Abstract
Sixty-two beagle dogs were given three doses of acetaminophen over a period of 24 hr in a fulminant liver failure model that is 70% lethal in 72 hr. Treatment of the animals with hepatic stimulatory substance alone or in a mixture with insulin, transforming growth factor-α and insulin-like growth factor II had no effect on mortality. Evidence of maximum regeneration with a mitotic index 20 to 25 times resting was the same in treated and untreated animals. Similarly, the biochemical and hematological indexes of liver injury were unaffected by therapy. These studies illustrate the futility of treating fulminant liver failure with exogenous growth factors that apparently are already present in large amounts in the natural response to liver injury. The results suggest that on-going liver injury by mechanisms other than lack of growth factors is the central problem of fulminant liver failure. If so, provision of regeneration-stimulating substance is an inappropriate therapeutic strategy.
A commonly proposed strategy for treatment of fulminant liver failure (FLF) has been the administration of factors that can initiate and sustain hepatocyte regeneration (1-4). In the last several years, purified growth factors to confirm or refute this hypothesis have become available (5). We report here the testing of the hepatic stimulatory substance (HSS) that is found in the livers of weanling rats or in the regenerating liver fragments after partial hepatectomy (6). The HSS was infused alone or in combination with other hepatic stimulatory factors into the portal veins of dogs in which FLF was caused by acetaminophen in 80% of untreated animals (7, 8).
MATERIALS AND METHODS
Chemicals
Acetaminophen and DMSO were purchased from Sigma Chemical Co., St. Louis, MO. Hepatocyte factors that have been shown to be stimulatory in our Eck’s fistula model for screening growth factors were transforming growth factor-α (Peninsula Laboratories Inc., Belmont, CA); human regular semisyntheic insulin (E.R. Squibb and Sons Inc., Princeton, NJ); insulin-like growth factor II (Collaborative Research, Inc., Bedford, MA); and HSS, produced in our laboratory with previously described methods (6). The HSS was purified 800,000 times from the cytosol of weanling rats (9) and had no known relation to hepatocyte growth factor of Zarnegar and Michalopoulos (10) and Nakamura et al. (11). We used doses of the continuously infused growth factors that were twice those shown with earlier testing in an Eck’s fistula model to give a maximum response (5). Murine (PCNA) monoclonal antibody (Signet Laboratory, Inc., Dedham, MA) was used to detect the protein cyclin, which appears on the nucleus of dividing cells.
Animals
Sixty-two beagles (Russel B. Hutton Farms, St. Thomas, P A) were housed in a large animal care facility and maintained at a constant temperature of 20° ± 1° C, with a 6 AM to 6 PM light cycle. The animals were quarantined and allowed to acclimatize in the animal facility for a minimum of 1 wk before being used. All dogs were given a standard dry dog chow and water ad libitum. Their body weights ranged from 9 to 14 kg.
Acetaminophen Intoxication
FLF was produced with three subcutaneous injections of acetaminophen in DMSO (7, 8) at a concentration of 600 mg/ml. The first dose (750 mg/kg) was at noon, the second (200 mg/kg) was 9 hr later and the third (200 mg/kg) was 24 hr after the initial injection. In the original validation of the methods, it was demonstrated that the methemaglobinemia and nephrotoxicity of the acetaminophen could be separated from the liver injury (7), performing multiple injections of drug. The death of all animals herein reported was caused by liver failure.
Blood or Serum Determinations
Multiple determinations of the serum or blood before and at various time intervals after acetaminophen administration were obtained. These determinations included levels of serum ALT, ammonia, albumin, bilirubin and cholesterol and the level of blood urea nitrogen. Amino acid profiles were determined with plasma deproteinized with 4% sulfosalicyclic acid. The resultant supernatant was applied to an amino acid analyzer (Beckman Instruments, Somerset, NJ), and the levels of free branched chain and aromatic amino acids (including tryptophan) were determined.
Three coagulation parameters were measured: fibrinogen, factor VII and factor X (12-15). In addition, the hematocrit and hemoglobinuria and methemoglobinemia (16) were measured at each time point.
Histological Conditions
All nonsurviving dogs underwent necropsies that included a full gross anatomic and microscopic examination of the liver, kidneys, lungs and heart. Tissues were fixed overnight in 10% neutral-buffered formalin, dehydrated, embedded in paraffin and cut into 5-μm sections. All sections were stained with hematoxylin and eosin for histological examination. Other specimens were used for hepatocyte proliferation study.
Hepatocyte Proliferation Measures
We determined an index of liver cell proliferation in a nonnecrotic area by counting the number of cyclin-labeled cells. PCNA/cyclin is a 36-kD nuclear protein that increases from the late G1 phase through the S phase of the cell cycle. The monoclonal antibody against PCNA/cyclin was used with the immunoperoxidase method. The positive-stained cells were counted per 1,000 cells (17-18).
Study Design
At laporatomy with dogs anesthetized with intravenous sodium pentobarbital, halothane and nitrous oxide, we inserted the tip of a 20-gauge catheter from ajejunal vein into the portal vein proximal to its bifurcation into right and left branches. The catheter was externalized by tunneling it through the body wall, and it was connected to a pump that delivered 20 ml saline every 24 hr. Five days later, the dogs were poisoned with acetaminophen by use of the protocol described above. Twenty hours after the acetaminophen injection, the animals were randomly divided into three groups to receive one of three different portal perfusion solutions (Table 1). Blood for analysis was obtained before the first acetaminophen injection at 24, 48 and 60 hr, subsequently. Liver specimens were obtained at necropsy, which was performed immediately after death or after euthanasia of animals that appeared to be dying.
Table 1.
Experimental groups and solutions infused
| Group | No. of animals |
Treatment |
|---|---|---|
| 1 | 20 | Vehiclea |
| 2 | 20 | HSS 100 ng/kg/day |
| 3 | 20 | HSS 100 ng/kg/day, insulin 0.4 U/kg/day, insulin-like growth factor II 100 ng/kg/day, transforming growth factor-α 100 ng/kg/day |
Saline modified by the addition of ammonium acetate 5 mmol/L and BSA 5 mg/L.
Statistical Analysis
Survival curves were generated for the three groups with the Kaplan-Meier method. Median survival times were compared with the generalized Wilcoxon (Breslow) test.
RESULTS
Clinical Behavior
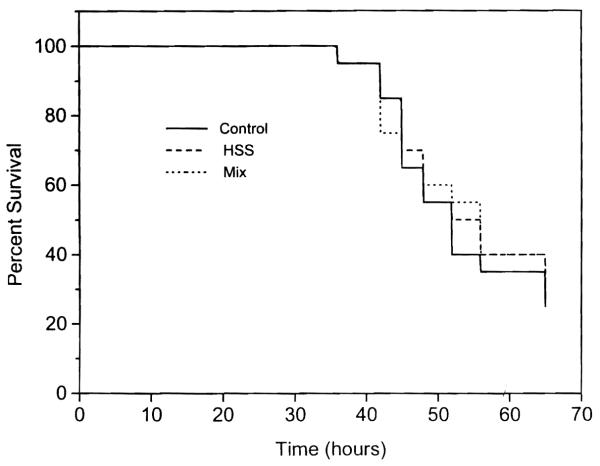
No animal died in the first 24 hr after acetaminophen administration. Two dogs that died immediately thereafter, without an increase in their serum level of transaminase but with very high levels of methemoglobinemia, were eliminated from the analysis. From 48 through 72 hr a progressive increase in mortality was observed in group 1 control animals, reaching 75% at 72 hr. These results were similar to those reported previously in untreated animals (7, 8). The survival rate in animals treated with HSS alone (group 2) or HSS plus a cocktail of other growth factors (group 3) was not significantly different from that in the group 1 controls (Fig. 1) (p = 0.934).
Fig. 1.
Survival rates of groups 1, 2 and 3 expressed in percentages.
All of the animals demonstrated a clinical picture consistent with FLF that paralleled the biochemical and histological data. Two different stages of the clinical course of these animals could be identified. The first stage was characterized by gastrointestinal signs and symptoms such as anorexia, vomiting and diarrhea. The second stage was characterized by the appearance of neurological symptoms that rapidly progressed to coma.
Biochemical and Hematological Changes
The evolution of biochemical (Table 2) and hematological abnormalities (Table 3) was similar in the control and treated animals of groups 2 and 3.
Table 2.
Biochemical features in nonsurviving dogs in groups 1, 2 and 3
| Groups | 0 hr | 24 hr | 48 hr | 60 hr | |
|---|---|---|---|---|---|
| 1 | |||||
| ALT (U/L) | 48 ± 5 | 105 ± 10 | 9,206 ± 3,100 | 24,218 ± 3,783 | |
| Bilirubin (mg/100 ml) | 1.2 ± 0.1 | 0.9 ± 0.2 | 1.8 ± 0.3 | 2.6 ± 0.8 | |
| Albumin (gm/100 ml) | 3.2 ± 0.4 | 2.6 ± 0.3 | 1.9 ± 0.1 | 1.8 ± 0.22 | |
| Ammonia (μmol/L) | 92 ± 15 | 310 ± 120 | 304 ± 60 | 625 ± 250 | |
| BCAA/AAA (μmol/L) | 3.14 | 0.88 | |||
| 2 | |||||
| ALT (U/L) | 45 ± 4 | 65 ± 6 | 8,140 ± 9,810 | 21,112 ± 6,250 | |
| Bilirubin (mg/100 ml) | 1.0 ± 0.1 | 0.8 ± 0.7 | 1.2 ± 0.12 | 2.0 ± 0.3 | |
| Albumin (gm/100 ml) | 3.0 ± 0.35 | 2.5 ± 0.3 | 2.0 ± 0.4 | 1.8 ± 0.18 | |
| Ammonia (μmol/L) | 86 ± 26 | 197 ± 2.8 | 270 ± 34 | 498 ± 121 | |
| BCAA/AAA (μmol/L) | 3.0 ± 0.45 | 0.94 ± 0.04 | |||
| 3 | |||||
| ALT (U/L) | 50 ± 5 | 78 ± 7 | 8,115 ± 3,325 | 20,083 ± 5,352 | |
| Bilirubin (mg/100 ml) | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.1 | 2.2 ± 0.25 | |
| Albumin (gm/100 ml) | 3.2 ± 0.5 | 2.8 ± 0.35 | 2.1 ± 0.35 | 1.9 ± 0.19 | |
| Ammonia (μmol/L) | 79 ± 23 | 186 ± 38 | 254 ± 62 | 465 ± 145 | |
| BCAA/AAA (μmol/L) | 2.9 ± 0.2 | – | – | 0.96 ± 0.07 | |
BCAA = branched chain amino acid; AAA = aromatic amino acid.
Table 3.
Coagulation profile in nonsurviving dogs of groups 1, 2 and 3
| Groups | 0 hr | 24 hr | 48 hr | 60 hr | |
|---|---|---|---|---|---|
| 1 | |||||
| Fibrinogen mg/100 ml | 294 ± 42 | 140 ± 32 | 45 ± 18 | 20 ± 3 | |
| Factor VII U/ml | 50.68 ± 0.9 | 0.80 ± 0.06 | 0.39 ± 0.02 | 0.20 ± 0.03 | |
| Factor X U/ml | 3.99 ± 0.3 | 0.74 ± 0.08 | 0.20 ± 0.02 | 0.19 ± 0.01 | |
| 2 | |||||
| Fibrinogen mg/100 ml | 300 ± 54 | 155 ± 28 | 60 ± 10 | 37 ± 6 | |
| Factor VII U/ml | 4.55 ± 0.9 | 0.89 ± 0.08 | 0.40 ± 0.06 | 0.20 ± 0.03 | |
| Factor X U/ml | 2.99 ± 0.8 | 0.90 ± 0.07 | 0.30 ± 0.04 | 0.21 ± 0.04 | |
| 3 | |||||
| Fibrinogen mg/100 ml | 320 ± 62 | 188 ± 30 | 60 ± 11 | 29 ± 8 | |
| Factor VII U/ml | 4.00 ± 0.7 | 0.90 ± 0.08 | 0.30 ± 0.04 | 0.28 ± 0.05 | |
| Factor X U/ml | 3.81 ± 0.2 | 0.96 ± 0.07 | 0.20 ± 0.03 | 0.25 ± 0.04 | |
Histological Examination and Hepatocyte Proliferation
Histological examination of livers obtained at the time of autopsy in dogs in groups 1, 2 and 3 demonstrated the same severe necrosis involving all of the lobes that we previously reported (7).
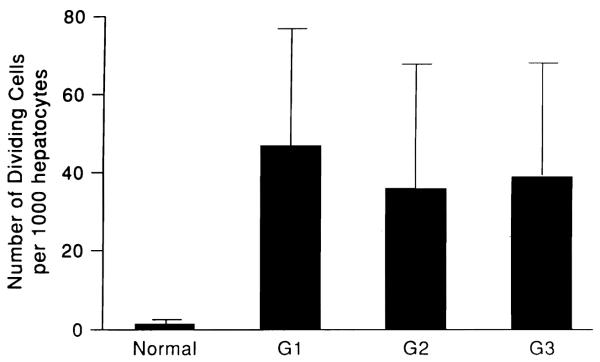
The number of cyclin-stained hepatocytes per 1,000 hepatocytes was 38 to 44 in all three groups, with no significant difference. This finding was 20 to 25 times higher than the number of hepatocytes in normal dog livers (1.5/1,000) (Fig. 2), which was approximately the same as the peak response in dogs after 70% hepatectomy (19) and three times greater than the maximal response to stimulation of the Eck’s fistula livers (5).
Fig. 2.
Number of dividing cells determined by immunocytochemical staining of PCNA/cyclin in the liver of the three groups of dogs and in the liver of normal dogs.
DISCUSSION
It has been estimated that recovery from FLF can be expected with 12% to 15% of the normal liver mass. The use of putative hepatic growth factors to achieve this critical functional level has been discouraging. After the demonstration that insulin is critical to a full expression of regeneration (20-23), this hormone alone (or usually with glucagon) has been tested for the treatment of FLF in mice (24), rats (25, 26) and human beings (27, 28). The animal studies, particularly in mice, have been encouraging, but the human trials have not shown a benefit from such treatment. The efficacy of noninsulin growth factors also has been controversial (29-30).
Although the number of putative growth factors has greatly increased in the last decade (5, 31, 32), evaluation of their therapeutic effectiveness for FLF has been limited by the excessive cost of testing them and the lack of discriminating models that are severe enough to preclude spontaneous recovery but not so severe that the liver injury produced cannot be reversed by any means.
The results described in this report could be an example of this quandary. In these experiments four of the most powerful known in vivo stimulators of hepatocyte proliferation were given in a mixture after liver injury by acetaminophen: HSS, transforming growth factor-α, insulin and insulin-like growth factor II. The rate of liver cell regeneration with this mixture, or when HSS was given as a single agent, was no greater than in the untreated animals; therefore the residual surviving cells were already maximally proliferating. Furthermore, the biochemical and hematological manifestations of liver injury were not ameliorated in either of the treatment groups nor was the mortality decreased.
The inability to demonstrate therapeutic efficacy of the growth factors could mean that their use is incorrectly conceived and therefore futile. A high rate of mitosis similar to that in our animals (33, 34) has been reported in human beings with FLF. Evidence in animals subjected to carbon tetrachloride injury (35) and in human beings with acute hepatitis (36-37) suggests that at least one growth promoter, hepatic growth factor, increases so much that it is difficult to imagine a benefit from exogenous supplementation of this and presumably other substances that modulate regeneration. Alternatively, our failure to see a therapeutic effect with a growth factor cocktail could mean that the proportions of the constituents were inappropriate.
However, it has been suggested recently that in FLF the inability of the liver to recover from injury may be a result of microcirculatory failure and consequential perpetuation of parenchymal injury, rather than a result of the inability of the hepatocytes to regenerate. This pathogenesis has been particularly evident in the ischemic injury of livers during preservation for use in transplantation (38). The primary cause of failure of these organs is damage to the sinusoidal endothelium that leads to capillary leak, complex coagulopathies and secondary damage to the hepatocytes by hypoperfusion (39). Protection of the microcirculation with prostaglandins (40) or measures that prevent diffuse intravascular coagulation (41) may be more appropriate than the provision of growth factors that already are present in excess.
Acknowledgments
Aided by Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland. This study has been supported by Consiglio Nazationale delle Richerche, ACRP Program, grant number 45-3-2.
REFERENCES
- 1.Saunders SJ, Hickman R, McDonald R, Terblanche J. The treatment of acute liver failure. In: Popper H, Schaffner F, editors. Progress in liver disease. IV. Grune and Stratton; New York: 1972. pp. 333–344. [PubMed] [Google Scholar]
- 2.Okuda K. Fulminant hepatic failure: a review. In: Picazo J, editor. Glucagon in gastroenterology and hepatology: pharmacological, clinical and therapeutic implications. MTP Press; Boston: 1982. pp. 127–140. [Google Scholar]
- 3.Kirsch RE, Cohen CD, Saunders SJ, van Hoorn-Hickman R, Terblanche J, Alp M. Acute liver failure. In: Wright R, Millward-Sadler GH, Alberti KGMM, Karran S, editors. Liver and biliary disease. 3rd ed. Bailliere Tindall/WB Saunders; London: 1985. pp. 659–676. [Google Scholar]
- 4.Gove CD, Hughes RD. Liver regeneration in relationship to acute liver failure. Gut. 1991;32:92–96. doi: 10.1136/gut.32.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francavilla A, Starzl TE, Porter K, Scotti-Foglieni C, Michalopoulos GK, Carrieri G, Trejo J, et al. Screening for candidate hepatic growth factors by selective portal infusion after canine Eck’s fistula. Hepatology. 1991;14:665–670. doi: 10.1016/0270-9139(91)90055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francavilla A, Ove P, Polimeno L, Coetzee M, Makowka L, Rose J, Van Thiel DH, et al. Extraction and partial purification of a hepatic stimulatory substance in rats, mice, and dogs. Cancer Res. 1987;47:5600–5605. [PMC free article] [PubMed] [Google Scholar]
- 7.Francavilla A, Makowka L, Polimeno K, Barone M, Demetris J, Prelich J, Van Thiel D, et al. A novel model for acetaminophen-induced fulminant hepatic failure in the dog. Gastroenterology. 1989;96:470–478. doi: 10.1016/0016-5085(89)91573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panella C, Makowka L, Barone M, Polimeno L, Rizzi S, Demetris J, Bell S, et al. Effect of ranitidine on acetaminophen-induced hepatotoxicity in dogs. Dig Dis Sci. 1990;35:385–391. doi: 10.1007/BF01537419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francavilla A, Barone M, Van Thiel DH, Mazzaferro V, Prelich J, Starzl TE. Further steps of HSS (hepatic stimulatory substance) purification. Dig Dis Sci. 1991;36:674–679. doi: 10.1007/BF01297037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarnegar R, Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989;49:3314–3320. [PubMed] [Google Scholar]
- 11.Nakamura T, Nawa K, Ichihara H, Kaise N, Nishino T. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987;224:311–316. doi: 10.1016/0014-5793(87)80475-1. [DOI] [PubMed] [Google Scholar]
- 12.Bontempo FA, Lewis JR, Van Thiel DH. The relation of preoperative coagulation finding to diagnosis, blood usage, and survival in adult liver transplantation. Transplantation. 1985;39:532–536. doi: 10.1097/00007890-198505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis JH. Coagulation defects. JAMA. 1961;178:1014–1019. doi: 10.1001/jama.1961.73040490010008. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JH. Hemostasis and hemorrhage. Sci Clin. 1972;1:1–66. [Google Scholar]
- 15.Lewis JH, Spero JA, Hasiba U. Bleeding disorders. Medical Examination Publishing; Garden City, New York: 1978. Diagnostic methods: laboratory tests; p. 22. [Google Scholar]
- 16.Van Assendelfp OW. Spectrophotometry of hemoglobin derivatives. CC Thomas; Assen, The Netherlands: 1970. pp. 128–130. [Google Scholar]
- 17.Bravo R, Celis JE. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Bioi. 1980;84:795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228–2234. [PubMed] [Google Scholar]
- 19.Francavilla A, Porter KA, Benichou J, Jones AF, Starzl TE. Liver regeneration in dogs: morphologic and chemical changes. J Surg Res. 1978;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Francavilla A, Halgrimson CG, Francavilla FR, Porter KA, Brown TH, Putnam CW. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Porter KA, Kashiwagi N, Putnam CW. Portal hepatotrophic factors, diabetes mellitus and acute liver atrophy, hypertrophy and regeneration. Surg Gynecol Obstet. 1975;141:843–858. [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 23.Starzl TE, Francavilla A, Porter KA, Benichou J. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;146:524–531. [PMC free article] [PubMed] [Google Scholar]
- 24.Farivar M, Wands JR, Isselbacker KJ, Bucher NL. Effect of insulin and glucagon on fulminant murine hepatitis. N Engl J Med. 1976;295:1517–1519. doi: 10.1056/NEJM197612302952706. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara K, Ogata I, Mishiro Y, Kyta Y, Oka Y, Takatsuki K, Sato Y, et al. Glucagon and insulin for the treatment of hepatic failure in dimethylnitrosamine-intoxicated rats. Scand J Gastroenterol. 1988;23:567–573. doi: 10.3109/00365528809093913. [DOI] [PubMed] [Google Scholar]
- 26.Minuk GY, Sherman TA, Shaffer EA, Kelly SR. A comparative study of the effects of insulin/glucagon infusions, parenteral amino acids and high dose corticosteroids on survival in a rabbit model of acute fulminant hepatitis. Hepatology. 1986;6:73–78. doi: 10.1002/hep.1840060114. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Shimizu M, Kosaka M. Nationwide statistics of severe hepatitis (fulminant hepatitis) [in Japanese] Sashin Igaku. 1979;34:2285–2288. [Google Scholar]
- 28.Harrison PM, Hughes RD, Forbes A, Portmann B, Alexander GJM, Williams R. Failure of insulin and glucagon infusion to stimulate liver regeneration in fulminant hepatic failure. J Hepatol. 1990;10:332–336. doi: 10.1016/0168-8278(90)90141-d. [DOI] [PubMed] [Google Scholar]
- 29.Francavilla A, DiLeo A, Polimeno L, Gavaler J, Pellicci R, Todo S, Karn I, et al. The effect of hepatic stimulatory substance, isolated from regenerating hepatic cytosol, and 50,000 and 300,000 subfractions in enhancing survival in experimental acute hepatic failure in rats treated with d-galactosamine. Hepatology. 1986;6:1346–1351. doi: 10.1002/hep.1840060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki M, Makowka L, Falk RE, Falk JA, Falk W, Venturi D. Reversal of lethal, chemotherapeutically induced acute hepatic necrosis in rats by regenerating liver cytosol. Surgery. 1983;94:142–144. [PubMed] [Google Scholar]
- 31.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990;4:176–187. [PubMed] [Google Scholar]
- 32.Fausto N. Hepatology: a textbook of liver disease. 2nd ed. WB Saunders; Philadelphia: 1990. Epetic regeneration; pp. 49–64. [Google Scholar]
- 33.Milandri M, Gaub J, Ranek L. Evidence for liver cell proliferation during fatal acute liver failure. Gut. 1980;21:423–427. doi: 10.1136/gut.21.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakita N, Seki S, Sakaguchi H, Yanai A, Kuroki T, Mizoguchi Y, Kobayashi K, et al. Analysis of proliferating hepatocytes using a monoclonal antibody against proliferating cell nuclear antigen/cyclin in embedded tissues from various liver diseases fixed in formaldehyde. J Pathol. 1992;140:513–520. [PMC free article] [PubMed] [Google Scholar]
- 35.Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. Hepatology. 1991;13:743–750. [PubMed] [Google Scholar]
- 36.Gohda E, Tsubouchi H, Makayama H, Hirono S, Takahashi K, Koura M, Hashimoto S, et al. Human hepatocyte growth factor in plasma from patients with fulminant hepatic failure. Exp Cell Res. 1986;166:139–150. doi: 10.1016/0014-4827(86)90514-8. [DOI] [PubMed] [Google Scholar]
- 37.Tsubouchi H, Niitani Y, Hirono S, Nakayama H, Gohda E, Arakaki N, Sakiyama O, et al. Levels of the human hepatocyte growth factor in serum of patients with various liver diseases determined by an enzyme-linked immunosorbent assay. Hepatology. 1991;12:1–5. [PubMed] [Google Scholar]
- 38.Clavien PA, Morgan GR, Sanabria JR, Petrunka C, Levy GA, Robert P, Havey C, et al. Effect of cold preservation on lymphocyte adherence in the perfused rat liver. Transplantation. 1991;52:412–417. doi: 10.1097/00007890-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 39.McKeown CMB, Edward V, Phillips MJ, Harvey PRC, Petrunka CN, Strasberg SM. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988;46:178–191. [PubMed] [Google Scholar]
- 40.Abecassis M, Falk JA, Makowka L, Dendzans VJ, Falk RE, Levy CA. 16,16 Dimethyl prostaglandin E2 prevents development of fulminant hepatitis and blocks the induction of monocyte/macrophage procoagulation activity after murine hepatitis virus strain 3 infection. J Clin Invest. 1987;80:881–889. doi: 10.1172/JCI113147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiwara K, Ogata I, Ohta Y, Hirata K, Oka Y, Yamada S, Sato Y, et al. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut. 1988;29:1103–1108. doi: 10.1136/gut.29.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]