Abstract
We studied seven patients aged 14 to 40 years who received living-related kidney transplants and had allograft survivals of 26 to 29 years. The blood urea and creatinine were either within normal limits or marginally elevated. Histopathologic examination showed only mild mesangial expansion, interstitial fibrosis, and arteriosclerosis. Immunoperoxidase staining with anti-HLA antibodies or in situ hybridization with a Y chromosome probe showed persistence of donor tubular epithelium and vascular endothelium within the graft. Recipient-derived glomerular cells were seen in one case, and interstitial lymphocytic infiltrates were seen in all cases. A review of the clinicopathologic data available for these cases indicated that both central and peripheral immunologic mechanisms contributed to the maintenance of prolonged graft survival. This extended survival was independent of six antigen matching, down-regulation of donor HLA antigen expression, and ingrowth of host epithelium/endothelium into the allograft.
Keywords: Kidney, transplantation, donor, recipient, Y chromosome
RENAL transplantation is currently a well-accepted modality for the treatment of end-stage renal disease. Sequential histopathologic examination of allografts during the first several years posttransplant frequently reveals progressive interstitial fibrosis, tubular atrophy, arteriosclerosis, and glomerulosclerosis.1-4 These changes are generically referred to as chronic allograft nephropathy and are believed to result from the combined interplay of multiple factors, such as rejection, drug toxicity, hypertension, pyelonephritis, mismatched nephron mass, and hemodynamic changes in renal blood flow. 5-8 Deterioration of renal function occurs pari passu with these changes. The current estimated 10-year graft survival is 42%.9 Approximately half of these patients do exceptionally well, and several original graft survivals exceeding 25 years have been reported.10,11 A pathologic evaluation of allografts maintaining good renal function for such long periods has never been reported. It is not known whether immunologic tolerance completely protects these kidneys from the development of chronic allograft nephropathy, or whether initial chronic changes subsequently stabilize or perhaps become offset by compensatory glomerular enlargement. It also is not known whether replacement of donor vascular endothelium and tubular epithelium in the graft by recipient cells plays a role in maintaining the prolonged graft tolerance observed in these cases. To address these questions we performed light microscopy, in situ hybridization for the Y chromosome, and immunohistochemistry using anti-HLA antibodies in seven allografts that have survived from 26 to 29 years. These seven patients are the subject of this report.
MATERIALS AND METHODS
The seven patients whose allografts were sampled were part of a larger cohort of 25-year survivors from the early years of a kidney transplantation program at the University of Colorado, Denver.1 One of the specimens was obtained at autopsy when the recipient of an uncle’s kidney died of a stroke. The other six were needle biopsy specimens obtained during a recent investigation of chimerism,12 in which donor- or host-derived cells were identified in peripheral tissues by their HLA specificity or sex chromosome. Because the nature of this investigation biased the case mix to HLA incompatibility, there were no examples of two HLA haplotype matches in this series. Five cases had one haplotype mismatch, one case had both haplotypes mismatched, and data were not available for the remaining case.
In four cases, the biopsy specimens were snap frozen and sectioned at 3 μm in a cryostat. The sections were fixed in cold acetone for 5 minutes, rinsed in phosphate-buffered saline (pH 7.4), and blocked with 1:10 normal goat or horse serum. This was followed by a 1-hour incubation with undiluted mouse anti-human HLA antibodies directed against selected donor or host specificities (Table 1). Multiple antibody combinations were used in each case to ensure reliable results. The antibodies were a gift from either Genetic Systems (Seattle, WA) or One-lambda (Los Angeles, CA), or were purchased from C-six (Mequon, WI). Some antibodies were derived from hybridomas obtained through the American Type Culture Collection (Rockville, MD). The secondary antibody was peroxidase-labeled horse or goat anti-mouse immunoglobulin. Color development was done using Biomeda’s Peroxidase Chromogen Kit (Foster City, CA). The sections were counterstained with hematoxylin-eosin for routine light microscopy. Negative controls consisted of sections incubated with anti-HLA antibodies of irrelevant specificity, or mouse immunoglobulin G (IgG) and IgM supernatants.
Table 1.
Anti-HLA Antibodies Used in Immunohistochemical Studies
| Case No. 1 | Case No. 2 | Case No. 3 | Case No. 6 | |
|---|---|---|---|---|
| Donor HLA phenotype | A2,–B18,27 Bw4,6 DR1,4 DQw1,3 |
A1,3 B35,44 Bw4,6 DR7,15 DQw1,2 |
A3,– B7,– Bw6– DR4,2 DQw1,3 |
A1,11 B57,62 BW4,6 DR4,7 DQ2,3 DRW53 |
| Recipient HLA phenotype |
A2,3 B7,27 Bw4,6 DR1,13 DQw1,– |
A3,26 B35,– BW6,– DR1,15 DQw1,– |
A24,29 B44,55 Bw4,6 DR4,7 DQw3,– |
A1,28 B35,57 BW4,6 DR4,15 DQ 1,3 |
| Anti-donor antibodies | B18 | A1,36 Bw4, B44 | A3 B7,40 | A11 B62 DR 7 DQ2 |
| Anti-recipient antibodies | A2,69* B7,40 | A26 DR1,10 | B7,22/55,27* | B35 |
| Control antibodies | A1,36 A1,9,10,11 | A2,69 B7,22,17 B13 | A2,69 A2,28 | DR1,10 B18,51 |
Antibodies directed to specificities shared by donor and recipient.
Antibodies suitable for discriminating between donor and recipient specificities were not available in cases no. 4 and 5. In case no. 6 antibodies selected for their donor specificity cross-reacted with recipient lymph node and skin, rendering interpretation of the immunohistochemical preparations difficult. Sex mismatch between donor and recipient made it possible to circumvent these difficulties in cases no. 4 and 5: the donor and host genotypes in these two cases could be distinguished using in situ hybridization with a nucleic acid probe for the Y chromosome (Oncor, Gaithersberg, MD). The procedure used deparaffinized sections of formalin-fixed tissue and began with a 40-minute digestion of tissue sections at 37°C using 6 mg/mL pepsin in O.12 N HCL. Following three 1-minute rinses in 2 × sodium chloride sodium citrate buffer (SSC) and dehydration, 2 μL biotinylated probe (dissolved in 10 μL Hybrizol VIII [Oncor, Gaithersberg, MD]) were applied to each tissue section. Subsequent steps included denaturation (at 90.5°C for 12 minutes), hybridization (at 37°C overnight), postwash in 2 × SSC-50% formamide (at 37°C for 15 minutes two times), and washing with 0.1 × SSC (at 37°C for 15 minutes two times) followed by 1 × phosphate buffer detergent (PBD) (at 25°C for 5 minutes). Detection of the hybridized target involved sequential incubation with blocking reagent 1, fluorescein-labeled avidin, 1 × PBD, blocking reagent 2, and anti-avidin antibody, and then a second round of blocking reagent 1 and fluorescein-labeled avidin, following the manufacturer’s instructions. The preparations were mounted in a propidium iodide antifade solution and examined under the oil immersion lens of a Nikon Optiphot 2 immunofluorescence microscope. The Y chromosomes were visualized as a bright green nuclear signal against a red background.
Case no. 7 was a sex-matched recipient who could not be analyzed by the Y chromosome probe. Snap-frozen tissue was not available for immunohistochemical studies with anti-HLA antibodies.
RESULTS
The clinical data (Table 2) for patients no. 1, 2, 3, and 4 have been previously published in a report focusing on the functional immunologic parameters and chimeric status of these individuals.12 All cases received a kidney allograft from a living-related donor aged 22 to 58 years. Immunosuppression consisted of 0 to 100 mg/d azathioprine and 0 to 10 mg/d prednisone maintained at the levels indicated in Table 2. Patient no. 3 has been off antirejection drugs for the past 4 months. Renal function, as assessed by blood urea nitrogen and creatinine, was either normal or marginally compromised.
Table 2.
Clinical Data on Living-Related Kidney Transplant Recipients With Long-term Graft Survival
| Case No. |
Age at Time of Transplantation (yr) |
Sex | Donor | Donor Age (yr) |
Follow-up (yr) |
Allograft Age (yr)* |
AZA (mg) |
PRED (mg) |
BUN (mg/dL) |
Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | F | Mother | 41 | 29 | 70 | 100 | 0 | 15 | 1.2 |
| 2 | 14 | F | Mother | 36 | 29 | 65 | 75 | 0 | 6 | 0.7 |
| 3 | 19 | M | Aunt | 58 | 28 | 86 | 0 | 0 | 19 | 1.6 |
| 4 | 19 | F | Father | 53 | 28 | 81 | 0 | 7.5 | 13 | 0.9 |
| 5 | 24 | M | Sister | 22 | 27 | 49 | 100 | 10 | 21 | 1.0 |
| 6 | 18 | F | Mother | 38 | 28 | 66 | 50 | 7.5 | 21 | 1.8 |
| 7 | 40 | M | Uncle | 46 | 26 | 72 | 0 | 10 | NA | 3.0 |
Abbreviations: AZA, daily dose of azathioprine; PRED, daily dose of prednisone; BUN, blood urea nitrogen; NA, not available.
Age of donor at time of transplantation + years of recorded allograft function in recipient.
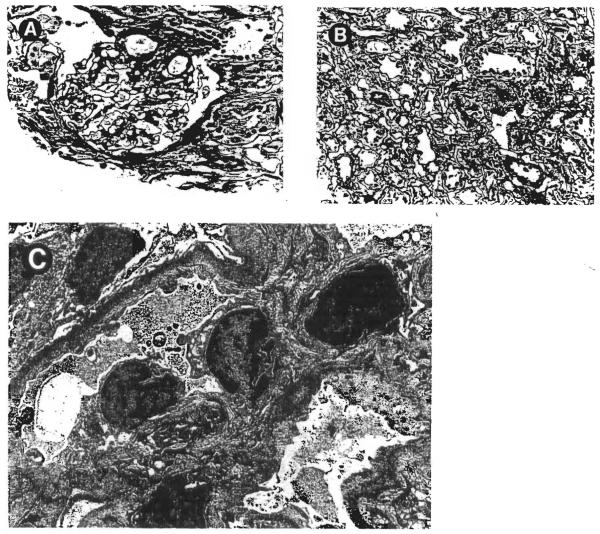
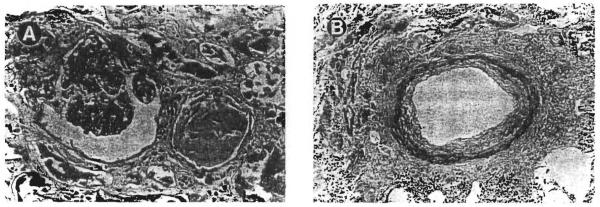
The histopathologic alterations seen in these cases were in the borderline or mild chronic allograft nephropathy categories, as defined in the Banff Working Classification of Kidney Transplant Pathology 4 Representative illustrations are provided in Figs 1 and 2. The interstitial compartment showed sparse to mild lymphocytic infiltrates, occasionally arranged in small clusters, without significant tubulitis. Mild interstitial fibrosis was seen in cases no. 4 and 7 (Fig 2A), but in other biopsy specimens, the renal tubules were quite closely packed together with little expansion of the sampled extracellular matrix (Fig 1B). The glomeruli showed either minor abnormalities (Fig 1A) or mild lobular accentuation, increased mesangial cellularity, and sclerosis of up to 10% of the total glomeruli (Fig 2A). Peritubular capillaries, interstitial venules, and arterioles were without significant pathologic change. The arcuate-sized vessels, when sampled, usually showed mild intimal thickening (Fig 2B). Electron microscopy could be performed in case no. 5. The subendothelial zone was mildly expanded by a granular electron lucent material consistent with early transplant glomerulopathy (Fig 1C).
Fig 1.
Morphologic alterations observed in a needle biopsy of the renal allograft in case no. 5. (A) The glomerulus is essentially unremarkable. (Magnification ×200.) (B) Minimal interstitial fibrosis was noted. (Magnification ×200.) (C) Electron microscopy showed a finely granular electron-lucent material deposited, at a subendothelial location, within the glomerular basement membranes. (Magnification ×5,400.)
Fig 2.
Case no. 7 showed more pronounced histopathologic changes than those illustrated in Fig 1. (A) The glomeruli showed an accentuation of the lobular pattern and a segmental increase in the mesangial cellularity. The degree of interstitial fibrosis was greater than that observed in case no. 5. (Magnification ×200.) (B) The arteries showed fibrous thickening of the intimal layer. (Magnification ×100.)
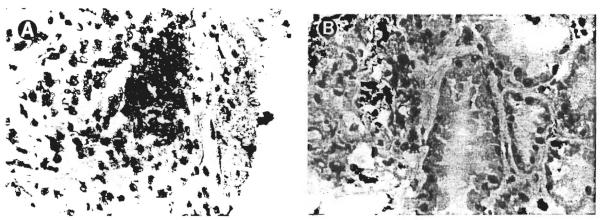
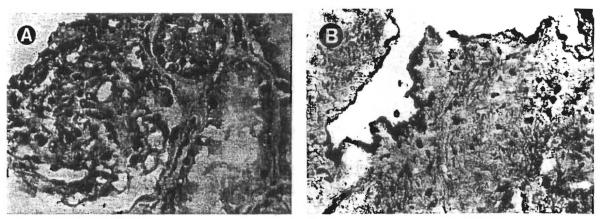
Immunohistochemical studies demonstrated the mononuclear cells infiltrating the interstitium to mark for recipient HLA antigens (Fig 3A). The endothelial lining of the peritubular and glomerular capillaries reacted strongly with anti-HLA antibodies of donor specificity (Fig 4). The tubular epithelium did not convincingly stain with any of the HLA antibodies tested, but hybridized with the Y sex chromosome probe. The Y chromosome was also demonstrable in the glomeruli and vascular endothelium. In case no. 1 the glomeruli contained numerous cells bearing recipient HLA antigens. Some of these cells seemed to line the glomerular capillary tufts while others appeared to be mesangial in location (Fig 3B).
Fig 3.
(A) Demonstration of recipient-specific HLA A3 antigen expression by the interstitial mononuclear cells in case no. 1. (Magnification ×200.) (B) This patient was unusual in that some cells with the recipient phenotype had also infiltrated the glomeruli. (Magnification ×200.)
Fig 4.
Needle biopsy of the allograft kidney in case no. 1 stained with the anti-HLA A2,69 antibody. Cells in the glomeruli (A) as well as the vascular endothelium (B) retained donor specificity 29 years after transplantation. (Magnification ×200.)
DISCUSSION
The cases reported here have some of the longest graft survivals ever documented in the history of kidney transplantation.11 All patients except no. 7 were younger than 25 years at the time of transplantation. The mildness of the histopathologic changes seen is remarkable; the chronologic age of the allografts at last biopsy was 49 to 86 years (Table 2), and one might have expected more prominent age-related glomerulosclerosis, interstitial fibrosis, and arteriosclerosis in these tissues. The mild glomerular alterations observed are similar to those reported in these and other well-functioning grafts biopsied 7 to 27 months posttransplant.1 It is possible that senile changes in the kidney, instead of being preprogrammed within the organ itself, occur concomitantly with generalized metabolic changes in the individual as a whole. This would explain our observation that older kidneys transplanted into younger individuals involuted at a rate apparently determined by the age of the recipient. It must, however, be pointed out that the rate of progression of age-related changes varies considerably from individual to individual. Definitive conclusions on the mechanisms of renal allograft aging should await prospective studies based on a large series of patients not specifically selected for prolonged graft survival.
Our experience illustrates that continuous azathioprine administration for up to 29 years does not in itself lead to any morphologic change attributable to the drug. The relative resistance of the kidney to clinically significant azathioprine toxicity is well known, but actual histopathologic examination of allografts maintained on this drug for such a prolonged period has not been previously reported. Azathioprine has only occasionally been implicated in interstitial nephritis.13 In contrast, the long-term administration of cyclosporine (which was not used in any of these patients) results in interstitial fibrosis and arteriolar hyalinosis even if plasma drug levels are maintained in a therapeutically acceptable range. 5,6
The specific mechanisms associated with the prolonged graft survival observed in this study are not clear. Six-antigen HLA matching was not achieved in any of the donor-recipient pairs. Downregulation of endothelial class I antigen expression is also excluded as a significant mechanism by the data presented: all biopsy specimens examined showed strong staining with the appropriate donor-specific antibodies. In the past, replacement of donor vascular endothelium and tubular epithelium by recipient cells was conceptualized as a possible mechanism of tolerance. Studies seeking to test this hypothesis documented partial replacement in a small percentage of cases, but the clinical follow-up was relatively short.14-16. The patients reported here, who had a much longer period of observation, confirm that graft tolerance is not predicated on renal cell turnover occurring after transplantation. The glomeruli in one case did show endothelial/mesangial cells of recipient origin, but the bulk of the vascular and renal tubular epithelium retained donor phenotype. The limited replacement observed probably followed injury to the original donor cells during an episode of acute rejection. Seeding of the allograft glomeruli by recipient cells could have been mediated by circulating mononuclear/endothelial progenitor cells or by migration of recipient cells across the surgical anastomosis sites. The former mechanism is favored after heart transplantation, since areas of recipient mediated re-endothelialization tend to be close to previous biopsy sites.17 Partial replacement of allograft coronary arteries by recipient myointimal cells also has been reported.18
Influx of scattered recipient lymphocytes into the graft interstitium was seen in all cases studied. The absence of associated tubulitis or vascular injury may be due to a variety of modulating influences, such as effector cell blockade, suppressor cells, or antibody-mediated tolerance.19 Concomitantly with the migration of recipient lymphocytes into the allograft, small numbers of donor-derived lymphocytes could be detected in recipient lymph nodes and skin of several cases.12 The importance of the development of such a chimeric state in ensuring prolonged graft survival is reinforced by parallel observations made by us in liver and small intestine transplant recipients.20 The actual mechanism by which this low-level chimerism exerts its effects is currently unknown. Mutual engagement, activation, and ultimately clonal silencing of the immunocytes of both parties seems to occur. Mixed lymphocyte reactivity assays between donor peripheral blood lymphocytes (stimulator cells) and the corresponding recipient cells (responder cells) are depressed in long-term survivors of kidney transplantation.12 Furthermore, donor cells in these cases fail to generate cytotoxic effector cells when cultured with host lymphocytes in vitro. Thus, there is evidence that prolonged graft survival after solid organ transplantation is the result of tolerogenic mechanisms at both peripheral (intragraft) and central (lymph node, peripheral blood) levels.
ACKNOWLEDGMENT
The authors thank Joyce Marcoz for fine secretarial assistance.
Supported by the Pathology Education and Research Fund, Pittsburgh, PA, and by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Porter KA, Dossetor JB, Marchioro TL, Peart WS, Rendall JM, Starzl TE, Terasaki PI. Human renal transplants I. Glomerular changes. Lab Invest. 1967;16:153–181. [PubMed] [Google Scholar]
- 2.Maryniak RK, First MR, Weiss MA. Transplant glomerulopathy. Evolution of morphologically distinct changes. Kidney Int. 1985;27:799–806. doi: 10.1038/ki.1985.83. [DOI] [PubMed] [Google Scholar]
- 3.Busch GJ, Galvanek EG, Reynolds ES. Human renal allografts—Analysis of lesions in long-term survivors. Hum Pathol. 1971;2:253–298. doi: 10.1016/s0046-8177(71)80037-0. [DOI] [PubMed] [Google Scholar]
- 4.Solez K, Axelson RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Keown PA, Marcussen N, Mihatsch MJ, Morozumi K, Myers BD, Nast CC, Olsen S, Racusen LC, Ramos EL, Rosen S, Sachs DH, Salomon DR, Sanfilippo F, Verani R, von Willebrand E, Yamaguchi Y. International standardization of criteria for the histologic diagnosis of rejection: The Banff Working Classification of Kidney Transplant Pathology. Kidney Int. 1993;44:411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 5.Porter KA. Renal transplantation. In: Heptinstall H, editor. Pathology of the Kidney. Little, Brown; Boston, MA: 1992. pp. 1799–1934. [Google Scholar]
- 6.Croker BP, Salomon DR. Pathology of the renal allograft. In: Tisher CC, Brenner BB, editors. Renal Pathology With Clinical and Functional Correlations. Lippincott; Philadelphia, PA: 1989. pp. 1518–1554. [Google Scholar]
- 7.Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease. N Engl J Med. 1982;307:652–660. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, Cohen RA, Milford EL. In renal transplantation, one size may not fit all. J Am Soc Nephrol. 1992;3:162–169. doi: 10.1681/ASN.V32162. [DOI] [PubMed] [Google Scholar]
- 9.Terasaki P, Mickey MR, Iwaki Y, Cicciarelli J, Cecka M, Cook D, Yuge J. Long-term survival of kidney grafts. Transplant Proc. 1989;21:615–617. [PubMed] [Google Scholar]
- 10.Starzl TE, Schroter GPJ, Hartmann NJ, Barfield N, Taylor P, Mangan TL. Long-term (25-year) survival after renal homotransplantation—The world experience. Transplant Proc. 1990;22:2361–2365. [PMC free article] [PubMed] [Google Scholar]
- 11.Graver B. World transplant records. In: Terasaki PI, Cecka JM, editors. Oinical Transplants. UCLA Press; Los Angeles, CA: 1991. pp. 431–434. [Google Scholar]
- 12.Starzl TE, Demetris AJ, Trucco M, Zeevi A, Ramos H, Terasaki P, Rudert WA, Kocova M, Ricordi C, Idlstad S, Murase N. Chimerism and donor specific non-reactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos EL, Tolney NL, Ravenscraft MD. Clinical aspects of renal transplantation. In: Brenner BM, Rector FC, editors. The Kidney. Saunders; Philadelphia, PA: 1991. pp. 2366–2367. [Google Scholar]
- 14.Sinclair RA. Origin of endothelium in human renal allografts. BMJ. 1972;4:15–16. doi: 10.1136/bmj.4.5831.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedmak DD, Sharma HM, Czajka CM, Ferguson RM. Recipient endothelialization of renal allografts: An immunohistochemical study utilizing blood group antigens. Transplantation. 1988;46:907–909. [PubMed] [Google Scholar]
- 16.Andersen CB, Ladefoged DS, Larsen S. Cellular inflammatory infiltrates and renal cell turnover in kidney allografts: A study using in-situ hybridization and combined in-situ hybridization and immunohistochemistry with a Y-chromosome-specific DNA probe and monoclonal antibodies. APMIS. 1991;99:645–652. doi: 10.1111/j.1699-0463.1991.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell JB, Renlund DG, Bristow MR, Hammond EH. Detection of allograft endothelial cells of recipient origin following ABO-compatible, nonidentical cardiac transplantation. Transplantation. 1991;51:438–442. doi: 10.1097/00007890-199102000-00033. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy LJ, Weissman IL. Dual origin in cardiac allograft arteriosclerosis. N Engl J Med. 1971;285:884–887. doi: 10.1056/NEJM197110142851603. [DOI] [PubMed] [Google Scholar]
- 19.Siskind GW. Immunologic Tolerance. In: Paul WE, editor. Fundamental Immunology. Raven; New York, NY: 1984. pp. 537–553. [Google Scholar]
- 20.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ, Nalesnik M, Zeevi A, Rudert WA, Kocova M. Cell migration and chimerism after whole organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]