Abstract
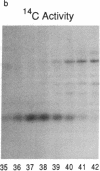
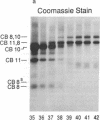
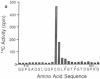
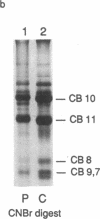
In a family who expressed severe dominantly inherited osteoarthritis, the underlying mutation was traced by genomic sequencing to a single base change which predicts an amino acid substitution of cysteine for arginine at residue 519 of the triple-helical domain of the type II collagen molecule (Ala-Kokko, L., C. T. Baldwin, R. W. Moskowitz, and D. J. Prockop. 1990. Proc. Natl. Acad. Sci. USA. 87:6565-6568). In the present study we examined whether this predicted protein phenotype was evident in articular cartilage obtained from an affected family member who underwent hip surgery. The cartilage collagen was solubilized by CNBr digestion. Cysteine residues were labeled by reduction and alkylation with 14C-iodoacetate. Collagen CNBr-peptides were fractionated by ion exchange and reverse phase column chromatography. One peptide from the alpha 1(II) chain, alpha 1(II) CB8, was found to be radiolabeled. Tryptic peptides were prepared from it and identified by microsequence analysis. The results show that approximately one-quarter of the alpha 1(II) chains present in the polymeric extracellular collagen of the patient's cartilage contained the Arg519-to-Cys substitution. The protein exhibited other abnormal properties including disulfide-bonded alpha 1(II)-dimers and signs of posttranslational overmodification. The premature cartilage failure and osteoarthritis are presumably a result of the abnormal type II collagen being expressed in the cartilage matrix.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. T., Reginato A. M., Smith C., Jimenez S. A., Prockop D. J. Structure of cDNA clones coding for human type II procollagen. The alpha 1(II) chain is more similar to the alpha 1(I) chain than two other alpha chains of fibrillar collagens. Biochem J. 1989 Sep 1;262(2):521–528. doi: 10.1042/bj2620521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Rapid fractionation of collagen chains and peptides by high-performance liquid chromatography. Anal Biochem. 1986 Apr;154(1):338–344. doi: 10.1016/0003-2697(86)90534-8. [DOI] [PubMed] [Google Scholar]
- Brown A. R., Rose B. S. Familial precocious polyarticular osteoarthrosis of chondrodysplastic type. N Z Med J. 1966 Jul;65(407):449–461. [PubMed] [Google Scholar]
- Eyre D. R., Koob T. J., Van Ness K. P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984 Mar;137(2):380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975 Dec;151(3):595–602. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Wu J. J., Apone S. A growing family of collagens in articular cartilage: identification of 5 genetically distinct types. J Rheumatol. 1987 May;14(Spec No):25–27. [PubMed] [Google Scholar]
- Eyre D. Collagen cross-linking amino acids. Methods Enzymol. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Nagase H., Woessner J. F., Jr Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989 Feb 15;258(1):115–119. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- KELLGREN J. H. THE EPIDEMIOLOGY OF RHEUMATIC DISEASES. Ann Rheum Dis. 1964 Mar;23:109–122. doi: 10.1136/ard.23.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein P. L., Malemud C. J., Pathria M. N., Carter J. R., Sheon R. P., Moskowitz R. W. Early-onset primary osteoarthritis and mild chondrodysplasia. Radiographic and pathologic studies with an analysis of cartilage proteoglycans. Arthritis Rheum. 1990 May;33(5):674–684. doi: 10.1002/art.1780330510. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Katzenstein P. L., Moskowitz R. W., Weaver E. J., Malemud C. J., Pathria M. N., Jimenez S. A., Prockop D. J. Genetic linkage of a polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990 Feb 22;322(8):526–530. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Maroudas A. I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976 Apr 29;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Woodall D. L., Vail M. S. Biosynthesis of cartilage collagen. Use of pulse labeling to order the cyanogen bromide peptides in the alpha L(II) chain. J Biol Chem. 1973 Mar 10;248(5):1666–1671. [PubMed] [Google Scholar]
- Murray L. W., Bautista J., James P. L., Rimoin D. L. Type II collagen defects in the chondrodysplasias. I. Spondyloepiphyseal dysplasias. Am J Hum Genet. 1989 Jul;45(1):5–15. [PMC free article] [PubMed] [Google Scholar]
- Palotie A., Väisänen P., Ott J., Ryhänen L., Elima K., Vikkula M., Cheah K., Vuorio E., Peltonen L. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989 Apr 29;1(8644):924–927. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- Starman B. J., Eyre D., Charbonneau H., Harrylock M., Weis M. A., Weiss L., Graham J. M., Jr, Byers P. H. Osteogenesis imperfecta. The position of substitution for glycine by cysteine in the triple helical domain of the pro alpha 1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest. 1989 Oct;84(4):1206–1214. doi: 10.1172/JCI114286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]