INTRODUCTION
Embryonic stem (ES) cells, originated from the inner cell mass (ICM) of a blastocyst embryo, are self-renewable (Evans and Kaufman, 1981; Martin, 1981). The pluoripotent nature of ES cells endows them as a great tool for dissecting cell lineage development in mammals and they can be used as a limitless source for producing specialized cells for potential cell therapy. Application of gene targeting technology in mouse ES cells has allowed the cloning of mice with modified genomes (Frohman and Martin, 1989; Koller et al., 1989), thus significantly widening the use of mice as a vertebrate experimental model in biological research today. Forced overexpression or knockout of critical genes by genetic modification has become a routine technique in mouse ES cells to create transgenic mice for analyzing gene function and genetic pathways in the context of intact animals and in cell lines derived from such animals. Gene targeting in mouse ES cells also allows the creation of mouse models of human diseases (Bedell et al., 1997a; Bedell et al., 1997b), which offers insights into the genetic, biochemical and pathological basis of the diseases and may help developing treatments.
Like their murine counterparts, human ES cells (Reubinoff et al., 2000; Thomson et al., 1998) offer invaluable and perhaps the only system for directly studying early human development as manipulation of human embryos is prohibited and information gained from model organisms (Anderson and Ingham, 2003) does not always reflect what is occurring in humans. The ability of human ES cells to produce almost any cell types in our body brings hopes to many incurable diseases affecting the central nervous system (CNS) (Dunnett et al., 2001; Silani et al., 2004), pancreas (Bonner-Weir and Weir, 2005), and heart (Srivastava and Ivey, 2006), etc. The potential use of human ES cells can be further enhanced by genetic modification (Drukker, 2005; Kobayashi et al., 2005). Application of currently available genetic modification techniques in human ES cells will provide researchers with even more versatile tools to study early human development and to expand the potential use in medicine. Basic mechanisms underlying early human development can be dissected through interfering with specific signaling pathways by forced gene overexperssion or silencing. Genetic defects may be corrected in human ES cells by site directed gene modifications, which may lead to the development of treatment for many inheritable diseases (Chang et al., 2006; Rideout et al., 2002). By labeling cells with tissue specific markers, genetic modification may help us obtain pure functional cells of desired types after differentiation, which can be used as more effective transplant medicine.
Unlike their murine counterparts, genetic modification in human ES cells has been technically challenging. Standard chemical or mechanical methods for gene delivery exhibit very low efficiency in human ES cells, with the most effective approaches yielding only approximately one stable transfectant per 105 transfected cells (Eiges et al., 2001; Zwaka and Thomson, 2003). After transfection, transgene expression appears to be suppressed more severely in human ES cells than in mouse ES cells (Xia, 2006). Thus successful examples are still rare. In this review, we will summarize the current methodologies for genetic modification of human ES cells from very limited literatures and propose future directions to overcome the present problems in genetic manipulation of human ES cells.
Techniques for genetic modification of human ES cells
TRANSFECTION
(i) Electroporation
Electroporation is the most commonly used mechanical method for introducing DNA into a wide range of cell types. This technique allows the passage of foreign DNA through pores formed transiently in the cell membrane caused by electric pulses. While the method has been proven very efficient for mouse ES cells (Thomas and Capecchi, 1987), early trials have shown that human ES cells did not survive traditional electroporation conditions well, with a cell survival rate of only about 1% (Eiges et al., 2001; Zwaka and Thomson, 2003). This was improved to some extent by using modified electroporation parameters combined with isotonic protein-rich electroporation solution (Zwaka and Thomson, 2003). However, the survival rate remained low. We have observed a survival rate of about 10% using the same improved conditions (unpublished observation). A systematic study of the electroporation parameters for human ES cell transfection indicated that by optimizing the voltage, pulse duration, and the number of pulses, high cell viability (>60%) and relatively high percentage of transfected cells (14%) can be achieved (Mohr et al., 2006). While it is not proven whether transfected cells retain their ES cell state, the study indicated that the low transfection efficiency and survival may not be inherent to human ES cells. Technical improvement may ultimately lead to efficient transgene delivery to human ES cells.
Despite the high cell death rate and relatively low transfection efficiency, electroporation is so far the only method that has been reported to achieve homologous recombination in human ES cells (Zwaka and Thomson, 2003). Considering the essentiality of homologous recombination for site specific gene modification, electroporation will remain to be indispensable until more effective novel methods are devised.
(ii) Chemical reagents
In the first attempts of human ES cell transfection, three commonly used chemical transfection reagents (LipofectAmine plus, FuGene6 and ExGen 500) were tested by Eiges et al. (Eiges, 2006; Eiges et al., 2001). Although the survival rate for human ES cell was not reported, we observed very few cell deaths (unpublished observation) following the protocol described by them. Among the different chemical reagents Eiges et al. tested, ExGen 500 showed significantly higher transient transfection rate than others. The stable transfection rate for ExGen 500, as reported by the authors, was about 10−5. Zwaka et al. (Zwaka and Thomson, 2003) re-evaluated two of the chemical reagents (FuGene6 and ExGen 500) and reported that the stable transfection rate for FuGene-6 was about twice that of ExGen 500, both in the magnitude of 10−5.
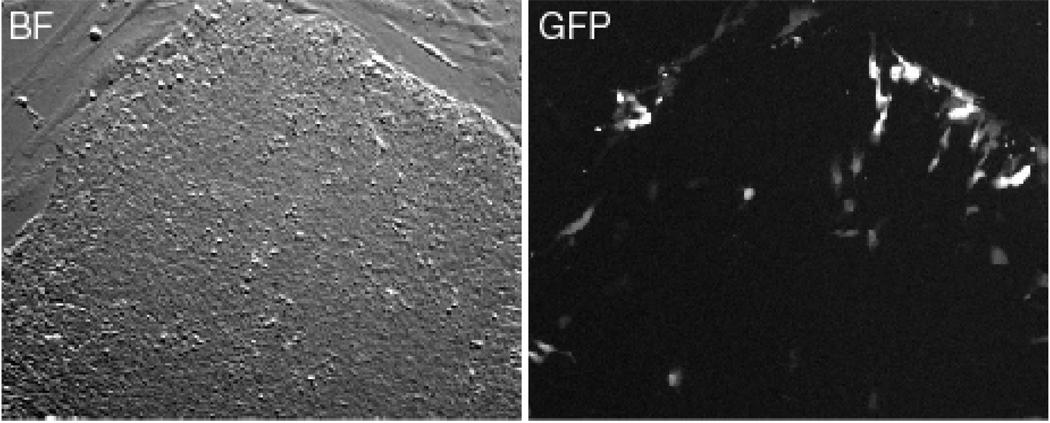
Taken together, the efficiency of standard transfection methods (electorporation and chemical reagents) in human ES cells is much lower, as compared to other cells, both in transient and stable transfection. We also observed that most successfully transfected cells tend to locate on the edge of the colony, and usually appear flat and show early signs of differentiation, as shown in Figure 1. This may result in overestimation of transfection efficiency in human ES cells. Therefore it is important to validate whether the transfected cells retain the expression of ES cell markers such as SSEA1 and Oct 4.
Figure 1. Location of transfected cells in a hES colony.
Cells were transfected with a GFP construct using FuGene6 following manufacturer’s instruction. Most of the transfected green cells were located on the edge of the colony.
VIRAL TRANSDUCTION
Viral transduction generally is more efficient and results in less damage to many types of cells than transfection. Several types of virus have been tested, to enable transient or stable expression of exogenous genes in human ES cells. For transient expression, adenovirus (Ad) has been shown to be efficient in mouse ES cells when appropriate promoters are used (Kawabata et al., 2006; Kawabata et al., 2005). Ad5 based adenoviral vectors have been proven to be capable of mediating transient expression in human ES cells under both undifferentiated and differentiated states (Smith-Arica et al., 2003). Because adenoviral vectors do not insert into the host genome and remain epichromosomal in the host cells (Russell, 2000), they are ideal for therapeutic applications in which no interruption of the endogenous genes by the transfected genes is desired.
Another type of virus, lentivirus, is derived from human immunodeficiency virus (HIV), which has the property of being able to stably infect both nondividing and dividing cells (Naldini et al., 1996), including human ES cells (Gropp et al., 2003; Ma et al., 2003; Xiong et al., 2005). It is probably the most promising gene delivery vehicle for stable expression of transgenes in human ES cells for the following reasons. (1) High transduction efficiency. Up to 70% of transduction efficiency has been achieved in human ES cells using a high titer of self-inactivating lentiviruses (Xiong et al., 2005). (2) The transgenes are integrated permanently into the host genome so that gene expression is stable and inheritable (Cockrell and Kafri, 2003). (3) Less vector-associated immunogenicity was observed for lentivirus mediated transduction than other viral vectors (Kordower et al., 2000). Lentiviral and adenoviral vectors can be used in genetic modifications of human ES cells to complement each other, depending on the purpose of modification.
Besides safety concerns, several other disadvantages should be considered when using viruses for genetic modification of human ES cells. First, viral integrations are random and unpredictable, which makes it unsuitable for site-directed genetic modification. Secondly, virus delivered transgenes usually undergo gene silencing in the host cells, meaning that expression of the genes will be gradually turned off during propagation and/or differentiation (Cherry et al., 2000; Laker et al., 1998). The extent of silencing differs among various promoters. In general, β-actin based promoters are less silenced during propagation and differentiation (Costa et al., 2005; Muotri et al., 2005), whereas many other commonly used promoters are eventually turned off completely, such as following terminal neuronal differentiation (Guillaume, 2006). Thirdly, besides gene silencing, lentivirus delivered transgenes may be suppressed immediately after transduction; therefore the transgenes are never expressed in the host cells (Xia, 2006). This gene suppression phenomenon is also highly promoter dependent. Unfortunately, promoters relatively exempt from gene suppression (like EF1α and PGK promoter) usually suffer from severe gene silencing during later differentiation, whereas afore-mentioned β-actin based promoters (e.g., CAG) are seriously suppressed in human ES cells. Therefore, cautions should be taken to choose the appropriate promoter based on the experimental purpose (Xia, 2006).
NUCLEOFECTION
Nucleofection is a mechanical method for DNA delivery developed by AMAXA Biosystems (AMAXA Biosystems, Gaitherburg, MD). The technique uses combinations of electric current and solutions tailored for specific cell types and enables introduction of plasmid DNA directly into the nucleus, giving rise to high gene delivery efficiency even in those hard-to-transfect cells such as non-dividing primary cells (Gresch et al., 2004). This technique has been recently applied to human ES cells and shown to be more superior than other transfection methods (Lakshmipathy et al., 2004; Siemen et al., 2005). Compared to traditional non-viral methods like electroporation, the authors showed that nucleofection appeared to cause significantly reduced cell death (<30%) and increased efficiency (66%). The technique has not been widely used in human ES cells and the transfection efficiency remains to be confirmed by more research groups. Although the transfected cells have been shown to retain the ES cell specific markers including Tra-1-60, Tra-1-81 and Oct4, it is not shown whether the transfected cells retain the same potential for terminal differentiation.
As a summary, the methodologies used for genetic modification of human ES cells are listed in Table 1.
Table 1.
Methodologies for genetic modification of human ES cell
| Method | Cell survival% | Transient transfection % |
Stable transfection % |
|---|---|---|---|
| Electroporation | ~5%(Lakshmipathy et al., 2004) >60% (Mohr et al., 2006) | 14% | 10−5 |
| LipofectAMINE plus | Low cell death | <10% | N/A |
| FuGene6 | Low cell death | <10% | 10−5 |
| ExGen 500 | Low cell death | <10% | 10−5 |
| Adenovirus | Low cell death | ~ 30% | N/A |
| Lentivirus | Low cell death | N/A | >70% |
| Nucleofection | >70% | 66% | N/A |
Applications of genetic modification in human ES cell research
Genetic modification of human ES cells is still at its early stage. Successful establishment of stable transgenic human ES cell lines are at present limited to a couple of laboratories. Judging from the impact of genetically modified mouse ES cells and resultant animals, we predict that genetically modified human ES cells will have a major impact on our ability to elucidate the mystery of early human development and pathogenesis of genetic disorders, to identify therapeutic targets, as well as to develop technology for more efficient stem cell maintenance and directed differentiation.
TRANSGENIC CELL LINES FOR ELUCIDATING PLUORIPOTENCY AND FOR ES CELL MAINTENANCE
One question central to stem cell biology is how the pluoripotency of ES cells is maintained. Answer to this question is instrumental to developing technology for effectively maintaining ES cells. Studies from mouse ES cells have shown that a network of transcription factors including Oct4, Sox2, Nanog and others are essential to the maintenance of stemness (Boiani and Scholer, 2005; Ivanova et al., 2006; Takahashi and Yamanaka, 2006). This transcription factor network in mouse ES cells can be maintained by a relatively simple factor, leukemia inhibitory factor (LIF). However, LIF cannot support self renewal of human ES cells (Thomson et al., 1998). Instead, high concentrations of basic fibroblast growth factor (bFGF) and suppression of bone mophogenetic protein (BMP) signaling by noggin have been shown to sustain undifferentiated proliferation of human ES cells (Levenstein et al., 2006; Xu et al., 2005), suggesting a possible discrepancy in the regulatory networks maintaining the stemness of human and mouse ES cells. Using a genetic modification approach, Darr et al established a human ES cell line overexpressing one of the transcription factors important for mouse ES cell pluripotency, Nanog, and showed that the cell line can be expanded under a feeder-free system in an undifferentiated state, indicating that common transcription factors are involved in the pluripotency of both mouse and human ES cells, even though they may be regulated by different sets of extracellular signals (Darr et al., 2006). Establishment of such human ES cell lines not only enables us to elucidate the mechanism underlying pluripotency, but also may help us to ultimately develop chemically defined systems for maintaining human ES cells, which will be a significant step toward potential clinical use of human ES cells.
Genetically marked undifferentiated human ES cells can be separated from differentiated cells, which can facilitate mechanistic analyses. Using the murine Rex1 promoter to drive the EGFP gene, a mouse ES cell line has been established, in which GFP is expressed at high levels in undifferentiated ES cells but at dramatically lower levels when cells are differentiated (Eiges et al., 2001). This allows sorting and analyzing a pure population of undifferentiated ES cells. In a more sophisticated manner, a cassette containing an internal ribosomal entry site (IRES) and an EGFP gene was knocked into the 3’ untranslated region (UTR) of the Oct4-encoding gene POU5F1 in human ES cells, which is also expressed exclusively in the pluripotent cells (Zwaka and Thomson, 2003). These fluorescently marked stem cells will greatly facilitate our understanding of stem cell pluoripotency and developing technology for stem cell maintenance in chemically defined conditions.
TRANSGENIC CELL LINES TO PROMOTE DIRECTED DIFFERENTIATION
Directed differentiation of human ES cells toward a specific fate is a key to potential therapeutic applications. Protocols have been devised for differentiating and/or enriching specific cell types from differentiated human ES cells (Kaufman et al., 2001; Kehat et al., 2001; Levenberg et al., 2002; Reubinoff et al., 2001; Xu et al., 2002; Zhang et al., 2001). Many other cell types, such as pancreatic cells, have not been as successfully differentiated from ES cells, partly because the extracellular signaling pathways that lead to pancreatic specification are not known. Genetic alteration (forced expression or silencing) in genes that determine specific cell fates can aid in the generation of specialized cells and potentially help identifying extrinsic factors that facilitate specification of a particular cell lineage. Forced expression of Pdx1 and Foxa2, transcription factors involved in pancreas development, in human ES cells indicates that Pdx1 enhances the expression of pancreatic enriched genes (Lavon et al., 2006), although additional signals are required for differentiation of cells that are capable of insulin induction. This preliminary study indicates the potential of using genetically modified human ES cells to identify extracellular signals that promote lineage differentiation.
Although not yet reported, interference with signaling pathways during differentiation using similar genetic manipulation will also likely to help identify conditions for directing the commitment of cells towards specific lineages.
TISSUE SPECIFIC FLUORESCENT CELL LINES TO FACILITATE PURIFICATION
Differentiation of ES cells usually results in a mixed population of cells. For example, the presently most effective protocols for differentiating human ES cells to spinal motor neurons or dopaminergic neurons yield about 30% of the target cells among the total differentiated progenies (Li et al., 2005; Perrier et al., 2004; Yan et al., 2005). For mechanistic analyses or potential future clinical applications, a pure or highly enriched population of differentiated cells is desirable. By transiently transfecting with a GFP construct driven by the motor neuron specific Hb9 homeobox gene promoter (Arber et al., 1999; Nakano et al., 2005) in human ES cells that are differentiated toward spinal motor neurons, Singh et al show that differentiated motor neurons turn on GFP. The fluorescent cells were then sorted out by fluorescence activated cell sorting (FACS), which led to more than 10-fold enrichment of motor neurons (Singh Roy et al., 2005).
Stable cell lines have also been established to trace specific cell types (Lavon et al., 2004, , 2006). The reporter EGFP gene driven by the albumin promoter, which is relatively specifically expressed in the hepatic lineage, has permitted detection of a small fraction (6±2%) of hepatic-like cells in human ES differentiated cells. Human ES cells stably transfected with EGFP driven by the pancreatic-specific Pdx1 gene promoter has similarly allowed researchers to trace a small fraction (5±2%) of pancreatic β-like cells from human ES differentiated cells. These stable cell lines eases transfection burdens considerably. Considering the difficulty in efficiently directing human ES cells to such lineages as pancreatic β cells and hepatic cells, these fluorescently marked cells under lineage-specific promoters will be extremely useful in isolating sufficient cells through sorting. The fluorescent marker is also a great readout for identifying or optimizing conditions for differentiating human ES cells to these particular lineages.
Future directions
The most successful application of mouse ES cells is perhaps the generation of knockout mice, by using mouse ES cells with ablated genes. The technique to achieve gene ablation in mouse ES cells, i.e. homologous recombination, has been brought to human ES cells with success using modified transfection methods (Zwaka and Thomson, 2003). However the in vitro success can not be extended to in vivo for obvious reasons. As a result, complete gene knockout can not be easily accomplished in human research unless the gene is located in one of the sex chromosomes, since homozygotes are typically generated by animal breeding in non-human research. For this reason homologous recombination may not be as valuable for gene knockout in human ES cells, although it is still a crucial technique for other site-specific genetic modification. An alternative way to achieve gene silencing is through RNA interference (RNAi) (Hannon, 2002). RNAi bypasses the need for homozygote since only one copy of small interference RNA transcribing gene is required. Gene knockdown by RNAi appears technically feasible in human ES cells (Liu et al., 2005; Zaehres et al., 2005). However, development of stable RNAi human ES cell lines has been hampered by technical hurdles described above, mainly the low gene delivery efficiency. So far no human ES cell line with stable RNAi knockdown of endogeneous gene has been reported yet. Another issue is whether the RNA polymerase III promoters (such as U6 and H1) commonly used to drive the transcription of small hairpin RNA will be silenced during the differentiation of human ES cells, as many of the RNA polymerase II promoters are. Although there are still many unsolved issues, RNAi is expected to be used broadly in human ES cells in the near future. A database of human ES cell lines with specific genes silenced will be a great asset to the research community, as its counterpart of knockout mice.
Cell lines which allow inducible gene overexpression or silencing are probably even more valuable than those constitutively overexpressing or silencing genes. Using the tet-on technique (Gossen et al., 1995), Kyba et al. have established a founder mouse ES cell line which has greatly facilitated the construction of inducible mouse ES cell lines (Kyba et al., 2002). Recently the tet technique was further successfully applied to primate ES cells (Adachi et al., 2006). All these progresses suggest that the extension of this technique to human ES cells is merely a matter of time.
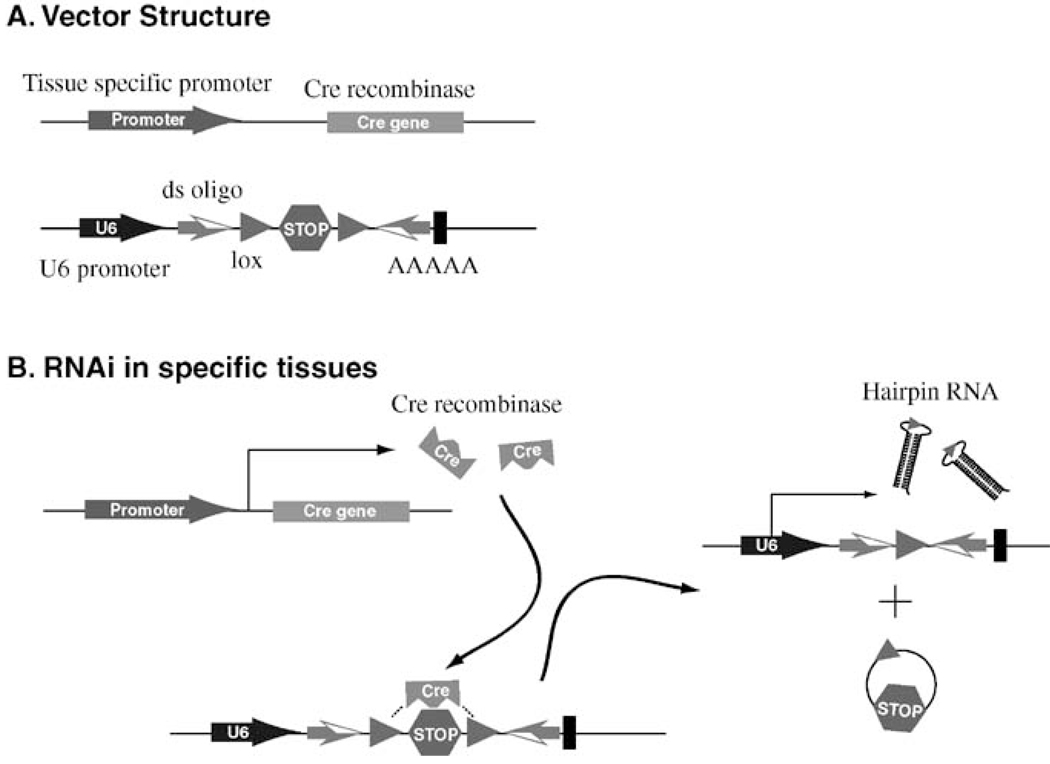
The conditional knockout technique developed in the 1990s has further expanded the scope of the traditional knockout technique (Gu et al., 1993). Using tissue specific promoters and Cre/loxp recombination, a specific gene can be selectively knocked out in certain types of tissues, which will enable researchers to determine gene function in different tissues. The conditional knockout techinique requires generation of a homozygous floxed exon, which as we discussed above, is not applicable to human research in most cases. However, a combination of RNAi, Cre/loxp recombination and tissue specific promoter still allows tissue specific gene silencing in human ES cells and derivatives (Figure 2). The strategy employs a tissue specific promoter to drive the expression of cre recombinase. As a result, the floxed stop codon is removed in the particular tissue through cre/loxp recombination so that the full length hairpin RNA is transcribed, which leads to silencing of the target gene. The first step is the establishment of founder human ES cell lines expressing the cre recombinase under a tissue specific promoter. Many founder mouse lines expressing the cre recombinase under various tissue specific promoters have been created and are made available through non-profit institutions like the Jackson Laboratory. Similar human ES cell lines expressing the cre recombinase under tissue specific promoters will undoubtedly change our research in human biology.
Figure 2. Strategy to achieve tissue specific gene slicing in hES cells and derivatives.
A, structures of the two constructs used in the strategy. One construct drives the expression of cre recombinase under a tissue specific promoter. The other construct uses U6 promoter to drive the expression of small hairpin RNA. The two reverse complementary double strand oligos were separated by a floxed stop codon. B, In the specific tissue where the promoter is active, cre recombinase is expressed so that the floxed stop codon is removed through cre/loxp recombination. This enables the transcription of the full self-complementary oligo so that small hairpin RNAs are generated which lead to knockdown of the target gene.
Abbreviations
- Ad
adenovirus
- bFGF
basic fibroblast growth factor
- BMP
bone morphogenetic protein
- CNS
central nervous system
- ES
embryonic stem
- FACS
fluorescence activated cell sorting
- HIV
human immunodeficiency virus
- ICM
inner cell mass
- LIF
leukemia inhibitory factor
- MEF
mouse embryonic fibroblast
- RNAi
RNA interference
- SCNT
somatic cell nucleus transfer
- UTR
untranslated region
References
- Adachi K, Kawase E, Yasuchika K, Sumi T, Nakatsuji N, Suemori H. Establishment of the Gene-Inducible System in Primate Embryonic Stem Cell Lines. Stem Cells. 2006 doi: 10.1634/stemcells.2005-0659. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Ingham PW. The transformation of the model organism: a decade of developmental genetics. Nat Genet. 2003;33 Suppl:285–293. doi: 10.1038/ng1105. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Jenkins NA, Copeland NG. Mouse models of human disease. Part I: techniques and resources for genetic analysis in mice. Genes Dev. 1997a;11:1–10. doi: 10.1101/gad.11.1.1. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Largaespada DA, Jenkins NA, Copeland NG. Mouse models of human disease. Part II: recent progress and future directions. Genes Dev. 1997b;11:11–43. doi: 10.1101/gad.11.1.11. [DOI] [PubMed] [Google Scholar]
- Boiani M, Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- Chang JC, Ye L, Kan YW. Correction of the sickle cell mutation in embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:1036–1040. doi: 10.1073/pnas.0510177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry SR, Biniszkiewicz D, van Parijs L, Baltimore D, Jaenisch R. Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol Cell Biol. 2000;20:7419–7426. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell AS, Kafri T. HIV-1 vectors: fulfillment of expectations, further advancements, and still a way to go. Curr HIV Res. 2003;1:419–439. doi: 10.2174/1570162033485104. [DOI] [PubMed] [Google Scholar]
- Costa M, Dottori M, Ng E, Hawes SM, Sourris K, Jamshidi P, Pera MF, Elefanty AG, Stanley EG. The hESC line Envy expresses high levels of GFP in all differentiated progeny. Nat Methods. 2005;2:259–260. doi: 10.1038/nmeth748. [DOI] [PubMed] [Google Scholar]
- Darr H, Mayshar Y, Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- Drukker M, Dhara SK, Benvenisty N. Genetic engineering of human embryonic stem cells. Human Embryonic Stem Cells. 2005:215–229. [Google Scholar]
- Dunnett SB, Bjorklund A, Lindvall O. Cell therapy in Parkinson's disease - stop or go? Nat Rev Neurosci. 2001;2:365–369. doi: 10.1038/35072572. [DOI] [PubMed] [Google Scholar]
- Eiges R. Genetic manipulation of human embryonic stem cells by transfection. Methods Mol Biol. 2006;331:221–239. doi: 10.1385/1-59745-046-4:221. [DOI] [PubMed] [Google Scholar]
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Frohman MA, Martin GR. Cut, paste, and save: new approaches to altering specific genes in mice. Cell. 1989;56:145–147. doi: 10.1016/0092-8674(89)90887-8. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gresch O, Engel FB, Nesic D, Tran TT, England HM, Hickman ES, Korner I, Gan L, Chen S, Castro-Obregon S, Hammermann R, Wolf J, Muller-Hartmann H, Nix M, Siebenkotten G, Kraus G, Lun K. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–163. doi: 10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Gropp M, Itsykson P, Singer O, Ben-Hur T, Reinhartz E, Galun E, Reubinoff BE. Stable genetic modification of human embryonic stem cells by lentiviral vectors. Mol Ther. 2003;7:281–287. doi: 10.1016/s1525-0016(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Guillaume DJ, Johnson MA, Li XJ, Zhang SC. Human ES cell-Derived neural progenitors develop into neurons and integrate into the host brain. Journal of Neuroscience Research. 2006 doi: 10.1002/jnr.21022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Sakurai F, Koizumi N, Hayakawa T, Mizuguchi H. Adenovirus vector-mediated gene transfer into stem cells. Mol Pharm. 2006;3:95–103. doi: 10.1021/mp0500925. [DOI] [PubMed] [Google Scholar]
- Kawabata K, Sakurai F, Yamaguchi T, Hayakawa T, Mizuguchi H. Efficient gene transfer into mouse embryonic stem cells with adenovirus vectors. Mol Ther. 2005;12:547–554. doi: 10.1016/j.ymthe.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Rivas-Carrillo JD, Soto-Gutierrez A, Fukazawa T, Chen Y, Navarro-Alvarez N, Tanaka N. Gene delivery to embryonic stem cells. Birth Defects Res C Embryo Today. 2005;75:10–18. doi: 10.1002/bdrc.20031. [DOI] [PubMed] [Google Scholar]
- Koller BH, Hagemann LJ, Doetschman T, Hagaman JR, Huang S, Williams PJ, First NL, Maeda N, Smithies O. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci U S A. 1989;86:8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Laker C, Meyer J, Schopen A, Friel J, Heberlein C, Ostertag W, Stocking C. Host cis-mediated extinction of a retrovirus permissive for expression in embryonal stem cells during differentiation. J Virol. 1998;72:339–348. doi: 10.1128/jvi.72.1.339-348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, Verfaillie CM. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22:531–543. doi: 10.1634/stemcells.22-4-531. [DOI] [PubMed] [Google Scholar]
- Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- Lavon N, Yanuka O, Benvenisty N. The effect of overexpression of pdx1 and foxa2 on the differentiation of human embryonic stem cells into pancreatic cells. Stem Cells. 2006;24:1923–1930. doi: 10.1634/stemcells.2005-0397. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liu YP, Dambaeva SV, Dovzhenko OV, Garthwaite MA, Golos TG. Stable plasmid-based siRNA silencing of gene expression in human embryonic stem cells. Stem Cells Dev. 2005;14:487–492. doi: 10.1089/scd.2005.14.487. [DOI] [PubMed] [Google Scholar]
- Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117. doi: 10.1634/stemcells.21-1-111. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JC, de Pablo JJ, Palecek SP. Electroporation of human embryonic stem cells: Small and macromolecule loading and DNA transfection. Biotechnol Prog. 2006;22:825–834. doi: 10.1021/bp0600334. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Windrem M, Zappavigna V, Goldman SA. Identification of a conserved 125 base-pair Hb9 enhancer that specifies gene expression to spinal motor neurons. Dev Biol. 2005;283:474–485. doi: 10.1016/j.ydbio.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Rideout WM, 3rd, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81:2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- Siemen H, Nix M, Endl E, Koch P, Itskovitz-Eldor J, Brustle O. Nucleofection of human embryonic stem cells. Stem Cells Dev. 2005;14:378–383. doi: 10.1089/scd.2005.14.378. [DOI] [PubMed] [Google Scholar]
- Silani V, Cova L, Corbo M, Ciammola A, Polli E. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet. 2004;364:200–202. doi: 10.1016/S0140-6736(04)16634-8. [DOI] [PubMed] [Google Scholar]
- Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Smith-Arica JR, Thomson AJ, Ansell R, Chiorini J, Davidson B, McWhir J. Infection efficiency of human and mouse embryonic stem cells using adenoviral and adeno-associated viral vectors. Cloning Stem Cells. 2003;5:51–62. doi: 10.1089/153623003321512166. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Xia X, Zhang Y, Zieth CR, Zhang SC. Transgenes Delivered by Lentiviral Vector are Suppressed in Human Embryonic Stem Cells in a Promoter-Dependent Manner. Stem Cells and Development. 2006 doi: 10.1089/scd.2006.0057. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C, Tang DQ, Xie CQ, Zhang L, Xu KF, Thompson WE, Chou W, Gibbons GH, Chang LJ, Yang LJ, Chen YE. Genetic engineering of human embryonic stem cells with lentiviral vectors. Stem Cells Dev. 2005;14:367–377. doi: 10.1089/scd.2005.14.367. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. doi: 10.1634/stemcells.2004-0252. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]