Summary
One of the most fundamental activities of the adaptive immune system is to kill infected cells and tumor cells. Two distinct pathways mediate this process, both of which are facilitated by a cytotoxic immunological synapse. While traditionally thought of as innate immune cells, natural killer (NK) cells are now appreciated to have the capacity for long-term in reactions to chemical and viral insults. These cells integrate multiple positive and negative signals through NK cell cytotoxic or inhibitory synapses. The traditional CD8+αβ T-cell receptor-positive cells are among the best models for the concept of an immunological synapse, in which vectoral signaling is linked to directed secretion in a stable interface to induce apoptotic cell death in an infected cell. Large-scale molecular organization in synapses generated a number of hypotheses. Studies in the past five years have started to provide clear answers regarding the validity of these models. In vivo imaging approaches have provided some hints as to the physiological relevance of these processes with great promise for the future. This review provides an overview of work on cytotoxic immunological synapses and suggests pathways forward in applying this information to the development of therapeutic agents.
Keywords: activation, cytotoxicity, synapse, actin, microscopy, adhesion
Introduction to the immunological synapse
Immunological synapses are antigen specific cell-cell junctions with a synaptic cleft stabilized by bona fide adhesion molecules for vectoral cell-cell communication between an immune cell and an antigen-presenting cell (APC) (1). We use the term synapse to describe junctions that meet these criteria. The cytotoxic synapse is one of the earliest and best defined of immunological synapse types based on a number of key findings in immunology in the 1970s and early 1980s that exploited this system, and clear functional importance that sustained interest even as other T-cell subsets were described. Zinkernagel and Doherty (2) defined the role of the major histocompatibility complex (MHC) in killing of virally infected cells by sensitized T lymphocytes, which are described as cytotoxic T lymphocytes (CTL) to distinguish them from helper T lymphocytes. The description of adhesion molecules like leukocyte function-associated antigen-1 (LFA-1) by Springer (3), the polarization of cytotoxic T cells (4, 5), and directed secretion of perforins and granzymes triggered by cytoplasmic Ca2+ elevation (6, 7) led to the proposal of a synaptic basis for T-cell killing (8). The cloning of the T-cell receptor (TCR) (9) and the definition of peptides bound to the groove of MHC molecules as TCR ligands (10, 11) allowed the generation of monoclonal T-cell mice and the biochemical preparation of defined TCR ligands that set the stage for further molecular dissection of the immunological synapse.
The study of natural killer (NK) cell synapses was on a parallel track to CTLs. Natural killing was described in the mid 1970s (12). Early studies on the cell biology of NK mediated killing noted dramatic secretory and cytoskeletal polarization that accompanied the cytotoxic process (13–16). The inverse relationship between natural killing and MHC class I expression was noted in 1986 by Kärre (17). Yokoyama described MHC class I binding inhibitory receptor Ly49 as a prototype molecular basis for ‘missing self’ recognition (18). Identification of the structurally unrelated but functionally equivalent MHC class I inhibitory receptors in human NK cells, the killer-cell Ig-like receptors (KIRs), led to the definition of the immunotyrosine inhibition motif (ITIM) sequence as V/IxYxxL, and the finding that tyrosine phosphatase SHP-1 is recruited by phosphorylated ITIMs to turn off activation signals (19). The description of many activating NK cell receptors and associated signal transduction modules suggested additional modes of positive signaling that are integrated with the negative signals in the NK synapse (20, 21). NK cells could also link into adaptive immunity via FcRs but were initially thought of as innate effector cells. Recently, NK cell ‘memory’ responses were described, which blur the line between adaptive CTLs and innate NK cells (22, 23). While both the CTL and NK synapses can be cytotoxic in nature, the distinct triggering mechanisms and checkpoints make the two cells synapse with potential targets quite differently. The analysis of the NK cell synapse includes both a cytotoxic synapse and an inhibitory synapse in which the negative regulatory receptors are dominant.
While the ‘synaptic basis of T-cell killing’ was first noted in 1984 (8) with a prominent review adopting the term in 1994 (24), this concept remained mostly latent until work by Kupfer on organization of molecules in the helper T cell-B cell interface and work from our group on the dynamics of pattern formation provided a molecular signature for an immunological synapse (25, 26). Studies by Kupfer revealed a striking segregation of adhesion receptors in the interface between T cells and antigen-presenting cells. These reconstructed images were presented at meetings in 1996, and they were so convincing of important underlying mechanism that Janeway introduced them into his Immunobiology textbook (27) as early as 1997, a year prior to peer-reviewed publication. The original publication in 1998 introduced the term supramolecular activation cluster (SMAC) into the immunology vocabulary to describe two distinct micron scale domains formed in a bull’s eye pattern: a central (c)SMAC rich in TCR and a peripheral (p)SMAC configured as a ring of LFA-1 adhesion receptors (25). Lck and protein kinase C-θ (PKCθ), a novel PKC isoform that is uniquely recruited to the T-B interface (28), were co-localized with TCR in the cSMAC. The widely expressed integrin-cytoskeletal linking protein talin was co-localized with LFA-1. This structure was observed under conditions of T-cell activation, and the organized SMACs were not observed when antagonistic MHC-peptide ligands were presented on the B-cell tumors used as APCs. In the same time frame, we were utilizing the supported planar bilayer system to investigate the organization of adhesive contacts formed by LFA-1 and CD2, a second important adhesion receptor utilized by human CTLs. Using a supported planar bilayer model, we demonstrated segregation of LFA-1 from CD2 and further demonstrated active concentration of CD2 through its interaction with the adapter CD2AP (26). In this paper and without knowledge of the SMAC nomenclature, we proposed the use of the term immunological synapse to describe the bull’s eye pattern of integrins around TCR and isometric adhesion systems like CD2 and CD28. That summer we succeeded in reconstituting T-cell activation by supported planar bilayers presenting ICAM-1 and MHC-peptide complexes. We again found the same end point as Kupfer but could watch the evolution of the patterns from inverted nascent structures in which TCR were engaged in peripheral clusters that translocated to the center of the interface to generate the SMACs (29). These results suggested that the immunological synapse functioned as a molecular machine to convert early TCR signals into a stable structure that would sustain signaling to achieve full activation (29). Thus, the mature immunological synapse was provisionally defined as a stable and antigen specific T cell-APC junction composed of SMACs.
The concept that actinomyosin-mediated transport plays an important role in forming the SMACs has been supported by studies in primary helper T cells and the Jurkat T-lymphoma model system. Supramolecular topology and membrane fluctuations can drive segregation of receptor-ligand interactions into microclusters, and this process may be particularly important in defining adhesion domains with the large integrin and small immunoglobulin superfamilly receptors (26, 30). These domains are typically sub-micron and can be organized into larger domains when coupled to cortical actin (31–33). Comparative studies demonstrate that the immunological synapse has parallels to integrin-mediated tissue cell spreading on planar substrates (34). One of the signatures of this process is membrane extension and retraction cycles (contractile oscillations) driven by actin polymerization and myosin II mediated contraction in the lamellipodium. This is considered a sensory process related to the ability of tissue cells to measure and eventually influence mechanical properties of the three dimensional (3D) tissue environment. These experiments demonstrated that the CD45-rich immunological synapse compartment defined by Kupfer as a distal (d)SMAC (35) is a radial lamellipodium that bestows the immune cells with the ability to sense both chemical and mechanical properties of the antigen-presenting cell (36). The predicted retrograde F-actin flow has been imaged directly in Jurkat cells (32) (Fig. 1A). Submicron TCR and LFA-1 microclusters that form in the dSMAC are transported through the pSMAC at ~40% of the rate of the actin flow. The actin flow dissipates at the inside edge of the cSMAC. Incorporation of TCR into the core of the cSMAC is dependent upon expression of Tsg101 (37), a protein that recognizes ubiquitinated cargo and mediates transport into small vesicle into the interior of endosomes (multivesicular bodies) or into the extracellular space in the context of retroviral budding (38). Small, dynamic microclusters have been observed exclusively with the planar bilayer system using total internal reflection fluorescence microscopy due to sensitivity and contrast issues. Methods to improve imaging at cell-cell interfaces to resolve such faint structures do not currently exist, but some promising ideas are in development (39).
Fig. 1. Schematic of CTL synapse and kinapse.
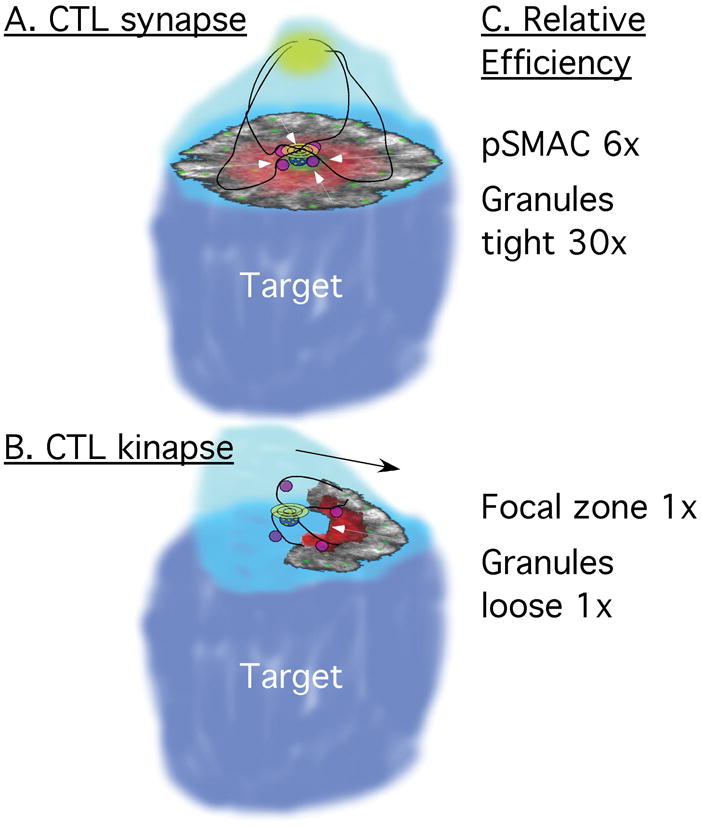
(A). CTL synapse. Strong TCR signal and CD8. The key feature is the symmetric actin pattern with centripetal flow (white arrows). This forms the pSMAC (red, ICAM-1) and positions the TCR for Tsg101 dependent movement in the cSMAC (green, TCR). In this efficient system the granules (purple) are targeted to the MTOC along microbutules (heavy black lines) prior to movement of the MTOC and Golgi to the cSMAC secretory domain. Some of the cSMAC-associated TCR is in multivesicular bodies (blue with green dots). (B). CTL kinapse. Weak TCR signal or CD4. The key feature is a asymmetric actin pattern with net retrograde actin flow (white arrow) inducing forward motion of the cell (black arrow). This forms the asymmetric focal zone (red, ICAM-1), whereas TCR microclusters do not accumulate in a cSMAC. The MTOC moves to the actin-depleted secretory domain, but the granules reach the domain more slowly. (C). Relative efficiency of the two configurations. The presence of an intact cSMAC gains ~6× increase in killing efficiency. The tight granule packing around the MTOC results in a ~30× increase in killing efficiency compared to the loose granule distribution.
The adhesion ring of the immunological synapse is established based on centripetal actin flow. This radial symmetry allows the T cell to dramatically slow or stop its motility without losing the sensory advantages of the lamellipodium in detection of MHC peptide complexes (40, 41) and the interpretation of mechanical cues (42). This symmetrical actin flow creates the pSMAC. Breaking the symmetry of the synapse restores motility and this process has been directly observed during T-cell priming on supported planar bilayers (36). This asymmetrical retrograde actin flow creates an LFA-1 focal zone that drive motility (43). It was surprising that even during antigen recognition T cells use the PKCθ signaling pathway to induce symmetry breaking and bursts of migration followed by Wiskott Aldrich syndrome protein (WASp)-dependent reestablishment of the symmetric synapse. We have proposed that the motile phases be referred to as a kinapse (Fig. 1B), with the distinct, but etymologically related name to reflect the functional implications of signal integration and effector functions executed while migrating (44). Similar symmetry breaking and kinaptic behavior have been observed in NK cells receiving a combination of activating and inhibitory signals (45).
The implications of the immunological synapse and SMACs for cytotoxic cells were immediately evident. It was already known that cytolytic granules of CTLs move to the interface with the microtubule-organizing center (MTOC) prior to lethal hit delivery by the CTL. The bull’s eye-like pattern suggested a central secretory target with a ring of adhesion molecule to prevent leakage of cytolytic cargo and spare bystander cells. However, the next synapse to be described was not the cytotoxic synapse but the inhibitory NK cell synapse. We review the data on NK and CTL synapses and particularly focus on the role of synapse organization in function where there are mechanistic insights.
Inhibitory NK cell synapses
NK cells look similar to T lymphocytes but lack antigen receptors and instead express an array of activating and inhibitory receptors and the Fc receptor CD16. Most host cells are protected from NK cells by expressing MHC-peptide complexes on their surface. In mice, these molecules are recognized by members of the Ly49 family, a group of dimeric type 2 transmembrane proteins with C-type lectin domains, which nonetheless recognize protein determinants of MHC class I (46). Just how rapidly this system is evolving is underscored by the fact that humans have a completely different family of immunoglobulin superfamily receptors, the KIRs, to serve the same function (47). In both cases, the inhibitory receptors have cytoplasmic domains with ITIMs. Mice and men express also the more conserved lectin-like inhibitory receptor CD94-NKG2A, which binds to the non-classical MHC class I molecule Qa1 and HLA-E, respectively. ITIMs are phosphorylated by Src family kinases, recruit Src homology (SH) domain-containing phosphate 1 (SHP-1), and terminate signaling (48) at a proximal step through dephosphorylation of Vav1 (49, 50). There are activating members of all three NK cell inhibitory receptor families. The ability of the activating receptors to bind host MHC class I is crippled by mutations in the binding sites, but it seems that they are evolved for recognition of MHC class I like viral antigens that may have initially evolved to engage inhibitory receptors (51). It is likely that such ‘arms races’ with viruses drive the rapid evolution of NK cell receptor families (52).
sModel systems with NK cell lines and target cells transfected to express green fluorescence protein (GFP)-tagged forms of human leukocyte antigen C (HLA-C) and KIR were developed to visualize NK cell inhibitory synapses (53). When NK cell lines contact cells expressing the MHC class I (HLA-C) recognized by a KIR, these molecules undergo dramatic accumulation in the contact area, and the NK cell migrates past the putative target (53). Typically, KIR and HLA-C molecules accumulate in a central area surrounded by LFA-1 and ICAM-1 (54, 55). Interestingly, the interaction of KIR with HLA-C and their central accumulation was F-actin, temperature, and energy independent, in striking contrast to the F-actin, temperature, and energy-dependent interactions in the helper T-cell immunological synapse. Although F-actin can accelerate KIR recruitment to the synapse, it is not absolutely required (56). Furthermore, inhibitory KIR does not block the actin-dependent accumulation of activation receptors but promotes an actin-independent accumulation of activation receptors at inhibitory synapses, where it prevents their phosphorylation (55). This observation suggested that the interactions driving inhibition would operate under a broader range of physical conditions than activating systems like the TCR and thus would dominantly inhibit NK killing under any condition where the NK cells contact another cell expressing the appropriate ligand. Rather than targeting activation receptors and their associated signaling subunits for dephosphorylation, ITIM-containing receptors appear to block activation by different types of receptors and signaling pathways through actin-independent inactivation of the Vav1-Rac1 pathway (20). This actin-independence is a unique situation in immune synapse formation. Surprisingly, phosphorylated KIR is not evenly distributed at inhibitory synapses but is concentrated in a few microclusters (57). Live imaging of the dynamics of NK cell inhibitory synapses should provide insights into the unique and unusual mechanism of inhibition by ITIM-containing receptors. It remains to be determined if inhibitory receptors expressed on T cells, such as programmed death-1 (PD-1), and other cells behave similarly or have distinct biophysical mechanisms.
NK cell effector functions are controlled by a balance –or rather an integration– of multiple activating and inhibitory signals. A recent study examined in detail how different signals control NK cell motility and shape (45). NKL cells stimulated by glass slides coated with the NKG2D ligand MICA spread and contracted, and a symmetrical ring of F-actin formed. In contrast, NKL cells spread asymmetrically and moved over the LFA-1 ligand ICAM-1. In the presence of both ligands, NKG2D engagement imposed a stop signal and a symmetrical synapse with peripheral F-actin formed. Interestingly, addition of HLA-E, the ligand of inhibitory receptor CD94-NKG2A, reversed the stop signal. This migratory behavior of NK cells under conditions where inhibitory signals dominate, which is reminiscent of the T-cell kinapses described above (44), may facilitate disengagement from cells that have to be spared, thus allowing NK cells to sample target cells more rapidly.
Cytotoxic NK cell synapse
NK cell-mediated killing can be triggered by a number of pathways, and in most cases, activation actually requires integration of multiple signals (58). The most potent mechanism for triggering degranulation is linked to antibody recognition through the Fc receptor CD16, but this mechanism does not induce polarity of granule release on its own. The transmembrane isoform of CD16 that mediates killing is a classical immunoreceptor in which signal transduction is accomplished by forming a complex between the ligand binding transmembrane receptor with an ITAM-containing signal transduction module on a separate transmembrane protein, most significantly FcR-γ, which is non-covalently associated through a process requiring charged residues in the transmembrane domain (59). CD16 signals can trigger killing by human NK cells without other signals and have the potential to overcome inhibition by KIRs despite the advantages of KIRs noted above. Other NK cell-activating receptors must be engaged in combinations in order to trigger cytotoxicity and the most synergistic combinations are activating receptors with different types of motifs (60). For example, several activating receptors associate with DAP12, which has an ITAM, while NKG2D associates with DAP10, which has a YINM motif shared with costimulatory receptors like CD28. Activating receptors in the SLAM family possess phosphotyrosine motifs that link to the small adapter SAP [SLAM (signaling lymphocytic activating molecule)-associated protein] to deliver activating signals through recruitment of Fyn. Several of the activating NK cell receptors have unknown but widely expressed ligands, such that not all of the receptors engaged in an activating NK synapses can be known at present.
Recruitment of signal transduction molecules to cytotoxic and inhibitory synapses were compared by Vyas and Dupont using NK cell lines, NK clones, and primary NK cells (61–64). NK cells readily formed cSMAC and pSMAC-like compartments in 1–10 min, regardless of whether the synapses would lead to cytotoxicity or inhibition. Thus, in these studies, the LFA-1-talin system seemed to be uniformly activated in this time frame to allow for sampling of signals present on apposed cells. The significant difference between cytotoxic and inhibitory synapses was the ratio of activating tyrosine kinases, like Syk, ZAP70, and Lck, to the tyrosine phosphatase SHP-1 in the cSMAC. This ratio was high in cytotoxic synapses and low in inhibitory synapses. This finding is consistent with the model that SHP-1 recruitment by inhibitory receptors must act locally on key phosphorylated tyrosines generated by activating receptors to prevent the tyrosine kinase cascade from propagating. Cytotoxic NK synapses are similar in many respects to T-cell synapses in that the active signaling molecules are recruited to the synapse and a well-defined pSMAC is formed. Delivery of lytic granules to the cytotoxic synapse requires actin cytoskeleton remodeling and microtubule-dependent transport. While actin rearrangement is required for polarization of NK cells, cortical F-actin forms a barrier that lytic granules must traverse in order to reach and fuse with the plasma membrane. Recent studies have reported that myosin IIA is not required for the formation of an organized and polarized NK cell synapse but is essential for the final step of lytic granule exocytosis (65, 66). Myosin IIA is associated with lytic granules and promotes their transport through the final layer of F-actin at the cytotoxic synapse (66).
Several groups working on NK cell synapses have converged on the conclusion that classical NK-mediated killing results form a multistage process with respect to patterning, cytoskeletal polarization and killing (67, 68). Early studies suggested that NK cells sustained nascent immunological synapses over longer periods compared to T cells (53). This prolonged nascent synapse, although not observed by all, suggested that NK cells might use the nascent synapse over time to test the ratio of activating to inhibitory inputs prior to commitment. The relationship between receptor accumulation, actin polarization, and killing suggested that NK cells use formation of a mature synapse as a checkpoint, the passage of which is dependent upon the ratio of activating and inhibitory signals (69, 70). While a few NK cells rapidly committed to the mature synapse and killed the target cell, as many as half of the cells took many minutes after contact to form a synapse and kill the target, if the target would be killed at all (54). This finding suggests that with a population of normal NK cells and nominally susceptible targets, there is a probability that the balance of activating and inhibitory synapses will fail to pass checkpoints for synapse formation and cytotoxic triggering or may be delayed in passing these checkpoints. Thus, compared to CTL synapses described below, the NK cytotoxicity commitment process is prolonged.
Two recent concepts in NK cell development have not been extensively studied with respect to synapse formation. At an early developmental stage, NK cells must be ‘licensed’ by interactions with MHC class I (71). This process, which generates mature NK cells, appears to be akin to positive selection in T cells. An alternative hypothesis to explain this phenomenon of NK cell ‘tolerance’ is that NK cells that do not receive inhibitory signals through MHC class I receptors become desensitized, or ‘disarmed’, through persistent activation signals (72). Epigenetic factors that are not well understood define the array of activating and inhibitory receptors that are expressed in any particular NK cell. Once these cells develop, only the NK cells that express sufficient levels of inhibitory receptors that recognize host MHC class I gain functional competence and are considered licensed, self-tolerant effector cells. Mouse models and human systems in which inhibitory receptor expression can be linked to specific MHC class I alleles demonstrate that licensed and unlicensed cells both express perforin and granzymes, but only licensed cells can engage in missing self recognition. The epigenetic mechanisms that control expression of inhibitory receptor also generate NK cells (10–15%) that lack MHC class I-binding inhibitory receptors, and these cells are hypoesponsive (73–76). The licensing interactions and cytotoxic synapse formation have not been examined in models where licensing can be controlled, and the signaling differences between licensed and unlicensed cells are not well defined. For example, it is not known if unlicensed cells form a nascent synapse similar to the bulk of primary NK cells, which are licensed.
A second process that is of great interest in NK cells is based on recent observations that virus-specific NK cells can engage in adaptive responses with primary expansion, contraction, memory, and recall phases (23). While populations of mouse cytomegalovirus (mCMV)-specific NK cells expressing the Ly49H activating receptor are much more abundant than mCMV-specific naïve T cells, the Ly49H+ NK cells nonetheless undergo a 100-fold expansion during infection. These cells then contract back to near pre-infection levels to initiate a memory phase. Recall responses are more efficient, as memory Ly49H+ NK cells elaborate a 10-fold enhanced ability to protect NK cell-deficient mice from mCMV infection compared to naive Ly49H+ NK cells. The characteristics of synapse formation by memory and naive NK cells are not known, but this could certainly be addressed in the mouse models. While most mice are raised in specific pathogen-free conditions, humans experience many viral infections, and it is likely that peripheral blood NK populations contain both naive and memory NK cells. It remains to be determined how much of the heterogeneity in human NK cell behavior is related to differences in these subsets.
The cytotoxic NK cell synapse has been modeled using supported planar bilayers (77). Bilayers containing CD48 and ULBP1 trigger synergistic granule release in primary human NK cells. In human NK cells, the receptor for CD48 is 2B4, a member of the SLAM family that associates with signaling adapter SAP, and ULBP1 is a ligand of NKG2D. Whereas both CD48 and ULBP-1 are needed to trigger degranulation, CD16 engagement with immunoglobulin G (IgG) alone is sufficient for degranulation. lysosomal membrane glycoprotein-1 (LAMP-1) (CD107a) molecules that have been delivered to the cell surface upon degranulation are not allowed to diffuse at the plasma membrane but are retrieved into a stable and central endocytic compartment. Formation of an organized cytotoxic synapse and retrieval of LAMP-1 at the center were absolutely dependent on LFA-1 interaction with ICAM-1 on the bilayer. Thus, an unanticipated function of an LFA-1 pSMAC in the cytotoxic NK synapse may be for the efficient recycling of granule membrane to form new cytolytic granules–a process that may be critical for serial killing. Another surprise in this study was that although both 2B4-CD48 and NKG2D-ULBP interactions span less then 15 nm between membranes and might be expected to co-cluster based on size rules for microcluster formation (78), these molecules were dramatically segregated. The 2B4-CD48 interactions were positioned at the center, perhaps as expected, whereas the NKG2D-ULBP-1 interactions were co-localized with the LFA-1-ICAM-1 interactions in a peripheral region. Thus, NK cells integrate NKG2D and 2B4 signals from different compartments of the synapse to trigger degranulation into the cSMAC.
Cytotoxic T cells
Cajal described the neural synapse as a ‘The protoplasmic osculation (kiss) … the final saga in an epic love story.’ Following on this precedent, the cytotoxic synapse of CTL has been aptly described as a kiss of death (79), certainly the final saga for the target. There are two modes of CTL-mediated killing: Ca2+-dependent killing by perforin and granzymes and Ca2+-independent killing mediated by Fas ligand (FasL) binding to Fas (CD95) on target cells. Perforin-mediated killing can also be diagnosed with the vacuolar acidification inhibitor concanamycin A (80). Both pathways trigger death by apoptosis, but the perforin pathway is typically faster. Perforin-mediated killing is more general, since it is based on a highly conserved membrane injury response and endosomal lysis leading to introduction of granzymes into the target cell cytoplasm (81), and thus, no specific receptor is needed. Since perforin and granzymes are released from the cell, the synaptic cleft has been assumed to enhance function through high local concentration and also to prevent bystander exposure to active perforin. Fas must also function in a cell-cell contact, since FasL and Fas are membrane proteins with a limited reach (~15 nm). FasL has been reported to be present in the granules of CTLs that co-express perforin and granzymes and to be exocytosed into the synaptic cleft in response to TCR triggering (82). This is in part explained by the sorting of FasL into multivesicular body core vesicles that are released from cells during degranulation (83). It has been observed that in transplant rejection, early CTL express perforin and FasL, while during the retraction phase the CTL lose the perforin pathway, but continue to be active killers using FasL. In CTLs utilizing only FasL, the pathway is not dependent upon protein synthesis, suggesting the FasL is stored in an intracellular compartment such as cytotoxic granules, but FasL-mediated killing is selectively inhibited by brefeldin A (84, 85). Brefeldin A inhibits both endoplasmic reticulum to Golgi and some regulated trans-Golgi to plasma membrane secretion pathways, but not the release of granules (secretory lysosome) or recycling endosomes (86). Thus, FasL-mediated killing can be diagnosed by anti-FasL antibodies and by acute treatment with brefeldin A (long-term treatment will eventually limit the availability of MHC-peptide complexes on the target).
The CTL immunological synapse
Studies on the CTL synapse have focused primarily on understanding of the perforin and granzyme-mediated killing. CTLs are known to engage in a killing cycle that involves tenacious adhesion, release of cytotoxic agents, and detachment from the target (79). CTLs undergo dramatic polarization changes with movement of the CTLs’ microtubule organizing center (MTOC) and linked cytotoxic granules to the synapse (4). Electron microscopy demonstrated that the MTOC is moved directly to the membrane, much like the basal body of a primary cilium (87). Some of the mechanisms mediating granule movement in the CTL synapse have been illuminated by study of patients and mouse models selected based on defects in pigmentation arising from melanocyte granule movement defects, which in some cases predict CTL granule movement defects (88–90). Molecules involved in this process include Rab27a, Munc13–4, and myosin V. Separating CTLs from target cells during the peak of adhesion is difficult and traumatic to both cells suggesting strong cell adhesion (91). LFA-1 and CD2 adhesion pathways mediate human CTL adhesion to an array of targets (92). It is likely that other pathways contribute in different contexts. How CTLs know to let go after targets are programmed for lysis is not clear. It is possible that some chemical or mechanical feedback is involved, as membranes of cells undergoing apoptosis undergo loss of phospholipid asymmetry and dramatic blebbing based on changes in the cortical cytoskeleton (93).
Molecular patterns in the immunological synapse were first described for CD4+ helper T cells as described above. Naive CD8+ T cells were subsequently found to form similar bull’s eye synapses during priming in vitro (94) and in mature CTLs during target lysis (95). The cytotoxic synapses were characterized by a well-defined pSMAC (LFA-1 ring) and cSMAC (TCR, CD8 and Lck). This process could be reconstituted with ICAM-1 and MHC class I peptide complexes in supported planar bilayers (96). Surprisingly, CTLs were very efficient at forming pSMAC-like adhesion rings and would do so transiently even in the absence of specific MHC-peptide complexes (96). Griffiths (95) described the cSMAC as being divided into signaling and secretory domains. Such functional compartmentalization of the cSMAC is likely to be important in integration of costimulatory signals with TCR signal and receptor degradation in helper cells (97). While both LFA-1 and CD2 adhesion pathways contribute to conjugate formation, only the LFA-1 pathway leads to formation of organized SMACs (98). Recently, the secretion of granules directly into the model cytotoxic synapse with a planar bilayer has been observed by TIRFM microscopy based on the detection of CD107a+ foci at the cSMAC (99, 100). These studies confirm that there is a secretory domain in the cSMAC. It has been reported that target lysis can take place without an immunological synapse (101). The quantitative advantages of forming a synapse versus other modes of interaction are only beginning to be understood.
The competitive edge offered by the synapse
The model that secretion of granule contents into the immunological synapse is important for efficiency of killing has recently been tested and an unexpected new parameter governing efficiency has been revealed. Sykulev and colleagues (99, 100) initiated studies comparing human cytotoxic T-cell clones expressing CD8 and CD4. The CD8+ T cells are 100-fold more efficient than the CD4+ CTLs in terms of the time and granule investment needed to kill targets. Both types of CTLs kill primarily using the perforin/granzyme pathway, and both cell types are equivalently armed at the level of granule activity. Comparison of synapse formation by both cell types revealed that the CD8+ CTLs form more stable synapses (80% vs 40% of total conjugates), whereas CD4+ CTLs have more prevalent kinapses (the asymmetric junctions that lead to migration and absence of a protected secretory domain). As described above, the generation of kinapses in CD4+ T cells is promoted by activation of PKCθ, such that inhibition of PKCθ stabilized the synapses of CD4+ CTLs. Synapse stabilization by this mechanism resulted in a threefold increase in killing efficiency. Since ~40% of CD4+ CTLs did form synapses without the inhibitor, it is suggested that stable synapses are sixfold more efficient than kinapses (100). The remaining 30-fold difference in efficiency between CD4+ and CD8+ T cells was due to a different, unexpected mechanism (Fig. 1C).
When triggered by agonist MHC-peptide complexes, CD8+ CTLs release granules faster than CD4+ CTLs. Triggering using the same anti-CD3 antibody eliminated the kinetic difference, and eliminating CD8+ CTL interactions slowed granule release from CD8+ CTLs. Examination of the movement of the MTOC and the granules to the synapse revealed that strong TCR signals promoted by CD8 led to fast granule recruitment to the MTOC and movement of that MTOC-granule complex to the secretory domain, such that the granules were tightly localized in the cSMAC. Signals generated by weaker agonist MHC-peptide complex, CD8 blockade, or use of CD4 as the coreceptor led to movement of the MTOC to the secretory domain without the granules, which then needed to slowly make there way to the secretory domain via the pSMAC such that the granules appears loosely arrayed in the synapse or kinapse (99). Similar results were obtained by the Griffith laboratory utilizing altered peptide ligands in a CD8 system, suggesting that both the CD8 and TCR-MHC-peptide interaction strength can determine the early signaling kinetics and pathway for granule delivery (102). Granule exocytosis rates and the diffusion rate for perforin and granzymes from the synaptic cleft will determine the efficiency of granzyme delivery to the cytoplasm. Therefore, the formation of a cytotoxic synapse based on pSMAC integrity and polarized granule delivery to the secretory domain are two components that offer a significant competitive advantage in the race with pathogens.
Cytotoxic synapses in vivo
In vitro studies have provided insights into the function of the immunological synapse, but in vivo studies have the potential to put this system in a more physiological context. Histological analysis supports a role of organized immunological synapses, particularly in the central nervous system (CNS) (103, 104). Two photon laser scanning microscopy particularly has enabled imaging of micron scale details hundreds of microns deep in live tissues to capture the dynamics of interactions (105). Priming of CD8+ T cells involves prolonged interactions with dendritic cells (106, 107). The effector phase involves stable interactions, but CTLs could kill 2.4 specific B-cell targets per hour. Interestingly, the presence of regulatory T cells reduced the killing rate to 0.4 per hour, a sixfold decrease in the killing rate (108). The tumor microenvironment further decreases the rate of killing to <0.2 per hour (109). Less has been done at this point with CTL in the context of infection. Recently, we examined the dynamics of LCMV-specific CTLs in the meninges during fatal LCMV meningitis (110). While histological analysis suggested the presence of stable synapses in this model, dynamic imaging revealed that the CTLs are constantly in motion, suggesting that they are forming kinapses. It is difficult in fixed images to distinguish a synapse with a transient break in the pSMAC from kinapse (103). It is only through dynamic imaging that the stability of interactions can be assessed. The kinapse formation by CTLs in the meninges precedes massive recruitment of myelomonocytic cells that is fatal to the host (110). It is not clear why more stable synapses leading to efficient killing of virally infected cells is not observed in this site. Future advances in intravital imaging should also provide more insight into cytotoxic synapse functioning in vivo. In vivo imaging methods with the required molecular resolution are already in use in the nervous system (111) and may soon illuminate immune cell function in physiological settings.
Conclusions
The immunological synapse defined by formation of an intact adhesion ring and the movement of cytolytic granules to a secretory domain provides a site for signal integration in NK cells and contributes to the efficiency of CTL-mediated killing. Studies of NK cells have revealed a central role of integrin LFA-1 in the organization and dynamics of NK cytotoxic synapses. Imaging of T-cell cytotoxic synapses has demonstrated physical differences between CD4+ and CD8+ cells that result in vastly superior cytotoxic activity of CD8+ cells. Outstanding problems in the field remain. For example, the permeability of the pSMAC ring is not known, and studies in CD4+ T cells suggest that it is surprisingly permeable to antibodies and Fab fragments (112). In fact, the anti-LAMP-1 CD107a Fab-based method used to rapidly detect granule exocytosis in the synapse with planar bilayers and TIRFM is dependent upon the ability of the labeled Fab to diffuse freely into the synaptic cleft for both NK cells and CTLs (77, 99, 100). Another issue is the minimal signal integration time for NK cells versus CTLs. It had been argued that CTLs were much more decisive than NK cells, but this apparently depends upon the ligand of the CTL. NK cells may make decisions in a more tentative process, but this seems to be followed by an efficient killing process. In contrast, the rate of commitment with CTLs depends upon ligand strength, with lower potency ligands leading to an inefficient killing process. This factor may be one that leads to slow killing of tumor cells.
Acknowledgments
Supported by NIH grants PN2 EY016586 (M.L.D.), R01 AI052812 (M.L.D.) and the Intramural Research Program of the NIH, NIAID (E.O.L.).
References
- 1.Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- 3.Davignon D, Martz E, Reynolds T, Kurzinger K, Springer TA. Lymphocyte function-associated antigen 1 (LFA-1): a surface antigen distinct from Lyt-2,3 that participates in T lymphocyte-mediated killing. Proc Natl Acad Sci USA. 1981;78:4535–4539. doi: 10.1073/pnas.78.7.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryser JE, Rungger-Brändle E, Chaponnier C, Gabbiani G, Vassalli P. The area of attachment of cytotoxic T lymphocytes to their target cells shows high motility and polarization of actin, but not myosin. J Immunol. 1982;128:1159–1162. [PubMed] [Google Scholar]
- 6.Weiss A, Imboden J, Shoback D, Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci USA. 1984;81:4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podack ER, Lowrey DM, Lichtenheld M, Hameed A. Function of granule perforin and esterases in T cell-mediated reactions. Components required for delivery of molecules to target cells. Ann NY Acad Sci. 1988;532:292–302. doi: 10.1111/j.1749-6632.1988.tb36347.x. [DOI] [PubMed] [Google Scholar]
- 8.Norcross MA. A synaptic basis for T-lymphocyte activation. Ann Immunol (Paris) 1984;135D:113–134. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- 10.Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987;329:512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- 12.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 13.Carpen O, Virtanen I, Lehto VP, Saksela E. Polarization of NK cell cytoskeleton upon conjugation with sensitive target cells. J Immunol. 1983;131:2695–2698. [PubMed] [Google Scholar]
- 14.Carpen O, Virtanen I, Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982;128:2691–2697. [PubMed] [Google Scholar]
- 15.Carpen O, Virtanen I, Saksela E. The cytotoxic activity of human natural killer cells requires an intact secretory apparatus. Cell Immunol. 1981;58:97–106. doi: 10.1016/0008-8749(81)90152-0. [DOI] [PubMed] [Google Scholar]
- 16.Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 18.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 19.Burshtyn DN, et al. Recruitment of tyrosine phosphatase HCP (SHP-1) by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 23.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 25.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 26.Dustin ML, et al. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA, Travers P. Immunobiology: the immune system in health and disease. 3. New York: Garland; 1997. [Google Scholar]
- 28.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 29.Grakoui A, et al. The immunological synapse: A molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 30.Weikl TR, Lipowsky R. Pattern formation during T-cell adhesion. Biophys J. 2004;87:3665–3678. doi: 10.1529/biophysj.104.045609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Hori Y, Groves JT, Dustin ML, Chakraborty AK. The synapse assembly model. Trends Immunol. 2002;23:500–502. doi: 10.1016/s1471-4906(02)02325-6. [DOI] [PubMed] [Google Scholar]
- 32.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaizuka Y, Douglass AD, Vardhana S, Dustin ML, Vale RD. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J Cell Biol. 2009;185:521–534. doi: 10.1083/jcb.200809136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobereiner HG, et al. Lateral membrane waves constitute a universal dynamic pattern of motile cells. Phys Rev Lett. 2006;97:038102. doi: 10.1103/PhysRevLett.97.038102. [DOI] [PubMed] [Google Scholar]
- 35.Freiberg BA, et al. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 36.Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 37.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of Ubiquitin and TSG101 in formation and function of the central supramolecular activation cluster. Immunity. 2010 doi: 10.1016/j.immuni.2010.04.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 39.Oddos S, et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008;95:L66–L68. doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Tromberg BJ, Cahalan MD. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca(2+) signaling. Proc Natl Acad Sci USA. 1999;96:8471–8476. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 42.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 43.Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dustin ML. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol. 2008;24:577–596. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 45.Culley FJ, et al. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7:e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 47.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 48.Binstadt BA, Brumbaugh KM, Leibson PJ. Signal transduction by human NK cell MHC-recognizing receptors. Immunol Rev. 1997;155:197–203. doi: 10.1111/j.1600-065x.1997.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 49.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adaptor Crk. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voigt V, et al. Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc Natl Acad Sci USA. 2003;100:13483–13488. doi: 10.1073/pnas.2233572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abi-Rached L, Parham P. Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J Exp Med. 2005;201:1319–1332. doi: 10.1084/jem.20042558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almeida CR, Davis DM. Segregation of HLA-C from ICAM-1 at NK cell immune synapses is controlled by its cell surface density. J Immunol. 2006;177:6904–6910. doi: 10.4049/jimmunol.177.10.6904. [DOI] [PubMed] [Google Scholar]
- 55.Schleinitz N, March ME, Long EO. Recruitment of Activation Receptors at Inhibitory NK Cell Immune Synapses. PLoS ONE. 2008;3:e3278. doi: 10.1371/journal.pone.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Standeven LJ, Carlin LM, Borszcz P, Davis DM, Burshtyn DN. The actin cytoskeleton controls the efficiency of killer Ig-like receptor accumulation at inhibitory NK cell immune synapses. J Immunol. 2004;173:5617–5625. doi: 10.4049/jimmunol.173.9.5617. [DOI] [PubMed] [Google Scholar]
- 57.Treanor B, et al. Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol. 2006;174:153–161. doi: 10.1083/jcb.200601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanier LL, Yu G, Phillips JH. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989;342:803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- 60.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vyas YM, Maniar H, Lyddane CE, Sadelain M, Dupont B. Ligand binding to inhibitory killer cell Ig-like receptors induce colocalization with Src homology domain 2-containing protein tyrosine phosphatase 1 and interruption of ongoing activation signals. J Immunol. 2004;173:1571–1578. doi: 10.4049/jimmunol.173.3.1571. [DOI] [PubMed] [Google Scholar]
- 62.Vyas YM, Maniar H, Dupont B. Visualization of signaling pathways and cortical cytoskeleton in cytolytic and noncytolytic natural killer cell immune synapses. Immunol Rev. 2002;189:161–178. doi: 10.1034/j.1600-065x.2002.18914.x. [DOI] [PubMed] [Google Scholar]
- 63.Vyas YM, Maniar H, Dupont B. Cutting edge: differential segregation of the SRC homology 2-containing protein tyrosine phosphatase-1 within the early NK cell immune synapse distinguishes noncytolytic from cytolytic interactions. J Immunol. 2002;168:3150–3154. doi: 10.4049/jimmunol.168.7.3150. [DOI] [PubMed] [Google Scholar]
- 64.Vyas YM, et al. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–4367. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 65.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–2291. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanborn KB, et al. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol. 2009;182:6969–6984. doi: 10.4049/jimmunol.0804337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci USA. 2003;100:7767–7772. doi: 10.1073/pnas.1336920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 72.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 73.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 76.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choudhuri K, Wiseman D, Brown MH, Gould K, Van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 79.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 80.Kataoka T, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 81.Keefe D, et al. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Lettau M, et al. The adaptor protein Nck interacts with Fas ligand: Guiding the death factor to the cytotoxic immunological synapse. Proc Natl Acad Sci USA. 2006;103:5911–5916. doi: 10.1073/pnas.0508562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuccato E, et al. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci. 2007;120:191–199. doi: 10.1242/jcs.03315. [DOI] [PubMed] [Google Scholar]
- 84.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–1079. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 85.Li JH, et al. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998;161:3943–3849. [PubMed] [Google Scholar]
- 86.Miller SG, Carnell L, Moore HH. Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J Cell Biol. 1992;118:267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 88.Baetz K, Isaaz S, Griffiths GM. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J Immunol. 1995;154:6122–6131. [PubMed] [Google Scholar]
- 89.Pastural E, et al. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genet. 1997;16:289–292. doi: 10.1038/ng0797-289. [DOI] [PubMed] [Google Scholar]
- 90.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 91.Sung K-LP, Sung LA, Crimmins M, Burakoff SJ, Chien S. Determination of junction avidity of cytoloytic T cell and target cell. Science. 1986;234:1405–1408. doi: 10.1126/science.3491426. [DOI] [PubMed] [Google Scholar]
- 92.Shaw S, Luce GE, Quinones R, Gress RE, Springer TA, Sanders ME. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986;323:262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- 93.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 94.Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci USA. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 96.Somersalo K, et al. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beal AM, et al. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beal AM, et al. Protein kinase CΘ regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 102.Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31:621–631. doi: 10.1016/j.immuni.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barcia C, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 106.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 107.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 108.Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 109.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


