FK 506 (Tacrolimus-Prograf) was first used clinically in February 1989.1 and approved by the United States Food and Drug Administration in April 1994 as a new immunosuppressive agent for use after liver transplantation.2-4 It has also been utilized as the primary immunosuppressive agent after heart.5 lung,6 intestinal7 and islet8 transplantation. In renal transplantation, it has been studied extensively as a primary and rescue agent in adults and children at a single center, the University of Pittsburgh9-12; it also has been studied as a primary agent in multicenter trials in Japan, Europe, and the United States.13,14 The most recent publications from the University of Pittsburgh have reported on a prospective, randomized trial comparing two FK 506-based regimens, with and without azathioprine.10 This report presents, for the first time, results with all 397 cases entered into this trial.
PATIENTS AND METHODS
Between August 1, 1991, and December 9, 1993, a total of 395 patients undergoing 397 renal transplants were entered into a prospective, randomized trial comparing FK 506/prednisone and FK 506/azathioprine/prednisone (Table 1). The details of randomization, the immunosuppressive protocols, and the inclusion and exclusion criteria have been previously described: the only additional exclusion criterion was for four patients who were undergoing their third or fourth transplant after October 11, 1992, and who received FK 506, prednisone, and 1 week of cyclophosphamide as part of a pilot trial. The mean recipient age was 44.6 ± 14.1 years (range 17.3 to 78.0). One hundred (25%) patients were undergoing retransplantation, and 53 (13%) had panel reactive antibody (PRA) levels greater than 40%. There were 72 (18%) recipients over 60 years of age at the time of transplantation, and 61 (15%) were either black (51% to 13%), Hispanic (6% to 2%), or Asian (4% to 1%). The most common cause of end-stage renal disease was diabetes mellitus, which accounted for 98 (25%) cases.
Table 1.
Recipient and Donor Demographics
| FK 506/Prednisone | FK 506/Aza/Pred | Total | |
|---|---|---|---|
| Patients | 198 | 197 | 395 |
| Transplants | 199 | 198 | 397 |
| Recipient age (yrs) | 43.6 ± 14.0 | 45.7 ± 14.1 | 44.6 ±14.1 |
| Range | (17.3–72.8) | (17.4–78.0) | (17.3–78.0) |
| First transplant | 147 (74%) | 150 (76%) | 297 (75%) |
| Retransplant | 52 (26%) | 48 (24%) | 100 (25%) |
| PRA ≥ 40% | 25 (13%) | 28 (14%) | 53 (13%) |
| Age ≥ 60 | 31 (16%) | 41 (21%) | 72 (18%) |
| Black | 23 (12%) | 28 (14%) | 51 (13%) |
| Donor age (yrs) | 35.3 ± 19.8 | 32.7 ± 21.2 | 34.0 ± 20.5 |
| Range | (0.1–69.0) | (0.1–75.0) | (0.1–75.0) |
| Cold ischemia time (hrs) | 31.6 ± 6.6 | 31.4 ± 9.4 | 31.5 ± 9.0 |
| Cadaver | 173 (87%) | 185 (93%) | 358 (90%) |
| Living donor | 26 (13%) | 13 (7%) | 39 (10%)* |
| En bloc | 19 (11%) | 31 (17%) | 50 (14%) |
| HLA match | 2.7 ± 1.4 | 2.5 ± 1.3 | 2.6 ± 1.3 |
| HLA mismatch | 3.0 ± 1.5 | 3.0 ± 1.4 | 3.0 ± 1.4 |
P < .04.
The mean donor age was 34.0 ± 20.6 years (range 0.1 to 75.0). There were 39 (10%) living donors. Fifty (14%) of the cadaveric cases were with pediatric en bloc kidneys from donors 3 years of age or younger. The mean cold ischemia time was 31.5 ± 9.0 hours. The mean number of HLA matches and mismatches was 2.6 ± 1.3 and 3.0 ± 1.4; there were 18 (5%) 6-antigen match, and 30 (8%) 0-antigen mismatch cases.
No significant differences existed between the double and triple therapy group with regard to the recipient and donor characteristics, with one exception; there were more living donor cases in the double therapy group (13% vs 7%, P = .03).
Statistical Analysis
The standard two-sample t test was used to test differences in means, while differences in proportions were tested using Pearson's chi-square test of association.
Patient survival was calculated from the date of kidney transplantation until death, and graft survival from the date of kidney transplantation until graft failure, retransplantation, or patient death. Survival curves were generated using the Kaplan-Meier (product-limit) method and were compared using the generalized Wilcoxon (Breslow) test. A multivariate Cox's regression analysis was performed using a stepwise procedure to identify high-risk patients for graft failure. A P-value less than .05 was considered statistically significant. All analyses were performed according to intention-to-treat, unless otherwise stated.
This trial was approved by the Institutional Review Board of the University of Pittsburgh.
RESULTS
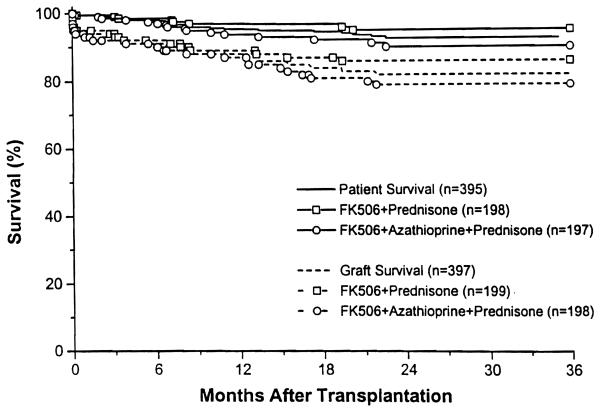
The mean follow-up was 20.0 ± 8.3 months (range 6.7 to 35.0). The overall 1- and 2-year actuarial patient survival was 95% and 93%. The overall 1- and 2-year actuarial graft survival was 89% and 83% (Fig 1). There was no difference in patient survival between the double and triple therapy groups, nor was there any difference in graft survival. For first cadaver transplants, the 1- and 2-year graft survival was 91% and 82%. Graft survival in selected subgroups is shown in Table 2. There was no difference in outcome between primary and retransplantation, low and high PRA patients, blacks and non-blacks, and patients under or over 60 years of age. Patients experiencing acute rejection after transplantation had a 1- and 2-year graft survival of 87% and 79%, respectively, while patients not experiencing rejection had a 1- and 2-year graft survival of 91% and 86% (P = NS).
Fig 1.
Actuarial patient and graft survival.
Table 2.
Actuarial Survival Data
| FK 506/Prednisone (%) |
FK 506/Aza/Pred (%) |
Total (%) |
|
|---|---|---|---|
| Patient survival | |||
| 1 yr | 97 | 94 | 95 |
| 2 yr | 95 | 90 | 93 |
| Graft survival overall | |||
| 1 yr | 90 | 88 | 89 |
| 2 yr | 86 | 79 | 83 |
| First Cadaver | |||
| 1 yr | 90 | 91 | 91 |
| 2 yr | 85 | 79 | 82 |
| Retranspiant | |||
| 1 yr | 88 | 77 | 83 |
| 2 yr | 86 | 75 | 80 |
| PRA ≥ 40% | |||
| 1 yr | 92 | 82 | 87 |
| 2 yr | 92 | 78 | 85 |
| Age ≥ 60 | |||
| 1 yr | 87 | 88 | 87 |
| 2 yr | 83 | 71 | 75 |
| Black | |||
| 1 yr | 96 | 82 | 88 |
| 2 yr | 84 | 66 | 74 |
| Rejection | |||
| 1 yr | 88 | 86 | 87 |
| 2 yr | 84 | 73 | 79 |
| No rejection | |||
| 1 yr | 92 | 89 | 91 |
| 2 yr | 89 | 84 | 86 |
| ATN | |||
| 1 yr | 80 | 76 | 78* |
| 2 yr | 76 | 64 | 71 |
| No ATN | |||
| 1 yr | 95 | 94 | 94 |
| 2 yr | 92 | 87 | 89 |
P< .0001.
A multivariate analysis was performed to identify specific factors associated with an increased risk of graft failure, and the only significant variable was the presence or absence of initial graft function. The 1- and 2-year actuarial graft survival for patients with initial function was 94% and 89%, respectively, while that for patients without initial function was 78% and 71% (relative risk 3.53, P < .0001. range 2.09 to 5.91).
The mean serum creatinine was 2.0 ± 1.1 mg/dL, and the calculated creatinine clearance was 56 ± 27 mL/min. The mean BUN was 32 ± 16 mg/dL. No differences existed between the two groups with regard to these parameters.
The incidence of acute rejection was 49%, and was higher in the double therapy than in the triple therapy group (54% vs 44%, P < .05). The incidence of steroid-resistant rejection requiring OKT3 or ATG was 10%, and again was higher in the double therapy group than in the triple therapy group (14% vs 7%, P < .04). A multivariate analysis revealed several risk factors for rejection, including double therapy (relative risk 1.68, range 1.10 to 2.55, P < .02); cadaveric donor (relative risk 2.18, range 1.06 to 4.50, P < .04); black recipient (relative risk 3.14, range 1.62 to 6.13, P < .001); PRA > 40% (relative risk 2.02, range 1.01 to 4.03, P < .03); and recipient age over 60 (relative risk 0.51, range 0.20 to 0.88, P < .02). In spite of their higher risk of rejection, and the reduction of this risk by triple drug therapy, there was a paradoxical trend (NS) to better results in black patients treated with the two drug regimen (Table 1).
The incidence of initial nonfunction and/or requirement for dialysis in the first week after transplantation was 35%, and this was not significantly different between the two groups.
The incidence of cytomegalovirus disease or infection was 16% and was not different between the double and triple therapy groups.
The incidence of posttransplant lymphoproliferative disorder (PTLD) was 1%. There were 5 cases of PTLD, 3 in the double therapy group, and 2 in the triple therapy group. All cases of PTLD resolved after reduction or cessation of immunosuppression and treatment with gancyclovir. One patient lost his allograft. In addition, there was one case of Kaposi's sarcoma in a patient on triple therapy who had been temporarily lost to follow-up. The lesion resolved after cessation of immunosuppression, but the allograft was eventually lost.
The incidence of initial new onset diabetes mellitus was 18%; over 40% of these patients were able to be weaned off insulin after the dosages of FK 506 and steroids were reduced, and the final incidence of new onset diabetes was 10%. There was no difference between double and triple therapy.
The mean FK 506 dosage was 0.15 ± 0.12 mg/kg per day at most recent follow-up, and the mean FK 506 plasma level was 0.83 ± 0.60 ng/mL. No significant differences existed between the double and triple therapy groups with regard to these parameters.
Forty-nine percent of successfully transplanted patients have been weaned off prednisone. The mean prednisone dose was 3.8 ± 5.2 mg/d; for those patients still on prednisone, the mean dose was 7.4 ±5.0 mg/d.
The mean azathioprine dose in the patients on triple therapy was 84 ± 41 mg/d, or 1.1 ± 0.5 mg/kg per day.
Thirty-nine (20%) patients randomized to double therapy had azathioprine added to their immunosuppressive regimen at one time or another because of rejection, and 33 (17%) have remained on triple therapy. A total of 111 (56%) patients randomized to triple therapy had azathioprine discontinued at one time or another, and 79 (40%) have remained on double therapy. The overall final incidence of crossover was thus 28%. Patient and graft survival after crossover were comparable between the two groups.
The mean serum cholesterol was 195 ± 49 mg/dL and was not different between the two groups.
Thirty-five percent of patients are off antihypertensive medications, and another 39% are taking one antihypertensive medication. There was no difference between double and triple therapy.
DISCUSSION
This paper presents data on the largest number of renal transplant recipients to date receiving FK 506 as the primary immunosuppressive agent. The data confirm and extend recent reports on the excellent outcomes achievable with this agent.10-12 One- and 2-year actuarial patient survival of 95% and 93%, and 1- and 2-year actuarial graft survival of 89% and 83%, respectively, have been obtained, and 49% of the successfully transplanted recipients have been weaned off prednisone. A previously reported trend toward inferior results under triple therapy was still evident,10 but this was no longer significant in the analysis of the larger group. The only difference associated with triple therapy was a slightly lower incidence of rejection, both steroid-sensitive and steroid-resistant. These data, together with the crossover incidence from double to triple therapy of 17%, suggest that there are a number of patients who can benefit from azathioprine as a third agent with FK 506. Unfortunately, both the high crossover incidence from triple to double therapy of 40% and the overall lack of improved patient or graft survival in the triple therapy group raise questions about the overall benefit of azathioprine with FK 506. We are at present evaluating the role of a short course of cyclophosphamide with FK 506 and steroids in an effort to abort cryptic or anamnestic antibody-mediated rejection, which is known to be nonresponsive to FK 506.9,15 As new immunosuppressive agents designed to replace azathioprine, such as Mycophenolate Mofetil,16 Brequinar,17 or other new agents, including Rapamycin18 and Leflunomide,19 become available, it will be worthwhile to evaluate them in combination with FK 506.
The toxicities of FK 506 have been well-described and include nephrotoxicity,20-24 neurotoxicity,25 and diabetoge-nicity;26 all of the side effects have been seen to a comparable degree with conventional cyclosporine-based regimens. For the same degree of toxicity, we have seen better outcomes under FK 506-based therapy than under cyclosporine-based regimens.
In summary, FK 506 is an effective immunosuppressive agent in patients undergoing kidney transplantation and is associated with 1- and 2-year actuarial patient survival of 95% and 93%, and 1- and 2-year actuarial graft survival of 89% and 83%, respectively. Also, 49% of successfully transplanted patients have been weaned off steroids. The addition of azathioprine resulted in comparable patient and graft survival, and somewhat less rejection, but with a need to discontinue the agent in 40% of cases. The effect of other third agents will be investigated as they become available.
ACKNOWLEDGMENTS
We would like to thank Regina Fenton, RN, BSN, CCTC; Loraine Kaminski, RN; Deborah Good, RN, BSN, CCTC; Holly Woods, RN, CCTC; Jareen Flohr, RN, BSN; Sue Bauder, RN; Janice Zagari, RN, BSN; Jennifer Ovesney, RN, BSN: Sharon Orlofske. RN; and Mark Paynter, RN, for their help with patient care; Janet Schmelzer for her assistance with data entry and organization: David Krakosky for his help with graph and slide preparation; Kate Carr for her help with slide preparation; Dolly Martin, Cynthia Eubanks, and Barbara Naples for their help in data collection; and Karen Toler for her assistance with typing the manuscript and table and slide preparation.
REFERENCES
- 1.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todo S, Fung JJ, Starzl TE. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung JJ, Abu-Elmagd K, Jain A, et al. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Fung JJ, Starzl TE, et al. Ann Surg. 1994;220:297. doi: 10.1097/00000658-199409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage JM, Kornos RL, Fung JJ, et al. Transplant Proc. 1991;23:3054. [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith BP, Bando K, Hardesty RL, et al. Transplantation. 1994;57:848. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todo S, Tzakis A, Reyes J, et al. Transplantation. 1994;57:840. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzakis AG, Ricordi C, Alejandro R, et al. Lancet. 1990;336:402. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Fung JJ, Jordan M, et al. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro R, Jordan ML, Scantlebury VP, et al. Transplantation. in press. [Google Scholar]
- 11.Shapiro R, Tzakis A, Scantlebury V, et al. J Am Coll Surg. in press. [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan M, Shapiro R, Vivas C, et al. Transplantation. 1994;57:860. doi: 10.1097/00007890-199403270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese FK 506 Study Group Transplant Proc. 1991;23:3085. [PubMed] [Google Scholar]
- 14.Japanese FK 506 Study Group Transplant Proc. 1993;25:649. [Google Scholar]
- 15.Murase N, Starzl TE, Demetris, et al. Transplantation. 1993;55:701. doi: 10.1097/00007890-199304000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sollinger HW, Deierhoi MH, Belzer FO, et al. Transplantation. 1992;53:428. doi: 10.1097/00007890-199202010-00031. [DOI] [PubMed] [Google Scholar]
- 17.Makowka L, Chapman F, Cramer DV. Transplant Proc. 1993;25:2. [PubMed] [Google Scholar]
- 18.Morris RE. Immunol Today. 1991;12:137. doi: 10.1016/S0167-5699(05)80040-4. [DOI] [PubMed] [Google Scholar]
- 19.Chong AS-F, Gebel H, Finnegan A, et al. Transplant Proc. 1993;25:747. [PubMed] [Google Scholar]
- 20.McCauley J, Takava S, Fung J, et al. Transplant Proc. 1991;23:1444. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Abu-Elmagd K, Tzakis A, et al. Transplant Proc. 1991;23:914. [PubMed] [Google Scholar]
- 22.Starzl TE. Transplant Proc. 1993;25:511. [PMC free article] [PubMed] [Google Scholar]
- 23.Demetris AJ, Banner B, Fung JJ, et al. Transplant Proc. 1991;23:944. [PMC free article] [PubMed] [Google Scholar]
- 24.Randhawa PS, Shapiro R, Jordan ML, et al. Am J Surg Pathol. 1993;17:60. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro R, Fung JJ, Jain AB, et al. Transplantation Proc. 1990;22(Suppl 1):35. [PMC free article] [PubMed] [Google Scholar]
- 26.Scantlebury V, Shapiro R, Fung JJ, et al. Transplant Proc. 1991;23:3169. [PMC free article] [PubMed] [Google Scholar]