Abstract
While recent laboratory-based studies have substantially advanced our understanding of the action of vitamin D in the brain, much is still unknown concerning how vitamin D relates to mood. The few epidemiologic studies of vitamin D and depression have produced inconsistent results and generally have had substantial methodologic limitations. Recent findings from a randomized trial suggest that high doses of supplemental vitamin D may improve mild depressive symptoms but important questions persist concerning how vitamin D may affect monoamine function and hypothalamic-pituitary-adrenal axis response to stress, whether vitamin D supplementation can improve mood in individuals with moderate to severe depression, and whether vitamin D sufficiency is protective against incident depression and recurrence. At this time, it is premature to conclude that vitamin D status is related to the occurrence of depression. Additional prospective studies of this relationship are essential.
Keywords: vitamin D, 25-hydroxyvitamin D, depression, review
Introduction
A small body of research has evaluated whether vitamin D may play a role in the occurrence of depression. While recent laboratory-based studies have substantially advanced our understanding of the action of vitamin D in the brain, much is still unknown concerning how vitamin D may relate to mood. The few epidemiologic studies of vitamin D and depression have produced inconsistent results and have generally had substantial methodologic limitations. However, a recent randomized trial1 has addressed many of the concerns raised by previous studies and offers provocative results suggesting that vitamin D indeed improves depressive symptoms. This review presents background information on vitamin D and depression, summarizes evidence from a variety of sources both suggesting and refuting their relationship, discusses the epidemiology of vitamin D and depression, and proposes important directions for future research to address the many unanswered questions.
Sources and metabolism of vitamin D
There are two sources of vitamin D: dietary consumption and endogenous production. Vitamin D may be consumed in the diet as either ergocalciferol (D2) from plant sources or cholecalciferol (D3) from animal sources. With the exception of fatty fish, relatively few foods are naturally rich in vitamin D. In the US, the predominant dietary sources of vitamin D are fortified foods, such as milk, yogurt, orange juice and cereals, and dietary supplements. However, in individuals with ample sunlight exposure the greater source is endogenous vitamin D produced when 7-dehydrocholesterol in the epidermis and dermis of the skin is converted into vitamin D3 after exposure to ultraviolet B radiation.
Vitamins D2 and D3 from dietary sources are transported to the liver on chylomicrons, while D3 from cutaneous production is carried through plasma bound to vitamin D binding protein. In the liver, both forms are hydroxylated to 25-hydroxyvitamin D (25(OH)D). This metabolite used to assess an individual’s vitamin D status, as it well reflects both dietary intake and sunlight exposure. 25(OH)D is hydroxylated to 1,25(OH)2D by 1-alpha-hydroxylase enzymes (CYP27B1) in kidney nephrons and a variety of other tissues. 1,25(OH)2D is the biologically active metabolite that binds to nuclear vitamin D receptors (VDR) in target tissues to regulate gene transcription. 1,25(OH)2D also binds to VDRs on cell membranes to mediate a variety of non-genomic responses.2 Because one of the primary function of 1,25(OH)2D is the maintenance of calcium levels in tissues, its metabolism from 25(OH)D is closely regulated in order to promote calcium homeostasis.
During sunny months in most parts of the world, vitamin D sufficiency can be achieved by minimal sun exposure. Holick (2003)3 has estimated that vitamin D levels resulting from daily exposure of 50% of the skin without sunscreen for 12 minutes during mid-day hours at mid-US latitudes are equivalent to those produced by dietary intake of 3,000 IU per day. During winter months and for those living at high latitudes, dietary intake is essential to maintain healthy 25(OH)D levels. Many experts feel that current recommendations for vitamin D intake of 200 – 600 IU per day are inadequate to prevent deficiency and a host of related health consequences.4,5 Instead, individuals without sunlight exposure likely require 800 – 1000 IU per day to produce 25(OH)D levels in the optimal range (30–40 ng/mL).5 This level of intake is well below the threshold for vitamin D toxicity (≥150 ng/mL); in fact, toxicity has not been observed with daily intakes up to 10,000 IU per day.5
Pathophysiology of depression
Depression is a condition characterized by depressed mood or loss of interest or pleasure in nearly all activities most of every day for a period lasting at least 2 weeks.6 Additional common symptoms include decreased energy; changes in appetite, sleep and psychomotor function; irritability; feelings of worthlessness, guilt and/or hopelessness; and suicidal ideation and/or action. To meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)6 diagnostic criteria for Major Depressive Disorder, symptoms must cause significant impairment in regular life activities and social relationships, and evidence of mania, psychosis or substance-induced depression must be ruled out. Lifetime incidence of MDD is higher in women (10–25%) than men (5–12%), and the average age of onset is in the mid 20’s. At any given time, 5 – 9% of women and 2 – 3% of men are currently experiencing MDD. Recurrence occurs in 50–60% of those experiencing a single depressive episode. Depression often occurs co-morbidly with anxiety.7
In a recent review, Belmaker and Agam (2008) elegantly summarize current hypotheses on the pathophysiology of depression.8 In addition to the potential contributions of genetic and epigenetic factors, few of which have been clearly identified, two predominant hypotheses have been the focus of research. First, depression has been closely linked with the availability and function of monoamine neurotransmitters in the brain (i.e., the monoamine deficiency hypothesis). Of the monoamines, serotonin and norepinephrine have been studied in greatest detail. Perhaps the greatest evidence implicating monoamines in depression comes from observations of the efficacy of anti-depressant medications. The three main classes of anti-depressants, monoamine oxidase inhibitors, tricyclics, and selective serotonin reuptake inhibitors (SSRI)/selective norepinephrine reuptake inhibitors (SNRI), act through a variety of mechanisms to increase serotonin and norepinephrine levels in synapses and to increase postsynaptic neuron firing. While few studies have demonstrated that serotonin and norepinephrine metabolite levels are lower in depressed patients than healthy controls, results from a variety of sources suggest that impairments in monoamine synthesis, release, reuptake, and/or receptor binding may be related to depression. For example, experimental studies in which levels of neurotransmitter substrates are intentionally depleted can produce depression in subjects with a history of the disorder, though not in healthy controls.9 This finding suggests an underlying predisposition to depression in some individuals related to monoamine function. Furthermore, some investigators have suggested that a “dopaminergic dysfunction” subtype of depression may exist, in which symptoms related to dopamine function, including anhedonia, slowed motor functions, low motivation and difficulty in concentrating, predominate.10 These individuals tend to respond less well to SSRIs and SNRIs than others.
A second, related hypothesis for the development of depression involves the impact of stress on psychological functioning.8 In response to stress, the hypothalamus releases corticotropin releasing hormone (CRH), which stimulates the pituitary to release corticotropins, which in turn stimulate the adrenals to release cortisol. Impairment in hypothalamic-pituitary-adrenal (HPA) axis functioning may be directly involved in depression as well as anxiety, but as with monoamines, the relationship is complex. In clinical studies, patients with depression have not consistently demonstrated abnormal cortisol levels compared to healthy controls, or evidence of impaired HPA function. However, some patients have demonstrated elevated CRH levels in cerebrospinal fluid, chronically increased cortisol levels in blood, and impaired negative feedback. Additionally, Eisch et al. (2008)11 recently highlight evidence supporting the hypothesis that decreased neurogenesis in the adult hippocampus in response to stress is important in the etiology of depression, including observations of reduced hippocampal volume in depressed patients. The efficacy of antidepressants also supports stress as a contributor to depression, as monoamines affect action of the HPA axis in response to stress, and antidepressants may improve mood by countering the effects of stress,8 perhaps by increasing neurogenesis.11
While impressive gains have been made in our understanding of the pathophysiology of depression, inconsistencies in findings from clinical studies underscore the fact that depression is a complex disorder that likely has multiple subtypes and multiple causes, including perhaps a role for vitamin D.
Vitamin D, the brain and depression
While ample evidence suggests that vitamin D likely has important functions in the human brain and many investigators consider it a neurosteroid,12,13 it remains unclear whether these functions may be related to the occurrence of major depression. Numerous recent studies have identified VDR in nearly all tissues in the body, including both neuronal and glial cells in the central nervous system.12 Eyles and colleagues (2005)13 identified VDR in multiple areas of the human brain, including the prefrontal cortex, hippocampus, cingulate gyrus, thalamus, hypothalamus, and substantia nigra, many of which have been implicated in the pathophysiology of depression.14 The majority of these regions also demonstrated substantial immunoreactivity for 1alpha-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D,13,15 suggesting that in these regions 1,25(OH)2D likely has autocrine and/or paracrine activity.13 Vitamin D metabolites can cross the blood-brain barrier, though animal studies suggest that there may be little uptake of 1,25(OH)2D in the brain;16,17 25(OH)D uptake also appears low but sufficient to provide substrate for local conversion to 1,25(OH)2D.18
A substantial amount of current evidence supporting a role of vitamin D in brain development and behavior has come from animal studies involving rodents experimentally deprived of vitamin D in utero or after birth and those lacking functional VDR. In a study of the effect of vitamin D deficiency on the developing rat brain, pups deprived of vitamin D in utero developed brains with thinner neocorticies, greater cell proliferation, heavier weight, and decreased levels of both nerve growth factor and glial cell-line derived neurotrophic factor than vitamin D-sufficient controls.19 It has been suggested that the increased brain weight is due to reduced apoptosis.20 Interestingly, in one study in humans the variant allele of the common fok1 polymorphism in the VDR gene, leading to production of a less active transcription factor than the wild-type, was associated with increased head size.21 Additional studies have indicated that in utero vitamin D deficiency results in dysregulation of cellular differentiation in the developing rat brain. Results from experiments by Féron et al. (2005)20 suggest that many of the effects of maternal vitamin D deficiency on brain development persist into adulthood even if vitamin D levels are adequate after birth.
Studies of mice lacking functioning VDR (i.e., VDR knock-out mice) have reported that the absence of VDR is associated with substantial behavioral impairment and increased anxiety (Minasyan, 2007).22 Kalueff et al. (2006)23 reported that “Tokyo” VDR knock-out mice demonstrated significant reductions in grooming (e.g., barbering), aggression, nest building, and maternal behaviors, all of which are related to anxiety, compared to wild-type controls. However, these mice did not demonstrate greater frequency of depressive-like behaviors, such as greater immobility during the tail suspension test, than wild-type controls. Other studies of VDR knock-out mice have reported behavioral differences indicative of greater anxiety in these animals; however, these may also be explained by greater impairment in musculoskeletal functioning.24,25 Behavioral impairments demonstrated by VDR knock-out mice appear to be different from those of infant and adult mice deprived of vitamin D in utero, suggesting that transient vitamin D deficiency is more closely related to behavioral change than sustained deficiency.26 However, these changes appear more closely related to symptoms of psychosis than depression. In fact, much of the research on vitamin D and brain function has been in the area of schizophrenia. Results from a variety of population studies suggest that schizophrenia is more common in individuals born during the winter and spring when maternal vitamin D levels are low,26 and that vitamin D supplementation in early life may reduce risk.19
At this time, there is little available evidence concerning how vitamin D relates to the monoamines likely to be involved in depression. 1,25(OH)2D appears to increase expression of genes encoding for tyrosine hydroxylase, the precursor of norepinephrine, in adrenal glands.12 Animal studies suggest that that 1,25(OH)2D may protect neurons against the effects of dopaminergic toxins by upregulating glial cell line-derived neurotropic factor (GDNF).27,28 In a recent study, rats exposed to methamphetamine, a dopaminergic toxin, experienced significant decreases in serotonin and dopamine in the striatum and accumbens; rats treated with 1,25(OH)2D in addition to the toxin did not demonstrate these effects.27 1,25(OH)2D treatment did not increase serotonin or dopamine concentrations in controls, but did increase concentrations of 3,4-dihydroxyphenylacetic acid (DOPAC), a major dopamine metabolite, in the striatum and accumbens. The authors hypothesize that this may be due to increased dopamine synthesis and metabolism or by upregulation of GDNF. Similarly, in a study of 4-week vitamin D deprivation in weanling rats, vitamin D deficiency was associated with significant increases in dopamine and DOPAC in the cortex and hypothalamus, suggesting that dopamine synthesis and utilization were both increased;29 cortical norepinephrine levels were also elevated. Furthermore, it has been suggested that vitamin D deficiency may be related to the development of Parkinson’s disease, which is characterized by the death of dopaminergic neurons in the substantia nigra of the brain.18
Evidence suggesting a relationship between vitamin D, stress and cortisol is also limited. Vitamin D receptors are present in hippocampal neurons, which also demonstrate 1-alpha-hydroxylase activity and thus the ability to metabolize 25(OH)D into 1,25(OH)2D for local use.13 It is unclear whether vitamin D is involved in neurogenesis in response to stress, but in vitro evidence suggests that there is cross-talk between VDR and glucocorticoid receptors in the hippocampus,30 and that vitamin D is involved in neuron differentiation and/or apoptosis in this region. Clearly, additional information on potential relationships between monoamines, HPA function and vitamin D are needed to determine whether vitamin D may be physiologically related to the occurrence of depression.
Epidemiologic studies of vitamin D status and depression
At this time, epidemiologic evidence of a relationship between vitamin D and depression is limited. Relatively few epidemiologic studies have evaluated this relationship, and results have been inconclusive (table 1).1,31–38 In all but one study,1 investigators assessed vitamin D metabolite levels and mood status cross-sectionally.
Table 1.
Major results from epidemiologic studies of vitamin D status and depression.
| Study/Location | Design | Population | Depression assessment |
Vitamin D measure | Main Finding |
|---|---|---|---|---|---|
| Jorde, 20081 Norway |
Randomized trial, 1 year follow-up |
334 men and women, 21– 70 years |
Total BDI, BDI subscale 1–13 (cognitive-affective) and 14–21 (somatic- vegetative) |
Treatment groups: 40,000 IU/wk 20,000 IU/wk placebo |
Mean total BDI scores at baseline and 12 months follow-up: 40,000 IU/wk = 4.5 to 3.0 (P < 0.01 vs. placebo) 20,000 IU/wk = 5.0 to 4.0 (P < 0.01 vs. placebo) placebo = 4.0 to 3.8 Results similar for BDI subscale 1–13 |
| Pan, 200936 China |
Cross- sectional |
3262 men and women, 50–70 years |
CES-D scale | 25(OH)D |
RR and 95% confidence interval for depressive symptoms by tertile of 25(OH) (nmol/L): 1 (mean = 26.1) = referent 2 (mean = 41.1) = 1.38 (1.00 – 1.90) 3 (mean = 65.1) = 1.35 (0.94 – 1.96) P for trend = 0.08 |
| Hoogendijk, 200835 Netherlands |
Cross- sectional |
1282 men and women, 65–95 years; 26 with major and 169 with minor depression |
CES-D scale and psychiatric evaluation |
25(OH)D |
Mean 25(OH)D (ng/mL): major depression = 19 vs. minor depression = 19 vs. nondepressed = 22 (P < 0.001) |
|
Eskandari, 200734 United States |
Cross- sectional |
Premenopausal women; 89 with major depressive disorder,44 controls |
Psychiatric evaluation |
25(OH)D | Mean 25(OH)D (ng/mL) Cases = 27 vs. Controls = 34 (P = 0.002) |
| Jorde, 200633 Norway |
Cross- sectional |
Men and women, > 29 years; 21 with secondary hyperparathyroidism (SHPT), 63 controls |
BDI; General Health Questionnaire-30 |
SHPT Case/control status; 25(OH)D |
Mean BDI scores: SHPT = 5.05 vs. Controls = 4.45 (P > 0.05) Mean BDI (items 1–13): SHPT = 2.67 vs. Controls = 2.03 (P < 0.05) |
| Wilkins, 200632 United States |
Cross- sectional |
Men and women >60 years; 40 with mild Alzheimer’s Disease, 40 non-demented |
Depressive symptom inventory |
25(OH)D (ng/mL): Sufficient ≥20 Insufficient 10–<20 Deficient <10 |
Prevalent mood disorder (n) Sufficient: 4 vs. Insufficient: 7 vs. Deficiency: 7 (P = 0.007) P for depressive features score = 0.75 |
|
Schneider, 200031 Germany |
Cross- sectional |
Men and women; 25 inpatient with major depression, 31 controls |
Clinical status in inpatient facility |
25(OH)D 1,25(OH)2D |
Mean 25(OH)D (ng/mL) Cases = 37.3 vs. Controls = 45.9 (P > 0.05) Mean 1,25(OH)2D (pg/mL) Cases = 29.2 vs. Controls = 39.4 (P < 0.01) |
| Herrán, 200037 Spain |
Cross- sectional |
Women; 19 with first depressive episode, 19 controls |
Psychiatric evaluation |
25(OH)D | Mean 25(OH)D (ng/mL) Cases = 27.3 vs. Controls = 23.0 (P = 0.2) |
|
Michelson, 199638 United States |
Cross- sectional |
Women; 24 with major depression, 24 controls |
Psychiatric evaluation |
25(OH)D 1,25(OH)2D |
Mean 25(OH)D (ng/mL) Cases = 39 vs. Controls = 35 (P = 0.44) Mean 1,25(OH)2D (pg/mL) Cases = 50 vs. Controls = 44 (P = 0.25) |
Abbreviations 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D = 1,25 dihydroxyvitamin D; BDI = Beck Depression Inventory; CES-D: Center for Epidemiologic Studies-Depression; RR = relative risk; CI = confidence interval
In one evaluation including 25 inpatients with major depression, cases had significantly lower mean 1,25(OH)2D levels and non-significantly lower 25(OH)D levels than 31 healthy controls.31 While these results were unadjusted for other factors, metabolite levels were not significantly correlated with age and didn’t vary significantly by gender. A second study assessed 25(OH)D levels and evidence of a mood disorder, as assessed by a 9-item depressive symptom inventory, in 40 individuals with mild Alzheimer’s disease and 40 non-demented persons, all over 60 years.32 After adjustment for age, sex, race and season, subjects with 25(OH)D <20.0 ng/mL were significantly more likely to have a mood disorder (P = 0.02) than those with higher levels. However, mean depressive features score did not vary by vitamin D status (P = 0.75). Jorde et al. (2006)33 reported a modestly higher prevalence of mood symptoms in 21 men and women with secondary hyperparathyroidism compared to 63 age and sex-matched controls, all of whom were members of the Tromso 5th study. In this study serum 25(OH)D levels were inversely related to scores on the total Beck Depression Inventory (BDI)39 scale (P = 0.04) and BDI cognitive-affective subscale (P = 0.01), though low mean scores on these scales overall suggest that few cases or controls were experiencing clinically significant levels of depressive symptoms. 25(OH)D levels were also significantly lower in 89 premenopausal women with MDD than in 44 controls matched on age and body mass index in a study of depression and bone mass (34 vs. 27 ng/mL, P = 0.002).34
In a recent substudy among participants in the Longitudinal Aging Study Amsterdam, Hoogendijk and colleagues (2008)35 evaluated 25(OH)D levels and prevalent depression, as assessed by the Center for Epidemiologic Studies – Depression (CES-D) scale and psychiatric evaluation, in men and women 65 years and older. Mean 25(OH)D levels in 26 participants with MDD and 169 with minor depression were comparable, and 14% lower than those of 1087 non-depressed individuals (19 vs. 22 ng/mL; P < 0.001). After adjustment for other factors including age, sex, smoking status and body mass index, depression severity as measured by continuous CES-D score was significantly associated with 25(OH)D (P = 0.03).
Other studies have not observed a relationship between vitamin D and depression. After adjustment for a variety of factors including geography, body mass index, physical activity and smoking, 25(OH)D levels were not associated with depressive symptoms in a recent study of 3262 older men and women in China.36 Two small studies of depression and bone health did not suggest that vitamin D metabolite levels were lower in women with depression than in healthy controls.37,38 Furthermore, cases and controls also did not differ in the distribution of alleles of 3 common vitamin D receptor polymorphisms (Apa1, Bsm1 and Taq1).38
While the findings of these studies are provocative, several important questions are raised. First, because vitamin D was evaluated with respect to current mood status, it cannot be ascertained whether observed relationships between low 25(OH)D levels and depression are likely causal. It is unknown whether low 25(OH)D levels preceded the development of depression or were a consequence of dietary and/or behavioral changes resulting from depression. For example, it is plausible that individuals developing depression may reduce their time spent outdoors, participate less in physical activity, change their diet, or increase smoking, all of which may result in lower 25(OH)D levels.5,40,41 To determine if 25(OH)D is etiologically related to depression, prospective studies that assess changes in mood over time in individuals with low vs. sufficient vitamin D levels are necessary.
Secondly, the potential for confounding in studies of vitamin D and depression is great. A variety of factors influence 25(OH)D levels, including age, time spent outdoors, latitude, physical activity, body mass index, smoking and alcohol use.5,40–44 Many of these factors are also associated with the incidence of depression.45–49 However, most of the cross-sectional studies conducted to date have presented unadjusted results not taking into consideration one or more of these important factors.31–33,37,38 It is thus difficult to determine if observed associations are accurate or may be explained instead by confounding.
A randomized trial of vitamin D supplementation and depression
To address methodologic concerns raised by these prior investigations, additional studies are needed that assess the relationship of vitamin D and depression prospectively over time and that control for a variety of confounding factors by multivariable statistical analysis or through randomization of vitamin D exposure. Recently, results from a randomized clinical trial by Jorde and colleagues (2008)1 have provided important additional support for a possible causal relationship between vitamin D status and depression. Participants in the trial were men and women 21 – 70 years, and all were overweight or obese (i.e., BMI ≥ 28.0 kg/m2). After exclusions for history of cardiovascular disease, previous antidepressant use, and other characteristics, 441 participants were enrolled.
At the time of enrollment, serum samples were collected and used to assess 25(OH)D, parathyroid hormone and calcium levels. The BDI was used to evaluate mood status at baseline.39 This well-validated scale asks respondents to choose the most applicable of 4 statements about their feelings over the previous two weeks, and includes 21 sets of statements. For example, item 1 concerning sadness includes the following statements: 0) “I do not feel sad”; 1) “I feel sad much of the time”; 2) “I feel sad all the time”; 3) “I am so sad or unhappy that I can’t stand it.” Each item is assigned a point score ranging from 0–3, with points summed across all items to derive a total score. The BDI also may be evaluated as two subscales, with items 1–13 addressing cognitive-affective symptoms of depression and 14–21 addressing somatic-vegetative symptoms. Total scores are classified as follows: 0 – 9 = not depressed; 10 – 18 = mild-moderate depression; 19 – 29 = moderate-severe depression; 30 – 63 = severe depression. The authors also collected information on other factors including physical activity, age, gender, BMI, smoking status.
The trial compared three treatments administered over 1 year: 1) two 20,000 IU capsules of vitamin D3 per week (40,000 IU/wk total); 2) one 20,000 IU capsule of vitamin D3 + one placebo capsule per week (20,000 IU/wk total); and 3) 2 placebo capsules per week. Participants were randomized to a treatment group after stratification by gender and smoking status. Blood samples were collected every 3 months during follow up to monitor calcium status and evaluate 25(OH)D changes. At the end of the 1-year follow-up, the BDI was readministered. Compliance with the intervention was similar in all 3 groups (95%), as was the drop-out rate (range of 22.7% – 25.3%). Overall, 334 participants completed the trial.
The authors assessed the correlation between baseline BDI scores and 25(OH)D levels prior to randomization and treatment. Continuous 25(OH)D levels were not significantly correlated with either total BDI scores or scores on either subscale. However, when participants were instead divided into those with sufficient and insufficient 25(OH)D levels (<40 vs. ≥ 40 nmol/L), those with insufficient 25(OH)D had significantly higher total BDI scores (6.0 vs. 4.5; P < 0.05) and BDI items 1–13 scores (2.0 vs. 1.0; P< 0.05) than those with sufficient 25(OH)D. Scores on BDI 14–21 were comparable (3.5 vs. 3.0).
In the 334 participants who completed the study, there was significant evidence of modest improvement in BDI scores after 1 year of vitamin D supplementation (figure 1). In the group receiving 40,000 IU/wk, median total BDI score decreased from 4.5 to 3.0 (P < 0.01), while in those receiving 20,000 IU/wk median score decreased from 5.0 to 4.0 (P < 0.01). In contrast, in the placebo group, median scores did not decrease significantly (4.0 vs. 3.8; P > 0.05). Both groups receiving vitamin D supplements also demonstrated significant improvement in the BDI cognitive-affective subscale, while no difference was observed in the placebo group. Differences in baseline vs. 12 month follow-up score distributions on the somatic-vegetative subscale were significant in all 3 groups.
Figure 1.
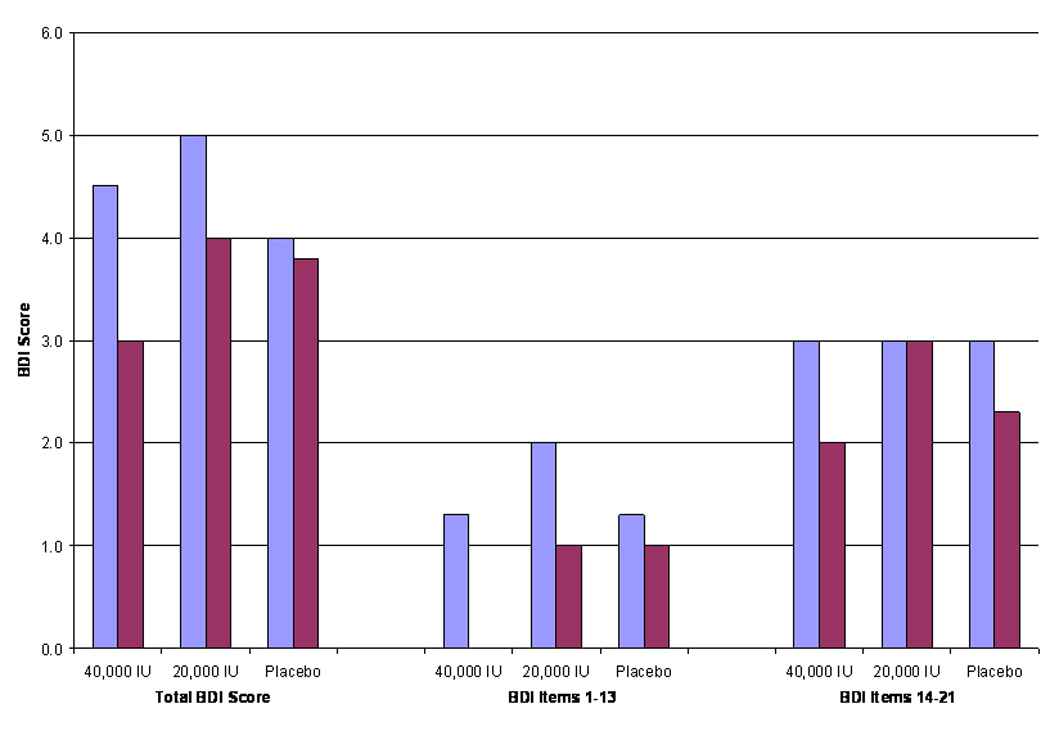
In addition, the authors observed greater improvement in cognitive-affective scores in several sub-groups of those receiving 40,000 IU/wk group including: women; older individuals; those with higher BMI; those with lower 25(OH)D at baseline; those with higher cognitive-affective BDI score at baseline; and those with lower physical activity at baseline. The frequency of adverse events was similar between groups. Only 1 subject had a persistently elevated serum calcium level in response to 20,000 IU/wk and had to withdraw from the study.
Results from this relatively large randomized trial contribute substantially to our growing knowledge of how vitamin D may relate to depression and has several important strengths. Given the randomized assignment of vitamin D supplementation, these results are not likely attributable to confounding by physical activity and other factors related to both depression and vitamin D. The prospective nature of this study also addresses the question of temporality of the vitamin D-depression relationship, as supplementation preceded evaluation of change in BDI scores; this lends support for a causal relationship between vitamin D and mood.
Despite these interesting findings, there are several important points requiring further consideration, many of which the authors themselves cite as limitations. First, as all participants were overweight or obese at randomization, it is unclear whether these findings are applicable to a normal weight population. Secondly, mean BDI scores in this population were low (4.5 – 5.0 at baseline), suggesting that few participants were clinically depressed. It is unclear whether these findings would be replicated in a population experiencing moderate to severe depression. It is also unclear whether the results suggesting differences in effect by gender, age and other factors indicate true physiologic differences or whether they are instead reflective of higher baseline BDI scores in women and other sub-groups, with more room for improvement in mood.
Thirdly, the doses of vitamin D tested were very large. 40,000 IU per week is equivalent to 5714 IU per day, while 20,000 IU is equivalent to 2,857 IU per day; after 1 year, 25(OHD) levels in these two groups were 112.1 nmol/L and 87.8 nmol/L, respectively. Despite this high level of supplementation, the observed improvements in mood were quite modest. Mean total scores on the BDI decreased by 1.5 points in the group receiving the highest supplementation. This is equivalent to a 1–2 level change in response category on a single item. It is unclear whether this improvement, though statistically significant, is clinically significant.
Lastly, the authors do not directly address how spontaneous regression of depressive symptoms over the 1 year of follow-up may have influenced results. Statistical comparisons are made within intervention groups, so it is not clear whether the improvements in BDI scores in those randomized to vitamin D still would be significant after taking into account the level of remission experienced in the placebo group. It is also unknown how quickly improvement in mood occurred after supplementation began.
Supporting evidence: vitamin D and related conditions
In addition to investigations of vitamin D and major depression or depressive symptoms, several studies have evaluated how vitamin D may relate to other affective disorders such as seasonal affective disorder, premenstrual syndrome/premenstrual dysphoric disorder, and fibromyalgia. Results from these studies are also inconsistent, but provide modest support for a possible effect of vitamin D on mood.
Seasonal Affective Disorder
Seasonal affective disorder (SAD) is a disorder characterized by symptoms of depression, anxiety, irritability, appetite changes, hypersomnia and fatigue that occur during winter months and abate in the spring and summer.6 Women are affected more than men and the average age at onset is comparable to that of major depression. Some evidence suggests that incidence increases with latitude and thus reduced sun exposure, and phototherapy with broad spectrum bright artificial light (>2500 lux) may improve symptoms within days in some patients.50
A role of vitamin D in the etiology of SAD was first suggested by Stumpf and Privette (1989).51 In the last two decades, several small randomized trials have tested this hypothesis, but have provide mixed evidence that vitamin D supplementation significantly improves SAD symptoms. Vieth and colleagues (2004)52 randomized 82 adults with evidence of vitamin D deficiency to the equivalent of 100 mcg (4000 IU) or 15 mcg (600 IU) of vitamin D3 per day for 3 months over 2 consecutive winters. Changes in wellbeing between December and February of each year were evaluated with a short questionnaire based on standard depression scales. The investigators observed some evidence of improved wellbeing in those assigned to the higher dose regimen compared to the lower, though results were not significant for all comparisons. Improvements in mood symptoms were also observed in two other small trials.53,54
Three other trials did not observe any improvement in SAD symptoms with vitamin D treatment.55–57 In a randomized trial of vitamin D supplementation to prevent seasonal bone loss, Harris and colleagues (1993)55 randomized 250 women aged 43–72 to either 400 IU of vitamin D + 377 mg of calcium per day or calcium alone for 1 year. Randomization took place in June and July, and follow-up visits were conducted multiple times over 1 year, including during December-January, when depressive symptoms were hypothesized to be most prevalent. At each follow-up visit, the Profile of Mood States (POMS) scale was used to assess depressive symptoms and blood samples were collected to assess 25(OH)D and 1,25(OH)2D levels. The authors did not find any difference in POMS score between women randomized to vitamin D vs. placebo. In addition, POMS scores were not correlated with vitamin D metabolite levels, and changes in vitamin D did not correlate with changes in POMS over time. Similarly, after 6 months, Dumville et al (2006)56 did not observe improvement in the Mental Component Score from the 12-item Short Form Health Survey in 1621 women randomized to 800 IU of vitamin D and 1000 mg/day of calcium compared to controls.
Two trials involving phototherapy as a treatment for SAD did not find that regular exposure to light at standard therapeutic doses affected 25(OH)D levels,57,58 suggesting that improvement in mood associated with phototherapy are unlikely to be through vitamin D-related pathways. Taken together, there is only modest evidence that vitamin D is effective at treating the symptoms of SAD.
Premenstrual Syndrome and Premenstrual Dysphoric Disorder
PMS is a disorder characterized by moderate to severe physical and emotional symptoms in the luteal phase of the menstrual cycle that substantially interfere with normal life activities and interpersonal relationships.59,60 Some of the most common emotional symptoms are depression, irritability, mood swings, and anxiety.61–63 Women in whom affective symptoms predominate may also meet criteria for PMDD, a more severe form of PMS that is associated with significant impairment of normal functioning.6,63 PMDD is recognized as a mood disorder in the DSM-IV.6
A small body of research suggests that circulating levels of vitamin D, calcium and PTH may be involved in the development of menstrual symptoms, PMS and PMDD. It has been suggested that women with luteal phase symptoms consistent with PMS may be experiencing vitamin D deficiency, calcium dysregulation, and hyperparathyroidism.64 A small number of studies have explored the relationship between these vitamin D related biomarkers and menstrual symptoms. Thys-Jacobs et al. (1995)65 reported significant differences in levels of these biomarkers in 7 PMS cases compared to 5 symptom-free controls. 1,25(OH)2D and PTH were higher in PMS cases than controls in the follicular, ovulatory and luteal phases of the menstrual cycle, while 25(OH)D levels were significantly lower at all phases. However, a second recent study by the same investigators reported that mean serum levels of 25(OH)D and 1,25(OH)2D were similar in women with 68 PMDD and 47 healthy control women.66
Vitamin D supplementation for the prevention or treatment of PMS has not been explored directly. However, low dietary vitamin D intake has also been associated with the initial development of PMS. A study by Bertone-Johnson et al. (2005)67 in a subset of the Nurses’ Health Study II cohort found that women with the highest reported intake of vitamin D from food sources, equivalent to approximately 400 IU per day, had a significant 41% lower risk of developing PMS over the next 2–4 years compared to women with the lowest intake (P for trend = 0.01). Additional evidence concerning the role of vitamin D deficiency in PMS/PMDD is clearly needed.
Fibromyalgia
Fibromyalgia is a disorder associated with chronic pain and tenderness at a variety of muscular skeletal points throughout the body.68 It often occurs concurrently with MDD, premenstrual dysphoric disorder and other psychiatric conditions, and is considered by many to be an affective spectrum disorder.69 Only one study to date has assessed whether vitamin D may be associated with depression and anxiety in fibromyalgia. Armstrong and colleagues (2007)70 compared serum 25(OH)D levels and Hospital Anxiety and Depression scores in 75 fibromyalgia patients. Significantly higher scores, indicating greater mood impairment, were observed in those with 25(OH)D deficiency (<25nmol/L) than those with sufficient levels. Replication of this finding is needed to better understand on the impact of vitamin D on mood in fibromyalgia.
Conclusion
At this time, the evidence linking vitamin D to the development of depression remains largely circumstantial. Findings of mechanistic studies suggest that vitamin D plays an important role in brain development and function. It is however unknown whether these actions directly affect monoamine levels, HPA axis functioning in response to stress, or other mechanisms involved in depression. Relatively few epidemiologic studies have robustly evaluated whether vitamin D levels sufficiency may prevent or treat depression. Recent findings of Jorde and colleagues (2008)1 lend some support for a relationship between vitamin D and depressive symptoms, but important questions persist. Additional information from several areas is clearly needed to answer the following:
-
▪From mechanistic studies:
-
○Does vitamin D affect monoamine and/or HPA axis functioning?
-
○Is vitamin D directly involved in hippocampal neurogenesis?
-
○Does vitamin D deficiency in adulthood affect brain function differently than deficiency in utero and/or during development?
-
○
-
▪From epidemiologic studies:
-
○Is vitamin D status related to all types of depression or to specific subtypes, such as depression co-occurring with symptoms of anxiety or with evidence of dopaminergic dysfunction?
-
○Can vitamin D supplementation substantially improve mood in a clinically depressed population, over and above what would occur spontaneously?
-
○Can maintaining sufficient vitamin D levels reduce the incidence of first major depression and prevent recurrence?
-
○What supplemental or sunlight exposure dose of vitamin D is most effective?
-
○
At this time, it is premature to conclude that vitamin D status is related to the occurrence of depression. However, until results from additional prospective studies are available, there is little harm in recommending that individuals with depressive symptoms consume the newly recommended dose of 1,000 – 2,000 IU of vitamin D per day and attain modest sun exposure, given the overall health benefits of vitamin D and low risk of toxicity at these doses.
Acknowledgements
This work was supported by Public Health Services Grant MH076274 from the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services. The author wishes to thank Drs. Sally Powers, Nancy Forger, and Mary Harrington for their helpful advice and insightful comments.
References
- 1.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;263:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 2.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88 suppl:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 4.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66 suppl2:S182–S194. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: DSM-IV; 1994. [Google Scholar]
- 7.Cerdá M, Sagdeo A, Galea S. Comorbid forms of psychopathology: Key patterns and future research directions. Epidemiol Rev. 2008;30:155–177. doi: 10.1093/epirev/mxn003. [DOI] [PubMed] [Google Scholar]
- 8.Belmaker RH, Agam G. Major depressive disorder. N Eng J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 9.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Molec Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 11.Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: Hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 13.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehnder D, Bland R, Williams MC, et al. Extrarnal expression of 25-hydroxyvitamin D3-1alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 16.Gascon-Barré M, Huet PM. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244:E266–E2671. doi: 10.1152/ajpendo.1983.244.3.E266. [DOI] [PubMed] [Google Scholar]
- 17.Pardridge WM, Sakiyama R, Coty WA. Restricted transport of vitamin D and A derivatives through the rat blood-brain barrier. J Neurochem. 1985;44:1138–1141. doi: 10.1111/j.1471-4159.1985.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 18.Newmark HL, Newmark J. Vitamin D and Parkinson’s Disease- A hypothesis. Mov Disord. 2007;22:461–468. doi: 10.1002/mds.21317. [DOI] [PubMed] [Google Scholar]
- 19.McGrath JJ, Feron FP, Burne THJ, Mackay-Sim A, Eyles DW. Vitamin D3- implications for brain development. J Steroid Biochem Molec Biol. 2004:557–560. doi: 10.1016/j.jsbmb.2004.03.070. 89–90. [DOI] [PubMed] [Google Scholar]
- 20.Féron F, Burne THJ, Brown J, et al. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65:141–148. doi: 10.1016/j.brainresbull.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Handoko HY, Nancarrow DJ, Mowry BJ, McGrath JJ. Polymorphisms in the vitamin D receptor and their associations with risk of schizophrenia and selected anthropometric measures. Am J Hum Biol. 2006;18:414–417. doi: 10.1002/ajhb.20504. [DOI] [PubMed] [Google Scholar]
- 20.Minasyan A, Keisala T, Lou YR, Kalueff AV, Tuohimaa P. Neophobia, sensory and cognitive functions, and hedonic responses in vitamin D receptor mutant mice. J Steroid Biochem Molec Biol. 2007;104:274–280. doi: 10.1016/j.jsbmb.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Kalueff AV, Keisala T, Minasyan A, Kuuslahti M, Miettinen S, Tuohimaa P. Behavioral anomalies in mice evoke by “Tokyo” disruption of the Vitamin D receptor gene. Neurosci Res. 2006;54:254–260. doi: 10.1016/j.neures.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Burne THJ, McGrath JJ, Eyles DW, Mackay-Sim A. Behavioural characterization of Vitamin D receptor knockout mice. Behav Brain Res. 2005;157:299–308. doi: 10.1016/j.bbr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Burne THJ, Johnston ANB, McGrath JJ, Mackay-Sim A. Swimming behavior and post-swimming activity in Vitamin D receptor knockout mice. Brain Res Bull. 2006;69:74–78. doi: 10.1016/j.brainresbull.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Harms LR, Eyles DW, McGrath JJ, Mackay-Smith A, Burne THJ. Developmental vitamin D deficiency alters adult behavior in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074:261–271. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 28.Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31:533–539. doi: 10.1007/s11064-006-9048-4. [DOI] [PubMed] [Google Scholar]
- 29.Baksi SN, Hughes MJ. Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res. 1982;242:387–390. doi: 10.1016/0006-8993(82)90331-6. [DOI] [PubMed] [Google Scholar]
- 30.Obradovic D, Gronemeyer H, Lutz B, Rein T. Cross-talk of vitamin D and glucocorticoids in hippocampal cells. J Neurochem. 2006;96:500–509. doi: 10.1111/j.1471-4159.2005.03579.x. [DOI] [PubMed] [Google Scholar]
- 31.Schneider B, Weber B, Frensch A, Stein J, Fritze J. Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm. 2000;107:839–842. doi: 10.1007/s007020070063. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 33.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J The Tromsø study. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. J Neurol. 2006;253:464–470. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 34.Eskandari F, Martinez PE, Torvik S, et al. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007;167:2329–2336. doi: 10.1001/archinte.167.21.2329. [DOI] [PubMed] [Google Scholar]
- 35.Hoogendijk WJG, Lips P, Dik MG, Deeg DJ, Beekman ATF, Penninx BWJH. Depression is associated with decreased 25-hydroxyvitmain D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65:508–512. doi: 10.1001/archpsyc.65.5.508. [DOI] [PubMed] [Google Scholar]
- 36.Pan A, Lu L, Franco OH, Yu Z, Li H, Lin X. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009 doi: 10.1016/j.jad.2009.02.002. doi:10.1016/j.jad2009.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Herrán A, Amado JA, García-Unzueta MT, Vázquez-Barquero JL, Perera L, Gonzáles-Macías J. Increased bone remodeling in first-episode major depressive disorder. Psychosom Med. 2000;62:779–782. doi: 10.1097/00006842-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Michelson D, Stratakis C, Hill L, et al. Bone mineral density in women with depression. N Eng J Med. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:225–236. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 40.Brot C, Jørgensen NR, Sørensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 41.Gerdheim P, Ringsberg KAM, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int. 2005;16:1425–1431. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 42.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 43.Hintzpeter B, Mensink GBM, Thierfelder W, Müller MJ, Scheidt-Nave C. Vitamin D status and health correlated among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 44.Santori D, Ceccanti M, Diacinti D, et al. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31:321–326. doi: 10.1007/BF03346365. [DOI] [PubMed] [Google Scholar]
- 45.Bjerkeset O, Romundstad P, Evans J, Gunnell D The HUNT study. Association of adult body mass index and height with anxiety, depression, and suicide in the general population. Am J Epidemiol. 2008;167:193–202. doi: 10.1093/aje/kwm280. [DOI] [PubMed] [Google Scholar]
- 46.Pasco JA, Williams LJ, Jacka FN, et al. Tobacco smoking as a risk factor for major depressive disorder: population-based study. Brit J Psychiatry. 2008;193:322–326. doi: 10.1192/bjp.bp.107.046706. [DOI] [PubMed] [Google Scholar]
- 47.Roberts RE, Kaplan GA, Sherma SJ, Strawbridge WJ. Does growing old increase the risk for depression? Am J Psychiatry. 1997;154:1384–1390. doi: 10.1176/ajp.154.10.1384. [DOI] [PubMed] [Google Scholar]
- 48.Galper DI, Trivedi MH, Barlow CE, Dunn AL, Kampert JB. Inverse association between physical inactivity and mental health in men and women. Med Sci Sports Exerc. 2006;38:173–178. doi: 10.1249/01.mss.0000180883.32116.28. [DOI] [PubMed] [Google Scholar]
- 49.Jané-Llopis E, Matytsina I. Mental health and alcohol, drug and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Alcohol Rev. 2006;25:515–536. doi: 10.1080/09595230600944461. [DOI] [PubMed] [Google Scholar]
- 50.Lurie SJ, Gawinski B, Pierce D, Rousseau SJ. Seasonal affective disorder. Am Fam Physician. 2006;74:1521–1524. [PubMed] [Google Scholar]
- 51.Stumpf WE, Privette TH. Light, vitamin D and psychiatry: role of 1,25-dihydroxyvitamin D3 (soltiol) in etiology and therapy of seasonal affective disorder and other mental processes. Psychopharmacol. 1989;97:285–294. doi: 10.1007/BF00439440. [DOI] [PubMed] [Google Scholar]
- 52.Vieth R, Kimball S, Hu A, Walfish PG. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gloth FM, III, Alam W, Hollis B. Vitamin D vs. broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3:5–7. [PubMed] [Google Scholar]
- 54.Lansdowne ATG, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacol. 1998;135:319–323. doi: 10.1007/s002130050517. [DOI] [PubMed] [Google Scholar]
- 55.Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49:77–87. doi: 10.1016/0165-1781(93)90031-b. [DOI] [PubMed] [Google Scholar]
- 56.Dumville JC, Miles JV, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10:151–153. [PubMed] [Google Scholar]
- 57.Partonen T, Vakkuri O, Lamberg-Allardt C, Lönnqvist Effects of bright light on sleepiness, melatonin, and 24-hydroxyvitamin D3 in winter seasonal affective disorder. Biol Psychiatry. 1996;39:865–872. doi: 10.1016/0006-3223(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 58.Oren DA, Schulkin J, Rosenthal NE. 1,25(OH)2 D3 levels in seasonal affective disorder: Effects of light. Psychopharmacol. 1994;116:515–516. doi: 10.1007/BF02247486. [DOI] [PubMed] [Google Scholar]
- 59.The American College of Obstetricians and Gynecologists. Clinical Management Guidelines. 2000 April;(No. 15) [Google Scholar]
- 60.Mortola JF., Jr Issues in the diagnosis and research of premenstrual syndrome. Clin Obstet Gynecol. 1992;35:587–598. doi: 10.1097/00003081-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 61.Johnson SR. The epidemiology and social impact of premenstrual symptoms. Clin Obstet Gynecol. 1987;30:367–376. doi: 10.1097/00003081-198706000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Mortola JF, Girton L, Beck L, Yen SS. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: the calendar of premenstrual experiences. Obstet Gynecol. 1990;76:302–307. [PubMed] [Google Scholar]
- 63.Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: Definitions and diagnosis. Psychoneuroendocrinology. 2003;28:25–37. doi: 10.1016/s0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 64.Thys-Jacobs S. Micronutrients and the premenstrual syndrome: the case for calcium. J Am Coll Nutr. 2000;19:220–227. doi: 10.1080/07315724.2000.10718920. [DOI] [PubMed] [Google Scholar]
- 65.Thys-Jacobs S, Alvir MJ. Calcium-regulating hormones across the menstrual cycle: evidence of a secondary hyperparathyroidism in women with PMS. J Clin Endocrinol Metab. 1995;80:2227–2232. doi: 10.1210/jcem.80.7.7608284. [DOI] [PubMed] [Google Scholar]
- 66.Thys-Jacobs S, McMahon D, Bilezikian JP. Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. J Clin Endocrinol Metab. 2007;92:2952–2959. doi: 10.1210/jc.2006-2726. [DOI] [PubMed] [Google Scholar]
- 67.Bertone-Johnson ER, Hankinson SE, Bendich A, Johnson SR, Willett WC, Manson JE. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246–1252. doi: 10.1001/archinte.165.11.1246. [DOI] [PubMed] [Google Scholar]
- 68.Ablin J, Neumann L, Buskila D J. Pathogenesis of fibromyalgia- A review. Joint Bone Spine. 2008;75:273–279. doi: 10.1016/j.jbspin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Bradley LA. Pathophysiologic mechanisms of fibromyalgia and its related disorders. J Clin Psychiatry. 2008;69:S6–S13. [PubMed] [Google Scholar]
- 70.Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551–554. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]


