Abstract
Initiation of highly active antiretroviral therapy (HAART) for HIV-infected individuals is associated with control of viremia, improved CD4 counts, and declining systemic HIV-specific immune responses. While HAART effectively reduces plasma viremia, it remains unclear how effectively antiretroviral drugs reach mucosal surfaces, such as those of the genital tract. The aim of this study was to determine the effect of HAART on genital tract CD4 T cell reconstitution, HIV shedding, and HIV-specific T cell responses. Cervical cytobrush and blood specimens were obtained from 35 HIV-infected, HAART-naïve women and 27 women on HAART in order to investigate HIV Gag-specific T cell responses by intracellular gamma interferon (IFN-γ) staining. Interleukin 1β (IL-1β), IL-6, and IL-8 concentrations were measured by enzyme-linked immunosorbent assays (ELISA). We show that for HIV-infected women, HAART is associated with significantly improved CD4 T cell counts both in blood and at the cervix. While HAART effectively suppressed both blood and cervical viremia, HIV-specific CD8 T cell responses in blood were lost, while those at the cervix were preserved.
South Africa currently has the largest number of individuals in the world infected with HIV (5.7 million), and the risk of HIV infection for young South African women is almost 6-fold that of young men (28. Highly active antiretroviral therapy (HAART) suppresses HIV replication and restores peripheral CD4 T cell counts in most HIV-infected individuals (2, 4, 57), and wide access to HAART has greatly reduced HIV-related morbidity and mortality rates worldwide (26). In South Africa, the eligibility criteria for the initiation of antiretroviral therapy are a blood CD4 count of less than 350 cells/μl or stage IV (AIDS) (59), and the current national recommendations for first-line treatment include the nucleoside reverse transcriptase inhibitors (NRTIs) lamivudine (3TC) and stavudine (d4T) combined with the nonnucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV) or nevirapine (38). In 2007, it was estimated that of the ∼889,000 HIV-infected individuals in South Africa needing HAART, only 371,731 (42%) were receiving treatment (50).
While HAART clearly reduces plasma viremia, it is unclear how various antiretroviral drugs influence HIV replication, virus shedding, and host immune reconstitution at mucosal surfaces of the genital tract, where HIV transmission occurs. NRTIs have been shown to reach the female genital tract more efficiently than protease inhibitors (PIs) and NNRTIs (16, 33). Although individuals on HAART (HAART+ individuals) shed fewer HIV virions from mucosal surfaces than women not on HAART (HAART− women) (13, 18, 20), it is apparent that HAART, even when administered early during infection, does not reliably result in reconstitution of T cell populations at these surfaces (22, 29, 37). This is perhaps not surprising, since in the absence of HAART, CD4 T cell restoration and preservation during HIV infection are generally less efficient at mucosal sites than in blood (23, 38). Ongoing low levels of HIV replication, the persistence of proviral DNA within infected cells of the mucosa (19, 43, 52, 56), and the poor penetration of drugs through to the mucosa have been implicated in the incomplete reconstitution of mucosal CD4 counts seen in individuals receiving HAART (17, 34).
Many studies have demonstrated that prolonged HAART is associated with a waning of HIV-specific cytotoxic T-lymphocyte (CTL) responses in blood, suggesting that ongoing antigenic stimulation provided by low-level HIV replication is necessary for the persistence of HIV-specific immune responses (1, 22, 31, 42, 60). However, other studies report the opposite findings, where blood HIV-specific responses increase after the initiation of HAART (2, 46). These discrepancies could be attributable to variations in both the time of treatment initiation and the degree to which viral replication is suppressed (44).
The influences of HAART on mucosal anti-HIV responses may be similar to those seen in blood. In the rectal mucosa, for example, the total suppression of HIV replication by HAART significantly reduced HIV-specific CD8 T cell responses at this site (12). This supports the hypothesis that persistent antigenic stimulation may be needed at mucosal surfaces in order to support cytotoxic responses at these sites.
HIV-specific T cell responses have also been identified in the genital tracts of HAART-naïve HIV-infected women (25, 52). The female genital tract has a large proportion of activated CD4 T cells expressing CCR5 that are preferentially infected by HIV (26). Little is known about either fluctuations in genital tract CD4 T cell numbers during HAART or the impact of therapy on the immune function of these cells. We have previously shown significantly reduced CD4/CD8 ratios at the cervices of chronically HIV infected women compared to uninfected women, suggesting that CD4 T cells are significantly depleted in the female genital tract during HIV infection (41). Previous studies have found near-complete restoration of CD4 T cell numbers in the gut mucosa following HAART (7, 38, 54) and have suggested that the differences observed in post-HAART CD4 T cell numbers may be attributable to differences in pre-HAART CD4 counts, the stage of HIV infection, or the specific gut regions where biopsy specimens were taken (54). The aim of this study was to investigate the impact of HAART on female genital tract CD4 T cell reconstitution, inflammation, local HIV shedding in genital secretions, and HIV-specific T cell immunity in the genital tracts of HAART-compliant women in South Africa.
MATERIALS AND METHODS
Study participants.
One hundred and eight women with chronic HIV infections who attended the Nyanga Day Hospital, Cape Town, South Africa, were recruited for this study. Of these women, 45/108 (41.7%) had initiated antiretroviral therapy and 63/108 (58.3%) were therapy naïve (see Fig. S1 in the supplemental material). Women who either were menstruating at the time of sampling, were postmenopausal, had undergone a hysterectomy, had vaginal discharge, or had visible or reported sexually transmitted infections (STIs) were excluded from the study. The study was approved by the Faculty of Health Sciences Human Research Ethics Committee of the University of Cape Town, South Africa. Written informed consent was obtained from all women before initiation of the study.
Collection and processing of cervical specimens.
Cervical lymphocytes were collected using a Digene cervical sampler according to a previously described method (25, 41). Briefly, cells were collected by using a single gentle 360° rotation of a cytobrush at the cervical os. The cytobrush was placed in 3 ml transport medium (R10, consisting of RPMI-1640 medium supplemented with 5 mM glutamine, fungin, penicillin-streptomycin, and 10% fetal calf serum [FCS]). Processing was performed within 4 h of collection to maximize recoverable cell yields and to maintain cellular viability. Cervical samples that had visible red blood cell contamination were discarded. Each cytobrush was flushed ∼30 times with R10 in the collection tube using a Pasteur pipette. The cell suspension was then transferred to a clean 15-ml tube and centrifuged at 2,300 rpm (1,000 × g) for 10 min. The supernatant was stored at −80°C for analysis of HIV shedding and genital inflammation. The pelleted cells were resuspended in R10 prior to processing for counting and phenotyping using an automated Guava cell counter (41) (Guava Technologies, Hayward, CA).
PBMC isolation.
Blood was collected by venipuncture into sterile acid-citrate-dextrose anticoagulated Vacutainer tubes (Becton Dickinson). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque density gradient centrifugation with Leucosep tubes and were counted using an automated Guava cell counter and Viacount staining (Guava). Plasma was stored at −80°C for viral load determination. Cell concentrations were adjusted to 2 × 106/ml, and the cells were rested for 16 h at 37°C under 5% CO2. All experiments were carried out on fresh PBMCs.
Intracellular cytokine staining.
To investigate the function and frequency of HIV Gag-specific responses by CD8 and CD4 T cells, PBMCs (1 × 106/ml) or cervical cells (∼0.15 × 106 to 1 × 106 lymphocytes/ml) were split into three BD Falcon tubes (500 μl/tube) and were either (i) stimulated with a single pool of 121 HIV subtype C (Du422) Gag overlapping peptides (each peptide was used at a final concentration of 1 μg/ml; peptides were kindly provided by the NIH AIDS Reagent Repository), (ii) stimulated with phorbol myristate acetate (PMA)-ionomycin (10 μg/ml each; Sigma-Aldrich) as a positive control, or (iii) left untreated for 4 to 6 h at 37°C under 5% CO2. Brefeldin A (10 μg/ml; Sigma, St. Louis, MO) was added after the first hour. In these experiments, cell fluorescence was measured by flow cytometry using a FACSCalibur (for cells from 11/45 HAART+ and 22/63 HAART− women) or LSRII (for cells from 34/45 HAART+ versus 41/63 HAART− women) flow cytometer (BD Immunocytometry Systems [BDIS]). For experiments using the LSRII flow cytometer, PBMCs and cervical cells were first incubated with the LIVE/DEAD Fixable Violet Dead Cell Stain for 20 min at room temperature (RT) and were then washed twice with phosphate-buffered saline (PBS). This step was not included for samples analyzed using the FACSCalibur flow cytometer. All staining was done in 96-well plates. Extracellular staining was performed for 30 min at RT with phenotypic markers using anti-CD8 conjugated to fluorescein isothiocyanate (FITC) or peridinin chlorophyll protein (PerCP) Cy5.5 (Becton Dickinson, San Jose, CA) and, for LSRII experiments only, anti-CD4 labeled with phycoerythrin (PE) Cy5 or PE Cy7 (BD) (this stain was omitted for FACSCalibur experiments). Cells were washed once by addition of 2 ml of 1% FCS-PBS, centrifuged (5 min, 300 × g [1,300 rpm], RT), fixed, permeabilized using BD CytoFix/CytoPerm for 10 min at room temperature, and washed once with Perm wash (BD). Cells were stained with allophycocyanin (APC)- or APC-H7-labeled anti-CD3, PE-conjugated anti-gamma interferon (anti-IFN-γ), or Alexa Fluor 700-conjugated anti-IFN-γ (both from BD Biosciences) for 30 min on ice. Cells were finally washed with 2 ml of 1% FCS-PBS, centrifuged (5 min, 300 × g, 1,300 rpm, room temperature), and fixed with BD Cell Fix. Acquisition was performed using either a FACSCalibur or an LSRII flow cytometer (BD Immunocytometry Systems), with FlowJo (Tree Star, Inc.) for data analysis. Figure S2 in the supplemental material shows the gating strategy used to define IFN-γ production by either CD4 or CD8 T cells in response to Gag.
Cervical cytobrush samples were excluded if the positive control (PMA-ionomycin) failed (samples from 10/45 HAART+ versus 18/63 HAART− women) or if the samples contained less than 250 CD3+ events by flow cytometry (8/45 HAART+ versus 10/63 HAART− samples [see Fig. S1 in the supplemental material]). Of the 62 samples remaining, 27/62 were from HAART+ women and 35/62 were from HAART− women. An average (± standard deviation [SD]) of 1,247 (±1,404) CD3+ T cells were acquired per cervical cytobrush sample during intracellular cytokine staining.
Measurement of HIV loads in cervical secretions and plasma.
Viral loads were determined in cervical secretions and plasma samples using Nuclisens Easyq HIV 1, version 1.2. Cervical secretions were obtained after flushing of the cytobrush 30 times in 3 ml transport medium and removal of cells by centrifugation (250 × g for 10 min). Plasma was obtained from ACD-anticoagulated whole blood following Ficoll density gradient centrifugation. The detection limit of this assay was 50 HIV RNA copies/ml. Women were considered to be shedding HIV in their genital secretions if they had cervical viral loads of ≥300 HIV RNA copies/ml of cervical wash fraction.
Measurement of antiretroviral drug concentrations in plasma.
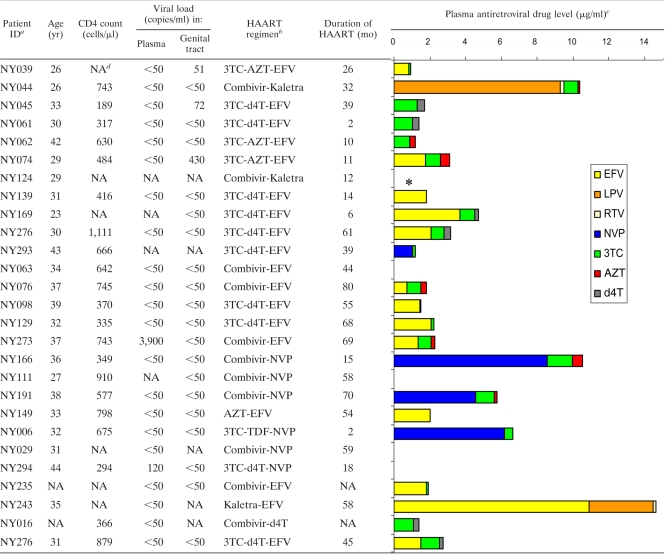
Antiretroviral drug concentrations were determined by liquid chromatography-tandem mass spectrometry (LC-MS-MS) using the modified methods described by Chi et al. (10) and Mistri et al. (39). Phenomenex Gemini C18 (5 by 2 by 5 μm) and Synergy Fusion C18 RP (50 by 2 by 4 μm) columns were used for NNRTIs/PIs and NRTIs, respectively. The plasma calibration curves of efavirenz, nevirapine, lopinavir, and ritonavir were linear over the range of 0.20 to 15 mg/liter, 0.25 to 10 mg/liter, 0.05 to 20 mg/liter, and 0.025 to 5 mg/liter, respectively. The plasma calibration curves of lamivudine, zidovudine, and stavudine were linear over the range of 0.02 to 6 mg/liter. Details of the lower limit of quantification (LLOQ), accuracy, and intraday and interday precision for each antiretroviral drug are included in Table S1 in the supplemental material.
Measurement of inflammatory cytokine concentrations in genital secretions.
The concentrations of interleukin 1β (IL-1β), IL-6, and IL-8 in cervical supernatants were determined using Quantikine immunoassay enzyme-linked immunosorbent assay (ELISA) kits (detection limits, 0.70 pg/ml for IL-6, 1 pg/ml for IL-1β, and 1.5 pg/ml for IL-8) (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The plates were read on a VersaMax ELISA microplate reader, and data were analyzed using SoftMax Pro software (version 4.3.1; Molecular Devices, CA).
Statistical analysis.
A Mann-Whitney U test for nonparametric unmatched data was used for independent sample comparisons; Wilcoxon rank tests were used for nonparametric dependent sample comparisons; Chi-square tests were used for comparison of proportions, and Spearman tests were used for correlations, using GraphPad Prism, version 5.0. P values of ≤0.05 were considered significant.
RESULTS
Of the 108 HIV-infected women enrolled in the study, 27/45 women receiving HAART (HAART+) and 35/63 women naïve to HAART (HAART−) were eligible for inclusion in this study to investigate the impact of HAART on HIV shedding and T cell immunity in the female genital tract (Table 1; see also Fig. S1 in the supplemental material). The majority of HAART+ women were on first-line combination HAART (88.9%) (40), which includes two nucleoside reverse transcriptase inhibitors (NRTIs) and one nonnucleoside reverse transcriptase inhibitor (NNRTI) (Table 2). Of these women, 37% were on lamivudine (3TC)-stavudine (d4T)-efavirenz (EFV) triple-drug therapy and 25.9% were on 3TC-zidovudine (AZT)-EFV (Table 2). Only 11.1% of the women in this study (NY044, NY124, and NY243) were on second-line therapy, which included two NRTIs and two protease inhibitors (Combivir [3TC-AZT] and Kaletra [ritonavir-lopinavir]). The HIV-infected women receiving HAART had been on therapy for a median of 35.5 months (range, 2 to 80 months). The majority of HAART+ HIV-infected women (85.1%) were found to have antiretroviral drugs detectable in plasma at the time of study, indicating drug compliance (Table 2).
TABLE 1.
Clinical characteristics of HIV-infected women included in this study
| Patient characteristic | Value for groupa |
P | |
|---|---|---|---|
| HAART− | HAART+ | ||
| No. | 35 | 27 | |
| Age (yr) | 32 (24-44) | 34 (23-44) | 0.3 |
| CD4 count (cells/μl) | 408 (206-919) | 630 (294-1,111) | 0.03 |
| Plasma viral load (copies/ml) | 7,800 (<50-61,000) | <50 (<50-3,900) | 0.006 |
| No. with detectable plasma HIV RNA/total no. (%) | 14/19 (73.7)b | 1/24 (4.2)c | <0.0001 |
| Genital viral load (copies/ml) | 325 (<50-28,000) | <50 (<50-430) | 0.002 |
| No. with detectable cervical HIV RNA/total no. (%) | 15/22 (68.2)b | 1/22 (4.5)c | <0.0001 |
| Duration on ARV (months) | − | 35.5 (2-80) | |
Except where otherwise indicated, values are medians (ranges).
Only 19/35 plasma and 22/35 genital supernatant samples from HAART− women were available for evaluation of plasma and cervical HIV loads, respectively.
Only 24/27 plasma and 22/27 genital supernatant samples from HAART+ women were available for evaluation.
TABLE 2.
Details of the antiretroviral drug regimen and plasma concentrations for HIV-infected women on HAART included in this study
ID, identification number.
3TC, lamivudine; AZT, zidovudine; EFV, efavirenz; d4T, stavudine; NVP, nevirapine; TDF, tenofovir. Combivir is a coformulation of lamivudine and zidovudine in one pill. Kaletra is a coformulation of the HIV protease inhibitors lopinavir (LPV) and ritonavir (RTV).
NA, not available.
*, no plasma supernatant was available for determination of antiretroviral drug concentrations.
Impact of HAART on CD4 reconstitution in blood and in the female genital tract.
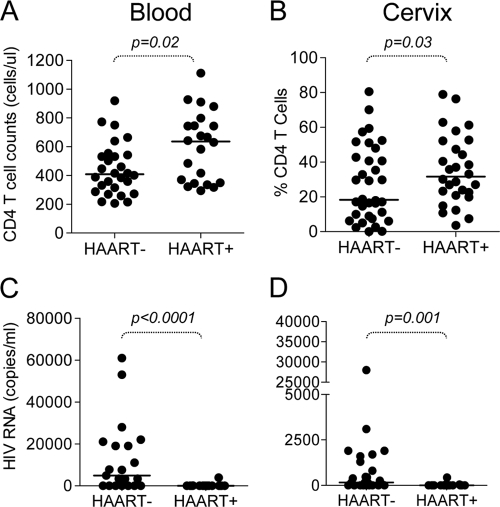
HIV-infected women receiving HAART had significantly higher CD4 counts in blood than HAART-naïve women (Table 1 and Fig. 1 A) (P, 0.02). We observed a significant positive correlation between time on therapy and blood CD4 cell counts (P, 0.05) (data not shown). For the majority of women on HAART, CD4 counts increased over time on therapy at an average rate of 32 CD4 cells/year of therapy (data not shown).
FIG. 1.
CD4 T cell reconstitution and HIV suppression in the blood and genital tracts of HIV-infected HAART− and HAART+ women. (A) CD4 T cell counts in the blood of HIV-infected HAART− and HAART+ women. (B) Percentages of CD4+ T cells measured in the genital tracts for HIV-infected HAART− and HAART+ women. (C) Plasma HIV loads (RNA copies/ml) for HAART− and HAART+ women. (D) HIV loads (RNA copies/ml in genital secretions from women on and off HAART. Each dot represents an individual woman's CD4 count or viral load; each horizontal line represents the median for the group. Statistical comparisons between HAART+ and HAART− women were performed using the Mann-Whitney U test for unmatched nonparametric data. P values of <0.05 were considered significant.
We have previously shown that HIV-infected women have significantly reduced CD4/CD8 ratios at the cervix compared to uninfected women, indicating a skewing toward CD8 T cell dominance in the genital tracts of HIV-infected women (41). To investigate the impact of HAART on CD4 reconstitution in the genital tract, we compared the percentages of CD4 T cells at the cervices of HAART− and HAART+ women (Fig. 1B). We found that HAART+ women had significantly higher frequencies of CD4 T cells in their genital tracts than HAART− women (P, 0.03), and correspondingly higher cervical CD4/CD8 ratios (data not shown), suggesting that HAART was associated with improved CD4 T cell numbers at the genital mucosa.
Impact of HAART on suppression of viremia in blood and at the cervix.
In HAART− women, the amount of HIV RNA detected in genital secretions was significantly associated with the amount of HIV RNA circulating in the blood (Rho, 0.7; P, 0.003 [data not shown]), indicating that genital tract HIV shedding was related to the level of plasma viremia.
Whereas we found that 73.7% of HAART− and 4.2% of HAART+ women had detectable HIV RNA in plasma, 68.2% of HAART− and 4.5% of HAART+ women had detectable HIV RNA in their genital secretions (Table 1). HAART− women had significantly higher plasma viral loads than HAART+ women (Table 1 and Fig. 1C) (P, <0.0001), while only one HAART+ woman had detectable HIV RNA in plasma (NY273, with 3,900 RNA copies/ml [Table 2]). Similarly, HAART− women had significantly higher concentrations of HIV detectable in genital secretions than HAART+ women (Table 1 and Fig. 1D) (P, 0.001), while only one HAART+ woman (NY074) had detectable virus in genital secretions (430 RNA copies/ml [Table 2]). It should be noted that the woman with incomplete plasma suppression (NY273) was not shedding HIV in the genital tract, while the woman shedding HIV in the genital tract (NY074) exhibited complete suppression of HIV in plasma and was HAART compliant (Table 2).
Genital tract inflammation and HIV shedding.
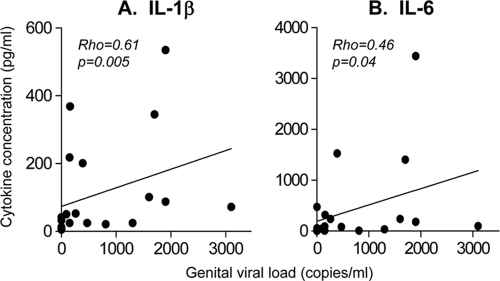
We have previously shown that HIV-infected women who shed HIV into their genital secretions had significantly higher genital IL-1β, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) concentrations than women who were not shedding HIV (25). Here we found that HAART− women had significantly higher concentrations of IL-8 (but not IL-1β and IL-6) than HAART+ women (P, 0.02) (Table 3). For HAART− women, concentrations of IL-1β (P, 0.005; Rho, 0.61) and IL-6 (P, 0.04; Rho, 0.46) in the genital tract were significantly associated with elevated HIV RNA concentrations in genital fluid (Fig. 2). Despite similar concentrations of IL-1β and IL-6 in genital secretions from HAART− and HAART+ women, only one HAART+ woman was shedding HIV, and this did not appear to be associated with elevated inflammation in the genital tract (data not shown).
TABLE 3.
Inflammatory cytokine concentrations in the genital tracts of HIV-infected HAART− and HAART+ women
| Cytokine | Median cytokine concn (range) for group |
P | |
|---|---|---|---|
| HAART− | HAART+ | ||
| IL-1β | 50.7 (5.1-535.3) | 28.9 (0.07-247.4) | 0.5 |
| IL-6 | 92.1 (6.3-3,442) | 63.6 (1.9-316.8) | 0.3 |
| IL-8 | 1,368 (85.0-5,899) | 435 (0.6-4756) | 0.02 |
FIG. 2.
Relationship between HIV shedding in the female genital tract and inflammatory cytokine concentrations. Concentrations of IL-1β (A) and IL-6 (B) in genital secretions from HIV-infected HAART− women were compared with the numbers of HIV RNA (copies/ml of cervical supernatant) detected in their genital tracts. Correlations were tested using the Spearman rank test, and P values of <0.05 were considered significant.
Impact of HAART on HIV-specific T cell responses in the genital tract.
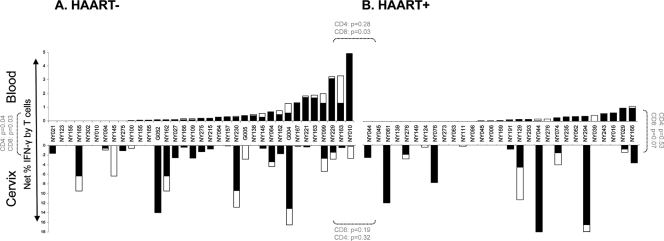
To evaluate the magnitude of T cell responses to HIV Gag in the genital tracts of HIV-infected women and to determine the impact of HAART on the reconstitution of T cell immunity in this compartment, we evaluated the frequencies of IFN-γ+ CD8 and CD4 T cells derived from the cervices of HAART+ and HAART− women (Fig. 3). We found that HAART− women had significantly higher frequencies of HIV-specific CD8 T cell IFN-γ responses detectable in blood than HAART+ women (P, 0.03) (Fig. 3A and B, top), suggesting that HAART significantly reduces the magnitude of CD8 T cell responses in blood to HIV (21, 29). In contrast, HAART− and HAART+ women did not differ in the magnitude of their cervical CD8 T cell responses to Gag (P, 0.2) (Fig. 3A and B, bottom), suggesting that genital tract CD8 T cell responses were not diminished by HAART. In addition, we found that Gag-specific CD8 responses in blood were significantly lower than those detected at the cervix for HAART− women (P, 0.03; Fig. 3A, top versus bottom), and this comparison approached significance for HAART+ women (P, 0.07) (Fig. 3B, top versus bottom).
FIG. 3.
HIV Gag-specific IFN-γ T cell responses detected in the blood and genital tracts of HIV-infected HAART− (A) or HAART+ (B) women. The responses of CD8 (filled stacked bars) and CD4 (open stacked bars) T cells in blood (top) and at the cervix (bottom) to Gag were measured in HAART− (A) and HAART+ (B) women. Each bar represents an individual woman's response at the cervix or in blood. Background-corrected net frequencies of IFN-γ+ CD8 T cells are shown. The Mann-Whitney U test was used to compare unmatched samples, while the Wilcoxon rank test was used to compare matched samples. P values of ≤0.05 were considered significant. P values for CD8 and CD4 T cell comparisons are shown separately in panels A and B.
HIV-specific CD8 responses detected in blood were not predictive of cervical CD8 T cell responses in either HAART− (Rho, 0.16; P, 0.40) or HAART+ (Rho, 0.11; P, 0.63) women (data not shown). For HAART+ women, we also detected no correlation between the frequency of Gag-specific IFN-γ+ CD8 T cells either in blood or at the cervix and improved CD4 counts in these compartments (data not shown). This indicates that CD4 reconstitution is probably not associated with an altered CD8 HIV-specific response in either compartment.
Previous studies have shown that CD4 T cell immunity in blood is impaired during HIV infection (3, 48) but is partially restored after the initiation of HAART (2, 4, 53). In this study, few HIV-infected women had detectable CD4 T cell responses to HIV Gag at the cervix or in blood (Fig. 3, open bars), although the magnitude of these responses was significantly higher at the cervix than in blood in HAART− women (P, 0.04) (Fig. 3A, top and bottom). No relationship was observed between the frequencies of IFN-γ+ CD4 T cells specific to Gag at the cervix and in blood, confirming that the compartments were independent (data not shown). In contrast to the differences we observed between Gag-responsive CD8 T cell frequencies between HAART− and HAART+ women, there were no obvious differences between these groups with respect to Gag-responsive CD4 T cell frequencies (P, 0.3) (Fig. 3A and B, top, open bars). There was no association between CD4 T cell reconstitution in blood or in the genital tract and the frequency of Gag-specific IFN-γ+ CD4 T cells detected in the genital tract or blood (although these were of low magnitude).
Together, these data suggest that reduced plasma viral loads in women receiving HAART are presumably due to decreased antigen loads, associated with significantly reduced Gag-specific CD8 T cell responses in blood. While we found that HAART fully suppressed HIV shedding in the majority of HAART-compliant women in this study, this suppression (which is analogous to a reduction in plasma viral loads) was not associated with a reduction in Gag-specific CD8 T cell responses in the genital tract.
DISCUSSION
Suboptimal bioavailability of antiretroviral drugs at mucosal surfaces has been associated with incomplete immune reconstitution in individuals receiving HAART (17, 34). Several studies speculate that this is a result of low levels of HIV replication within persistently infected cells, in the inflammatory environment associated with mucosal tissue (20, 24, 45, 54, 58). In this study, we show that in one of the regions of greatest HIV endemicity in the world, HAART effectively suppressed both plasma viral loads and genital shedding of viral particles. Furthermore, we found that women receiving HAART had significantly better CD4 counts both in blood and at the cervix than women who were not receiving treatment. However, HAART− women had significantly higher frequencies of Gag-specific CD8 T cell responses in blood than HAART+ women, a finding that most likely reflects the fact that the latter group experienced reduced antigen loads. Despite showing significantly reduced CD8 T cell responses in blood, we found that cervical CD8 T cell responses to Gag were preserved in women on HAART and were similar in magnitude to those observed in HIV-infected women not on HAART.
We have previously shown that more than 10% of HIV-infected South African women receiving HAART who have undetectable plasma HIV RNA concentrations have >400 HIV RNA copies/ml in their genital secretions (41). In that study, however, HAART compliance was not evaluated. Here we have shown that <5% of HAART-compliant women had any detectable HIV in their genital secretions, an observation that clearly indicates the potential effectiveness of both the national HAART program and treatment adherence in slowing the spread of HIV.
Pharmacokinetics studies have shown that NRTIs penetrate the female genital tract more efficiently than either PIs or NNRTIs (17, 34), achieving higher drug concentrations at the female genital tract than in blood (16, 11, 34). In this study, all of the HAART+ women were receiving NRTIs as part of either a first-line treatment regimen (24/27 women), together with NNRTIs, or a second-line regimen (3/27 women), together with PIs. The single HAART+ woman with detectable HIV RNA in her genital secretions (430 RNA copies/ml) was taking lamivudine (3TC)-zidovudine (AZT)-efavirenz (EFV) and had clearly detectable levels of all three antiretroviral drugs in her plasma. Although the concentrations of some of the drugs measured in plasma do not provide reliable information on adherence to therapy because of their short half-lives (less than 24 h) (5), it is unlikely that genital HIV shedding in this woman was related to poor adherence. Continued HIV shedding in the presence of fully suppressed plasma viremia in this woman may instead reflect ineffective penetration of drugs to the genital tract. This woman was on a regimen of zidovudine, lamivudine, and efavirenz. While zidovudine and lamivudine have been shown to achieve higher concentrations in the female genital tract than in blood (200% and 400% higher, respectively), efavirenz has comparatively poorer genital exposure (0.6% relative to concentrations in blood) (11).
Several studies have reported that CD4 T cells residing at the mucosa of the gastrointestinal tract (8, 23), lung (6), and male genital tract (48) are massively and persistently depleted during HIV infection. Furthermore, HAART has been shown to be ineffective at reconstituting CD4 T cell counts in the gut mucosa (36, 57), although the impact of therapy on CD4 reconstitution at other mucosal sites is unclear. We confirm in this study that women on HAART had significantly higher CD4 T cell counts in blood than HAART− women, and the extent of CD4 reconstitution was associated with the time on therapy. In the female genital tract, we also found a significantly higher proportion of CD4 T cells for HAART+ women than for HAART− women, indicating that HAART was also associated with the restoration of CD4 T cell proportions at the genital tract.
Although a number of studies have shown that the suppression by HAART of HIV loads in blood diminishes the magnitude of HIV-specific T cell responses after therapy (1, 22, 31, 43, 60), certain other studies have not found this effect (2, 46). We found that HAART+ women had significantly lower HIV-specific CD8 T cell responses in blood than HAART− women. It has been suggested that low levels of ongoing viral replication (antigenic stimulation) are necessary for the maintenance of immune responses in HIV-infected individuals (1).
While we found significantly lower blood CD8 T cell responses to HIV Gag in HAART+ women than in HAART− women, Gag-specific CD8 T cell responses in the genital tract were detected in more than one-third of HAART+ women and were not significantly different in magnitude from the responses detected in HAART− women. These Gag-specific CD8 T cell responses at the cervix were found to persist despite almost-complete suppression of genital HIV shedding in HAART+ women. In women receiving HAART, genital tract CD8 T cell responses against Gag were significantly higher in magnitude at the cervix than in blood. This finding is in contrast to those of other studies evaluating HIV-specific T cell responses at the cervix (52) and in the gut (12), which reported that mucosal T cell immunity to HIV is diminished in HAART+ individuals compared to those not on HAART, with only 1/9 (52) and 3/13 (12) HAART+ individuals having detectable HIV-specific IFN-γ T cell responses at the cervix and gut, respectively. It should be stressed, however, that these other studies evaluated fewer participants than were evaluated here and included participants on HAART regimens that differed from those of the women included in our study.
HIV-specific CTLs are known to occur in the genital tracts of HIV-infected women and have been shown to be protective in long-term nonprogressors (35, 47) and HIV-exposed but persistently seronegative (HEPS) women (32, 33). The maintenance of these responses could therefore potentially block any breakthrough HIV shedding at the cervices of HIV-infected women undergoing therapy. We have previously shown that cervical cytobrush-derived T cells are dominated by effector memory subsets, with central memory and transitional memory being present at lower frequencies (41). While effector memory cells are short-lived, central and transitional memory cells are more persistent (51). Previous studies have shown that resting memory CD4 cells can support low levels of intracellular HIV replication and can therefore act as “reservoirs” of HIV infection (55). It is therefore plausible that the presence of HIV-specific CD8 T cells in the female genital tract, even during HAART, is the result of ongoing low levels of virus replicating within cells that are HIV reservoirs. Future studies should aim to evaluate HIV reservoirs in the genital tract of HIV-infected women on and off HAART.
In conclusion, we have shown that by effectively reducing blood and cervical viremia, HAART reconstitutes CD4 T cell counts within these compartments. Whereas we confirm previous reports of decreased blood CD8 T cell responses to HIV Gag following HAART, induced by decreases in viral loads, HIV-specific CD8 T cell responses were preserved in the genital tracts of women receiving HAART. We propose that suboptimal antiretroviral drug concentrations in an environment rich in inflammatory signals may allow for the persistence within this compartment of latent HIV reservoirs within which intermittent local HIV replication persistently induces HIV-specific T cell responses.
Supplementary Material
Acknowledgments
We thank the women from the Nyanga Day Hospital who kindly participated in the study, Janine Jones for collecting the specimens, and Darren Martin for reviewing this article and offering constructive comments to improve it.
This study was supported by grants from the Wellcome Trust and the Medical Research Council of South Africa. N.N.M. and J.S.P. received training in the United States as part of the Columbia University-Southern African Fogarty AITRP Program. N.N.M. is funded by the National Research Foundation (South Africa) African Scholarships Program and is a L'Oréal-UNESCO sub-Saharan regional fellow. J.S.P. is a Wellcome Trust Intermediate Fellow in Infectious Diseases.
Footnotes
Published ahead of print on 4 August 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alatrakchi, N., C. Duvivier, D. Costagliola, A. Samri, A. G. Marcelin, G. Kamkamidze, M. Astriti, R. Agher, V. Calvez, B. Autran, and C. Katlama. 2005. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS 19:25-33. [DOI] [PubMed] [Google Scholar]
- 2.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 5.Bazzoli, C., V. Jullien, C. Le Tiec, E. Rey, F. Mentré, and A.-M. Taburet. 2010. Intracellular pharmacokinetics of antiretroviral drugs, and their correlation with drug action. Clin. Pharmacokinet. 49:17-45. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., K. S. Knox, A. I. Asher, D. A. Price, L. M. Kohli, E. Gostick, B. J. Hill, C. A. Hage, Z. Brahmi, A. Khoruts, H. L. Twigg III, T. W. Schacker, and D. C. Douek. 2008. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 1:49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., L. E. Ruff, J. P. Casazza, R. A. Koup, D. A. Price, and D. C. Douek. 2006. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J. Virol. 80:6801-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Chi, J., A. L. Jayewardene, J. A. Stone, et al. 2002. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J. Pharm. Biomed. Anal. 30:675-684. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, M. S., C. Gay, A. D. M. Kashuba, S. Blower, and L. Paxton. 2007. Antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann. Intern. Med. 146:591-601. [DOI] [PubMed] [Google Scholar]
- 12.Critchfield, J. W., D. H. Young, T. L. Hayes, J. V. Braun, J. C. Garcia, R. B. Pollard, and B. L. Shacklett. 2008. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One 3:e3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Cu-Uvin, S., A. M. Caliendo, S. Reinert, A. Chang, C. Juliano-Remollino, T. P. Flanigan, K. H. Mayer, and C. C. Carpenter. 2000. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS 14:415-421. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Dumond, J. B., R. F. Yeh, K. B. Patterson, A. H. Corbett, B. H. Jung, N. L. Rezk, A. S. Bridges, P. W. Stewart, M. S. Cohen, and A. D. Kashuba. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Reference deleted.
- 19.Fiore, J. R., B. Suligoi, A. Saracino, M. Di Stefano, R. Bugarini, A. Lepera, A. Favia, L. Monno, G. Angarano, and G. Pastore. 2003. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS 17:2169-2176. [DOI] [PubMed] [Google Scholar]
- 20.George, M. D., E. Reay, S. Sankaran, and S. Dandekar. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 79:2709-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham, S. M., S. E. Holte, N. M. Peshu, B. A. Richardson, D. D. Panteleeff, W. G. Jaoko, J. O. Ndinya-Achola, K. N. Mandaliya, J. M. Overbaugh, and R. S. McClelland. 2007. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS 21:501-507. [DOI] [PubMed] [Google Scholar]
- 22.Gray, C. M., J. Lawrence, J. M. Schapiro, J. D. Altman, M. A. Winters, M. Crompton, M. Loi, S. K. Kundu, M. M. Davis, and T. C. Merigan. 1999. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J. Immunol. 162:1780-1788. [PubMed] [Google Scholar]
- 23.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guadalupe, M., S. Sankaran, M. D. George, E. Reay, D. Verhoeven, B. L. Shacklett, J. Flamm, J. Wegelin, T. Prindiville, and S. Dandekar. 2006. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J. Virol. 80:8236-8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumbi, P. P., N. N. Nkwanyana, A. Bere, W. A. Burgers, C. M. Gray, A. L. Williamson, M. Hoffman, D. Coetzee, L. Denny, and J. A. Passmore. 2008. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. J. Virol. 82:8529-8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hladik, F., P. Sakchalathorn, L. Ballweber, G. Lentz, M. Fialkow, D. Eschenbach, and M. J. McElrath. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtgrave, D. R. 2005. Causes of the decline in AIDS deaths, United States, 1995-2002: prevention, treatment or both? Int. J. STD AIDS 16:777-781. [DOI] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Joint United Nations Programme on HIV/AIDS (UNAIDS). March 2008. Sub-Saharan Africa AIDS epidemic update: regional summary, 2007. UNAIDS, Geneva, Switzerland. http://data.unaids.org/pub/Report/2008/jc1526_epibriefs_subsaharanafrica_en.pdf.
- 30.Kader, M., W. M. Hassan, M. Eberly, M. Piatak, J. D. Lifson, M. Roederer, and J. J. Mattapallil. 2008. Antiretroviral therapy prior to acute viral replication preserves CD4 T cells in the periphery but not in rectal mucosa during acute simian immunodeficiency virus infection. J. Virol. 82:11467-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalams, S. A., P. J. Goulder, A. K. Shea, N. G. Jones, A. K. Trocha, G. S. Ogg, and B. D. Walker. 1999. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J. Virol. 73:6721-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 33.Kaul, R., S. L. Rowland-Jones, J. Kimani, K. Fowke, T. Dong, P. Kiama, J. Rutherford, E. Njagi, F. Mwangi, T. Rostron, J. Onyango, J. Oyugi, K. S. MacDonald, J. J. Bwayo, and F. A. Plummer. 2001. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol. Lett. 79:3-13. [DOI] [PubMed] [Google Scholar]
- 34.Kwara, A., A. Delong, N. Rezk, J. Hogan, H. Burtwell, S. Chapman, C. C. Moreira, J. Kurpewski, J. Ingersoll, A. M. Caliendo, A. Kashuba, and S. Cu-Uvin. 2008. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin. Infect. Dis. 46:719-725. [DOI] [PubMed] [Google Scholar]
- 35.Lambotte, O., F. Boufassa, Y. Madec, A. Nguyen, C. Goujard, L. Meyer, C. Rouzioux, A. Venet, J. F. Delfraissy, and the SEROCO-MEMOCO Study Group. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1694. [DOI] [PubMed] [Google Scholar]
- 36.Macal, M., S. Sankaran, T. W. Chun, E. Reay, J. Flamm, T. J. Prindiville, and S. Dandekar. 2008. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 1:475-488. [DOI] [PubMed] [Google Scholar]
- 37.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehandru, S., M. A. Poles, K. Tenner-Racz, P. Jean-Pierre, V. Manuelli, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2006. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 3:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mistri, H. N., A. G. Jangid, A. Pudage, N. Gomes, M. Sanyal, and P. Shrivastav. 2007. High throughput LC-MS/MS method for simultaneous quantification of lamivudine, stavudine and nevirapine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 853:320-332. [DOI] [PubMed] [Google Scholar]
- 40.National Department of Health, South Africa. 2004. National antiretroviral treatment guidelines. National Department of Health, Pretoria, South Africa.
- 41.Nkwanyana, N. N., P. P. Gumbi, L. Roberts, L. Denny, W. Hanekom, A. Soares, B. Allan, A. L. Williamson, D. Coetzee, A. J. Olivier, W. A. Burgers, and J. A. Passmore. 2009. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology 128:e746-e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 43.Ogg, G. S., X. Jin, S. Bonhoeffer, P. Moss, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, A. Hurley, M. Markowitz, D. D. Ho, A. J. McMichael, and D. F. Nixon. 1999. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 73:797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortiz, G. M., J. Hu, J. A. Goldwitz, R. Chandwani, M. Larsson, N. Bhardwaj, S. Bonhoeffer, B. Ramratnam, L. Zhang, M. M. Markowitz, and D. F. Nixon. 2002. Residual viral replication during antiretroviral therapy boosts human immunodeficiency virus type 1-specific CD8+ T-cell responses in subjects treated early after infection. J. Virol. 76:411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrowski, S. R., T. L. Katzenstein, P. T. Thim, B. K. Pedersen, J. Gerstoft, and H. Ullum. 2005. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J. Infect. Dis. 191:348-357. [DOI] [PubMed] [Google Scholar]
- 46.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 97:3382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereyra, F., S. Palmer, T. Miura, B. L. Block, A. Wiegand, A. C. Rothchild, B. Baker, R. Rosenberg, E. Cutrell, M. S. Seaman, J. M. Coffin, and B. D. Walker. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Politch, J. A., K. H. Mayer, and D. J. Anderson. 2009. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 50:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reference deleted.
- 50.Rehle, T., O. Shisana, V. Pillay, K. Zuma, A. Puren, and W. Parker. 2007. National HIV incidence measures—new insights into the South African epidemic. S. Afr. Med. J. 97:194-199. [PubMed] [Google Scholar]
- 51.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 52.Shacklett, B. L., S. Cu-Uvin, T. J. Beadle, C. A. Pace, N. M. Fast, S. M. Donahue, A. M. Caliendo, T. P. Flanigan, C. C. Carpenter, and D. F. Nixon. 2000. Quantification of HIV-1-specific T-cell responses at the mucosal cervicovaginal surface. AIDS 14:1911-1915. [DOI] [PubMed] [Google Scholar]
- 53.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 54.Sheth, P. M., D. Chege, L. Y. Shin, S. Huibner, F. Y. Yue, M. Loutfy, R. Halpenny, D. Persad, C. Kovacs, T. W. Chun, G. Kandel, M. Ostrowski, and R. Kaul. 2008. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. 1:382-388. [DOI] [PubMed] [Google Scholar]
- 55.Siliciano, J. D., and R. F. Siliciano. 2005. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol. Biol. 304:3-15. [DOI] [PubMed] [Google Scholar]
- 56.Reference deleted.
- 57.Tincati, C., M. Biasin, A. Bandera, M. Violin, G. Marchetti, L. Piacentini, G. L. Vago, C. Balotta, M. Moroni, F. Franzetti, M. Clerici, and A. Gori. 2009. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir. Ther. 14(3):321-330. [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Wendland, T., H. Furrer, P. L. Vernazza, K. Frutig, A. Christen, L. Matter, R. Malinverni, and W. J. Pichler. 1999. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 13:1857-1862. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson, J., J. J. Zaunders, A. Carr, and D. A. Cooper. 1999. CD8+ anti-human immunodeficiency virus suppressor activity (CASA) in response to antiretroviral therapy: loss of CASA is associated with loss of viremia. J. Infect. Dis. 180:68-75. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization (WHO), HIV/AIDS Programme. 2006. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach (2006 revision). World Health Organization, Geneva, Switzerland. http://www.who.int/hiv/pub/arv/adult/en/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.