VP35 IID fusion protein interactions with NP.
Forty-five micrograms of pcDNA3 EBOV NP was transfected into 3 × 107 HEK293T cells. At 24 h posttransfection, the cells were lysed in 4 ml NP-40 lysis buffer (50 mM Tris-HCl [pH 8], 280 mM NaCl, 0.5% NP-40, 0.2 mM EDTA, 2 mM EGTA, and 10% glycerol with protease inhibitors). Three hundred fifty microliters of clarified lysate was incubated with the VP35 IID domain fused with MBP or equivalent amounts of the MBP-mutant IID fusion proteins at 4°C. Twenty-four hours later, MBP fusion proteins were bound with 40 μl of amylose resin for 30 min. The resin was washed three times, and MBP fusion proteins were eluted with 100 μl of 10 mM maltose. A fraction of the elution was analyzed by Western blotting. MBP was detected with an anti-MBP antibody (New England Biolabs), and NP was detected with the previously described (see above) anti-NP mouse monoclonal antibody.
To determine if the MBP-VP35 IID fusion proteins could interact with NP in the presence of dsRNA, lysates expressing NP were generated as described above. Before addition of the MBP-VP35 IID protein to the cellular lysate, increasing amounts of poly(I:C) (Invivogen) were first added to the lysate. Poly(I:C) was added at a concentration of either 16 or 98 nM, while either WT or mutant VP35 IID was used at a concentration of 2.4 μM. Following a 24-h incubation period, samples were processed as described above.
RESULTS
First basic patch residues retain dsRNA binding activity.
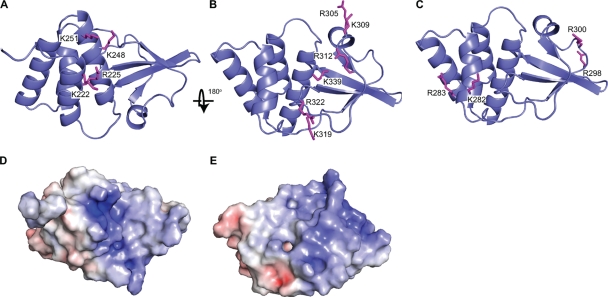
The locations of the first basic patch, central basic patch, and basic residues that border the central basic patch are illustrated in Fig. 1. We previously determined that mutation to alanine of VP35 IID central basic patch residues or of end-cap residues impairs IID dsRNA binding activity and impairs the IFN-antagonist function of full-length VP35 (25). The same mutations, however, had little impact on VP35 polymerase cofactor function (25). In contrast, mutation to alanine of first basic patch residue K222, R225, K248, or K251 did not impair IFN-antagonist function (25). To determine whether the first basic patch mutants affect either the global structure of the VP35 IID or its dsRNA binding activity, we performed a series of nuclear magnetic resonance (data not shown) and ITC experiments. First, 1H/15N heteronuclear single quantum coherence (HSQC) spectra were collected for each mutant from the first basic patch and compared to the corresponding WT spectrum. The results indicated that while some local changes result from the mutation, no global changes occur upon introduction of the alanine substitution. Consistent with these observations, our dsRNA binding studies, using ITC, show that all WT and mutant proteins bind to dsRNA with a similar high affinity, further demonstrating that residues within the first basic patch are not important for dsRNA binding (Fig. 2). In contrast, mutation of residues in the central basic patch, such as R305, K309, R312, K319, R322, and K339, was previously shown to diminish dsRNA binding (25). Altogether, these observations are consistent with previous data indicating that the extent of dsRNA binding by VP35 strongly correlated with its ability to inhibit IFN-α/β production, but it is unlikely that the first basic patch contributes to the IFN antagonism (25).
FIG. 1.
Highly conserved basic residues are located in three regions within VP35 IID. Ribbon representation of VP35 IID highlighting residues from the first basic patch (A) and central basic patch (B) and border basic residues located outside the central basic patch (C). Electrostatic surface representation of the first basic patch (D) and central basic patch and bordering basic residues (E) (scale, −10 kT/e to +10 kT/e).
FIG. 2.
ITC binding isotherms and corresponding raw data for 8-bp dsRNA binding to WT (A) and mutant R225A (B), K248A (C), and K251A (D) VP35 IIDs. The corresponding average KD values for WT, R225A, K248A, and K251A are 0.7, 1.0, 2.4, and 0.8 μM, respectively.
First basic patch residues are critical for VP35 polymerase cofactor function.
To determine whether the first basic patch mutants are critical for VP35 to function in the viral polymerase complex, a minigenome assay was employed (25, 29, 30, 33). This assay measures expression of a fused GFP-CAT reporter gene expressed from a noninfectious, model viral RNA. Expression of GFP-CAT is entirely dependent upon the presence of a functional EBOV RNA polymerase complex, which is reconstituted by coexpression of the viral NP, VP35, VP30, and L. In the absence of VP35, no reporter gene expression is detected, while in the presence of optimal concentrations of VP35, maximal reporter gene activation is detected (25, 33). These plasmids are cotransfected with a constitutively expressed Renilla luciferase plasmid which serves as a control for transfection efficiency (25, 33). The mutation of R225, K248, or K251 to alanine completely abrogated GFP-CAT reporter gene expression, while the K222A mutant retained activity similar to that of WT VP35, with optimal reporter activation occurring at 125 to 250 ng of VP35 plasmid DNA (Fig. 3). These data demonstrate that three of four first basic patch residues in VP35 are important for viral polymerase cofactor function.
FIG. 3.
Viral polymerase cofactor function of basic residue-to-alanine first basic patch mutants. Viral polymerase activity was assessed in the minigenome assay. Empty vector (−), WT VP35 (VP35), or the indicated VP35 mutants were assessed. Cells were transfected with empty vector or with increasing amounts of wild-type or mutant VP35 plasmid and expression plasmids for ZEBOV NP, VP30, L, T7 RNA polymerase, and luciferase. A luciferase expression plasmid was cotransfected as a control for transfection efficiency. A plasmid expressing a negative-sense minigenome RNA and reporter construct was also transfected. Minigenome activity was quantified by measuring CAT activity, and this was normalized to the level of expression of luciferase. Error bars represent 1 standard deviation for three experiments. (Lower panel) Western blots (WB) for VP35 proteins. The concentrations of VP35 plasmids used were 63 ng, 125 ng, and 250 ng. WT VP35 at 250 ng was set as 100% minigenome activity.
We also individually substituted glutamic acid residues for the first basic patch residues R225, K248, and K251. For this analysis, we also generated an H240E mutant, since H240 is located near the first basic patch. Previously, an H240A mutant was found to exhibit marginal IFN-antagonist function, but because H240A was poorly expressed in the minigenome assay, the ability of this mutant to function as a polymerase cofactor was uncertain (25, 33). Each of the mutants with the glutamic acid substitution expressed VP35 comparably to WT VP35 by both the minigenome assay and the IFN-antagonist assay (Fig. 4). As was seen with mutation to alanine, mutation to glutamic acid in each of the first basic patch residues also abrogated VP35 polymerase cofactor function, as evidenced by loss of expression from the minigenome (Fig. 4A). Although H240A was poorly expressed in assays for polymerase function (25), the H240E mutation was readily expressed but the mutant exhibited no detectable activity (Fig. 4A). Despite a lack of polymerase cofactor activity, the R225E, K248E, and K251E mutants did retain the ability to inhibit IFN-β promoter activation (Fig. 4B). Consistent with data obtained with the H240A mutant, the H240E mutant exhibited, at best, a minimal ability to inhibit IFN-β promoter activation (IFN-β promoter activity was approximately 90% of that of the no-VP35 control; Fig. 4B).
FIG. 4.
Viral polymerase cofactor function and IFN-antagonist function of basic residue-to-glutamine first basic patch mutants. (A) Empty vector (EV), wild-type VP35 (VP35), or the indicated first basic patch VP35 mutants were assessed in the minigenome assay, as described in the legend to Fig. 3. (B) Inhibition of IFN-β (IFNb) promoter activation by wild-type and mutant VP35 proteins. HEK293T cells were transfected with empty vector (−) or increasing amounts (1, 2, or 4 μg) of WT VP35 (VP35) or mutant VP35 expression plasmid, an IFN-β promoter-firefly luciferase reporter plasmid, and a constitutively expressed Renilla luciferase reporter plasmid. Firefly luciferase activity was normalized to the level of Renilla luciferase activity. Data are presented such that the activity of the empty vector, SeV-infected samples (+), is set equal to 100%. Error bars represent 1 SD, and corresponding Western blots (WB) of cell lysates are shown below the graphs.
Mutation of basic residues bordering the central basic patch lose polymerase cofactor function.
To determine whether the first basic patch residues are unique in their importance for viral polymerase function, the basic residues K282, R283, R298, and R300 were individually mutated to alanine. These residues are located on the periphery of the central basic patch (Fig. 1C). The resulting minigenome data, shown in Fig. 5 A, revealed diminished polymerase cofactor function (Fig. 5A). In order to determine whether the border residue mutants are globally defective for VP35 functions, they were assayed for IFN-antagonist function (Fig. 5B). VP35 with the K282A mutation displayed a modest loss of activity relative to that of WT VP35, which is consistent with the electrostatic interactions between the K282 ɛ amino group and the phosphodiester backbone of the dsRNA in the VP35 IID/8-bp dsRNA complex structure (25). The remaining border residue mutants exhibited IFN-inhibitory activity comparable to that of WT VP35 (Fig. 5B).
FIG. 5.
Viral polymerase cofactor function and IFN-antagonist function of border residue mutants. (A) Empty vector (first bar), WT VP35 (VP35), or the indicated border basic residue mutants were assessed for function in the minigenome assay, as described in the legend to Fig. 3. (B) Inhibition of IFN-β promoter activation by WT and mutant VP35 proteins. HEK293T cells were transfected with empty vector (first bar) or increasing amounts (1, 2, or 4 μg) of WT VP35 or mutant VP35 expression plasmid, an IFN-β promoter-firefly luciferase reporter plasmid, and a constitutively expressed Renilla luciferase reporter plasmid. Firefly luciferase activity was normalized to the level of Renilla luciferase activity. Data are presented such that the activity of empty vector, SeV-infected samples, is set equal to 100%. Error bars represent one SD, and corresponding Western blots (WB) of cell lysates are shown below the graphs.
The basic nature of VP35 IID residues, not their identity, is the critical determinant for polymerase cofactor function.
To assess whether the basic charge or the identity of the residue is the critical determinant for VP35-mediated polymerase cofactor function, several arginine residues were mutated to lysine, while lysine residues were mutated to arginine in the first basic patch (R225K, K248R, K251R), in a representative border residue (R283K), and at two representative central basic patch positions (R312K and R322K). These mutations were assessed in the minigenome assay, revealing that all VP35 proteins with these mutations functioned similarly to wild-type VP35. Although VP35 proteins with the R283K, R312K, and R322K mutations displayed a moderate decrease in function, this is most likely due to differences in expression of the various plasmids (Fig. 6), as opposed to direct differences in function. These data suggest that having a basic charge at these particular sites is important for VP35 copolymerase function and that arginine and lysine residues can be used interchangeably at these positions with limited perturbation of the VP35 polymerase cofactor function.
FIG. 6.
Viral polymerase cofactor function of arginine-to-lysine and lysine-to-arginine mutations for representative residues from the first basic patch, border residues, and the central basic patch. Empty vector (−), WT VP35 (VP35), or the indicated mutants were assessed for function in the minigenome assay, as described in the legend to Fig. 3. Error bars represent SDs, and corresponding Western blots (WB) of cell lysates are shown below the graphs. The concentrations of VP35 plasmids used were 125 ng, 250 ng, and 500 ng. Data were expressed as the fold induction over that by the negative control (no VP35).
First basic patch mutants are impaired for interaction with the viral nucleoprotein.
VP35 interacts with NP, and this interaction is presumed to be essential for viral polymerase activity (4). To determine whether loss of polymerase activity could be explained by loss of VP35-NP interaction, co-IPs were performed with WT and mutant full-length VP35 proteins which were expressed with NP in HEK293T cells (Fig. 7). First basic patch mutants R225A and K248A displayed reduced levels of VP35 expression in this experiment relative to the level of WT VP35 expression and an unusual migration pattern in which a significant amount of each protein, as detected in the Western blot, ran at a lower rate than was expected. These mutants did not display any detectable interaction with NP. The K251A mutant displayed a greatly reduced interaction with NP. The H240A mutant was also assessed. When it was expressed from the plasmid used in the co-IP, the H240A mutant was detectably expressed, but a fraction of the protein exhibited aberrant migration, similar to that seen with the R225A and K248A mutants. This mutant also lacked the ability to efficiently interact with NP (Fig. 7). The VP35 mutants with a first basic patch mutation to glutamic acid were also tested for their interaction with NP. These VP35 mutants showed a significant reduction in NP binding compared to WT VP35 (data not shown). This reduced binding to NP most likely explains the lack of copolymerase activity demonstrated by these mutants in Fig. 4A. In contrast to the first basic patch mutants, the border residue mutants retained the capacity to interact with NP, despite their loss of polymerase cofactor function. The loss of polymerase cofactor function without the loss of NP interaction is not unique to mutants at these border basic residues. For example, we also assessed interaction with NP by mutants with mutations at either of two phenylalanines important for VP35 end capping of dsRNAs. Mutant F235A was previously demonstrated to lose function as a polymerase cofactor, while mutant F239A retained this function (25). However, both mutants retain the ability to interact with NP (Fig. 7).
FIG. 7.
Full-length WT and mutant VP35 interactions with EBOV NP. Cells were transfected with WT or mutant VP35 and EBOV NP. The left-most lane (−) corresponds to a sample in which NP was expressed in the absence of VP35. Lysates were subjected to IP with anti-VP35 antibody. Proteins were detected by Western blotting with anti-NP and anti-VP35 monoclonal antibodies.
VP35 IID is sufficient to interact with NP.
We next asked whether the VP35 IID is sufficient to interact with NP. Pull-downs were performed by mixing purified MBP-VP35 IID fusions with lysates of HEK293T cells transfected with the NP expression plasmid. MBP alone was used as a negative control. NP was not precipitated in the absence of added protein or in the presence of MBP alone. In contrast, WT MBP-VP35 IID precipitated NP, demonstrating that the VP35 IID is sufficient for interaction with NP (Fig. 8 A). When the first basic patch mutants were assessed for NP binding, only the MBP-VP35 IID K222A mutant retained binding to NP, consistent with the fact that this mutant retains its function in the polymerase complex. In contrast, the other first basic patch mutants, which lost function as a polymerase cofactor, also lost their interaction with NP. Specifically, MBP-VP35 IID R225A and K248A mutants lacked any detectable binding to NP, whereas the MBP-VP35 IID K251A mutant showed significantly reduced coprecipitation with NP (Fig. 8B). Also consistent with the results of the co-IP assays, MBP-VP35 IID F235A and F239A mutants retained the ability to bind to NP, while MBP-VP35 IID H240A did not pull down NP (Fig. 8B).
FIG. 8.
The VP35 IID is sufficient to interact with NP, and this interaction is impaired by either specific point mutations or the presence of dsRNA. (A) MBP or MBP-VP35 IID was added to NP-transfected cell lysates. MBP was immobilized by amylose resin, and proteins were detected with anti-MBP (α-MBP) or anti-NP (α-NP) antibodies. (B) MBP alone, MBP-VP35 IID WT, or mutants with point mutations were added to lysates containing EBOV NP. MBP was precipitated using amylose resin and was detected with NP and MBP antibodies. (C) MBP, WT MPB-VP35 IID, or MBP-VP35 IID mutants (R312A, K322A, or F239A) were added to NP-transfected lysates in the absence or presence of poly(I:C). The final concentration of each MBP was 2.4 μM, and poly(I:C) was present at either 16 or 98 nM. MBPs were pulled down, and both MBP and NP were detected in the same way as described for panels A and B. The input whole-cell extract lane (WCE) expressing NP represents 4% of the total lysate used for each group, while the MBP pull-down lane represents 15% of the total pull down.
Recent structural studies suggest that dsRNA binding can lead to high-order complex formation between dsRNA and VP35 (D. W. Leung, G. K. Amarasinghe, and C. F. Basler, unpublished observations). In order to assess if VP35-NP interactions are sensitive to the dsRNA binding functionality of VP35, we analyzed VP35-NP interactions in the presence of dsRNA. To this end, increasing amounts of poly(I:C) were added to a MBP-VP35 IID-NP coprecipitation experiment. The left panel of Fig. 8C represents the input amount of NP before the addition of each MBP. As the poly(I:C) concentration increased, the WT VP35 IID-NP interaction was impaired (Fig. 8C, right panel). In contrast, the ability of the central basic patch dsRNA binding mutants R312A and R322A to interact with NP was unaffected by the dsRNA (Fig. 8C, right panel). F239A is an end-cap residue mutant that also displays reduced dsRNA binding (25). As demonstrated above (Fig. 7), this mutant exhibits reduced binding to NP. Consistent with the results obtained with dsRNA binding mutants R312A and R322A, interaction of this mutant with NP was unaffected by addition of poly(I:C) (Fig. 8C). Therefore, these results suggest that dsRNA binding by the VP35 central basic patch can modulate the VP35-NP interaction.
Impact of basic residue mutations on VP35 interaction with L.
VP35 interaction with L is also required for filoviral RNA synthesis (28). Characterizing the interactions of filovirus L with other components of the viral RNA polymerase complex is complicated by difficulties in detecting the expression of full-length L. However, a previous study demonstrated that the marburgvirus (MARV) VP35 can interact with the amino-terminal 503 amino acids of the MARV L (28). This interaction required the presence of a functional coiled-coil oligomerization motif within the amino-terminal half of VP35 (28). We therefore screened WT and mutant full-length ZEBOV VP35 proteins for interaction with a similar construct, HA-tagged ZEBOV L (encompassing amino acids 1 to 505). Full-length VP35, as well as all mutants tested, retained interaction with L (Fig. 9). Therefore, loss of interaction between VP35 and L does not appear to explain why mutation of the basic residues surrounding the central basic patch causes loss of polymerase cofactor function.
FIG. 9.
Full-length WT and mutant VP35 interactions with EBOV L. Cells were transfected with WT or mutant VP35 and EBOV HA-L amino acids 1 to 505 (L1-505). Lysates were immunoprecipitated with anti-VP35 antibody and protein G beads. Proteins were detected with HA or VP35 monoclonal antibodies. WT and mutant full-length VP35 can coimmunoprecipitate with HA-L amino acids 1 to 505. WC, whole-cell extract.
DISCUSSION
The molecular basis for the high virulence of EBOVs is incompletely understood. Recent studies have, however, provided insight into the structure and function of VP35, a protein that functions as a virulence factor, that suppresses innate immunity, and that acts as an essential component of the viral polymerase (21, 23, 25, 26). To date, VP35 virulence factor function has been correlated with its ability to bind to dsRNA and to suppress IFN-α/β responses. Its function as part of the viral polymerase complex appears to require its interaction with the viral NP and L (4, 29, 30). Notable structural features of VP35 relevant to its IFN-antagonist function are a central basic patch and a set of hydrophobic residues that end cap dsRNAs within the carboxy-terminal IID (25). Mutations to either of these sets of residues can disrupt VP35-dsRNA binding and impair its ability to inhibit IFN-α/β production. Central basic patch mutations are also sufficient to severely attenuate virus replication (15, 33). The present studies have identified additional features within the VP35 IID that are also of functional importance. In contrast to the central basic patch and end-cap residues, the current study demonstrates that the residues of the first basic patch as well as other basic residues surrounding the central basic patch are not required for VP35 interaction with dsRNA. These residues are, however, essential for VP35 polymerase cofactor function.
VP35 is an essential component of the filoviral polymerase complex. This has been demonstrated with systems analogous to the minigenome system described above (28-30). The minimal set of filoviral proteins required for transcription and replication for MARV are NP, VP35, and L, although recovery of recombinant MARVs from cDNA also required expression of VP30 (9). Transcription of EBOV subgenomic replicon RNAs requires NP, VP35, VP30, and L; however, the replication reaction can proceed in the absence of VP30 (5, 30). Omission of VP35 from either system abolishes reporter gene expression, demonstrating an essential role for VP35 as a viral polymerase cofactor. The importance of VP35 for viral polymerase function is also supported by the observation that Zaire EBOV VP35 could not functionally substitute for Reston EBOV VP35 when the remaining nucleocapsid proteins, NP, VP30, and L, were Reston EBOV derived (5).
VP35 interactions with NP and with L are expected to be essential for viral polymerase function. Similar to rhabdovirus and paramyxovirus systems, where viral phosphoprotein is thought to be the functional equivalent of VP35, VP35 can complex with L, the enzymatic subunit of viral polymerase (4). Because viral genomes and antigenomes are NP associated, VP35 presumably brings L to its template RNAs. On the basis of similarity to rhabdoviruses and paramyxoviruses, VP35 may also chaperone NP to prevent its association with cellular RNAs. Consistent with these models, VP35 from either EBOV or MARV has been demonstrated to interact with NP and L by colocalization and coimmunoprecipitation studies (4, 28). Our current work demonstrates that the VP35 C-terminal IID is sufficient for interaction with NP and that the first basic patch residues are critical for both viral polymerase function and VP35-NP interaction. These studies also provide the first residue-specific interaction sites on VP35 that are important for VP35-NP binding. The behavior of these mutants in the minigenome system further demonstrates that the interactions between VP35 and NP are critical for viral polymerase function. Our data presented above indicate that the C-terminal VP35 IID is sufficient to interact with NP, suggesting that VP35 may possess a single NP-interacting interface, although this remains to be formally tested, as the VP35 N-terminal oligomerization domain may contain additional interaction elements (28). Additionally, our results from VP35-NP binding in the presence of dsRNA suggests that although residues in the central basic patch are not important for VP35-NP binding, this interaction may be sensitive to the oligomerization state(s) that results from VP35 binding to dsRNA. Several lines of evidence, including our own unpublished data, suggest that VP35 IID can form multimeric complexes upon dsRNA binding. Therefore, the observed loss of VP35-NP binding for WT VP35 but not for VP35 mutants that lack dsRNA binding suggests that the NP binding site is likely to include elements outside the first basic patch.
The basis for loss of polymerase function upon mutation of the basic residues bordering the central basic patch remains undefined. MARV VP35 was previously demonstrated to interact with amino acids 1 to 503 of MARV L by coprecipitation, and the amino-terminal 879 amino acids of Reston EBOV L were sufficient to interact with the Zaire or Reston EBOV NP-VP35 complex, as assessed by colocalization studies (5, 28). We therefore tested ZEBOV WT and mutant VP35 proteins for interaction with ZEBOV L amino acids 1 to 505. Because MARV VP35 requires an intact oligomerization function and because this oligomerization function is mediated by an amino-terminal coiled-coil motif, we tested full-length VP35 rather than monomeric VP35 IID for interaction with the L amino terminus. Our data demonstrate that the N-terminal region of L is sufficient to interact with full-length VP35 and that all of the mutants tested retain the ability to interact with this region of L. Therefore, the loss of polymerase cofactor function upon mutation of the basic residues that border the central basic patch is unlikely to be due to the loss of VP35-L interactions.
EBOV VP35 is reported to interact with a variety of other viral and host proteins (7, 20, 22, 32). For example, VP35 is reported to interact with NP to form filamentous nucleocapsids when these proteins are coexpressed with the viral VP24 protein (19, 36). EBOV VP35 interaction with the EBOV matrix protein VP40 has been demonstrated by a mammalian two-hybrid assay and by immunofluorescence studies, and this interaction could mediate packaging of a model viral genomic RNA into virus-like particles (20). VP35 interactions with cellular kinases IκB kinase ɛ and TANK binding kinase 1 (TBK-1) and with interferon regulatory factor 7 (IRF7), ubiquitin-conjugating enzyme 9 (Ubc9), and protein inhibitor of activated signal transducer and activators of transcription 1 (PIAS1) are reported to influence its capacity to inhibit IFN-α/β production (7, 32). VP35 also inhibits the IFN-induced, dsRNA-activated kinase PKR, potentially countering the antiviral effects of PKR (10, 35), and can also inhibit RNA silencing (12). Whether the basic residues studied here affect these interactions and functions remains to be determined.
Currently, there are no approved vaccines or treatment for the EBOVs, in large part due to the paucity of knowledge regarding the specific molecular features critical for the function of viral components. Therefore, the identification of structural features, such as those described here, can potentially facilitate development of antivirals. To this end, we recently demonstrated that mutation to alanine of two basic residues within the central basic patch, K319 and R322, abrogated VP35 dsRNA binding activity and greatly reduced its ability to inhibit IFN-α/β production. This mutation did not significantly impair VP35 function as a polymerase cofactor (33). However, when they were introduced into a recombinant EBOV, the same mutations in VP35 greatly attenuated virus replication in cells that produce IFN-α/β and rendered the virus avirulent in guinea pigs (33). Mutation of another central basic patch residue, R312, also impaired the ability of a recombinant EBOV to suppress IFN responses and greatly attenuated replication in mice (15-17). Together, these data validate the central basic patch as a potential target for antivirals. Similarly, the hydrophobic residues that mediate VP35 interaction with the ends of dsRNA are required for full IFN-antagonist function and are therefore candidate targets as well (25). The present study defines additional elements within the VP35 IID, including the first basic patch and residues that border the central basic patch, as specific targets for antiviral development. Strategies to target these residues through mutations or with inhibitors that interact with these residues would be predicted to impair virus polymerase function, thereby limiting virus replication.
Acknowledgments
This work is supported by NIH grants (grant 1R56AI089547 to C.F.B. and G.K.A., grant 1F32AI084324 to D.W.L., grants R01AI059536 and AI057158 [Northeast Biodefense Center-Lipkin] to C.F.B., grant 1F32AI084453 to R.S.S., and grant R01AI081914 to G.K.A.), an MRCE developmental grant (grant U54AI057160-Virgin to G.K.A.), and the Roy J. Carver Charitable Trust (grant 09-3271 to G.K.A.).
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Basler, C. F., and G. K. Amarasinghe. 2009. Evasion of interferon responses by Ebola and Marburg viruses. J. Interferon Cytokine Res. 29:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basler, C. F., A. Mikulasova, L. Martinez-Sobrido, J. Paragas, E. Muhlberger, M. Bray, H. D. Klenk, P. Palese, and A. Garcia-Sastre. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77:7945-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. U. S. A. 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, S., C. Rinne, U. Hofsass, H. D. Klenk, and E. Muhlberger. 1998. Interactions of Marburg virus nucleocapsid proteins. Virology 249:406-417. [DOI] [PubMed] [Google Scholar]
- 5.Boehmann, Y., S. Enterlein, A. Randolf, and E. Muhlberger. 2005. A reconstituted replication and transcription system for Ebola virus Reston and comparison with Ebola virus Zaire. Virology 332:406-417. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas, W. B., Y. M. Loo, M. Gale, Jr., A. L. Hartman, C. R. Kimberlin, L. Martinez-Sobrido, E. O. Saphire, and C. F. Basler. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, T. H., T. Kubota, M. Matsuoka, S. Jones, S. B. Bradfute, M. Bray, and K. Ozato. 2009. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 5:e1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiCarlo, A., P. Moller, A. Lander, L. Kolesnikova, and S. Becker. 2007. Nucleocapsid formation and RNA synthesis of Marburg virus is dependent on two coiled coil motifs in the nucleoprotein. Virol. J. 4:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enterlein, S., V. Volchkov, M. Weik, L. Kolesnikova, V. Volchkova, H. D. Klenk, and E. Muhlberger. 2006. Rescue of recombinant Marburg virus from cDNA is dependent on nucleocapsid protein VP30. J. Virol. 80:1038-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, Z., M. Cerveny, Z. Yan, and B. He. 2007. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J. Virol. 81:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, M., C. Spiropoulou, and P. E. Rollin. 2007. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub-populations in vitro. Virology 364:45-54. [DOI] [PubMed] [Google Scholar]
- 12.Haasnoot, J., W. de Vries, E. J. Geutjes, M. Prins, P. de Haan, and B. Berkhout. 2007. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog. 3:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habjan, M., I. Andersson, J. Klingstrom, M. Schumann, A. Martin, P. Zimmermann, V. Wagner, A. Pichlmair, U. Schneider, E. Muhlberger, A. Mirazimi, and F. Weber. 2008. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS One 3:e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harcourt, B. H., A. Sanchez, and M. K. Offermann. 1998. Ebola virus inhibits induction of genes by double-stranded RNA in endothelial cells. Virology 252:179-188. [DOI] [PubMed] [Google Scholar]
- 15.Hartman, A. L., B. H. Bird, J. S. Towner, Z. A. Antoniadou, S. R. Zaki, and S. T. Nichol. 2008. Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of Ebola virus. J. Virol. 82:2699-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman, A. L., J. E. Dover, J. S. Towner, and S. T. Nichol. 2006. Reverse genetic generation of recombinant Zaire Ebola viruses containing disrupted IRF-3 inhibitory domains results in attenuated virus growth in vitro and higher levels of IRF-3 activation without inhibiting viral transcription or replication. J. Virol. 80:6430-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman, A. L., L. Ling, S. T. Nichol, and M. L. Hibberd. 2008. Whole genome expression profiling reveals that inhibition of host innate immune response pathways by Ebola virus can be reversed by a single amino acid change in the VP35 protein. J. Virol. 82:5348-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartman, A. L., J. S. Towner, and S. T. Nichol. 2004. A C-terminal basic amino acid motif of Zaire Ebolavirus VP35 is essential for type I interferon antagonism and displays high identity with the RNA-binding domain of another interferon antagonist, the NS1 protein of influenza A virus. Virology 328:177-184. [DOI] [PubMed] [Google Scholar]
- 19.Huang, Y., L. Xu, Y. Sun, and G. Nabel. 2002. The assembly of Ebola virus nucleocapsid requires virion-associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol. Cell 10:307. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, R. F., S. E. McCarthy, P. J. Godlewski, and R. N. Harty. 2006. Ebola virus VP35-VP40 interaction is sufficient for packaging 3E-5E minigenome RNA into virus-like particles. J. Virol. 80:5135-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimberlin, C. R., Z. A. Bornholdt, S. Li, V. L. Woods, Jr., I. J. MacRae, and E. O. Saphire. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc. Natl. Acad. Sci. U. S. A. 107:314-319. [DOI] [PMC free article] [PubMed]
- 22.Kubota, T., M. Matsuoka, T. H. Chang, M. Bray, S. Jones, M. Tashiro, A. Kato, and K. Ozato. 2009. Ebolavirus VP35 interacts with the cytoplasmic dynein light chain 8. J. Virol. 83:6952-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung, D. W., N. D. Ginder, D. B. Fulton, J. Nix, C. F. Basler, R. B. Honzatko, and G. K. Amarasinghe. 2009. Structure of the Ebola VP35 interferon inhibitory domain. Proc. Natl. Acad. Sci. U. S. A. 106:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung, D. W., N. D. Ginder, J. C. Nix, C. F. Basler, R. B. Honzatko, and G. K. Amarasinghe. 2009. Expression, purification, crystallization and preliminary X-ray studies of the Ebola VP35 interferon inhibitory domain. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65:163-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung, D. W., K. C. Prins, D. M. Borek, M. Farahbakhsh, J. M. Tufariello, P. Ramanan, J. C. Nix, L. A. Helgeson, Z. Otwinowski, R. B. Honzatko, C. F. Basler, and G. K. Amarasinghe. 2010. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat. Struct. Mol. Biol. 17:165-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung, D. W., R. S. Shabman, M. Farahbakhsh, K. C. Prins, D. M. Borek, T. Wang, E. Muhlberger, C. F. Basler, and G. K. Amarasinghe. 2010. Structural and functional characterization of Reston Ebola VP35 interferon inhibitory domain. J. Mol. Biol. 399:347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez, O., C. Valmas, and C. F. Basler. 2007. Ebola virus-like particle-induced activation of NF-kappaB and Erk signaling in human dendritic cells requires the glycoprotein mucin domain. Virology 364:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller, P., N. Pariente, H. D. Klenk, and S. Becker. 2005. Homo-oligomerization of Marburgvirus VP35 is essential for its function in replication and transcription. J. Virol. 79:14876-14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlberger, E., B. Lotfering, H. D. Klenk, and S. Becker. 1998. Three of the four nucleocapsid proteins of Marburg virus, NP, VP35, and L, are sufficient to mediate replication and transcription of Marburg virus-specific monocistronic minigenomes. J. Virol. 72:8756-8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhlberger, E., M. Weik, V. E. Volchkov, H. D. Klenk, and S. Becker. 1999. Comparison of the transcription and replication strategies of Marburg virus and Ebola virus by using artificial replication systems. J. Virol. 73:2333-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 32.Prins, K. C., W. B. Cardenas, and C. F. Basler. 2009. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J. Virol. 83:3069-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prins, K. C., S. Delpeut, D. W. Leung, O. Reynard, V. A. Volchkova, S. P. Reid, P. Ramanan, W. B. Cardenas, G. K. Amarasinghe, V. E. Volchkov, and C. F. Basler. 2010. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J. Virol. 84:3004-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez, A., T. W. Geisbert, and H. Feldmann. 2007. Filoviridae: Marburg and Ebola viruses, p. 1410-1448. In D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 35.Schumann, M., T. Gantke, and E. Muhlberger. 2009. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J. Virol. 83:8993-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe, S., T. Noda, and Y. Kawaoka. 2006. Functional mapping of the nucleoprotein of Ebola virus. J. Virol. 80:3743-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]