Abstract
Bone marrow (BM)-derived dendritic cells (DC) are the most potent known antigen (Ag) presenting cell in vivo and in vitro. Detailed analysis of their properties and mechanisms of action requires an ability to produce large numbers of DC. Although DC have been isolated from several rat tissues, including BM, the yield is uniformly low. We describe a simple method for the propagation of large numbers of DC from rat BM and document cell yield with the rat DC marker, OX-62.
After depletion of plastic-adherent and Fc+ cells by panning on dishes coated with normal serum, residual BM cells were cultured in gelatin coated flasks using murine rGM-CSF supplemented medium. Prior to analysis, non-adherent cells were re-depleted of contaminating Fc+ cells. Propagation of DC was monitored by double staining for FACS analysis (major histocompatibility complex (MHC) class II+/OX-62+, OX-19−). Functional assay, morphological analysis and evaluation of homing patterns of cultured cells revealed typical DC characteristics. MHC class II and OX-62 antigen expression increased with time in culture and correlated with allostimulatory ability. DC yield increased until day 7, when 3.3 × 106 DC were obtained from an initial 3 × 108 unfractionated BM cells. Significant numbers of DC can be generated from rat BM using these simple methods. This should permit analysis and manipulation of rat DC functions in vivo and in vitro.
Keywords: Dendritic cell, Bone marrow, GM-CSF, OX-62, (Rat)
1. Introduction
Bone marrow (BM)-derived dendritic cells (DC) are the most potent stimulator cells of primary mixed leukocyte reactions (MLR) and also serve as powerful antigen-presenting cells in the priming of CD4+ T cells in vivo (Steinman, 1991; Steinman and Cohn, 1973). Furthermore, based on the recent appreciation of donor hematolymphoid cell chimerism persisting up to 30 years after kidney or liver transplantation (Starzl et al., 1992, 1993b), donor-derived cells with DC characteristics have been implicated in the induction of tolerance after solid organ transplantation (Demetris et al., 1993; Starzl et al., 1992, 1993b). Although a hypothesis by which DC might mediate the induction of allograft tolerance has been proposed (Demetris et al., 1994; Starzl et al., 1993a) (based on the permissive effect of immunosuppressants on leukocyte migration between graft and host), a definite mechanism has not yet been established.
The investigation of mechanisms by which DC function, both in vivo and in vitro, requires effective culture of these cells, with production of large numbers for analysis. With the availability of recombinant (r) murine GM-CSF, several reports have shown that it is possible to grow large numbers of DC from murine (Inaba et al., 1992a; Scheicher et al., 1992) and human (Reid et al., 1992) bone marrow (BM) and this cytokine has been used to promote the survival of lymph-borne DC for up to 72 h in the rat (MacPherson, 1989; MacPherson et al., 1989). Cytokines have not been used to enhance DC yield from fresh rat bone marrow cultures, even though the species is used extensively to investigate many immunological phenomena.
In this report, we describe a simple and highly reproducible technique for the propagation of rat BM-derived DC. Phenotypic analysis of these cells was enhanced by the use of OX-62, the new murine monoclonal antibody directed against an integrin-like rat DC surface marker (Brenan and Puklavec, 1992). This method utilises several of the previously described properties of rat DC precursors to maximise the yield of these cells in culture, name1y their lack of adherence to plastic flasks (Klinkert et al., 1980, 1982), FcR status (Klinkert et al., 1980, 1982) and their partial dependence on murine rGM-CSF (MacPherson, 1989; MacPherson et al., 1989). In addition to these measures, tissue culture flasks were coated with gelatin to augment DC growth (in a fashion analogous to the use of type 1 collagen to promote the maturation of liver-derived DC (Lu et al., 1994)). Using this approach, bulk cultures of DC can readily be generated from rat BM, as documented by their morphology, functional ability in mixed lymphocyte reactions (MLR) and their expression of MHC class II (Banuls et al., 1993; Bowers and Berkowitz, 1986; Hart and Fabre, 1981) and the DC-specific surface marker, OX62 (Brenan and Puklavec, 1992).
2. Materials and methods
2.1. Collection of rat bone marrow
7–9-week-old Lewis rats (LEW; RT11), purchased from Harlan Sprague Dawley (Indianapolis, IN) and maintained in conventional animal facilities, were used as BM cell donors. After killing, using methoxyflurane inhalational anesthesia (Pitman-Moore, Mundelein, IL), BM cells were removed from the femurs and tibias and washed twice with complete medium (RPMI 1640 medium containing 5% FBS, 5 µg/ml of gentamicin, 2 mM l-glutamine and 10 mM Hepes buffer). The medium and all supplements were purchased from Gibco (Life Technologies, Grand Island, NY).
2.2. Coating of tissue culture flasks with gelatin
A 1% solution of gelatin (cell culture tested, 300 bloom porcine skin gelatin; purchased from Sigma Cell Culture Reagents, St. Louis, MO) in PBS was used to coat tissue culture flasks at room temperature for 2 h (2 ml/flask, allowed to air-dry). Flasks were washed twice with PBS before use for BM culture.
2.3. Depletion of Fc + and plastic-adherent (FcPA) BM cells
To optimise the variety of Fc receptor binding sites with which to deplete FcPA cells, petri dishes (100 × 15 mm, Falcon #1029; Becton-Dickinson, Palo Alto, CA) were coated overnight at 4°C with a mixture of 10% normal goat serum and 5% normal human AB serum in PBS. After washing twice with PBS to remove any excess serum, 50 × 106 BM cells, suspended in 6 ml complete medium, were added to each petri dish and incubated at room temperature for 1 h with gentle swirling at 30 min. The non-adherent FcPA depleted cells were gently removed and the dishes were subjected to two further gentle washes to remove any remaining non-adherent cells. The pooled non-adherent cells were then used for culture. Using the same protocol, non-adherent cells removed from cultures were re-depleted of FcPA cells before functional assay and phenotypic analysis, as indicated below.
2.4. Propagation of DC from rat bone marrow
Approximately 120 × 106 FcPA depleted BM cells were cultured at a concentration of 3 × 106/ml in each 75 cm2 Falcon tissue culture flask, with or without gelatin coating. Complete medium, further supplemented with 10% FBS, 5 × 10−5 M 2-ME, 0.5 µg/ml of NMA (N-γ-monomethyl-l-argine, Schweizerhall, Piscataway, NJ) and an optimal concentration of mouse rGM-CSF (0.4 ng/ml; R&D System, Minneapolis, MN), was used as a culture medium. Cultures were fed every 2nd day by exchanging half the medium for fresh rGM-CSF containing medium, without discarding any cells (medium being exchanged was centrifuged and any cells were restored to the culture flask). In some experiments, rGM-CSF was further supplemented with recombinant human TNF-α (50 U/ml; Genzyme, Cambridge, MA).
2.5. Mixed leukocyte reactions (MLR)
Lymph node cells from syngeneic LEW (RTll) and allogeneic ACI (RTla) and PVG (RTlc) rats (Harlan Sprague Dawley) were used as responders. DC-rich cultured BM cells and fresh unfractionated LEW spleen cells (positive controls) were used as stimulators. One way MLR were performed in U-shaped 96-well microtest plates (Falcon #3077). A constant number of responders (9 × 104/well) was used against various numbers of γ-irradiated (20 Gy) stimulator cells in each assay. Stimulator to responder ratios ranged from 3:1 to 1:729 and with this system the peak response occurred between days 3 and 4. Each MLR was labelled with 1 µCi/ml final concentration of [3H]thymidine (2 Ci/mM; New England Nuclear, Beverly, MA) for 7 h prior to harvesting with an automatic harvester (Skatron, Lier, Norway). Thymidine incorporation was determined using a liquid scintillation counter (1205 Betaplate, Wallac, Gaithersburg, MD). Results are expressed as mean counts per minute (cpm) ± 1 standard deviation.
2.6. Immunofluorescence labelling and FACS analysis
DC yield from BM cultures was monitored by double staining with the mAbs OX-6 and OX-62 using flow cytometric analysis. Throughout these experiments, cultured DC were confirmed to be OX-19− to avoid the possibility of any cross-reaction between OX-62 and a γ/δ T cell population. OX-6 (MHC class II), OX-19 (pan-T cell), ED-1 (monocyte/macrophage) and ED-2 (a monocyte subset) mAbs were purchased from Accurate Chemical and Scientific Corp., Hicksville, NY. OX-62 (rat DC) was a generous gift donated by M.J. Puklavec (Serotec, Oxford, UK). Double immunofluorescence staining was performed as previously described (Chen-Woan and Goldschneider, 1991) and 104 gated events were analysed for each sample on an Epics Elite flow cytometer (Coulter, Hialeah, FL).
2.7. In vivo homing studies and immunocytochemistry
FcPA depleted BM was cultured for 6 days with rGM-CSF. Non-adherent cells were removed, re-depleted of FcPA cells, washed in RPMI 1640 and injected into the penile vein of anesthetised BN rats, treated with 1 mg/kg per day of FK506. 1–3 days later the spleen was removed, frozen in OCT (Miles, Elkhart, IN) and stored at −70°C. LEW cells were localised in the recipient BN tissue using Cy3-conjugated L-21-6 (a mAb which reacts with the invariant chain of class II MHC of LEW and other rat strains, but not BN (Murase et al., 1991)) with a standard direct immunofluorescent technique. Normal LEW and BN spleen sections were used as positive and negative controls, respectively.
2.8. Ultrastructural studies
Transmission electron microscopy (TEM) was performed on pellets of centrifuged cells after standard dehydration, embedding, sectioning and staining, using a JEOL 100-CX microscope (JEOL Technics, Tokyo, Japan). For scanning electron microscopy (SEM), cells were placed on cover-slips coated in poly-l-lysine (Sigma), critical point dried, sputter coated with gold and viewed using a JEOL JSM-T300 microscope.
All results are expressed as mean ± 1 standard deviation. Student’s t test was used where appropriate and p < 0.05 was accepted as statistically significant.
3. Results
3.1. Effect of GM-CSF and gelatin-coated flasks
The use of gelatin-coated tissue culture flasks increased the proliferation of cells in unfractionated BM cultures to 1.3–1.4 times that seen without gelatin (p = 0.05 at day 5) (Table 1). Further improvement could be achieved by the addition of murine rGM-CSF which significantly (p < 0.01) increased cell proliferation three and five days after commencing cultures of unfractionated rat BM (Table 1). Furthermore, we observed that using gelatin-coated flasks increased the number of small clusters of dendritic shaped cells floating throughout the culture medium, in comparison to cultures without gelatin, and that more adherent cells were present in gelatin-negative cultures. In three experiments the mean yield of non-adherent cells from day 6 cultures was 13.5 ± 2% and 8.2 ± 0.7% of the initial number used (for cultures with and without gelatin, respectively: p < 0.02). Since gelatin coated flasks improved the yield of non-adherent cells, all subsequent cultures used this technique. Further supplementation with human TNF-α, as may be required in cultures of human bone marrow (Reid et al., 1992), did not augment cell proliferation (Table 1).
Table 1.
The proliferation of cells in unfractionated rat BM cultures (as measured by level of DNA synthesis) can be increased by supplementation with recombinant murine GM-CSF and also by the utilisation of gelatin-coated culture flasks in the presence of GM-CSF
| Time | Cytokine | No gelatin a | Gelatin a | Amount of increase b |
|---|---|---|---|---|
| Day 0 c | No cytokine | 53.8 | 51.2 | |
| rGM-CSF d | 52.9 | 54.4 | ||
| rGM-CSF + TNFα e | 53.5 | 53.4 | ||
| Day 3 | No cytokine | 6.5 | 6.3 | × 1.0 |
| rGM-CSF | 9.2 * | 11.8 ** | × 1.3 (p = 0.07) | |
| rGM-CSF + TNFα | 9.7 | 12.6 | × 1.0 | |
| Day 5 | No cytokine | 2.1 | 2.9 | × 1.4 |
| rGM-CSF | 6.8 ** | 9.8 ** | × 1.4 (p = 0.05) | |
| rGM-CSF + TNFα | 6.1 | 8.1 | × 1.3 |
Further supplementation with human TNF-α does not improve the level of proliferation achieved.
Data in cpm × 10 −3. Cultures were pulsed with [3H]thymidine for 7 h.
Ratio of gelatin/no gelatin.
‘Day 0’ = fresh unfractionated rat BM.
Recombinant murine GM-CSF at a concentration of 0.4 ng/ml.
Recombinant human TNF-α at 50 U/ml.
p = 0.06 vs. no cylokine;
p < 0.0005 vs. no cytokine.
3.2. Effect of retaining non-adherent cells when re-feeding cultures
As removal of non-adherent cells during culture improves the yield of DC from mouse peripheral blood (Inaba et al., 1992b) and BM (Inaba et al., 1992a), we compared two different techniques of growing DC from rat BM. In one method we discarded the non-adherent cells every 2 days when adding fresh medium to the cultures, as previously described in murine DC systems (Inaba et al., 1992a, b). For the second method, we retained the non-adherent cells by resuspending any floating cells (in the medium being exchanged) in fresh culture medium and returning them to the culture flask. The allostimulatory ability of non-adherent cells obtained from GM-CSF-supplemented day 8 cultures of unfractionated rat BM was significantly improved by retaining the floating cells that would have been removed during medium exchanges using the ‘murine’ technique, at stimulator to responder ratios of 1:27 the MLR against PVG were 21747 ± 1484 and 6800 ± 1024, respectively (p < 0.01). These results are entirely consistent with previous reports that mature rat DC do not adhere to culture flasks (Klinkert et al., 1980, 1982). Thus the removal of non-adherent cells during culture discards many of the mature DC and lowers the MLR activity obtained. Non-adherent cells were therefore retained for all subsequent experiments.
3.3. Effect of depletion of FcPA cells from BM prior to culture
After depletion of FcPA cells by panning on serum-coated petri dishes, between 54 and 65% of the amount panned were recovered as non-adherent cells (60.5 ± 4.3% (mean ± 1 SD); n = 7). When uncoated plates were used, the percentage of non-plastic-adherent cells was approximately 80%.
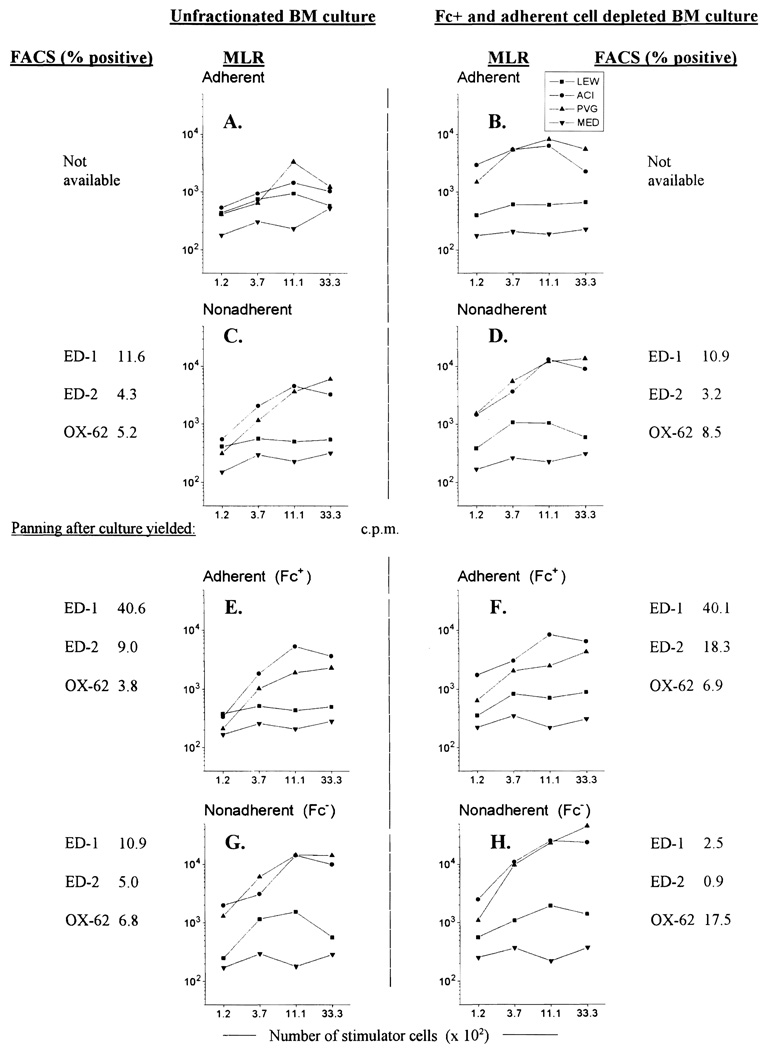
The upper panels in Fig. 1 (experiments performed three times, representative data shown) demonstrate that the stimulatory ability of cultured cells could be markedly increased by depleting FcPA cells before initiating BM cultures (B vs. A; D vs. C). Non-adherent cells from unfractionated cultures (C) showed 3.5 times greater stimulatory ability than unfractionated LEW splenocytes, while non-adherent cells from cultures of FcPA depleted cells (D) were found to be 31 times stronger than fresh spleen cells. Staining with the DC specific mAb, OX-62 revealed that the yield of OX-62+ cells could be increased from 5.2% to 8.5% by depleting BM of FcPA cells before culture (C vs. D). These results confirm that DC precursors are retained in a non-adherent and Fc− fraction of normal rat bone marrow. Although the initial number of cells in FcPA depleted BM cultures was only 60% of that in unfractionated cultures, the yield of non-adherent cells after 5 days of culture was similar (28.2 × 106 vs. 36 × 106), i.e., a particular cell yield could be obtained more efficiently after depletion of FcPA cells.
Fig. 1.
Depletion of Fc+ and adherent cells before culture of unfractionated rat BM (B, D, F, H) augments the stimulatory ability of nonadherent cells obtained from day 5 cultures supplemented with GM-CSF in gelatin-coated flasks (D) and increases the percentage of DC from 5.2% (C, unfractionated BM) to 8.5% of gated cells. Depletion of Fc+ and adherent cells from the NA cells obtained after culture can enrich the DC population up to 17.5% (H), with an associated increase in allostimulatory ability, while reducing ED-1+ contamination to 2.5%. Examination of the adherent cells remaining in the panning plates (E and F) revealed that 40% of these cells are ED-1+ while less than 7% are OX-62+ Throughout this experiment, nonadherent cells from cultures of Fc+ and adherent cell depleted BM showed consistently more OX-62+ cells and higher levels of stimulatory ability in MLR than were achieved with unfractionated BM (A, C, E, G). Results shown are representative data from three experiments. MLR = mixed lymphocyte reaction; FACS = flow cytometry.
3.4. Effect of depletion of FcPA cells at the end of the culture period
To further enrich the purity of DC at the end of the culture period, non-adherent cells from day 5 cultures of unfractionated BM, and FcPA depleted BM were panned using serum-coated petri dishes, as before (Fig. 1, lower panels). The phenotypic profiles and allostimulatory ability of the two populations were then compared. BM depleted of FcPA cells before culture yielded consistently better results (F and H vs. E and G). The results also showed that panning with serum coated petri dishes could efficiently remove contaminating monocytes from cultured BM cells. Adherent fractions after panning (E and F) consisted largely of ED-1+ and ED-2+ labelled monocytes, whereas very few of these cells remained in the non-adherent cell fractions (G and H). OX-62+ cells accounted for 6.9% and 17.5% of the adherent and non-adherent fractions, repectively (F and H).
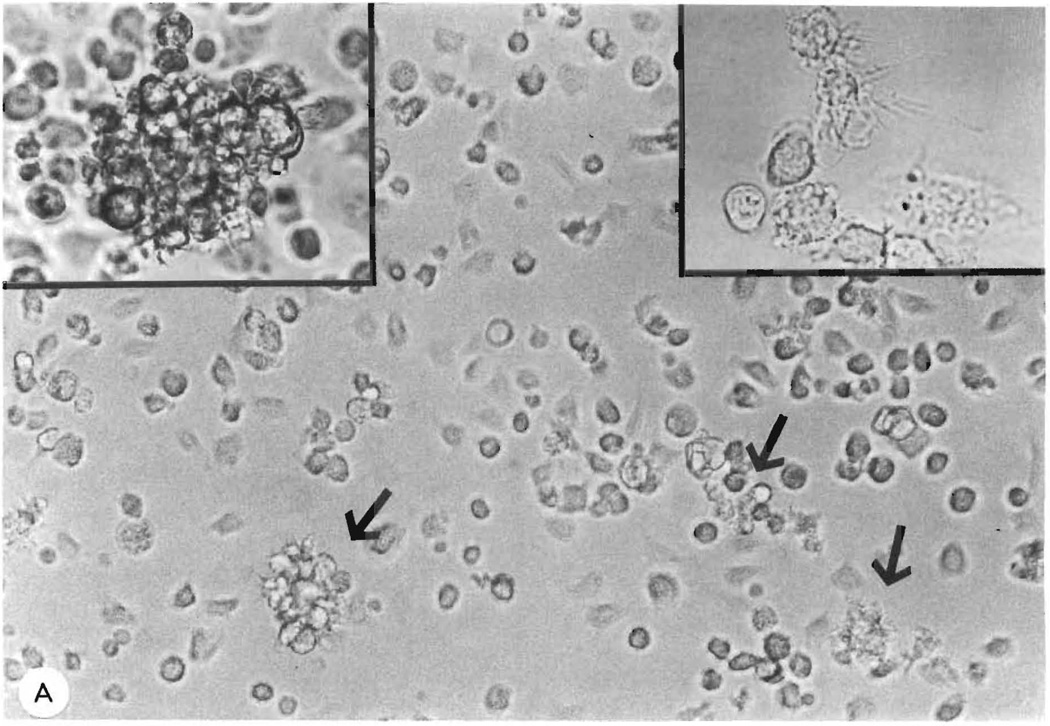
A correlation between the percentage of OX-62+ cells and the stimulatory ability of day 5 BM cultures was also clearly demonstrated throughout this experiment (Fig. 1, A–H). During the course of the experiment the stimulatory power of non-adherent and adherent fractions was 132 (H) and 32 (F) times greater than that of fresh spleen controls, respectively. Light microscopic examination of non-adherent fractions showed many of these cells to have dendritic morphology (Figs. 2A and 2B).
Fig. 2.
A: phase contrast microscopy (× 400) of a day 6 culture of Fc+ and plastic-adherent (FcPA) cell depleted BM. Cells with dendritic morphology can be visualised throughout the field and some lightly adherent, small clusters of growing cells (arrows) are also present. High power views (× 1000) are shown of a single growing cluster (left inset) and of non-adherent cells with characteristic dendritic morphology which have been aspirated from a day 5 culture and viewed on a microscopy slide. B: Giemsa staining (× 1000) of cytospin FcPA cells from day 7 culture.
3.5. Kinetics of growth/maturation of rat BM cultures
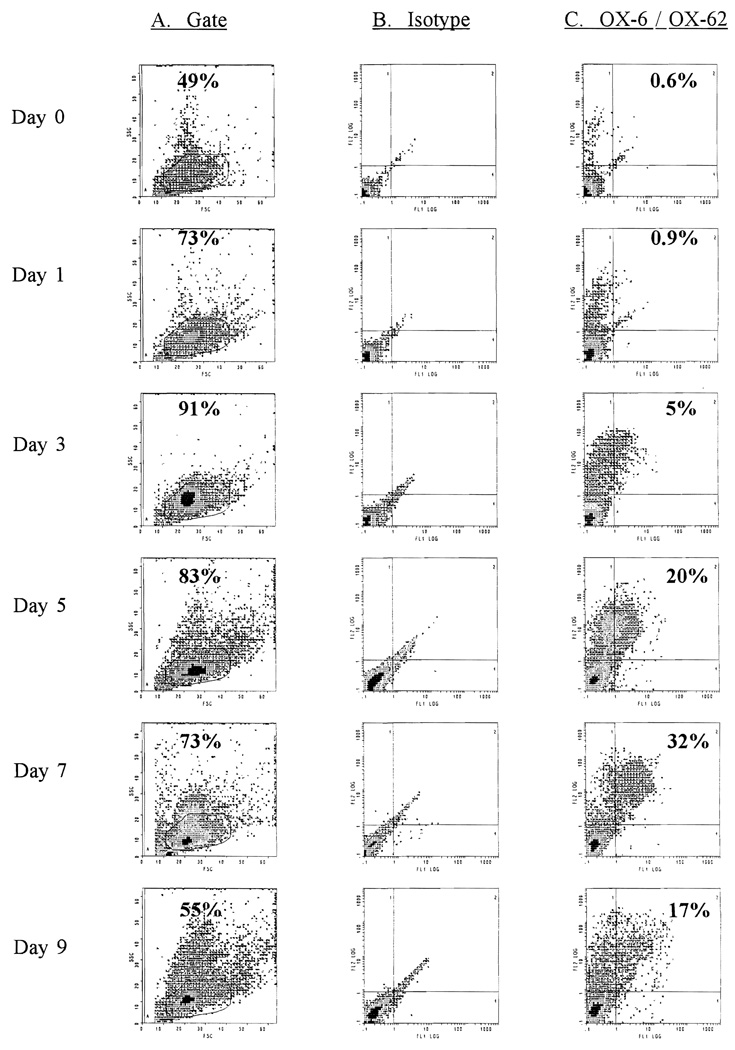
To ascertain the time of optimum DC yield, a detailed growth curve was performed. Since rat DC express both MHC class II and an α chain-like integrin recognised by OX-62 as surface markers, it was possible to study the kinetics of their development in BM culture by sequentially documenting the presence of double labelled cells. On days -9, -7, -5, -3 and -1, 240 × 106 FcPA depleted fresh BM cells were set up in culture using a standardised technique. All cells were harvested on day 0 and, with the exception of ‘day 0’ cells (FcPA depleted fresh BM), cultures were re-panned with normal serum coated plates to deplete monocytes before functional assessment and phenotypic analysis (Figs. 3 and 4). Assaying all cultures on the same day with identical batches of panning solutions, media, etc., minimised variations that might occur between different time points.
Fig. 3.
Flow cytometric analysis of Fc+ and plastic-adherent (FcPA) cell-depleted BM cultured with GM-CSF in gelatin-coated flasks. Cultures were commenced on days -9, -7, -5, -3 and -1. Non-adherent cells were re-depleted of FcPA cells before analysis. Column A shows the forward and side scatter profiles of cultured cells and gives the percentage of total cells which falls within the gate where DC are found. Many of the cells outside this gate are granulocytes. Column C shows results after double-staining with the rat DC-specific mAb OX-62 and OX-6 (MHC class II) (isotype controls in column B). ‘Day 0’ cells were obtained from fresh BM depleted of FcPA cells on the day of analysis. The maximum percentage of double OX-62+/OX-6+ cells was seen in day 7 cultures (32.1%).
Fig. 4.
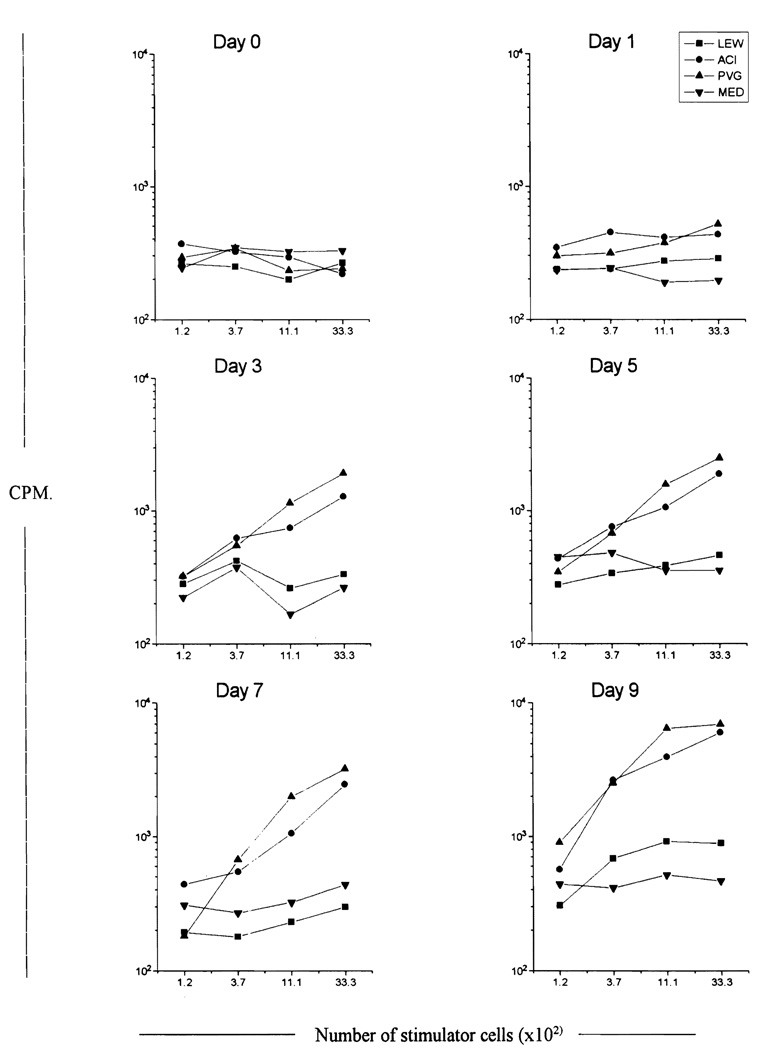
Allostimulatory data for the cells analysed by flow cytometric analysis in Fig. 3. After γ-irradiation, various numbers of cells were used to stimulate a constant number of responder lymphocytes (9 × 104/well) from syngeneic (LEW) and third party (ACI and PVG) cervical lymph nodes; MED contained medium in place of responder cells. Cultures were maintained for 3 days and labelled with [3H]thymidine 7 h before harvesting. A continuous increase in the allostimulatory ability of cultured cells was seen up to day 9, at which stage the cells were between 597 (PVG responder) and 665 (ACI responder) times more potent than fresh LEW splenocytes (between 2.7 × 105 and 1 × 104 LEW splenocytcs/well were used as positive controls).
Fig. 3 demonstrates that the emergence of OX6+/OX62+/OX19− DC using this culture technique is a function of the time spent in culture up to day 9. Although the yield of OX6+/OX62+/OX19− DC increased up to day 7, on day 9 it diminished (day 1 = 0.2 × 106; day 3 = 2.0 × 106; day 5 = 2.3 × 106 ; day 7 = 3.3 × 106 ; day 9 = 0.3 × 106). The stimulatory ability of these cultures at different time points also strongly correlated with the intensity of expression of class II and OX-62 (Fig. 4). Day 9 cultures were the most potent stimulators on a cell-for-cell basis. A simple count of OX-62+ cells does not accurately reflect DC potency or maturity (compare day 7 and day 9, Figs. 3 and 4). These findings demonstrate that the maturation of DC is a dynamic event during which cells upregulate surface marker expression in conjunction with their increasing allostimulatory ability.
The yield of DC is summarised in Table 2. After depletion of FcPA cells from 300 × 106 unfractionated BM cells, 0.6 × 106 DC are seen. When these cells are cultured for up to 7 days and redepleted of FcPA cells, a maximum of 3.3 × 106 OX6+/OX62+ cells can be obtained. The allostimulatory ability of these cells increases up to day 9, at which stage they are between 597 (PVG responder) and 665 (ACI responder) times more potent allostimulators than equivalent numbers of unfractionated LEW splenocytes.
Table 2.
The enhancement of cell yield using this protocol for the propagation of rat BM-derived DC (kinetics experiment data for day 1 and day 9, mean of three experiments for day 3, day 5 and day 7)
| Day | NA cells recovered from cultures (× 106) |
Mean yield of NA cells after panning (× 106) |
OX-6 +/OX-62 + cells after panning (× 106) |
Ratio of allostimulatory ability of DC to that of fresh spleen in MLR |
|
|---|---|---|---|---|---|
| ACI responder | PVG responder | ||||
| 0 a | 300 a | 180 | 0.6 | – | – |
| 1 | 69 | 29 | 0.2 | 68 | 34 |
| 3 | 72 | 44 | 2.0 | 124 | 105 |
| 5 | 38 | 14 | 2.3 | 178 | 145 |
| 7 | 27 | 14 | 3.3 | 177 | 183 |
| 9 | 7.2 | 2.5 | 0.3 | 665 | 597 |
After panning of unfractionated BM, 180 × 106 Fc + and plastic-adherent depleted cells were obtained (0.6 × 106 DC). Cells were cultured and analysed 1–9 days later. OX-6 +/OX-62 + cells increased from 0.2 × 106 to a maximum of 3.3 × 106 by day 7. The allostimulatory ability of these cells increased until day 9, at which stage they were approximately 600 times more potent allostimulators than fresh LEW splenocytes in MLR.
Fresh unfractionated bone marrow cells from LEW rat.
3.6. In vivo homing patterns of DC
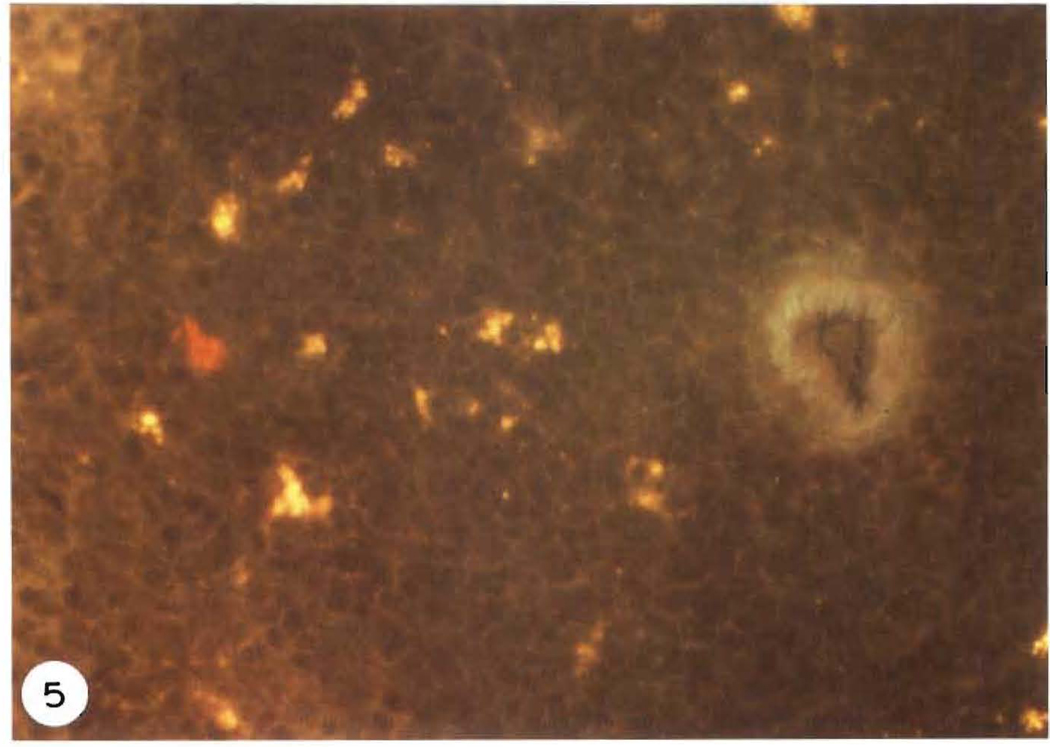
To confirm the homing properties of cultured DC, non-adherent cells from day 6 cultures of FcPA depleted BM were redepleted of contaminating monocytes using serum coated plates. Allogeneic recipient animals were then injected intravenously with 10 × 106 cultured cells. Examination of the recipient’s spleen 2–3 days after injection revealed homing of the cells to the periarteriolar lymphatic sheath, as expected for DC (Fig. 5).
Fig. 5.
Immunohistochemical staining of allogeneic spleen 3 days after intravenous injection of 10 × 106 cultured DC, depleted in Fc+ and plastic-adherent cells. LEW cells were localised in the recipient BN tissue using Cy3-conjugated L-21-6 (which reacts with the invariant chain of class II MHC of LEW, but not BN) using a standard direct immunofluorescent technique. A positive (red) stained LEW DC is seen in the area of the T-dependent periarteriolar lymphatic sheath.
3.7. Ultrastructure of DC
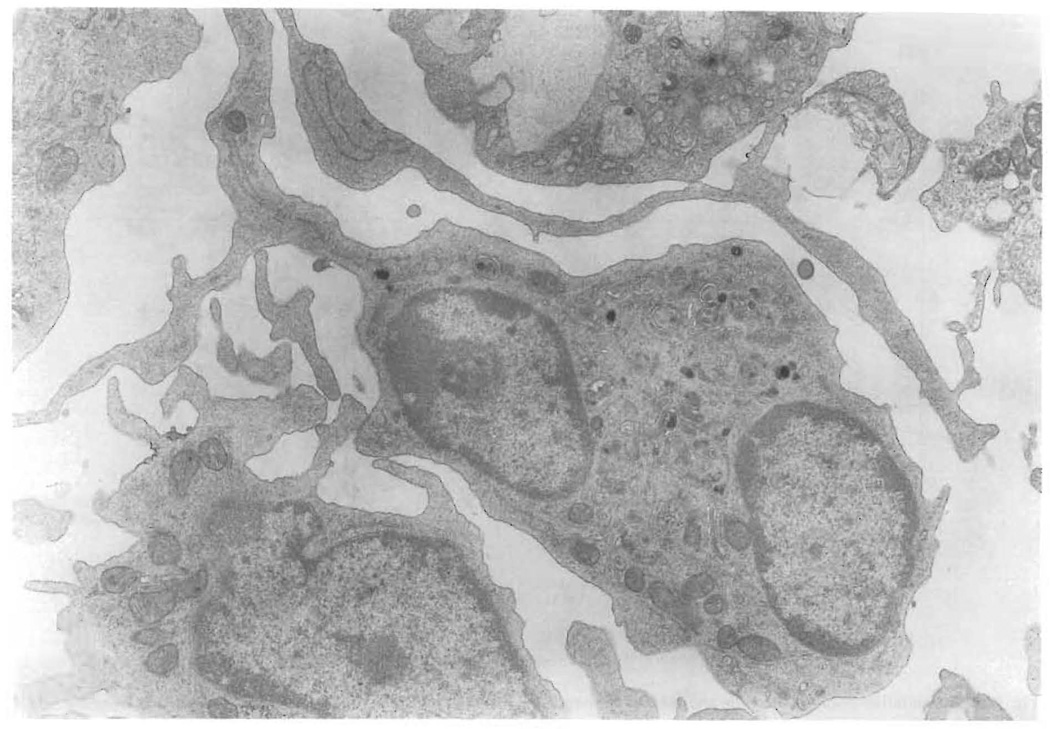
For TEM, day 6 BM cultures, depleted of monocytes by repanning, were further subjected to BSA gradient fractionation as previously described (Klinkert et al., 1980, 1982). The low density fraction (ρ = 1.048) consisted of greater than 90% DC, and representative cells are shown in Fig. 6. The allostimulatory capability of this low density fraction was approximately 1600 times that of fresh spleen cells (data not shown). As previously described, TEM demonstrated many cells with long dendritic processes, lobed nuclei and profuse mitochondria (Steinman and Cohn, 1973).
Fig. 6.
Transmission electron micrograph (× 6300) of nonadherent cells removed from day 6 cultures of BM with GM-CSF in gelatin-coated flasks. The cells were re-depleted of Fc+ and plastic-adherent cells and further purified on a BSA gradient before analysis. These cells exhibit numerous, long dendritic processes, have irregularly shaped nuclei with promioent nucleoli and have profuse mitochondria.
4. Discussion
Although short-term culture (1–3 days) to aid in the isolation of DC from various rat tissues, including lymph nodes, skin, thoracic duct lymph and peritoneal exudate cells, has been described previously (Bowers and Berkowitz, 1986; Klinkert et al., 1980, 1982; MacPherson, 1989; MacPherson et al., 1989), only two reports describe the culture of DC from rat bone marrow. In these papers, Bowers and Berkowitz (1986) used serum-free medium while Klinkert (1984) used medium supplemented with ConA-stimulated spleen cell supernatant to promote DC growth. With thc advances in molecular biology in the past decade, it is now possible to obtain recombinant cytokines for definitive studies of hemopoiesis, both in vivo and in vitro.
In murine systems, large numbers of BM derived DC have been generated using species-specific rGM-CSF alone (Inaba et al., 1992a). In contrast, propagation of DC from CD34+ stem cells from human BM and cord blood requires a combination of tumor necrosis factor (TNF) and GM-CSF (Caux et al., 1992; Reid et al., 1992). Recombinant murine GM-CSF has been used in the rat to improve recovery at 72 h of thoracic duct lymph-borne DC (MacPherson, 1989; MacPherson et al., 1989), however the cytokine has not been used to enhance DC yield from fresh rat bone marrow cultures. In this paper we demonstrate that murine rGM-CSF significantly promotes the proliferation of cells from rat BM (as measured by level of DNA synthesis). The addition of a low concentration of human rTNF-α (50 U/ml) does not improve proliferation, although this may be related to the inactivity of this cytokine in the rat (rat TNF-α is not currently available).
GM-CSF promotes the growth of monocytes, macrophages and granulocytes in cultures of BM, probably by stimulation of a common myeloid precursor cell. Apart from reducing DC purity, monocytes can also express class II Ag as a surface marker. Hence, simple determination of the number of class II+ cells in culture, as has been performed in previous reports (Klinkert et al., 1982; MacPherson, 1989), may not adequately differentiate between monocytes and DC. The recent availability of a specific mAb for the rat DC (OX-62) (Brenan and Puklavec, 1992) makes it possible to reliably monitor the development of BM-derived DC in culture. Although OX-62 does not stain all DC (Brenan and Puklavec, 1992), it does allow us to standardise culture techniques for the propagation of DC from the rat, an animal used widely for investigating allograft-recipient interactions. Therefore in this paper, putative DC (confirmed to be OX-19− and therefore not γ/δ T cells) are double labelled with OX-62 and the MHC class II marker, OX-6, allowing an accurate (although conservative) determination of cell phenotype and number. Using double labelling of cultured cells, we demonstrate that the yield of DC is a function of the time spent in culture. Furthermore, their stimulatory activity in MLR strongly correlates with the intensity of expression of these surface antigens. This is not surprising, because OX-62 identifies the α subunit of an integrin-like molecule (Brenan and Puklavec, 1992), up regulation of which would be expected under the influence of GM-CSF. Similarly, the increased expression of class II on mature DC is not unexpected, as DC are well known to be the most potent of Ag presenting cells (Steinman, 1991; Steinman and Cohn, 1973).
The question remains whether these DC are growing, or simply maturing from precursors present in the initial BM culture. Taken in conjunction, three separate pieces of evidence suggest that DC are actually being propagated in this culture system and are not only maturing. First, the number of DC gradually increases up to day 7 in culture (Table 2) in conjunction with a significant increase in the DNA synthesis of cells from BM in the presence of GM-CSF. Second, using light microscopy and TEM to examine day 7 cultures, we observed DC with different lengths of distinct cytoplasmic veils, probably reflecting different stages of activation and maturation, as previously reported for human DC (Patterson et al., 1991). Finally, discontinuous BSA gradient centrifugation showed that, even as late as day 7, many cells with the appearance of what may be immature DC (fuzzy border, short dendrites, small veils (Caux et al., 1992; Patterson et al., 1991)), were present in the high density fraction, possibly due to their immature status.
Gelatin (a water soluble, denatured form of collagen) has previously been used to support endothelial cell cultures in our laboratory (Colson et al., 1990). Because the propagation of murine DC on type 1 collagen-coated tissue culture plates has been found to promote the maturation of ‘immature’ liver-derived DC in the presence of rGM-CSF (Lu et al., 1994), we assessed the effect of gelatin on rat DC growth. Both the yield and the potency of DC were enhanced by culture in gelatin-coated flasks, although the mechanism by which gelatin might promote the growth and/or maturation of these cells is unknown (Lu et al., 1994). This effect may be mediated directly by gelatin, or indirectly through its binding to fibronectin in culture media. Gelatin has been used for many years for the purification of plasma and tissue fibronectins that in turn have been shown to play an important role in cell differentiation (D’Ardenne and McGee, 1984; Hynes and Yamada, 1982). Macrophages and neutrophils are producers of fibronectin (Beaulieu et al., 1987; Tsukamoto et al., 1981) and consequently, in cultures supplemented with GM-CSF, there is a continuous supply of fibronectin in the medium. Further study is required to determine how gelatin improves the yield of BM-derived DC.
Unfortunately the rat DC precursor is a floating cell. This eliminates the option of decanting off granulocytes and other contaminating cells at an early stage (as performed in mouse DC cultures). Previous studies have shown, however, that DC precursors in rat BM are Fc− and do not adhere to plastic dishes and that these cells can be enriched by removing the plastic-adherent cells and depleting Fc+ cells by using sheep red blood cell rosetting (Klinkert et al., 1982; Klinkert et al., 1980). To accomodate both properties of the DC precursors, we panned BM cell suspensions on normal serum-coated petri dishes prior to culture of rat BM in rGM-CSF supplemented medium and repanned the non-adherent cultured cells prior to analysis. This extremely simple technique allows the generation of a population of cells which are 32% double-positive for OX-6 and OX-62. Furthermore, these cells have more than 100 times more stimulatory capacity than fresh spleen cell controls in MLR. Simple depletion of the adherent BM cells prior to culture can increase the stimulatory ability of the cells obtained by 30-fold. Furthermore, culture of these cells in gelatin-coated flasks significantly increases the yield of DC obtained. This procedure can readily be adopted in any cell culture laboratory.
Acknowledgements
The authors thank Dr. A.S. Rao and Dr. A.W. Thomson for reviewing the manuscript prior to submission.
References
- Banuls MP, Alvarez A, Ferrero I, Zapata A, Ardavin C. Cell-surface marker analysis of rat thymic dendritic cells. Immunology. 1993;79:298. [PMC free article] [PubMed] [Google Scholar]
- Beaulieu AD, Lang F, Belles-Isles M, Poubelle P. Protein biosynthetic activity of polymorphonuclear leukocytes in inflammatory arthropathies: increase synthesis and release of fibronectin. J. Rheumatol. 1987;14:656. [PubMed] [Google Scholar]
- Bowers WE, Berkowitz MR. Differentiation of dendritic cells in cultures of rat bone marrow cells. J. Exp. Med. 1986;163:872. doi: 10.1084/jem.163.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J. Exp. Med. 1992;175:1457. doi: 10.1084/jem.175.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Chen-Woan M, Goldschneider I. Evidence that cyclosporine treatment causes the appearance in rat lymph node of T cells having the antigenic phenotypes of cortical thymocytes. Transplantation. 1991;51:661. doi: 10.1097/00007890-199103000-00023. [DOI] [PubMed] [Google Scholar]
- Colson YL, Markus BH, Zeevi A, Duquesnoy R. Increased lymphocyte adherence to human arterial endothelial cell monolayers in the context of allorecognition. J. Immunol. 1990;144:2975. [PubMed] [Google Scholar]
- D’Ardenne AJ, McGee JO. Fibronectin in disease. J. Pathol. 1984;142:235. doi: 10.1002/path.1711420402. [DOI] [PubMed] [Google Scholar]
- Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, chimerism and tolerance after liver, bone marrow and heart transplantation. Transplant. Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and ‘tolerance’ after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, et al., editors. Rejection and Tolerance. Dordrecht: Kluwer Academic Publishers; 1994. p. 325. [Google Scholar]
- Hart DNJ, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J. Exp. Med. 1981;154:347. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Yamada KM. Fibronectins: multi-functional modular glycoproteins. J. Cell Biol. 1982;95:369. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992a;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Steinman RM, Witmer Pack M, Aya H, Inaba M, Sudo T, Wolpe S, Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J. Exp. Med. 1992b;175:1157. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert WEF. Rat bone marrow precursors develop into dendritic accessory cells under the influence of a conditioned medium. Immunobiology. 1984;168:414. doi: 10.1016/S0171-2985(84)80127-8. [DOI] [PubMed] [Google Scholar]
- Klinkert WEF, LaBadie JH, O’Brien JP, Beyer CF, Bowers WE. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc. Natl. Acad. Sci. USA. 1980;77:5414. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert WEF, LaBadie JH, Bowers WE. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J. Exp. Med. 1982;156:1. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Woo J, Rao AS, Li Y, Watkins SC, Qian S, Starzl TE, Demetris AJ, Thomson AW. Propagation of dendritic cell progenitors from normal mouse liver using GM-CSF and their maturational development in the presence of type-l collagen. J. Exp. Med. 1994;179:1823. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson GG. Properties of lymph-borne (veiled) dendritic cells in culture. I. Modulation of phenotype, survival and function: partial dependence on GM-CSF. Immunology. 1989;68:102. [PMC free article] [PubMed] [Google Scholar]
- MacPherson GG, Fossum S, Harrison B. Properties of lymph-borne (veiled) dendritic cells in culture. II. Expression of the IL-2 receptor: role of GM-CSF. Immunology. 1989;68:108. [PMC free article] [PubMed] [Google Scholar]
- Murase N, Demetris AJ, Matsuzaki T, Yagihasi A, Todo S, Fung J, Starzl TE. Longterm survival in rats after multivisceral versus isolated small bowel allo-transplantation under FK506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- Patterson S, Gross J, Bedford P, Knight SC. Morphology and phenotype of dendritic cells from peripheral blood and their productive and non-productive infection with human immunodeficiency virus type 1. Immunology. 1991;72:361. [PMC free article] [PubMed] [Google Scholar]
- Reid CDL, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34 + progenitors in human bone marrow. J. Immunol. 1992;149:2681. [PubMed] [Google Scholar]
- Scheicher CM, Mehlig M, Zecher R, Reske K. Dendritic cells from mouse bone marrow: in vitro differentiation using low doses of recombinant granulocyte-macrophage-CSF. J. Immunol. Methods. 1992;154:253. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol. Today. 1993a;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi c, Ildstad S, Ramos H, Todo S, Tzakis A, Fung J, Nalesnik M, Zeevi A, Rudert WA, Kocova M. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993b;17:1127. [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Ann. Rev. Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantification, tissue distribution. J. Exp. Med. 1973;137:1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Helsel WE, Wahl SM. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J. Immunol. 1981;127:673. [PubMed] [Google Scholar]