Abstract
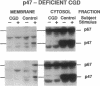
Two of the cytosolic NADPH oxidase components, p47-phox and p67-phox, translocate to the plasma membrane in normal neutrophils stimulated with phorbol myristate acetate (PMA). We have now studied the translocation process in neutrophils of patients with chronic granulomatous disease (CGD), an inherited syndrome in which the oxidase system fails to produce superoxide due to lesions affecting any one of its four known components: the gp91-phox and p22-phox subunits of cytochrome b558 (the membrane-bound terminal electron transporter of the oxidase), p47-phox, and p67-phox. In contrast to normal cells, neither p47-phox nor p67-phox translocated to the membrane in PMA-stimulated CGD neutrophils which lack cytochrome b558. In one patient with a rare X-linked form of CGD caused by a Pro----His substitution in gp91-phox, but whose neutrophils have normal levels of this mutant cytochrome b558, translocation was normal. In two patients with p47-phox deficiency, p67-phox failed to translocate, whereas p47-phox was detected in the particulate fraction of PMA-stimulated neutrophils from two patients deficient in p67-phox. Our data suggest that cytochrome b558 or a closely linked factor provides an essential membrane docking site for the cytosolic oxidase components and that it is p47-phox that mediates the assembly of these components on the membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolscher B. G., van Zwieten R., Kramer I. M., Weening R. S., Verhoeven A. J., Roos D. A phosphoprotein of Mr 47,000, defective in autosomal chronic granulomatous disease, copurifies with one of two soluble components required for NADPH:O2 oxidoreductase activity in human neutrophils. J Clin Invest. 1989 Mar;83(3):757–763. doi: 10.1172/JCI113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Volpp B. D., Leidal K. G., Nauseef W. M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest. 1990 Mar;85(3):714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Chronic granulomatous disease. Adv Hum Genet. 1987;16:229–297. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T. Classification of chronic granulomatous disease. Hematol Oncol Clin North Am. 1988 Jun;2(2):241–252. [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Dinauer M. C., Curnutte J. T., Rosen H., Orkin S. H. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest. 1989 Dec;84(6):2012–2016. doi: 10.1172/JCI114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Suzuki K., Suzuki S., Andrews P. C., Babior B. M. A possible role for protein phosphorylation in the activation of the respiratory burst in human neutrophils. Evidence from studies with cells from patients with chronic granulomatous disease. J Biol Chem. 1986 Jul 15;261(20):9109–9115. [PubMed] [Google Scholar]
- Heyworth P. G., Badwey J. A. Protein phosphorylation associated with the stimulation of neutrophils. Modulation of superoxide production by protein kinase C and calcium. J Bioenerg Biomembr. 1990 Feb;22(1):1–26. doi: 10.1007/BF00762842. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Shrimpton C. F., Segal A. W. Localization of the 47 kDa phosphoprotein involved in the respiratory-burst NADPH oxidase of phagocytic cells. Biochem J. 1989 May 15;260(1):243–248. doi: 10.1042/bj2600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I. M., Verhoeven A. J., van der Bend R. L., Weening R. S., Roos D. Purified protein kinase C phosphorylates a 47-kDa protein in control neutrophil cytoplasts but not in neutrophil cytoplasts from patients with the autosomal form of chronic granulomatous disease. J Biol Chem. 1988 Feb 15;263(5):2352–2357. [PubMed] [Google Scholar]
- Lomax K. J., Leto T. L., Nunoi H., Gallin J. I., Malech H. L. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989 Jul 28;245(4916):409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- Nunoi H., Rotrosen D., Gallin J. I., Malech H. L. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988 Dec 2;242(4883):1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- Okamura N., Babior B. M., Mayo L. A., Peveri P., Smith R. M., Curnutte J. T. The p67-phox cytosolic peptide of the respiratory burst oxidase from human neutrophils. Functional aspects. J Clin Invest. 1990 May;85(5):1583–1587. doi: 10.1172/JCI114608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Curnutte J. T., Roberts R. L., Babior B. M. Relationship of protein phosphorylation to the activation of the respiratory burst in human neutrophils. Defects in the phosphorylation of a group of closely related 48-kDa proteins in two forms of chronic granulomatous disease. J Biol Chem. 1988 May 15;263(14):6777–6782. [PubMed] [Google Scholar]
- Okamura N., Malawista S. E., Roberts R. L., Rosen H., Ochs H. D., Babior B. M., Curnutte J. T. Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood. 1988 Aug;72(2):811–816. [PubMed] [Google Scholar]
- Parkos C. A., Allen R. A., Cochrane C. G., Jesaitis A. J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J Clin Invest. 1987 Sep;80(3):732–742. doi: 10.1172/JCI113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Dinauer M. C., Jesaitis A. J., Orkin S. H., Curnutte J. T. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989 May 1;73(6):1416–1420. [PubMed] [Google Scholar]
- Quinn M. T., Parkos C. A., Walker L., Orkin S. H., Dinauer M. C., Jesaitis A. J. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989 Nov 9;342(6246):198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Kleinberg M. E., Nunoi H., Leto T., Gallin J. I., Malech H. L. Evidence for a functional cytoplasmic domain of phagocyte oxidase cytochrome b558. J Biol Chem. 1990 May 25;265(15):8745–8750. [PubMed] [Google Scholar]
- Segal A. W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987 Mar 5;326(6108):88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Heyworth P. G., Cockcroft S., Barrowman M. M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985 Aug 8;316(6028):547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- Teahan C. G., Totty N., Casimir C. M., Segal A. W. Purification of the 47 kDa phosphoprotein associated with the NADPH oxidase of human neutrophils. Biochem J. 1990 Apr 15;267(2):485–489. doi: 10.1042/bj2670485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahan C., Rowe P., Parker P., Totty N., Segal A. W. The X-linked chronic granulomatous disease gene codes for the beta-chain of cytochrome b-245. 1987 Jun 25-Jul 1Nature. 327(6124):720–721. doi: 10.1038/327720a0. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Clark R. A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988 Dec 2;242(4883):1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- Volpp B. D., Nauseef W. M., Donelson J. E., Moser D. R., Clark R. A. Cloning of the cDNA and functional expression of the 47-kilodalton cytosolic component of human neutrophil respiratory burst oxidase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7195–7199. doi: 10.1073/pnas.86.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]