Contrast-enhanced Imaging for Hepatocellular Carcinoma
Case 1
A 62-year-old woman has biopsy-proven cirrhosis that is presumed to be secondary to chronic hepatitis B because of her known history of hepatitis B. When the liver biopsy first diagnosed cirrhosis in 2005, she initiated tenofovir treatment. Tenofovir therapy provided satisfactory control of the patient's viral load for several years. During this time, she underwent routine follow-up with computed tomography (CT) imaging in 2005 and 2007, neither of which showed a liver mass. At the time of her follow-up in 2007, a liver panel was performed and came back as normal; however, she had slightly elevated levels of alpha-fetoprotein (AFP; 14 ng/mL). At a subsequent follow-up in January 2009, her CT scan again appeared normal but her AFP levels were now substantially elevated (85 ng/mL). She returned in November 2009 after an ultrasound revealed a mass (dimensions, 2.6 × 3.1 × 3.9 cm) in the liver. However, the mass was not evident in a contrast-enhanced CT scan (Figure 1).
Figure 1.
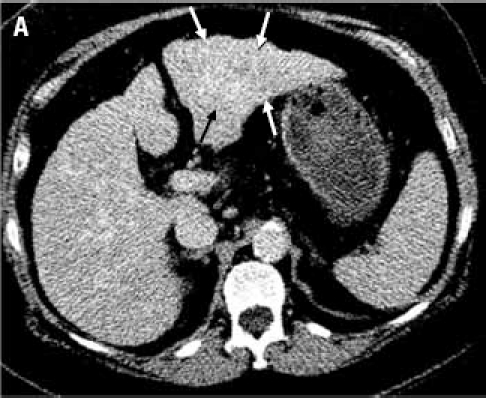
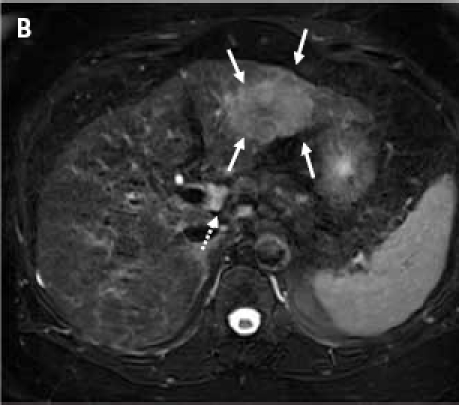
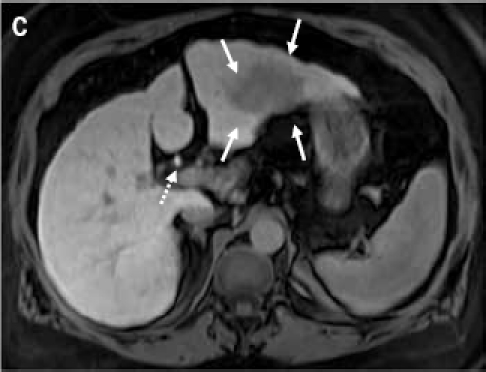
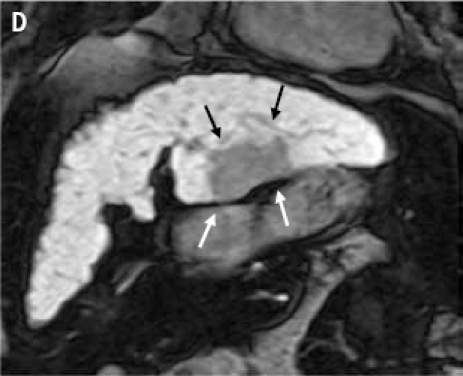
(A): Contrast-enhanced computed tomography scan through the liver (displayed with window center at 93 and window width of 109) acquired 7 minutes post-contrast initiation. The mass (arrows) in the left lobe cannot be delineated with confidence; the mass does not demonstrate any contrast washout. (B): Axial 2D fat suppressed fast spin echo respiratory triggered T2-weighted image shows a hyperintense mass in the left hepatic lobe (arrows) and enlarged lymph node in the liver hilum (dashed arrow). (C): Fat suppressed axial 3D T1-weighted gradient echo image (flip angle 10 degrees) acquired during the hepatocyte phase shows a hypointense mass in the left hepatic lobe (arrows) and contrast excretion into the common bile duct (dashed arrow). (D): Fat suppressed coronal 3D T1-weighted gradient echo image (flip angle 30 degrees) acquired during the hepatocyte phase shows a hypointense mass in the left hepatic lobe (arrows).
Because of the discrepancy in the imaging studies, a magnetic resonance imaging (MRI) study was performed to determine conclusively if a liver mass was present. MRI was performed before and after administration of the liver-specific contrast agent gadoxetate disodium. Prior to contrast agent delivery, a T2-weighted MRI revealed a hyperintense mass located in the left hepatic lobe, as well as an accompanying enlarged lymph node in the liver hilum (Figure 1B). An MRI following gadox-etate disodium delivery found that the mass did not take up the contrast agent (Figures 1C and 1D). By December 2009, the patient's AFP levels had risen to above 200 ng/mL. After a discussion of her treatment options, the patient opted to undergo a laparoscopic surgical resection of the left hepatic lobe, which was successfully performed. Pathologic analysis of the specimen determined it to be grade 2 hepatocellular carcinoma (HCC). At the time of resection, the tumor size was 6.4 × 4.5 × 3.7 cm. Fortunately, the tumor was confined to the liver, and the margins were negative; the distance to the closest margin was 1.4 cm. Additionally, pathologic findings included cirrhosis.
Figure 1B.
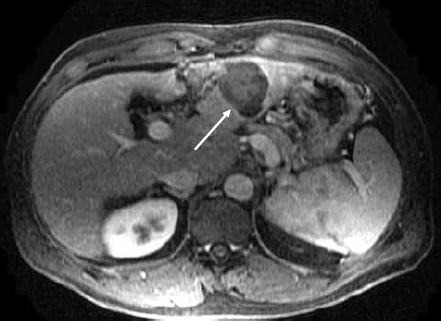
Contrast-enhanced magnetic resonance image of the liver shows decrease in size and no enhancement of the treated lesion in the left lobe of the liver (arrow).
Figure 1C.
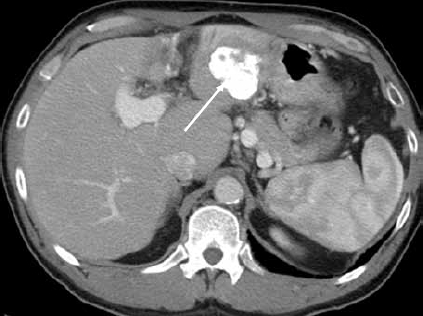
Contrast-enhanced computed tomography of the liver shows ethiodol uptake in the lesion in the left lobe of the liver. Enhancement is difficult to evaluate following transarterial chemoembolization (arrow).
Figure 1D.
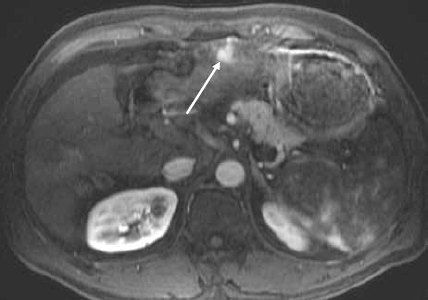
Contrast-enhanced magnetic resonance image of the liver shows a hypervascular lesion in the left lobe of the liver in keeping with hepatocellular carcinoma (arrow).
Discussion
This case provides a good example of a negative CT imaging test for HCC, despite rising AFP levels. Side-by-side comparison of the CT image versus the contrast-enhanced MRI found that the latter reveals the lesion much better. Although this is purely speculative, it is reasonable to consider that if a contrast-enhanced MRI had been performed at an earlier time point, such as when the AFP levels had first started to rise, the HCC could have been discovered at an earlier stage.
Notably, the lesion actually appears darker than the rest of the liver after administration of the gadoxetate disodium contrast agent (Figures 1C and 1D). After injection, gadoxetate disodium is taken up selectively by functioning hepatocyte cells, explaining why the lesion did not contain the contrast agent in these studies.1 Gadoxetate disodium is taken up into the hepatocyte cells via the organic anion-transporting polypeptide (OATP1B1) receptor, although the contrast agent is also a substrate of OATP1B3.2 The lack of OATP1B1 expression in the HCC cells prevents uptake of the dye into the tumor. The dye is excreted through the multi-drug-resistance–associated protein 2 (MRP2) transport mechanism into the biliary canaliculi.
Case 2
A 73-year-old male was referred for consultation regarding an abnormal liver panel and a focal liver lesion that was evident in abdominal imaging studies. The patient was in generally good health until December 2008, at which point he began to exhibit anorexia, became very weak, and had generalized malaise. He also described his abdomen as feeling bloated. The man was not jaundiced and had no systemic symptoms (fever, chills, sweats, or dark urine). Over a period of several months, the patient's weight fell from 244 to 205 pounds, which he attributed to decreased food intake. Subsequent abdominal imaging studies revealed multiple liver lesions. In March 2009, the patient underwent an ultrasound-guided liver biopsy, at which time a large (8.9 × 8.5 × 8.7 cm) heterogeneous mass in the right lobe of the liver was noted and sampled by fine-needle aspiration. The pathologic report found only steatosis with glycogenated nuclei and no evidence of malignancy. Since this point, the patient has been followed conservatively. Laboratory findings revealed elevated liver enzymes suggestive of possible cirrhosis—alkaline phosphatase (ALP) was 364 IU/L; alanine aminotransferase (ALT) was 82 IU/L; aspartate aminotransferase (AST) was 83 IU/L; albumin was 3.1 g/dL; bilirubin was 1.1 mg/dL; and creatinine levels were normal. The patient's cholesterol level (266) and triglycerides (274) were also elevated.
Discussion
Multiple liver lesions are evident in a 2D T1-weighted gradient dual echo sequence (Figures 2A and 2B); those lesions which appear darker in the posterior aspect compared with the in-phase image are indicative of fat-containing lesions. However, typical primary liver lesions generally are not fatty. More commonly, fat-containing lesions are suggestive of hepatic adenoma; in the setting of a cirrhotic background, the most common fat-containing liver lesion is HCC. However, the biopsy result of this patient showed no evidence of malignancy, prompting the physician to question if a sampling error was made during biopsy.
Figure 2.
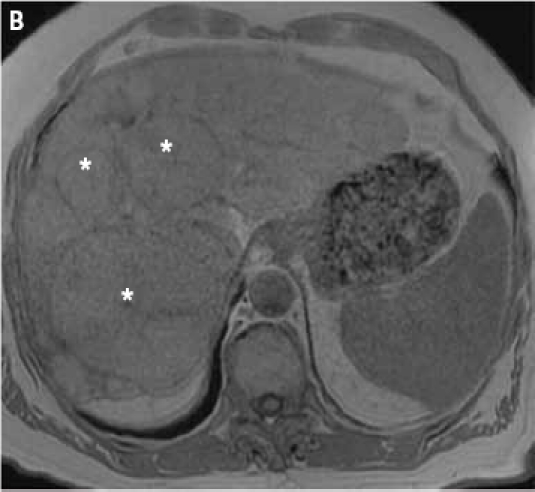
2D gradient dual echo opposed-phase (A) and in-phase (B) images demonstrate multiple liver lesions (asterisks). Of note, some areas of the largest lesion (white arrows) show a loss of signal intensity on the opposed-phase image (A) indicative of fatty components.
Administration of the contrast agent gadoxetate disodium allowed for vivid enhancement of the focal lesions (Figures 2C–2F). This is a classic finding for primary liver lesions such as focal nodular hyperplasia (FNH), HCC, and hepatic adenoma. An interesting phenomenon occurs when the portal venous and hepatocyte phases are observed—the 2 anterior lesions do not take up the contrast agent (Figures 2I and 2J). Again, this shows a section of tissue with either a dysfunctional OATP1B1 transport mechanism or enhanced excretion from the hepatocyte into the biliary canaliculi. In contrast, the larger lesion contains some areas which do take up the contrast agent while some areas do not. Overall, these lesions are highly suspicious for HCC. This is further supported with T2-based imaging (Figures 2K–2N), which show restricted diffusion. Based on the overwhelming evidence of HCC, which became apparent with imaging studies, another biopsy was ordered; these pathologic findings showed malignancy consistent with HCC.
Figure 2.
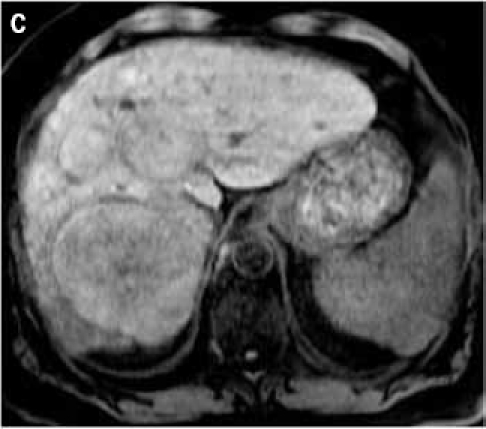
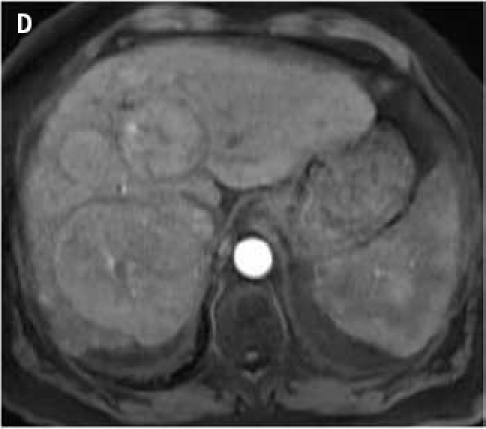
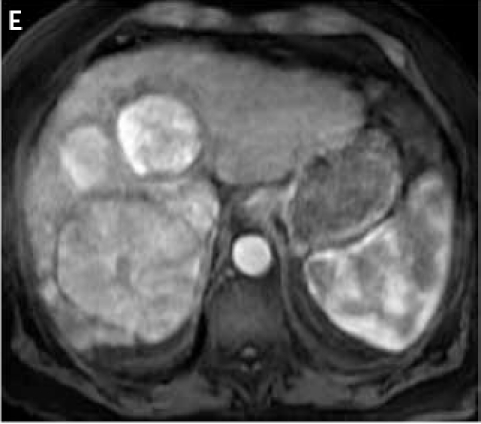
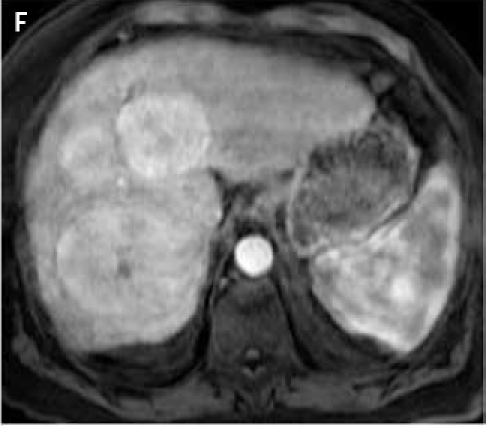
Fat suppressed 3D gradient echo images before (C) and during the early (D), mid (E), and late (F) arterial phase post-gadoxetate disodium administration demonstrate vivid uptake of the multiple liver lesions.
Figure 2.
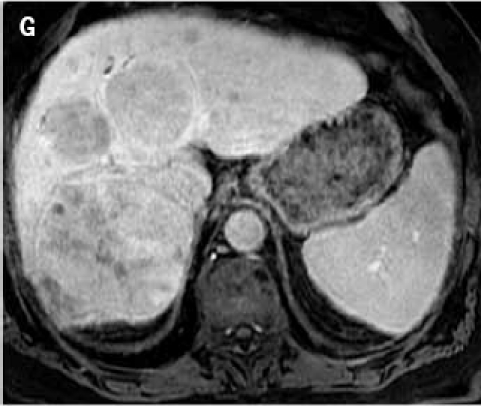
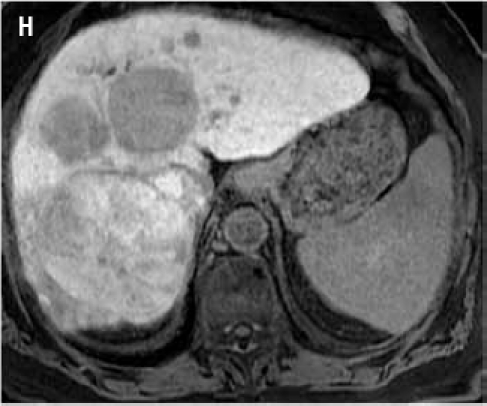
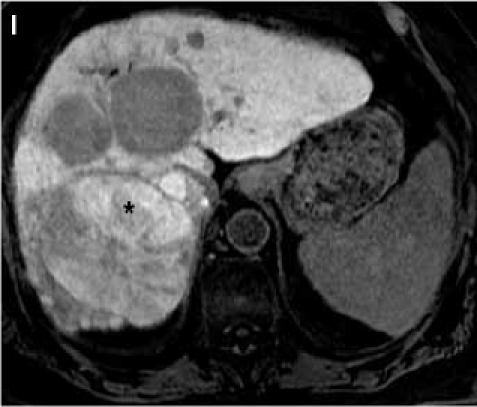
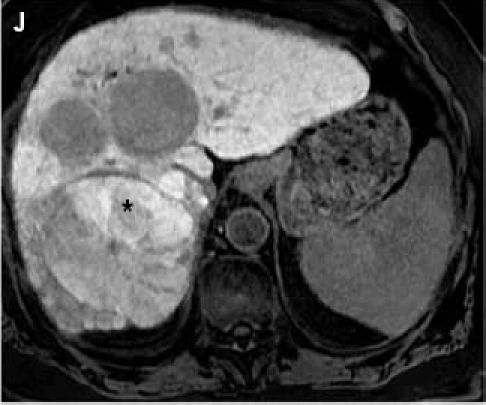
Fat suppressed 3D gradient echo images during the portal venous phase (G), and 10 min (H), 17 min (I) and 22 min (J) post–gadoxetate disodium administration demonstrate tumor components with persistent enhancement (asterisk; I, J), whereas other tumors are uniformly hypointense.
Figure 2.
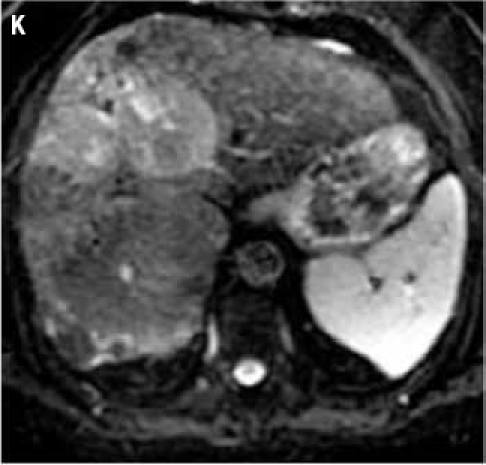
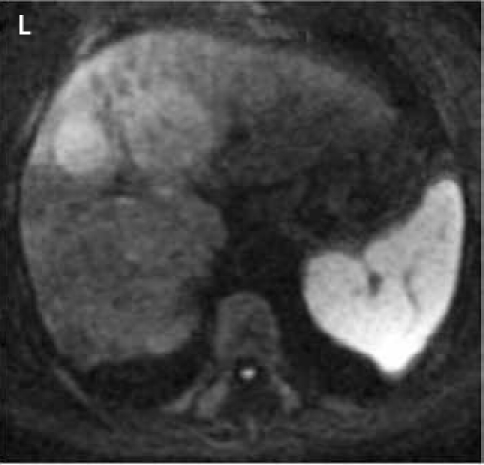
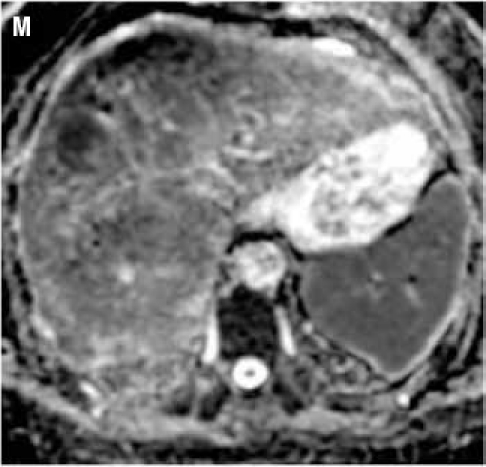
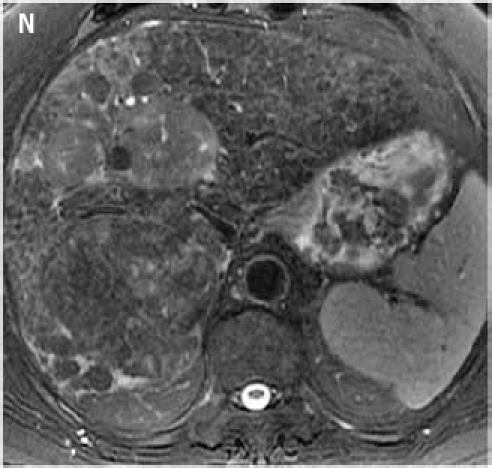
Fat suppressed 2D diffusion-weighted images. (K): low b-value; (L): high b-value; (M): corresponding apparent diffusion coefficient map shows restricted diffusion. Tumors demonstrate various signal intensities on fat suppressed 2D T2-weighted image (N).
Thus, this is an example in which different HCC lesions have different enhancement patterns in imaging studies. While the larger posterior lesion exhibited characteristics which allowed it in part to take up the contrast agent, neither of the 2 smaller anterior lesions took up the contrast agent. A retrospective study recently reported by Tsuboyama and colleagues may help to explain the differential appearance of HCC lesions observed here.3 This study correlated gadoxetate disodium–enhanced imaging studies with immunohistochemical staining. High enhancement was associated with positive expression of OATP1B1 and/or OATP1B3. In addition, 2 patterns of MRP2 expression that correlated with enhancement emerged—decreased total expression as well as increased expression at the luminal membrane of pseudoglands. Additionally, this study showed that nodules with bile pigment significantly appeared to be enhanced. Notably, this study suggests that uptake of the contrast agent gadoxetate disodium is actually dependent on the expression of transport proteins and receptors, which until now could only be assessed with a tumor biopsy followed by immunohistochemistry.
Adverse Events Associated With Gadolinium-based Contrast Agents
A potential risk associated with the use of gadolinium-based contrast agents is nephrogenic systemic fibrosis. In May 2007, the US Food and Drug Administration issued black-box warnings for all gadolinium-based contrast agents for MRI, based on an increased risk of nephrogenic systemic fibrosis in patients with kidney disease and patients with chronic liver disease, including those who are about to undergo or have recently undergone liver transplantation. The warning cites the possibility of nephrogenic systemic fibrosis in patients with severe kidney insufficiency. Gadolinium-based agents should not be used in patients with acute or chronic severe renal insufficiency (defined as a glomerular filtration rate of <30 mL/min/1.73 m2), patients with acute renal insufficiency of any severity due to the hepatorenal syndrome, or during the perioperative liver transplantation period.
In a single-center study of 18,142 MRI examinations administered with and without gadolinium-based contrast media, the incidence of acute adverse reactions to these agents was 0.48%.4 Among the adverse reactions, 96% were mild, 2% were moderate (1 patient developed shortness of breath that required oxygen supplementation and intravenous steroidal management), and 2% were severe (1 patient developed an anaphylactoid reaction, but successfully recovered with resuscitation). Among the 45 patients who developed adverse reactions, 3 patients (6.7%) had had previous adverse reactions to iodinated contrast media, 3 (6.7%) had had previous reactions to a different gadolinium-based contrast agent, 1 (2%) had asthma, and 9 (20%) had a history of drug/food allergy. The researchers noted that these rates of adverse events concurred with those previously reported.
Summary
Both of the cases presented here revolve around the use of gadoxetate disodium as a contrast agent. Personally, in our clinical practice, we now rely heavily on this agent in our imaging studies. However, we did not switch immediately to this agent when it first became available. Instead, we restricted its use at first to young females in whom it was necessary to differentiate FNH and adenoma. We then applied gadoxetate disodium in an off-label setting to support biliary imaging, which we found to be very helpful. Subsequently, we began to more routinely use this particular contrast agent in studies looking for metastatic or secondary liver lesions. Now, gadoxetate disodium has become our default agent for imaging studies unless there is a need to look for vascular issues.
There are cases for which other agents are more appropriate. For example, if there is reason to suspect hemangioma, an extracellular contrast agent such as gado-pentetate dimeglumine would be more useful. The same holds true if vascular issues are the main indication for the MRI. In this scenario, an extracellular contrast agent with a high relaxivity, such as gadobenate dimeglumine, provides excellent results. In patients with impaired hepatic function (bilirubin levels >5 mg/dL), hepatobiliary contrast agents, such as gadoxetate disodium, offer minimal to no benefit over extracellular contrast agents because the hepatobiliary contrast agent will not be excreted sufficiently via the biliary pathway. In our practice, however, gadoxetate disodium has proven to be the most useful contrast agent for the majority of the hepatic imaging studies that we perform.
References
- 1.Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007;6:43–52. doi: 10.2463/mrms.6.43. [DOI] [PubMed] [Google Scholar]
- 2.Leonhardt M, Keiser M, Oswald S, et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024–1028. doi: 10.1124/dmd.110.032862. [DOI] [PubMed] [Google Scholar]
- 3.Tsuboyama T, Onishi H, Kim T, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–833. doi: 10.1148/radiol.10091557. [DOI] [PubMed] [Google Scholar]
- 4.Li A, Wong CS, Wong MK, Lee CM, Au Yeung MC. Acute adverse reactions to magnetic resonance contrast media—gadolinium chelates. Br J Radiol. 2006;79:368–371. doi: 10.1259/bjr/88469693. [DOI] [PubMed] [Google Scholar]
Gadolinium-enhanced Imaging to Identify Metastatic Lesions
Case 1
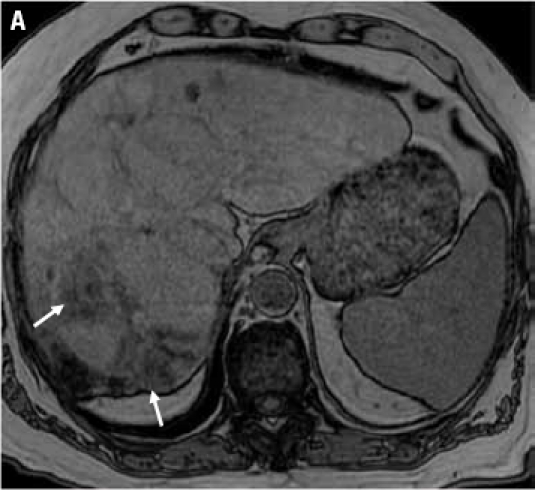
A 55-year-old male was found to have cirrhosis of the liver during a laparoscopic cholecystectomy. He was also diagnosed with hepatitis C. An ultrasound revealed a mass in the liver. These findings prompted his referral. His AFP was 4.6 ng/mL. An MRI of the abdomen was performed with and without gadolinium, which revealed a 7.6-cm mass in the left lobe of the liver with biliary dilatation of segments II and III (Figure 1A).
Figure 1A.
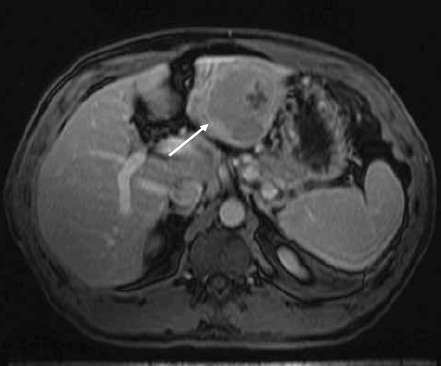
Contrast-enhanced magnetic resonance image of the liver shows a hypointense mass in the left lobe of the liver (arrow).
A 1.6-cm nodule seen in the right lobe of the liver was indeterminate. The nodule showed early enhancement on the arterial phase of contrast administration and was isointense to the liver on all other series. The patient went to the operating room to undergo a possible resection, at which time a biopsy of the lesion in the right lobe of the liver was consistent with HCC. At this time, the patient was not considered a candidate for resection or transplant. He proceeded with transarterial chemoembolization therapy (TACE). His initial treatment was with mitomycin, doxorubicin, and cisplatin, first to treat the lesion in the left lobe of the liver and followed by a second session, a month later, to treat the lesion in the right lobe of the liver. Follow-up imaging with CT and MR showed response to treatment with chemoembolization material in the lesions in the right and left lobes of the liver (Figures 1B and 1C).
Follow-up imaging with MR showed a vascular lesion in segment III of the liver; this lesion demonstrated washout and peripheral ring enhancement on delayed imaging, consistent with HCC (Figure 1D). Because this lesion measured approximately 1 cm, the patient again underwent transarterial chemoembolization. On follow-up MRI, there was no evidence of residual disease in the right and left lobes of the liver. Two months later, the patient underwent a liver transplant; he is currently undergoing routine follow-up and shows no evidence of disease.
Discussion
The surgical options for the treatment of HCC are resection and liver transplant. There are criteria for resectability that include the number and size of lesions and distribution (right and left lobe). MRI was useful to characterize lesions in the left lobe of the liver as HCC and was able to detect an indeterminate lesion in the right lobe of the liver. The surgeon was aware of this finding, and before major intervention was performed, laparoscopic biopsy proved that this second lesion represented HCC. At this time, the patient was not considered a surgical candidate. Instead, the patient underwent chemoembolization. The follow-up evaluation with CT was suboptimal in the post-TACE setting, as lesion enhancement is difficult to assess, due to the high attenuation of the chemoembolization material. MRI was able to assess response to treatment of the 2 treated lesions. In addition, MRI detected and characterized an additional lesion that prompted a third chemoembolization. The distinctive imaging features of HCC in this case included early enhancement, delayed washout, and ring enhancement of the capsule of the lesion. Importantly, even though the patient originally presented with a very large mass, liver-directed treatment was able to downstage the cancer to a point where the patient became a candidate for liver transplantation.
Case 2
A 53-year-old male with a clinical history of psoriatic arthritis presented with abdominal pain and weight loss of approximately 40 pounds. A CT scan revealed a mass in the colon and lesions in the liver (Figure 2A). There were multiple liver lesions in the right lobe of the liver and segment IV. At initial evaluation, the carcinoembryonic antigen (CEA) score was 229. The patient admitted to a history of smoking (6 packs per day for 30 years), alcohol abuse, and multiple chemical exposures. The liver lesions were biopsied and were consistent with metastatic disease from the colon primary. The patient initiated standard therapy with FOLFOX (fluorouracil, leucovorin, and oxaliplatin) plus bevacizumab. A follow-up MRI with and without gadolinium was performed (Figure 2B).
Figure 2A.
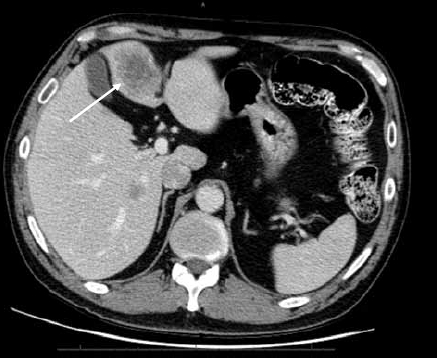
Post-contrast computed tomography examination of the liver shows hypointense masses in the left and right lobes of the liver (arrow).
Figure 2B.
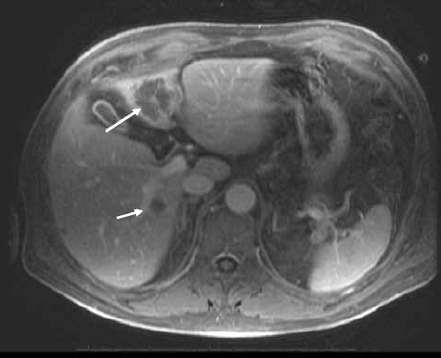
Post–gadolinium-enhanced magnetic resonance image of the liver shows masses in the left and right lobes of the liver (arrows). The response to treatment is assessed by the decreased enhancement of the masses.
The MRI also confirmed that there were no lesions in segments II and III of the liver. The patient completed the FOLFOX plus bevacizumab treatment. The patient was scheduled for surgical resection of the primary tumor and liver metastases in segment IV. Pathology examination reported that less than 25% of the residual tumor was present in the liver; 80% was present at the primary site. At this point, a CT scan was performed to evaluate for liver volume. These studies found that 29% of functional liver volume remained; portal vein embolization was performed, which caused an increase to 33%. The patient underwent a second surgery, at which time an extended right hepatectomy was performed. After the procedure, the patient continued to receive FOLFOX plus bevacizumab. Follow-up MRI evaluation detected lesions in the residual left lobe of the liver (Figures 2C and 2D). A radiofrequency ablation liver-directed procedure was performed on the lesion in the left lobe of the liver. The patient is now stable and due for another follow-up visit.
Figure 2C.
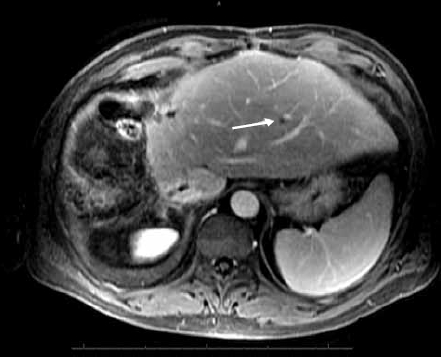
Axial post-gadolinium image of the liver shows a nodule in the left lobe of the liver. This new lesion is suspicious for metastatic disease.
Figure 2D.
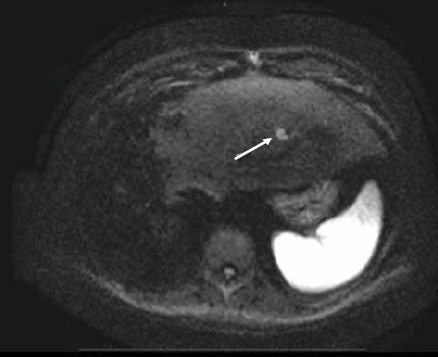
Axial diffusion-weighted image with B value of 500 mm2/sec shows a hyperintense nodule in the left lobe of the liver. The high signal on the diffusion-weighted image suggests a solid mass in keeping with metastatic disease.
Discussion
The most common measurement for the assessment of response to treatment of liver metastases is the change in size. One notable issue that was very apparent in this case was the ability of MRI to detect treatment response following systemic treatment, even in the setting where the lesion size had not significantly changed. The resection of the multiple liver lesions was split into multiple surgeries in order to ensure sufficient functional liver remnant. An important point in the management of this patient was the effort to increase the functional liver volume through portal vein embolization of the resected liver. On follow-up imaging, an indeterminate new lesion was detected in the residual left lobe of the liver. The combination of imaging features on post-gadolinium images and diffusion-weighted images (DWI) allowed these very small lesions to be detected and characterized as metastatic. This prompted early intervention with liver-directed therapy with radiofrequency ablation.
Summary
Both of these cases demonstrate the use of MRI to detect, characterize, and monitor response to treatment of patients who at first may not have been considered surgical candidates. In the presurgical setting, the use of extracellular contrast agents in combination with T2-weighted images, DWI, and noncontrast T1-weighted images permitted the detection and characterization of hypervascular lesions (in the setting of HCC) or hypo-vascular solid lesions (in the setting of metastatic disease).
As presented here, there are multiple therapeutic options for the treatment of liver malignancies. These include liver resection, transplantation, chemoembolization, radiofrequency ablation, and systemic chemotherapy. Both of these cases demonstrate the ability of MRI to detect the response to treatment of both liver-directed and systemic therapy of primary and metastatic liver lesions. In contrast to C T, MRI with gadolinium has higher sensitivity and does not suffer from some of the limitations of iodine-enhanced CT.
Finally, in the setting of systemic chemotherapy, where lesion size has been considered the standard for assessment of response to treatment, we suggest that the lack of enhancement proved useful for the characterization of response to treatment.
Increased Diagnostic Confidence With Use of Magnetic Resonance Contrast Agents
Case 1
A 60-year-old Asian male with a history of cirrhosis secondary to chronic hepatitis B was treated with transarterial chemoembolization for a right hepatic lobe HCC. He returned for follow-up CT 4 months later. Dual-phase contrast-enhanced multidetector CT showed several areas of arterial enhancement without washout in the right hepatic lobe, as well as areas of vague arterial enhancement with washout in the left lateral lobe, away from the treated tumor, suspicious for viable tumor (Figure 1). However, from the CT alone, it was unclear whether the areas of enhancement in the right lobe represented shunting versus tumors. Thus, a contrast-enhanced MRI was performed to further clarify the findings. A 1.5 Tesla MRI of the abdomen was performed approximately 6 weeks following the CT scan; no treatment was administered during the interim period. An extracellular gadolinium contrast agent, gadopentetate dimeglumine, was used. In addition to precontrast in-and out-of-phase T1-, T2-, diffusion-weighted imaging, 3D T1 GRE sequence was performed before and after dynamic administration of gadolinium contrast (dual arterial, portal venous, and equilibrium phases). Based on the T1, T2, and diffusion and contrast-enhanced imaging, a 4-cm tumor was clearly depicted in the left lateral hepatic lobe (segment 2; Figure 2). Additionally, MRI clearly showed that the areas of enhancement in the right lobe were compatible with arterioportal shunting and not with tumor. It was determined that the patient's initial tumor had had a good response to treatment (as it was completely necrotic) and did not require additional treatment, while the second left lobe tumor required a new chemoembolization.
Figure 1.
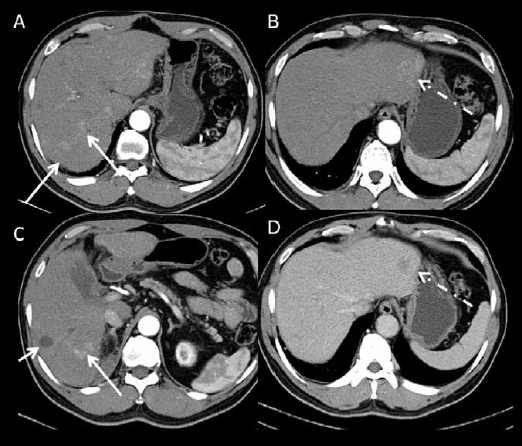
Dual-phase contrast-enhanced multidetector computed tomography images. (A, B, C): images acquired at the arterial phase. (D): image acquired at the portal venous phase at the same level as B. There is a treated non-enhancing hepatocellular carcinoma in the right hepatic lobe (short arrow on C). There is an enhancing lesion with washout, compatible with hepatocellular carcinoma in the lateral left lobe (dashed arrow). There are also multiple areas of arterial enhancement without washout in the right lobe (long arrows) that are difficult to characterize. Magnetic resonance imaging was performed to further characterize these findings.
Figure 2.
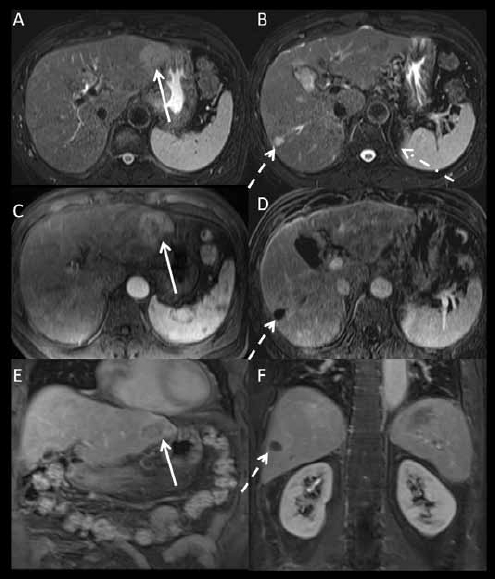
1.5 Tesla magnetic resonance imaging with an extracellular gadolinium contrast agent (gadopentetate dimeglumine) obtained 6 weeks after computed tomography in the same patient as in Figure 1. (A, B): axial fat suppressed T2-weighted images. (C): axial post-contrast fat suppressed T1-weighted image acquired at the arterial phase. (D): axial T1-weighted subtracted image acquired at the arterial phase at a different level. (E, F): coronal post-contrast fat suppressed T1-weighted images acquired at the portal venous phase. The treated right lobe hepatocellular carcinoma is T2 hyperintense and completely necrotic (dashed arrows on B and D). The left lateral lobe lesion (arrow) measures 4.3 cm and is T2 hyperintense with arterial enhancement and washout compatible with hepatocellular carcinoma. The vague areas of enhancement seen on computed tomography are likely areas of arterioportal shunting, as they are isointense on T2- and post-contrast T1-weighted images acquired at the portal venous and equilibrium phases.
Discussion
This case provides an example of a situation in which CT imaging showed indeterminate lesions, and subsequent contrast-enhanced MRI was needed to clarify the findings. MRI is superior to CT for lesion characterization due to better contrast resolution, especially on precontrast sequences, and also due to the fact that multiple postcontrast phases can be acquired since MRI is radiation-free compared to CT. Because generally only 2 postcontrast phases are acquired with CT, perfusion abnormalities and areas of shunting may be difficult to differentiate from tumors; conversely, the different phases of contrast enhancement available with MRI allow improved characterization.
Case 2
A 71-year-old Caucasian male with a long history of hepatitis C and cirrhosis was found to have liver tumors on triple phase CT. However, the exact location of these tumors was unclear, and it was not certain that tumors were present in either or both liver lobes; this prevented the ability for this patient to undergo surgery at this point because it was not clear that the entire tumor burden would be removed with partial resection. Therefore, MRI was used in order to better delineate the extent of tumor burden and to decide if the patient was a candidate for surgical resection or liver transplantation.
A 3T MRI was performed using gadoxetate disodium, a liver-specific agent with hepatocyte and biliary uptake, with a dynamic phase of enhancement. The protocol combined T1 in- and out-of-phase, fat-suppressed FSE T2, single shot T2 HASTE, diffusion imaging precontrast, and dynamic T1-weighted imaging before and after dynamic injection of contrast. Two arterial phases were performed, followed by a portal venous phase (1 min), equilibrium phase (3 min), and a delayed hepatocyte phase (at 10 and 20 min after contrast injection). The images demonstrated a large HCC in the left lobe with portal vein invasion, as well as multiple nodules in the right lobe (Figure 3). Based on the MRI findings, it was determined that the patient was outside transplant criteria and not a candidate for surgical resection, due to extensive tumor burden and portal vein invasion. The patient was then given the option of treatment with radioembolization.
Figure 3.
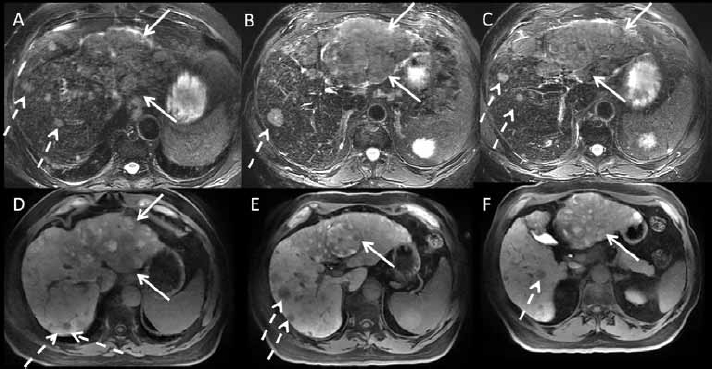
3 Tesla magnetic resonance imaging with liver-specific gadolinium contrast (gadoxetate disodium) in a patient with cirrhosis and hepatocellular carcinoma. (A, B, C): axial fat suppressed T2-weighted images. (D, E, F): hepatocyte phase T1 post-contrast images (20 min). There is a large infiltrative mass (arrows) of the left hepatic lobe, which is T2 hyperintense with heterogeneous signal on post-contrast hepatocyte phase images, with areas in low and high signal. Areas in high signal presumably represent well-differentiated hepatocellular nodules. In the right lobe, there are innumerable T2 hyperintense nodules (dashed arrows) with decreased gadoxetate disodium uptake on the hepatocyte phase, compatible with multifocal hepatocellular carcinoma. The patient is outside transplant criteria and cannot be resected, and will be treated with radioembolization.
Discussion
Gadoxetate disodium is currently approved by the FDA as a liver-specific contrast agent. Although this agent is administered at a quarter of the dose of extracellular contrast agents, the relaxivity of the agent is high and therefore produces acceptable contrast enhancement even with such a small dose. Unlike extracellular gadolinium agents, there is approximately 50% hepatocyte uptake, allowing for increased lesion detection using delayed images. When gadoxetate disodium is chosen as the contrast agent, the clinician must ensure that it is administered with the correct imaging protocol, which is slightly different from the one used with extracellular gadolinium agents.
Most HCCs appear typically hypointense on the delayed hepatocyte phase. However, well-differentiated HCCs may appear partially or completely iso- or hyper-intense compared to liver parenchyma, due to residual hepatocyte activity. In addition, hepatocyte uptake may be impaired in advanced cirrhosis and in biliary obstruction, making the contrast agent less useful. Therefore, gadoxetate disodium may not be the agent of choice in patients with decompensated cirrhosis or biliary obstruction.
Summary
MRI with the use of intravenous gadolinium contrast agents is extremely accurate for liver lesion detection and characterization. Liver MRI protocols combine the use of unenhanced sequences—such as T1 in-phase and out-of-phase, fat-suppressed T2, and diffusion-weighted imaging—with dynamic 3D contrast-enhanced T1-weighted sequences. One of the advantages of MRI over CT is that it can be used with a vast array of contrast agents for liver imaging. These agents include extracellular gadolinium chelates, such as gadopentetate dimeglumine, gadodiamide, gadoteridol, gadobenate dimeglumine (Gd-BOPTA), and gadoversetamide, which are the most frequently used agents, and liver-specific agents, including gadoxetate disodium (Gd-EOB-DTPA, which has approximately 50% liver uptake) and Gd-BOPTA (which has a 3–5% liver uptake). In addition, reticuloendothelial agents, such as superparamagnetic iron oxide particles, have been used by several groups, but they are currently not available in the United States. The choice of the contrast agent depends on availability, local expertise, and cost. There is considerable experience and literature on the use of extracellular agents for liver lesion detection and characterization.
Experience with liver-specific agents is growing. As in many other centers, we are increasingly using gadoxetate disodium as the first-line contrast agent for liver imaging, with a few exceptions. One exception is for patients with advanced liver disease (Child-Pugh C cirrhosis) or biliary obstruction. Due to decreased liver function (in cirrhosis) or cholestasis, the agent will not be taken up by hepatocytes as well as it would be in the normal liver. The second exception is for patients with suspected or known portal vein or hepatic vein occlusion, in whom we prefer the use of extracellular gadolinium agents, due to better dynamic venous phase. (As there is no increased liver signal, the vessels will appear bright with the extracellular agent and isointense to hypointense with gadoxetate disodium during the venous/equilibrium phases.) Additionally, at this time, gadoxetate disodium is more expensive compared with extracellular gadolinium agents, preventing its implementation in all liver MRIs used to screen and diagnose HCC. However, the value of gadoxetate disodium is especially evident in “problem-solving cases,” when it is necessary to differentiate between HCC and benign cirrhotic nodules, and to map liver metastases before surgery and/or local ablation.
Footnotes
Supported through an educational grant from Bayer Healthcare
Sponsored by Postgraduate Institute for Medicine
Disclosure of Conflicts of Interest:Postgraduate Institute for Medicine (PIM) assesses conflict of interest with its instructors, planners, managers, and other individuals who are in a position to control the content of CME activities. All relevant conflicts of interest that are identified are thoroughly vetted by PIM for fair balance, scientific objectivity of studies utilized in this activity, and patient care recommendations. PIM is committed to providing its learners with high-quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
The faculty reported the following financial relationships or relationships to products or devices they or their spouse/life partner have with commercial interests related to the content of this CME activity:
Dr. Elmar Merkle—Consulting fees: Bayer Healthcare, Bracco Pharmaceuticals, GE Healthcare; Fees for non-CME services: Bayer Healthcare; Contracted research: Siemens; Ownership interest: GE Healthcare Dr. Janio Szklaruk-Consulting fees: Bayer Healthcare; Fees for non-CME services: Bayer Healthcare
Dr. Bachir Taouli—Consulting fees: Bayer Healthcare; Contracted research: Bayer Healthcare
The following PIM planners and managers, Jan Hixon, RN, BSN, MA, Trace Hutchison, PharmD, Julia Kimball, RN, BSN, Samantha Mattiucci, PharmD, Jan Schultz, RN, MSN, CCMEP, and Patricia Staples, MSN, NP-C, CCRN, hereby state that they or their spouse/life partner do not have any financial relationships or relationships to products or devices with any commercial interest related to the content of this activity of any amount during the past 12 months.
Hazuki Aikawa: No real or apparent conflicts of interest.
Jacquelyn Matos: No real or apparent conflicts of interest.
Disclosure of Unlabeled Use:This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. Postgraduate Institute for Medicine (PIM), Gastroenterology & Hepatology, and Bayer Healthcare do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of PIM, Gastro-Hep Communications, or Bayer Healthcare. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer:Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient's conditions and possible contraindications or dangers in use, review of any applicable manufacturers product information, and comparison with recommendations of other authorities.
Funding for this case study compendium has been provided through an educational grant from Bayer Healthcare. Support of this monograph does not imply the supporter's agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporters, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.
Contributor Information
Elmar M. Merkle, Professor of Radiology Director of MR Imaging Medical Director, Center for Advanced MR Development Duke University Medical Center Durham, North Carolina.
Janio Szklaruk, Associate Professor Diagnostic Radiology UT MD Anderson Cancer Center Houston, Texas.
Bachir Taouli, Associate Professor of Radiology and Medicine Director of Body MRI Department of Radiology The Mount Sinai Medical Center New York, New York.


