Abstract
Sensitive analytical methods are needed for the separation and quantification of neurotransmitters obtained in microdialysate studies. This unit describes methods that permit quantification of nanomolar concentrations of monoamines and their metabolites (high-pressure liquid chromatography electrochemical detection), acetylcholine (HPLC-coupled to an enzyme reactor), and amino acids (HPLC-fluorescence detection; capillary electrophoresis with laser-induced fluorescence detection).
Keywords: high pressure liquid chromatography (HPLC), electrochemical detection, capillary electrophoresis, laser fluorescence, monoamines, amino acids, acetylcholine
INTRODUCTION
This unit describes methods for the separation and quantification of neurotransmitters in microdialysis samples by high-pressure liquid chromatography (HPLC) coupled to either electrochemical (EC) or fluorescence detection and a more recently developed technique, capillary electrophoresis laser-induced fluorescence detection, for detection of monoamines and amino acids. Other methods including gas chromatography (GC; Griffin et al., 2007), radioimmunoassay (RIA; Maidment et al., 1989), and mass spectrometry (Lanckmans et al., 2006) are used for quantification of drugs and other analytes. Methods of sufficient sensitivity for the routine quantification of neuropeptides are limited (Baseski et al., 2005; Babu et al., 2006) and are not discussed here.
When selecting a method for separation and quantification, several issues must be considered. First, the concentration of most neurochemicals in the extracellular space is quite low. Because temporal resolution of an analyte in a dialysis sample is inversely related to volume, the analytical method employed should be that which provides detection limits below the lowest concentration expected in the dialysate and that which requires the smallest sample volume. Because of the low volume of microdialysate samples, pipetting or sample-cleanup techniques are often impossible. Finally, the perfusion medium itself contains neurochemicals and inorganic ions that may interfere with the quantification method employed (unit 7.1). The protocols describe commonly used procedures for the detection of catecholamines and indoleamines (HPLC-EC detection; see Basic Protocol 1), detection of acetylcholine (HPLC-EC detection with enzyme reactor; see Basic Protocol 2 and Alternate Protocol 1), and detection of amino acids (HPLC-fluorescence detection; see Basic Protocol 3; and capillary electrophoresis laser-induced fluorescence, see Basic Protocol 4). Each of these protocols affords detection limits in the low nanomolar range.
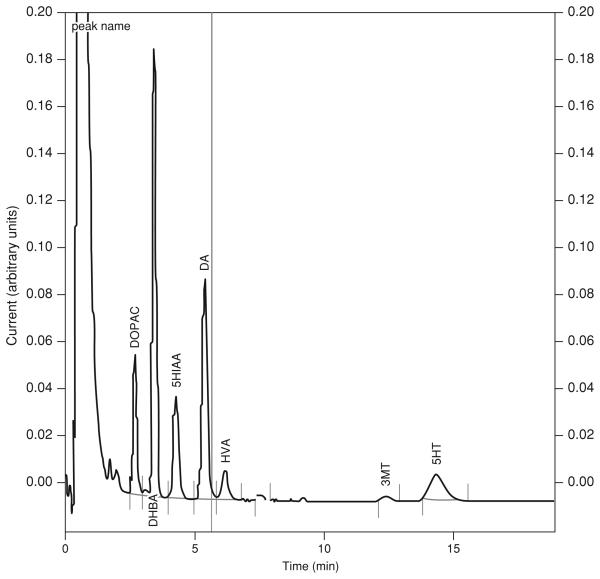
BASIC PROTOCOL 1: DETECTION AND QUANTIFICATION OF DOPAMINE AND SEROTONIN BY HPLC/EC
Ungerstedt and co-workers (Zetterstrom et al., 1983) were the first to combine HPLC with electrochemical detection (EC) to analyze dopamine and its metabolites in brain dialysate samples. This technique was refined by several laboratories (Church et al., 1987; Abercrombie et al., 1988; Church and Justice, 1989; Pettit and Justice, 1991) and provides a sensitive method with which to quantify catecholamines and indoleamines. In HPLC/EC, oxidation/reduction occurs at a fixed point along a flowing eluent (mobile phase). The mobile phase passes in a thin layer through the cell over the electrode. As the single layer of mobile phase passes over an electrode held at a fixed potential, it is oxidized or reduced. If the potential is greater than that required for oxidation/reduction of the analyte, a charge passes between the electrode and solute. The resultant current is directly proportional to the concentration of analyte passing through the cell. This current is amplified and sent to a recorder to yield a chromatogram.
There are two approaches to EC detection for HPLC: amperometric and coulometric. In the amperometric technique, some fraction (usually 5% to 15%) of the analyte is oxidized or reduced. This is in contrast to the coulometric detector, which enables essentially 100% conversion of the analyte. By placing an additional electrochemical cell before the analytical cell, it is possible to oxidize or reduce compounds that may co-elute with the analyte of interest, thereby preventing them from interfering with the analysis. Both the amperometric and coulometric EC methods depend on electron transfer between the mobile phase (solute) and the electrode surface. Therefore, mobile-phase composition is critical. The mobile phase must have a sufficiently high dielectric constant to permit ionization of the electrolyte and must be electrochemically inert at the electrode surface (i.e., background current should be low). Column size and composition are also important factors in determining sensitivity of the method. This protocol may be used for the amperometric detection of either catecholamines/indoleamines together with their metabolites (using mobile phase buffer I, running time: ~15 min) or dopamine alone (using mobile phase buffer II, running time: ~5 min).
Materials
Mobile phase buffer I or II (see recipes)
HPLC-grade water
10 μM monoamine standard working solutions (see recipe) and dilutions bracketing those estimated for the samples
Microdialysis samples to be analyzed (see units 7.2 & 7.3)
3,4 dihydroxybenzylamine as internal standard (10 μM stock, dilute 1/20 in sample or standard)
- Filtration apparatus consisting of:
- Borosilicate glass vacuum flask
- Fritted filter support and spring clamp
- 0.22-μm filter discs (e.g., Millipore)
- Vacuum source
- Ultrapure helium with regulator and Teflon tubing
- HPLC/EC system consisting of:
- HPLC dual-piston pump equipped with pulse dampener
- For mobile phase I, C18 column, 5-μm particle size, 100-mm length × 1-mm i.d. (Bioanalytical Systems)
- For mobile phase II, C18 column, 3-μm particle size, 100-mm length × 2-mm i.d. (UniJet LC column, Bioanalytical Systems)
- Manual injector (20-μl sample loop/gas-tight HPLC glass microsyringe) or autoinjector
- BAS LC-4C amperometric electrochemical detector (or equivalent) equipped with a radial flow cell with a glassy carbon working electrode (Bioanalytical Systems)
Pen recorder and integrator or equivalent computer software program
- Prepare 1 to 2 liters of either mobile phase buffer I or II using glassware that has been cleaned and rinsed with HPLC-grade water.
- Because contaminants can affect detection, the glassware and the HPLC-grade water employed should be checked for electroreactive species that may co-elute with the analytes to be measured. The system used by the authors requires HPLC-grade water because the deionized water from their filtration system results in a contaminant that co-elutes with dopamine. Typically, the mobile phase is recycled and replaced one to two times a week. If background noise is detected, however, the recycled mobile phase should be degassed once again.
- Filter mobile phase solution through 0.22-μm filter under vacuum, then degas by passing a gentle stream of ultrapure helium through the solution for 10 min at a pressure of 5 to 10 psi, using Teflon tubing.
- Filtration and complete degassing is essential for sensitive electrochemical detection of catecholamines. Particulate matter in the mobile phase can clog the HPLC/EC detector system, creating high pressure and baseline disturbances. Air bubbles from improper degassing are the most common problem in EC detection and can wreak havoc on an HPLC/EC system (see Critical Parameters and Troubleshooting). The mobile phase can simply be degassed under vacuum after filtration. However, even after vacuum degassing, the authors recommend that the mobile-phase solution be “sparged” with helium as described above. The degassed mobile phase will then be stable for 7 to 9 hr of an HPLC run. The helium serves to blanket the top of the mobile phase, thus preventing the entry of air. Absence of air bubbles will decrease baseline noise, thus increasing the signal-to-noise ratio.
- Purge any air bubbles from the mobile phase inlet lines and pump using a priming syringe. Assemble the HPLC/EC system, connecting the mobile phase to the inlet lines. Set the flow rate (Mobile Phase I: 0.160 ml/min; Mobile Phase II: 0.450 ml/min), and allow several hours (e.g., 14 to 16 hr) for equilibration of the mobile phase and column.
- This flow rate produces a back pressure of ~2200 to 2800 psi in the authors' system. The authors allow equilibration overnight.
Adjust cell potential to 650 mV or 750 mV for mobile phase I or II, respectively.
Adjust electrode sensitivity to 1 to 10 nA.
Inject 10 μlof10 μM monoamine standard working solution to determine retention times.
- Thaw microdialysis samples on ice. Inject 10 μl of sample manually or using an autoinjector and run HPLC/EC. Inject 10 μl of monoamine standard solutions (in concentrations bracketing those estimated for the samples) prior to and at the end of each series of samples.
- Because injections of samples from multiple subjects can take several hours, standards are typically injected onto the HPLC prior to and at the end of an animal's run. This enables determination of whether chromatographic conditions (e.g., sensitivity and retention times) have changed during the course of the run. If changes are noted, the average of these standard curves can be used for the linear regression to determine analyte concentration. An internal standard with a known retention time and concentration can also be included in the samples for quality control purposes. This method allows for resolution of the internal standard 3,4 dihydroxybenzylamine (DHBA) between the DOPAC and 5-HIAA peaks.
Determine dialysate concentrations of amines by comparing peak heights with those of known concentrations of external standards. Perform a linear regression analysis of the standards and use the resulting linear regression equation to determine actual concentrations in samples.
BASIC PROTOCOL 2: DETECTION AND QUANTIFICATION OF ACETYLCHOLINE BY HPLC/EC
A phosphate buffer allows fast elution and optimal separation selectivity for choline and acetylcholine on a cation exchange column. Acetylcholine is neither electroactive nor detectable by optical methods. The most frequently used method for acetylcholine detection is based on enzymatic conversion of acetylcholine into choline and acetate by acetylcholinesterase, and then subsequent oxidation of choline by choline oxidase to betaine and H2O2. The latter can be oxidized on a platinum electrode. Enzymes can be covalently attached to packing material to form an immobilized enzyme reactor (IMER). Bioanalytical Systems and ESA sell a kit that includes the analytical column and the IMER, both in microbore and normal bore versions, which greatly facilitates method development. This method allows detection of acetylcholine concentrations as low as 50 fmol on the column, permitting quantification of dialysate acetylcholine concentrations of 5 to 10 nM. However, these concentrations are higher than those in typical brain dialysates. Therefore, an acetylcholine esterase inhibitor (1 to 10 μM neostigmine) is frequently added to the perfusion buffer to increase dialysate acetylcholine to detectable levels. It is important to perform pilot experiments to determine the minimum concentration of neostigmine needed to reliably quantify dialysate acetylcholine under the experimental and analytical conditions employed. Artificially increasing acetylcholine levels by using acetylcholinesterase inhibitors alters the mechanisms responsible for the physiological regulation of extracellular acetylcholine levels and may interfere with the goals of the experiment. Recently, improvements in sensitivity have been reported by replacing the platinum electrode with a carbon electrode coated with peroxidase enzyme. The peroxidase oxidizes H2O2 and the resulting electrons are transferred to the electrode surface by a redox polymer and detected on the carbon surface operating in reduction mode. A kit containing the peroxidase and the redox polymer necessary to coat the carbon electrode is available from Bioanalytical Systems (see Alternate Protocol 1).
Materials
20% (v/v) nitric acid
30% (v/v) acetic acid
HPLC-grade water
Mobile phase buffer III (see recipe)
Acetylcholine/choline working standards (see recipe)
Frozen microdialysis samples to be analyzed (see Support Protocol 1 or 2)
- HPLC/EC system consisting of:
- HPLC dual-piston pump equipped with pulse dampener
- Analytical column (UniJet, MF8904 Bioanalytical Systems)
- Immobilized enzyme reactor (IMER, MF-8903, Bioanalytical Systems)
- Amperometric electrochemical detector equipped with platinum electrode and Ag/AgCl reference electrode (Bioanalytical Systems)
- 20-μl injection loop
Pen recorder and integrator or equivalent computer software program
- Filtration apparatus:
- Borosilicate glass vacuum flask
- Fritted filter support and spring clamp
- 0.22-μm filter discs (e.g., Millipore)
Vacuum source
Ultrapure helium with regulator and Teflon tubing
- Set up the HPLC system without the columns and detector cells. Passivate the pump and injector by flushing with the following solutions in the order indicated:
- 50 ml 20% nitric acid
- 50 ml 30% acetic acid
- 100 ml HPLC-grade water
- 100 ml mobile phase buffer III.
- It is important to passivate the HPLC pump thoroughly when starting the acetylcholine assay to prevent contamination from previous eluants. Passivation will remove previous bacterial contamination and also prevent corrosion of metal parts, which would result in increased background current. It is essential to remove prior bacterial contamination in the system. Bacteria produce catalase, which degrades H2O2, resulting in loss of sensitivity. Be sure to remove columns and detector cells from flow during this operation and to flush the injector/loop both in the load and inject positions. Check the pH of the mobile phase eluting from the system before reconnecting the column, IMER, and detector. Wait until pH is the actual pH of the mobile phase. More acidic pH indicates a need to flush system for a longer period of time.
Filter 1 liter of mobile phase buffer III through 0.22-μm filter discs under vacuum, then degas by passing a gentle stream of ultrapure helium through the solution for 10 min at a pressure of 5 to 10 psi using Teflon tubing (also see Basic Protocol 1, step 2, annotation).
- Connect the guard and analytical columns to the injector (but not the IMER). Rinse the guard column and the analytical column with 40 column volumes of mobile phase buffer III. Finally, connect the IMER to the column and rinse with 20 column volumes of mobile phase buffer III.
- If choline is of no interest, an additional IMER with choline oxidase and catalase (Bioanalytical Systems cat. no. MF8907) can be attached between the injector and the analytical column. This will degrade choline in the dialysate. This can be advantageous when there is poor resolution between the acetylcholine and choline peaks since the size of the choline peak can interfere with acetylcholine quantification.
- Connect the cell and detector and equilibrate the system with mobile phase buffer III for at least 1 hr at 0.140 ml/min. Set the detector range at the highest setting, then switch on the cell at +0.500 V oxidation potential (the oxidation current will jump and then will slowly begin to decrease and stabilize).
- The platinum electrode requires long stabilization times, it is better to let it stabilize overnight before sample analysis.
- Inject 20 μl of 1.0 μM acetylcholine standard and 20 μl of 1.0 μM choline standard in separate HPLC runs to determine retention times.
- Choline is very susceptible to bacterial degradation and acetylcholine is hydrolyzed under strong basic or acidic conditions. The standards should be prepared in 0.3% glacial acetic acid in HPLC-grade water (adjusted with NaOH to pH 5.0 to 5.5) containing 0.005% Proclin (bacteriostatic, Bioanalytical Systems) and stored for no more than 2 weeks at 4°C. Finally, acetylcholine and choline are extremely hygroscopic. They will adsorb moisture and their mass will increase. When weighing powder, allow the bottle to come to room temperature before opening. Seal the bottle and store in a desiccator in the freezer.
Thaw microdialysis samples. Inject 20 μl of sample at 0.140 ml/min. Inject 20 μl of 1.0 μM acetylcholine/choline standards, and standards bracketing concentrations estimated for the samples, prior to, during, and at the end of a batch of samples.
- Determine dialysate concentrations of acetylcholine using standards containing known concentrations of analyte (see Basic Protocol 1, step 8).
- The sensitivity of the platinum electrode decreases steadily over time. To maintain maximum sensitivity, the electrode can be polished (electrode polishing kit available from Bioanalytical Systems). Since the sensitivity of the electrode can change over the course of a complete experiment, it is common practice to inject a single standard concentration every five to ten samples and correct for changes in electrode sensitivity.
- Bacterial contamination is the biggest problem source with this method. Bacteria in the HPLC system produce catalase, which will interfere with H2O2 detection. The mobile phase described contains a bacteriostatic agent, ProClin (Bioanalytical Systems). It is recommended not to recycle the mobile phase and change to freshly prepared and filtered mobile phase at least once a week.
- If the HPLC system is not to be used for a prolonged period, the analytical column and IMER should be moistened with mobile phase and should not be allowed to dry out. Store column and IMER at 4°C.
ALTERNATE PROTOCOL 1: HPLC/EC DETECTION OF ACETYLCHOLINE USING A PEROXIDASE-WIRED ELECTRODE
H2O2 can also be detected on a glassy carbon electrode that has been “wired” with a redox polymer containing peroxidase instead of the platinum electrode used in Basic Protocol 2. The peroxidase oxidizes H2O2 and the resulting electrons are transferred to the carbon electrode surface by the redox polymer and detected there with the electrode in the reduction mode. Bioanalytical Systems supplies a kit that allows the coating of a regular glassy carbon electrode. The peroxidase-wired electrode has much shorter equilibration times than platinum electrodes, as well as lower background current, and allows an about five to ten times increase in assay detection limits (5 to 10 fmol in column), making it possible to detect acetylcholine in the dialysate with much lower concentrations of neostigmine (in the nanomolar range) or, depending on brain area and microdialysis conditions, no neostigmine at all.
Additional Materials (also see Basic Protocol 2)
Peroxidase/redox polymer coating kit (Bioanalytical Systems cat. no. MF-2095) Glassy carbon electrode
Prepare HPLC system
Passivate system (see Basic Protocol 2, step 1).
Cleanse the pump by flushing it at a flow rate of 2.0 ml/min, first for 25 min with 40% acetonitrile, then for 55 min with 100% acetonitrile, and finally for 25 min with 40% acetonitrile.
Filter 1 to 2 liters of mobile phase buffer III through a 0.22-μm filter under vacuum.
Flush the system with 50 ml of filtered mobile phase buffer III.
Prepare column and IMER
5. Install the analytical column and flush for 15 min with mobile phase buffer III at 0.140 ml/min.
6. Install the postcolumn IMER and flush overnight with mobile phase buffer III at 0.140 ml/min to equilibrate.
7. Clean and polish a glassy carbon electrode. Coat the electrode with the peroxidase/redox polymer solution according to the kit instructions. Let it cure overnight. Protect the electrode from dust during the curing process.
8. Mount the newly wired electrode and attach the cell to the HPLC system. Set the electrode range to the higher value and the potential at +0.100 V in the reduction mode. Switch on the electrode, allow it to equilibrate for 1 hr, and adjust sensitivity rangeto1to10nA.
Analyze samples and standards
9. Using acetylcholine/choline standards, determine retention times and detection limits. Construct an SD curve.
- 10. Thaw microdialysis samples. Inject 10 to 20 μl. Inject the same volume of a 50 nM acetylcholine/choline standard working solution every five to ten samples to monitor for changes in sensitivity.
- Typical running times are between 15 and 20 min.
- 11. Determine dialysate concentrations of acetylcholine.
- With this method, it should be possible to measure dialysate acetylcholine levels using very low concentrations of neostigmine (50 to 100 nM) in the perfusion buffer. Run pilot experiments to determine the lowest concentration of neostigmine needed to detect the levels of acetylcholine in the dialysate samples under the dialysis and analytical conditions employed. Depending on the brain area sampled and the sensitivity of the analytical system, it may be possible to detect acetylcholine in the absence of neostigmine.
BASIC PROTOCOL 3: DETECTION OF AMINO ACIDS IN MICRODIALYSATES BY HPLC AND FLUOROMETRIC LABELING WITH o-PHTHALDIALDEHYDE
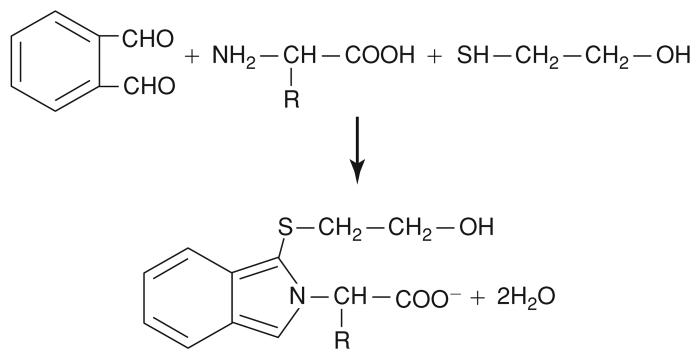
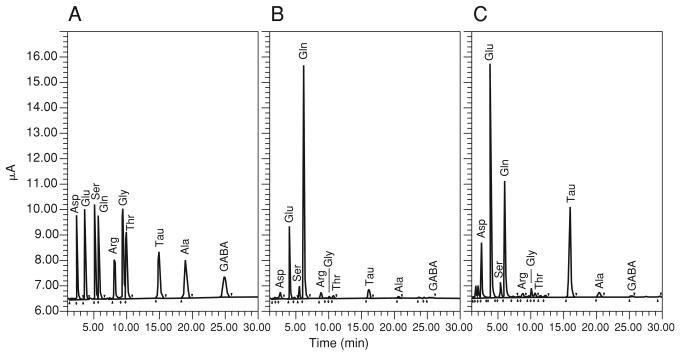
Amino acids in microdialysates can be readily separated using reversed-phase high-performance liquid chromatography (RP-HPLC). This protocol describes the detection of primary amino acids treated with o-phthaldialdehyde (OPA) in the presence of 2-mercaptoethanol at basic pH in an aqueous solution. The reaction yields isoindole derivatives of the primary amino acids, rendering them highly fluorescent (Fig. 7.4.1). Once injected into the HPLC system, the derivatized sample is carried at a constant flow rate through the chromatography column in an optimized buffer, which constitutes the mobile phase. Bonded to the matrix of the column are long carbon chains (C18 column), which form a hydrophobic stationary phase. Separation occurs as the derivatized amino acids in the sample are retained in the column on the basis of their relative hydrophobicity in the mobile phase. Retention times range from ~2 min for aspartate to 25 min for γ-aminobutyric acid (see Anticipated Results). The fluorescence detector can measure picogram quantities of the OPA-derivatized amino acids as they elute from the column.
Figure 7.4.1.
OPA (o-phthaldialdehyde) reaction. OPA reacts with a primary amine of an amino acid in the presence of 2-mercaptoethanol forming a substituted isoindole derivative, which is amenable to fluorimetric detection.
Materials
Mobile phase buffer IV (see recipe)
H3PO4
OPA stock reagent (see recipe)
Microdialysate sample or standard
Borax buffer (0.1 M sodium tetraborate, pH 10.4, adjusted with 10 M NaOH)
- Filtration apparatus:
- Borosilicate glass vacuum flask
- Fritted filter support and spring clamp
- 0.22-μm filter disks (e.g., Millipore)
- Vacuum source
- HPLC system consisting of:
- 10-ml gas-tight HPLC priming syringe (e.g., Kloehn or Waters)
- Chromatography pump: Waters 510 or comparable pump capable of delivering flow rate of 1.0 ml/min at pressures ≤4000 psi
- Injector (e.g., Waters 717 autosampler equipped with glass low-volume inserts or manual Injector)
- C18 column (Alltech Adsorbosphere cat. no. 287095)
Fluorescence detector: Waters 420 or comparable detector equipped with 338- and 450-nm excitation filter and 450-nm emission filter
Data acquisition software (Waters Millennium or equivalent)
- Adjust the pH of the mobile phase buffer IV to 5.88 (at 25°C) with H3PO4. Filter and degas solution through a 0.22-μm filter under vacuum.
- Precise formulation of mobile phase buffer IV is crucial for good chromatographic results. Slight changes in either pH or the water/methanol ratio can alter peak retention times. Particulate contaminants in the mobile phase can clog the small-diameter tubing and column, resulting in leaks, high back pressure, or system noise. Therefore, the procedures in this step must be performed before each chromatographic run.
- Purge any air bubbles from the mobile phase inlet lines and pump using a priming syringe. Assemble the HPLC system and column, attaching the mobile phase buffer IV to the inlet lines, set the flow rate to 1 ml/min. Allow the column to equilibrate with mobile phase buffer IV for at least 30 to 60 min. Turn on the fluorescence detector 30 min before the beginning of the run to warm up the lamp.
- Fluctuations in system pressure accompanied by a grinding noise in the pump head indicate the presence of an air bubble. Failure to either equilibrate the column with mobile phase buffer IV or warm up the fluorescence detector can result in an unstable or rising baseline. The mobile phase buffer IV can be recirculated following the equilibration period.
- Prepare OPA working solution. Add 6 μl working OPA solution to 4 μl microdialysate sample or standard and 12 μl of Borax buffer and mix by pipetting up and down several times. Incubate 2 min at room temperature protected from exposure to direct light. Inject 20 μl of this sample/OPA mix into the HPLC system.
- The chromatographic run time for each injection is 30 min (see Anticipated Results).
- Derivatization and injection of the sample can be automated through the incorporation of an autosampler (e.g., Waters 717) into the HPLC system. The autosampler must be set up to perform suitable mixing of the OPA solution with the sample for the derivatization reaction to occur. Collection of microdialysates into Waters glass, low-volume inserts, which are compatible with the Waters 717 autosampler carousels, reduces the risk of contamination and sample loss. Recovery of amino acids in microdialysates is optimal at low perfusion rates (e.g., 1 μl/min).
Determine dialysate concentrations of amino acids by comparing peak areas with those of known concentrations of external standards. Perform a linear regression analysis of the standards and use the resulting linear regression equation to determine actual concentrations in samples.
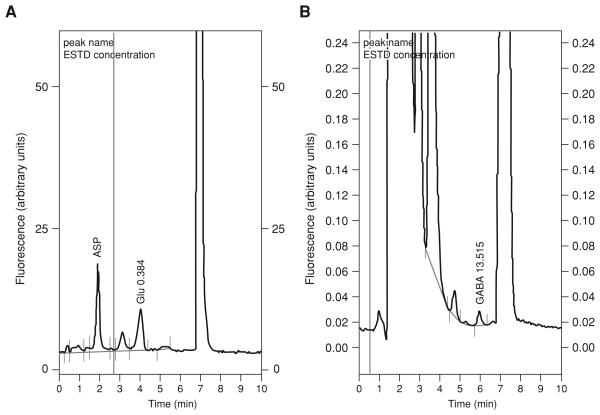
ALTERNATE PROTOCOL 2: QUANTIFICATION OF GLUTAMATE, ASPARTATE, OR GABA IN DIALYSATES
The method described in Basic Protocol 3 is able to resolve a mixture of 10 amino acids present in brain dialysates. However, the running time of the chromatographic method is ~30 min. This long running time can be a problem when many samples need to be analyzed, as is often the case in microdialysis experiments. If the amino acids of interest are limited to glutamate (Glu), aspartate (Asp), or GABA, there is the possibility to optimize the assay to improve detection of those amino acids and decrease running time at the expense of losing the rest of the amino acids. The two methods described here are based on those originally described by Kehr (1998a,b) and involve the use of microbore columns and an organic phase flush step. One of the problems with the chromatographic methods for amino acids is the existence of very late-eluting peaks that can contaminate chromatograms during repeated runs. One solution to this problem is to flush the column with a high concentration of organic solvent after the amino acids of interest have eluted. This causes all the late peaks to elute from the column. However, this normally involves the use of a gradient pump and more sophisticated HPLC equipment. A viable alternative is the use of microbore columns. The internal volume of those columns is so small that they can be flushed with a small volume of organic solvent (i.e., 20 μl). This can be achieved by injecting the solvent through the manual or automatic injector. The use of microbore columns enhances the assay sensitivity since the smaller column volume results in less dilution of the injected sample.
Additional Materials (also see Basic Protocol 3)
40% and 100% acetonitrile
Mobile phase buffer V (for GABA) or VI (for Asp/Glu) (see recipes)
Assemble the HPLC system. Install new column, disconnect column from detector, and run 40% acetonitrile for 20 min (adjust flow rate so pressure is between 2200 and 3000 psi). Flush the column with 100% acetonitrile and then again with 40% acetonitrile (20 min each step).
Prepare fresh mobile phase buffer, filter, and degas under helium for 20 min. Purge the pump with the fresh mobile phase buffer, re-attach the column to the detector, switch the detector on, and allow the column to equilibrate with the mobile phase buffer and allow the lamp to warm up for at least 30 min.
- Prepare fresh OPA working solution. Mix 4 μl of the sample or standard with 6 μl of OPA working solution and 12 μl of Borax buffer. Allow to react for 2 min and inject 20 μl into the HPLC. After the peaks of interest have eluted, flush the column by injecting 20 μl of acetonitrile and proceed with the next sample.
- It is important to keep the reaction time constant between samples. The use of an automatic injector facilitates the handling and derivatization of samples and keeps the processing times constant, thus enhancing the reproducibility of the method. The acetonitrile flushing injections can be achieved by intercalating vials containing acetonitrile between each sample. Some injectors have the option to inject a calibration solution from a fixed vial position. This feature can be exploited to program an acetonitrile flush for every other sample injection.
Calculate a concentration-fluorescence linear function for the Asp, Glu, and GABA standards and use this function to calculate the concentration of Asp, Glu, or GABA in dialysates.
BASIC PROTOCOL 4: DETECTION OF AMINO ACIDS IN MICRODIALYSATES BY CAPILLARY ELECTROPHORESIS WITH LASER-INDUCED FLUORESCENCE DETECTION
Although high-pressure liquid chromatography (HPLC) remains the most commonly used method for quantification of neurotransmitters in dialysate samples, it provides only moderate mass sensitivity. Consequently, HPLC analysis requires large injection volumes and, hence, long microdialysis sampling times (generally 5 to 15 min). As a consequence, the limited time resolution is often insufficient to monitor rapid chemical changes linked to neuronal activity. This temporal resolution, already poor when compared with the duration of neurobiological events, is further decreased when samples are split for the simultaneous determination of different classes of neurotransmitters. Temporal resolution can be improved by coupling microdialysis with capillary electrophoresis (CE), a separation technique, which not only requires lower sample volumes, possesses a high mass sensitivity (10−18 mol) and high separation efficiency, but also allows rapid separation of a large number of compounds. Due to its plug-like flow and minimal diffusion, CE possesses enormous resolving power and large peak capacities. With this approach, sampling times as short as 5 to 30 sec have been reported (Bowser and Kennedy, 2001; Kennedy et al., 2002; Parrot et al., 2004; Powell and Ewing, 2005), allowing the monitoring of rapid changes associated with neuronal events. Although many of these studies have been performed using home-made CE instruments, which cannot easily be set up in most biology laboratories, recent studies have shown that short sampling times can also be achieved by coupling microdialysis with commercially available CE laser-induced fluorescence (LIF) detection systems.
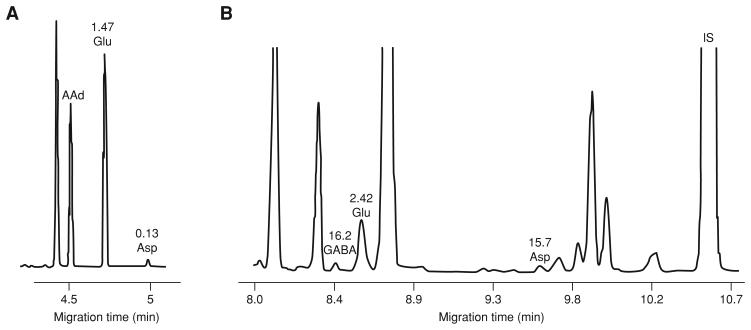
The separation of Glu and Asp is readily achieved by capillary zone electrophoresis (CZE), a separation mode in which the background electrolyte is a simple electrolyte solution such as phosphate, borate, or citrate buffer. The electrophoretic migration and the electroosmotic flow allow the separation of molecules. These forces, as well as the characteristics of the capillary (dimension, coating), differentiate each separation system. In this case, only charged molecules can be separated, since neutral compounds migrate together with the electroosmotic flow. In CE, detection of neurotransmitters can be made by LIF or by electrochemistry (EC). Though no EC detector is presently commercially available, some laboratories use home-made EC detectors to identify electroactive compounds such as catecholamines or derivatized amino acids. However, use of EC is complicated by the need to decouple the electrophoretic current from the amperometric detector. In contrast, LIF detectors are commercially available and user-friendly while providing detection limits in the same range as EC detectors.
As neurotransmitters are not fluorescent at available laser wavelengths, microdialysis samples must be derivatized with a tagging agent (Prata et al., 2001). Fluorescent reagents such as naphthalene-2,3-dicarboxyaldehyde (NDA), o-phthaldialdehyde, or fluorescein isothiocyanate, react with the primary amine function of neurotransmitters, allowing their detection following laser excitation at 442, 325, or 488 nm. Naphthalene-2,3-dicarboxyaldehyde (NDA) is a reagent of choice because (1) it is not fluorescent itself (in contrast with fluorescein isothiocyanate, for instance), (2) it reacts rapidly in presence of CN− to give stable fluorescent derivatives (cyanobenzo[f]isoindol or CBI products) with minute amounts of amino acids and biogenic amines, and (3) NDA derivatives can be detected after excitation with the 442-nm He-Cd laser or the more robust 410-nm diode laser. OPA is also an efficient tagging agent for amine neurotransmitters, but the derivatives formed are relatively unstable. Moreover, OPA derivatives are only detected after excitation by a 325-nm He-Cd laser, which may be less reliable.
This protocol describes a commonly used CZE-LIF procedure (Robert et al., 1998) for the detection of excitatory amino acids. Alternate Protocol 3 describes a procedure that enables detection of inhibitory and excitatory amino acids using another mode of CE, namely micellar electrokinetic chromatography with laser-induced fluorescence (MEKC-LIF, Sauvinet et al., 2003). Each of these protocols affords detection limits in the low nanomolar range.
Materials
Background electrolyte I (100 mM borate buffer, pH 9.2; see recipes)
HPLC-grade water
1% (w/v) NaOH
Derivatized microdialysis samples to be analyzed (see Support Protocol 1 or 2)
Derivatized excitatory amino acid standards and derivatized aCSF (see Support Protocol 1 or 2)
Internal standard: α-amino adipic acid
0.22-μm filter discs (e.g., Millipore)
- CE-LIF system consisting of:
- CE instrument with 1 to 30 kV voltage capabilities, refrigerated sampler
- (Beckman-MDQ; Agilent 3D)
- Fused silica capillary (50-μm i.d., 340-μm o.d.; Polymicro Technology)
- LIF detector (Picometrics Zetalif ) equipped with 410-nm diode-laser
- (Melles-Griot) or 442-nm He-Cd laser (Melles Griot)
NOTE: Because primary amine contaminants can affect detection, special care must be taken to avoid any dust or bacterial contamination. HPLC-grade water, pipet tips and microcentrifuge (PCR) tubes should be autoclaved. Gloves must be worn during manipulation of samples and reagents.
NOTE: Filtration of solutions is important for proper functioning of CE since particulate matter can clog the capillary lumen leading to baseline disturbances and current failure. Degassing in an ultrasonic bath is not essential, but strongly recommended since it will avoid formation of micro-bubbles inside the capillary due to Joule heating.
In the inlet buffer rack of the CE instrument place one vial containing filtered, autoclaved water, three vials containing filtered background electrolyte (one for washing the capillary, one for rinsing the inlet electrode and the outside of capillary inlet, and one for the separation), and one vial containing 1% (w/v) NaOH.
Place one vial containing filtered background electrolyte in the outlet buffer rack of the CE instrument.
- Perform washing and conditioning of the capillary (once at the beginning of each working day) by flushing it with the following order of reagents: 1% NaOH (20 psi, 10 min), HPLC-grade water (20 psi, 10 min), and background electrolyte (20 psi, 10 min). Check capillary and background electrolyte by applying a positive voltage of 25 kV between inlet and outlet electrodes. Note value of current (this value will depend on the total length and the inner diameter of the capillary).
- For example, the current should be ~30 μA when the total length of a 50-μm i.d. capillary is 60 cm. If the current is lower than expected, this may indicate a clogged capillary, resulting usually from salt deposits at the capillary inlet. Trimming the capillary inlet may rectify the problem. If not, the capillary should be changed. If the current is higher than expected, this may indicate a contaminated background electrolyte.
- Turn on the laser 5 min (for 410-nm diode laser) or 20 min (for 442-nm HeCd laser) before the beginning of the first analysis.
- It is recommend that a log-book be kept in which the power of the laser source (indicated on the fluorescence detector), level of background fluorescence, and the value of current when applying voltage is recorded for each session.
- Load derivatized samples in the sample rack of the CE instrument.
- Sample can be loaded in 500-μl polyethylene PCR tubes, plastic, or glass microtubes. Make sure that samples lie at the bottom of each tube; a brief centrifugation may help to ensure that small volume samples are deposited to the bottom of the tubes. Moreover, one must check that sample volume is sufficient so that the inlet of the capillary can plunge into the sample.
- Rinse capillary briefly with background electrolyte (20 psi, 0.5 min) and then inject sample (0.2 psi, 10 sec). After sample injection, briefly (0.01 min) dip the inlet electrode and capillary inlet into a second vial of background electrolyte. Plunge the inlet electrode and the inlet of the capillary into a third vial containing background electrolyte and apply voltage (25 kV) for 6 min. Repeat this sequence for each sample.
- A stacking procedure can be used for sample injection to increase the sensitivity and the separation efficiency of the assay. This procedure decreases the length of the analyte band within the sample plug, leading to sharper peaks, improving the separation and the limit of detection. In this case, injection of sample is followed by injection of 0.1 M phosphoric acid (0.2 psi, 5 sec).
- Determine dialysate concentration of Glu and Asp by comparing relative peak areas (i.e., relative to internal standard α-amino adipic acid) with those of known concentrations of external standards. Perform a linear regression analysis of the standards and use the resulting linear regression equation to determine actual concentrations in the samples.
- α-Amino adipic acid is used as an internal standard since this compound is not present in brain microdialysates, is chemically similar to Glu and Asp, and does not co-migrate with any endogenous compounds present in microdialysates.α-Amino adipic acid is added to samples during the derivitization step (see Support Protocols 1 and 2), at a final concentration per sample of 0.71 μM.
ALTERNATE PROTOCOL 3: DETECTION AND QUANTIFICATION OF AMINO ACIDS BY MICELLAR ELECTROKINETIC CHROMATOGRAPHY WITH LASER-INDUCED FLUORESCENCE (MEKC-LIF)
Addition of surfactant to the CE buffer generates an enhanced degree of resolution and aids in the separation of analytes with similar charges, such as biogenic amines. Surfactants, the most commonly used being sodium dodecyl sulfate (SDS), are added at a relatively high concentration and form micelles within the background electrolyte. Micelles exhibit a hydrophobic core and are considered a mobile “pseudo-stationary” phase, by analogy with the fixed stationary phase of chromatographic columns. This mode of separation is called micellar electrokinetic chromatography (MEKC). The analytes are resolved according to their hydrophobicity, as a consequence of their interaction with micelles and their charge-to-mass ratio. Consequently, compounds exhibiting similar charge-to-mass ratios, like NDA derivatives of monocarboxylic amino acids, can be separated.
The following protocol may be used for the determination of γ-aminobutyric acid (GABA), Glu, and L-Asp within microdialysates.
Additional Materials (also see Basic Protocol 4)
Background electrolyte II (75 mM borate buffer, pH 9.2, containing 10 mM hydroxypropy-β-cyclodextrine (HP-β-CD, Sigma) and 70 mM SDS; see recipes)
Derivatized Glu, L-Asp and GABA standards and derivatized aCSF
The procedure for MEKC-LIF analysis is similar to Basic Protocol 4 described above for CZE analysis of excitatory amino acids, with the following important modifications:
For each run: Rinse capillary briefly with 1% NaOH, HPLC-grade water, and background electrolyte II (20 psi, 0.5 min each) and then inject sample (0.6 psi, 10 sec). After sample injection, briefly (0.01 min) dip the inlet electrode and capillary inlet into a second vial of background electrolyte. Plunge the inlet electrode and the inlet of the capillary into a third vial containing background electrolyte and apply voltage (25 kV) for 10 min. Repeat this sequence for each sample.
- Capillary temperature is crucial for the separation of GABA in microdialysates. The optimal temperature is 36° to 38°C. When using a new batch of background electrolyte, an adjustment of the separation temperature with a 0.5°C precision is required. This is made by trial and error while analyzing a few microdialysis samples (such an adjustment cannot be made with standards). If the temperature is too low, GABA and Glu co-elute. Any increase in temperature increases the migration time of Glu and allows the resolution of GABA from Glu. If the temperature is too high, Glu may be poorly resolved from the following peak. When the working temperature has been optimized, the background electrolyte can be stored up to 2 weeks at room temperature and no further temperature adjustment is necessary.
- Since the background electrolyte contains 10 mM HP-β-CD, it leads to a splitting of the Asp peak, due to a partial resolution of D- and L- forms. Since only L-Asp is present in microdialysates from adult brain, L-Asp instead of the racemic DL amino acid is used as a standard.
- α-Amino adipic acid cannot be used as an internal standard, since it co-migrates with several endogenous compounds contained in brain microdialysate samples. Cysteic acid is used instead, since this acidic derivative of cysteine exhibits a high migration time and is therefore well separated from analytes of interest and other compounds present in the microdialysates.
SUPPORT PROTOCOL 1: MANUAL DERIVATIZATION OF MICRODIALYSATES WITH NDA/NaCN
Since amino acids are not fluorescent, microdialysis samples need to be derivatized with a reagent to give a fluorescent end product. The procedure for derivatization of samples with NDA is described here. NDA is not fluorescent itself but readily reacts with primary amines in the presence of CN to give stable fluorescent derivatives. After derivatization, samples can be analyzed by CE-LIF.
Materials
NaCN stock solution (see recipe)
Derivatization buffer (see recipe)
Microdialysis sample
NDA stock solution (see recipe)
Internal standard working solution, e.g., 10 μM α-amino adipic acid (see recipe) 1-ml glass vial
1. Prepare NaCN-borate solution by adding 111 μl NaCN stock solution to 556 μl derivatization buffer. Store in a sealed 1-ml glass vial up to 1 day at 4°C.
- 2a. In a tube containing 10 μl of microdialysis sample, add in the following order:
- 1 μl internal standard working solution, e.g., 10 μM α-amino adipic acid
- 2 μl NaCN-borate solution
- 1 μl NDA stock solution
- If volume of sample is > 10 μl, increase volume of each reagent proportionately.
- 2b. If volume of microdialysis sample is <10 μl, prepare the following stock reagent mixture immediately prior to use:
- 10 μl internal standard working solution
- 20 μl NaCN-borate solution
- 10 μl NDA stock solution
3. Add 0.8 μl of the reagent mixture to each tube containing 2 μl of microdialysis sample. If the sample volume is >2 μl, increase volume of each reagent accordingly.
4. Vortex and then let the reaction develop 15 min at room temperature. Store up to 6 hr at 4°C prior to analysis.
SUPPORT PROTOCOL 2: ONLINE DERIVATIZATION OF MICRODIALYSATES WITH NDA/NaCN
Manual derivatization of sample volumes <2 μl cannot be accomplished with good reproducibility. Since NDA reacts rapidly with the primary amine moiety of neurotransmitters, continuous flow derivatization (online derivatization) can be performed on nanovolumes of microdialysates in a microreactor placed at the outlet of a microdialysis probe. This procedure has the advantage of continuously adding very small volumes of reagents to the perfusate sample with good reproducibility, while preventing evaporation and sample loss. Systems for online derivatization can easily be constructed but must have a minimal dead volume to limit diffusion of solutes in the microdialysis tubing (Parrot et al., 2004). In this respect, the use of tubing with very small inner diameters and very slow perfusion rates allows submicroliter volumes to be collected with good reproducibility, and results in a high microdialysis sampling rate. The online derivatization of submicroliter volumes of sample and femtomole amounts of compounds does not decrease sensitivity as compared to manual derivatization (see Support Protocol 1). The following protocol describes the reagents and procedures for online derivatization. The procedures can be used with the CZE or MEKC protocols described above.
Materials
Epoxy glue
- Derivatization reagents (see recipes):
- Internal standard (α-amino adipic acid for CZE-LIF determination of excitatory amino acids, or cysteic acid for the MEKC-LIF determination of amino acids) in 0.117 M perchloric acid
- 14.66 mM NaCN in 0.5 M borate buffer, pH 8.7
- 2.925 mM NDA in acetonitrile-water (50:50, v/v)
aCSF or Ringer's solution (unit 7.2 & appendix 2a)
Fused-silica capillary tubing (Polymicro Technology): 40-μm i.d., 105-μm o.d.; 75-μm i.d., 150-μm o.d.
Microdialysis probe
Polyethylene tubing (300-μm i.d.)
50-, 100-, and 500-μl microsyringes
Microsyringe pump (Harvard Apparatus, Instech, CMA), equipped with a multiple-syringe holder
Construct system for online derivatization
Adapt a 40-μm i.d., 105-μm o.d. capillary (capillary no. 1) to the outlet of the microdialysis probe.
- Introduce and fix with epoxy glue three 75-μm i.d., 150-μm o.d. capillaries (capillary no. 2, 3, and 4) into one side of a 2.5-cm long, 300-μm i.d. polyethylene tubing.
- These capillaries will bring reagents and the internal standard into the lumen of the polyethylene tubing; the solutions contained within the capillaries will mix by passive diffusion inside this polyethylene tubing to produce the reaction mixture.
- Introduce and fix with epoxy glue another 75-μm i.d. capillary (capillary no. 5) onto the other side of the polyethylene tubing.
- The reaction mixture made inside the polyethylene tubing will flow out through capillary no. 5.
Glue capillary no. 1 (probe outlet) to capillary no. 5 (polyethylene tubing outlet) lengthwise.
Operate the system for online derivatization
- 5. Place the four microsyringes (two 50-μl, one 100-μl, and one 500-μl) on the rack of a microsyringe pump. Adjust the flow rate so that the 500-μl syringe delivers solution at a flow rate of 0.5 to 2 μl/min.
- The flow rate should be that used for perfusion of aCSF into the microdialysis probe.
- The 500-μl syringe contains aCSF and is connected to the inlet of the microdialysis probe.
- A 50-μl syringe contains the internal standard (α-amino adipic acid for CZE-LIF determination of excitatory amino acids, or cysteic acid for the MEKC-LIF determination of amino acids) and is connected to capillary no. 2.
- The 100-μl syringe contains the NaCN/borate mixture and is connected to capillary no. 3.
- The other 50-μl syringe contains the NDA solution and is connected to capillary no. 4.
- With this system, the microdialysate coming from the outlet of the probe (capillary no. 1) is delivered together with the derivatization mixture coming from capillary no. 5 into collecting vials in which the derivatization reaction takes place.
REAGENTS AND SOLUTIONS
Use HPLC-grade water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
Acetylcholine/choline standards
Stock solutions: Prepare diluent by adding 3.0 ml glacial acetic acid to 1 liter HPLC-grade water. Adjust pH to 5.25 with 1 M NaOH. Add 5 ml of commercial 1% Proclin solution (bacteriostatic, Bionalytical Systems), and store up to 1 month at 4°C. Prepare 2.0 mM acetylcholine stock solution by dissolving 36.3 mg acetylcholine chloride in 100 ml diluent and 2 mM choline stock solution by dissolving 27.9 g choline chloride in 100 ml diluent. Store up to 1 month at 4°C.
Working solutions: On day of assay, prepare fresh 20 μM working standards and other standard concentrations as needed by diluting the stock solutions with diluent.
Acetylcholine and choline are highly hygroscopic. Store powder in a desiccator and minimize the time the bottle is open.
Background electrolyte I
Prepare in autoclaved HPLC-grade water and use HPLC-grade chemicals:
100 mM H3BO3 (6.18 g/liter)
Adjust pH to 9.2 with 10 M NaOH
Store in a sealed glass container for up to 2 months at 4°C
Filter solution through a 0.22-μm filter before use
Background electrolyte II
Prepare in autoclaved HPLC-grade water and use HPLC-grade chemicals:
75 mM H3BO3 (2.32 g/500 ml)
Adjust pH to 9.2 with 10 M NaOH
10 mM hydroxypropyl-β-cyclodextrine (7.45 g/500 ml)
70 mM SDS (10.08 g/500 ml)
Store in a sealed glass container for up to 2 months at room temperature
Filter solution through 0.22-μm filter before use
Mobile phase buffer I
Prepare in HPLC-grade water and use HPLC-grade chemicals:
5.0 g/900 ml citric acid
3.8 g/900 ml NaH2PO4
0.350 g/900 ml sodium 1-octanesulfonic acid
0.037 g/900 ml EDTA
Add 60 ml methanol and 4 ml tetrahydrofuran (THF)
Adjust pH to 4.10 with 5 M NaOH or 85% H3PO4
Filter through a 0.2-μm filter and degas under helium
Store in a sealed container up to 1 week at 4°C
Correct pH is critical for the separation of the metabolites. Lower pH will cause 5-hydroxyindole acetic acid (5-HIAA) to elute before DHBA and homovanillic acid (HVA) to co-elute with dopamine. With a new column, it may be necessary to readjust the pH between 4.00 and 4.20. THF allows for the resolution of 3-methoxytyramine (3-MT) and serotonin, but can poison the carbon electrode by forming a film on the carbon surface. For this reason, keep THF concentrations as low as resolution between 3-MT and serotonin permits, and change mobile phase frequently.
Mobile phase buffer II
Prepare in HPLC-grade H2O and use HPLC-grade chemicals:
0.15 M NaH2PO4 (17.9 g/liter)
1 mM disodium EDTA dihydrate (0.372 g/liter)
2.28 mM sodium 1-octanesulfonic acid (0.535 g/liter)
Adjust pH to 5.0 with 5 M NaOH or 85% H3PO4
Add 13% (v/v) methanol
Filter through a 0.2-μm filter and degas under helium
Store in a sealed container up to 1 week at 4°C
Mobile phase buffer III
Prepare 30 mM NaH2PO4 in HPLC-grade water (3.6 g NaH2PO4 per liter)
Adjust pH to 8.52 using 10 M NaOH
Add 5 ml of commercial 1% Proclin
Filter through a 0.2-μm filter and degas under helium
Store in a sealed container for up to 1 week at 4°C
Use HPLC-grade chemicals. This mobile phase is for a cationic exchange column; never use organic solvents. To fine-tune acetylcholine and choline retention times, adjust the mobile phase ionic strength.
Mobile phase buffer IV
Prepare in HPLC-grade water and use HPLC-grade chemicals:
0.1 M Na2HPO4 (14.2 g/liter)
0.13 mM disodium EDTA (48 mg/liter)
33% (v/v) methanol
Store for up to several months at 4°C in a tightly capped glass container
The sodium phosphate component may require a brief period of mixing to completely dissolve. Adjust the pH of the buffer to 5.88 (25°C) with 40% H3PO4. Filter through a 0.2-μm filter and degas the buffer before use.
Mobile phase buffer V
Prepare in HPLC-grade water and use HPLC-grade chemicals:
0.1 M sodium acetate trihydrate (13.6 g/liter)
Adjust pH to 5.4 with glacial acetic acid
Add 25% (v/v) acetonitrile
Filter through a 0.2-μm filter and degas under helium
Store in a sealed container for up to 1 week at 4°C
Mobile phase buffer VI
Prepare in HPLC-grade water and use HPLC-grade chemicals:
0.1 M sodium acetate trihydrate (13.6 g/liter)
Adjust pH to 6.0 with glacial acetic acid
Add 6% (v/v) acetonitrile
Filter through a 0.2-μm filter and degas under helium
Store in a sealed container for up to 1 week at 4°C
Monoamine standards
Stock solutions (prepare in HPLC-grade water):
1 mM dopamine HCl (18.96 mg/100 ml)
1 mM serotonin HCl (21.27 mg/100 ml)
1 mM 3,4-dihydroxyphenylacetic acid (DOPAC; 16.81 mg/100 ml)
1 mM homovanillic acid (HVA; 18.22 mg/100 ml)
1 mM 5-hydroxyindoleacetic acid (5-HIAA; 19.12 mg/100 ml)
1 mM 3-methoxytyramine HCl (3-MT; 20.37 mg/100 ml)
1 mM 3,4-dihydroxybenzylamine HBr (DHBA; 22.01 mg/100 ml)
Prepare 1-ml aliquots and store up to 1 month at −20°C
Working solutions: Prepare 10 μM working standards on day of assay by diluting 1 ml of stock solutions in 100 ml of the perfusate buffer used to collect the microdialysate of interest. Prepare other concentrations as needed to bracket the estimated concentrations of the samples.
The monoamines can be obtained from a variety of sources including Sigma and Research Biochemicals. Keep powder and standard solutions protected from light.
NDA/NaCN derivatization stock solutions
To prepare 2.925 mM NDA (0.539 mg/ml of acetonitrile-water, 50:50) stock solution, accurately weigh an amount of NDA between 2 and 5 mg. Calculate the volume of acetonitrile and water to be added. Add acetonitrile first, then water. Store up to 1 week at 4°C in a foil-wrapped, capped glass container.
To prepare 87 mM NaCN (4.264 mg/ml of water) stock solution, weigh an amount of NaCN between 4 and 8 mg. Add the required volume of water. Store up to 1 week at 4°C in a capped glass container.
To prepare buffer for derivatization, mix 500 mM (3.1 g/100 ml) H3BO3 and 125 mM (4.8 g/100 ml) borax to obtain a pH 8.7 solution. Store in a sealed glass container up to 1 month at ambient temperature.
OPA stock solution
Prepare borax buffer (0.1 M sodium tetraborate, pH 10.4 with 10 M NaOH).
Prepare OPA stock solution (10 mg OPA, bring into solution in 100 μl methanol and add 9.9 ml of borax buffer).
Prepare β-mercaptoethanol stock solution (dilute 1/10 in methanol in a fume hood).
Keep the stocks in capped vials and protected from light. The stocks are stable for up to 1 month at room temperature. To prepare fresh working solution, mix 0.9 ml of borax buffer, 0.1 ml of OPA stock solution, and add 3 μl of β-mercaptoethanol stock.
The borax buffer tends to precipitate at 4°C. Filter and keep at room temperature.
Standards for CZE analysis of excitatory amino acids
Stock solutions: Prepare diluent by adding 2.07 ml of 37% HCl to 250 ml of HPLC-grade water.
Prepare 1 mM d,l-α amino adipic acid stock solution (16.1 mg in 100 ml diluent).
Prepare 1 mM d,l- glutamic acid stock solution (14.7 mg in 100 ml diluent).
Prepare 1 mM d,l- aspartic acid stock solution (13.3 mg in 100 ml diluent).
Dispense into 1.5-ml microcentrifuge tubes. Store up to 6 months at −20°C.
Working solutions: Prepare diluent for internal standard by adding 1.00 ml of 70% HClO4 to 100 ml of HPLC-grade water. On the day of assay, prepare fresh 10 μM working internal standard by diluting the α amino adipic acid stock solution with diluent. Prepare glutamic acid and aspartic acid standard concentrations as needed by diluting the respective stock solutions with aCSF.
Standards for MEKC analysis of amino acids
Stock solutions: Prepare diluent by adding 2.07 ml of 37% HCl to 250 ml of HPLC-grade water.
Prepare 1 mM L-cysteic acid stock solution (8.5 mg in 100 ml diluent).
Prepare 1 mM D,L-glutamic acid stock solution (14.7 mg in 100 ml diluent).
Prepare 1 mM L-aspartic acid stock solution (13.3 mg in 100 ml diluent).
Prepare 1 mM GABA stock solution (5.2 mg in 100 ml diluent).
Dispense into 1.5-ml microcentrifuge tubes. Store up to 6 months at −20°C.
Working solutions: Prepare diluent for internal standard by adding 1.00 ml of 70% HClO4 to 100 ml of HPLC-grade water. On the day of assay, prepare fresh 50 μM working internal standard by diluting the stock solution of L-cysteic acid with diluent. Prepare amino acid standard concentrations as needed by diluting the respective stock solutions with aCSF.
COMMENTARY
Background Information
Catecholamines
Liquid chromatography was the first method used for the measurement of catecholamines and indoleamines in biological samples. Isolation/purification of specific catecholamines was achieved by adsorption onto either acid-washed alumina (catecholamines) or cation-exchange resins (catecholamines and indoleamines; Anton and Sayre, 1962). Fluorescence analysis with or without chemical derivatization was then employed to identify specific amines (Welch and Welch, 1969). These methods, however, afforded limited sensitivity and selectivity. Three techniques that offered greater sensitivity and selectivity were subsequently developed. Gas chromatography with mass-spectrometric detection (Koslow et al., 1974) resulted in improved selectivity and detection limits. However, its use was limited by expense and the need to derivatize compounds. The second approach employed the enzyme catechol O-methyl-transferase and radioactive labeling to increase the selectivity of primary amine determination. This technique increased detectability relative to that attainable via fluorescence methods. However, safety concerns and expense precluded its routine use. The third approach, liquid chromatography combined with electrochemical detection (HPLC/EC; Kissinger et al., 1977), was developed in the early 1970s and is now the most commonly used technique for the quantification of amines and their metabolites. However, the use of HPLC with EC is often more problematic than with ultraviolet or fluorescence detection. Although detection limits are typically lower, noise can profoundly affect performance. Electrode connections, pump pulsations, bubbles on the electrode, and temperature must be rigorously controlled. The applied potential is a major determinant of the signal-to-noise ratio, and voltammograms can be used to determine the optimal setting to be applied. Mobile phase composition is critical for the separation of the various amines. Knowledge of pKa values and the effects of ion-pairing agents is essential for the development of a mobile phase that will provide optimal separation and detection of electroactive species.
Acetylcholine
Dialysate levels of acetylcholine can be readily quantified using reversed-phase high performance liquid chromatography coupled with electrochemical detection (HPLC/EC). The HPLC/EC method for acetylcholine analysis was originally developed by Potter et al. (1983). This technique was modified and further refined by Damsma et al. (1987), who converted the reversed-phase analytical column into a cation-exchange column by loading sodium lauryl sulfate (or sodium dodecyl sulfate; SDS) onto the reversed-phase column.
The typical assay involves an analytical column packed with a material based on silica, with covalently bonded cationexchange/reversed-phase groups. A phosphate buffer allows fast elution and optimal separation selectivity for choline and acetylcholine. The covalent binding of acetylcholinesterase and choline oxidase onto an immobilized enzyme reactor (IMER) allows the sequential and stoichiometric conversion of acetylcholine to acetate and choline, and of choline to betaine and hydrogen peroxide. The hydrogen peroxide is then electrochemically detected via oxidation on a platinum electrode. Recently, a new method has been developed involving coating of a glassy carbon electrode with a peroxidase/redox polymer, which allows for faster stabilization and lower background currents, resulting in lower detection limits (Kehr et al., 1998).
Amino acids
First described by Roth (1971), the reaction of primary amino acids with OPA in the presence of mercaptans yields strongly fluorescent isoindole derivatives (Fig. 7.4.1). Basic Protocol 3 describes a precolumn derivatization in which the reaction with OPA is performed before injection of the sample into the HPLC system. The reaction with OPA is rapid and amenable to automation. Retention of OPA-derivatized amino acids is governed by their relative hydrophobic and hydrophilic interactions with the hydrocarbon stationary phase and the aqueous-methanolic mobile phase (Lindroth and Mopper, 1979). Although amino acids derivatized with OPA can also be detected by coulometry (e.g., using a dual-electrode coulometric-amperometric analytical cell with potentials of −0.03V and +0.65V), fluorimetric detection offers a higher level of sensitivity, which is often necessary when quantifying low nanogram amounts of amino acids present in microdialysate samples.
CE-LIFD
Online versus manual collection
Microdialysates can be analyzed online, i.e., just after the outlet of the probe, through an analytical interface allowing the direct coupling of the microdialysis probe to the CE instrument, or offline, i.e., after sample collection in vials, which may be stored frozen before analysis.
Since dialysates are protein-free, online coupling between microdialysis and laboratory-made CE-LIF instruments have been described to prevent sample loss and evaporation. The online instrument removes the requirement of collecting, storing, derivatizing, and analyzing large numbers of nanoliter-volume dialysate fractions. Using various interfaces connected to a continuous flow derivatization device, 20-sec to 3-min sampling rates have been reported for the in vivo monitoring of excitatory amino acids (Robert et al., 1998; Bowser and Kennedy, 2001). Microdialysis sampling could be coupled via a flow-gated interface online to CE for in vivo monitoring of neuroactive amino acids and amines. In the instrument, analytes are derivatized precolumn with OPA and β-mercaptoethanol to form fluorescent isoindole products (Bowser and Kennedy, 2001). With online methods, however, temporal resolution becomes limited by the speed of the analytical method used. In addition, for an accurate monitoring of fast events, one must take into account the total delay in the response of the online system to a biochemical event.
The analysis of low-volume micro-dialysates can also be performed offline using a commercially available CE-LIF system, provided that online derivatization of sample is carried out (Parrot et al., 2004). Consequently, this technique can be set up in neuroscience laboratories that have no access to a specialized workshop for making custom-made analysis instruments. Furthermore, one advantage of the offline approach is to uncouple micro-dialysis sampling from the CE analysis. Off-line analysis can be useful since, if any breakdown of the CE-LIF system occurs, microdialysates can be stored up to 3 days prior to analysis. In contrast, if online analysis is used, samples cannot be saved and data are lost. Thus, offline analysis offers more flexibility for planning experiments.
Capability to handle small volumes of samples
In CE, the small inner diameter of the capillary tube is well adapted to the analysis of extremely small sample volumes: samples ranging from a few microliters down to nanoliters can routinely be injected.
When microdialysates are manually collected and analyzed offline, the minimal sample volume required for the CE analysis must be determined. Sample injections are currently made at one side of the capillary by applying pressure. The volume of the sample must be sufficient (1) to allow the capillary to plunge into it, preventing the injection of air microbubbles with the sample, and (2) to avoid any significant loss by evaporation when a series of dialysates (i.e., at least 30 samples) is placed in the CE sample rack before being injected. The authors have found that 940 nl is the minimal volume required for the analysis of a large series of sample with a good reproducibility. With a shorter series of samples, determination of amino acid neurotransmitter content can be performed using 500 nl of derivatized microdialysates.
The reduction of microdialysis sample volumes needed for neurotransmitter analysis using CE-LIF allows slower perfusion rates, thereby increasing temporal resolution. With slow perfusion rates, the concentration gradients developed by the probe are less marked, a characteristic that improves the spatial resolution and decreases disturbance of neural tissue. Moreover, the increased concentration of sample that is removed at slow perfusion rates improves the detection limit for concentration-limited measurements.
Finally, once it has become possible to handle even lower sample volumes, the use of slower perfusion rates (<0.1 μl/min) will allow direct measurement of the extracellular concentration of neurotransmitters in dialysates. As a consequence, in vivo calibration methods such as quantitative no-net-flux or extrapolation to zero flow rate, which are time consuming, could be avoided. However, if the “no-net-flux” method is used for determining the true extracellular concentration, short collection times allow the duration of these “no-net-flux” experiments to be shortened.
Potential for determining several neurotransmitters
Since a derivatization reaction with a fluorescent tag is needed to detect the molecules of interest and since most of the fluorescent reagents used react with primary amines, a large number of the neurochemical constituents of brain microdialysis samples can potentially be detected. In this respect, the use of CE-LIFD makes possible the high-sensitivity determination of various neurotransmitters in a single microdialysis sample with improved temporal resolution. This can be performed via several analyses. The low volume requirement of CE-LIFD allows several subsequent offline analyses to be performed on the same sample of microdialysate. The advantage of such multiple assays is the ability to study functional or drug-induced interactions between neurotransmitters.
Critical Parameters and Troubleshooting
Catecholamines
Measurement of dopamine and its metabolites in dialysates using an HPLC/EC detection method (see Basic Protocol 1) presents a number of challenges. Some of these are due to characteristics of the dialysate in general and some to characteristics of brain dopamine systems. These issues are as follows.
To maximize diffusion into the dialysis probe, the perfusate is pumped through the probe at slow rates (0.5 to 2 μl/min) resulting in small-volume samples (on the order of 10 to 15 μl or even less if automated autosamplers are used). The entire sample is usually needed for analysis, which allows only one opportunity to assay each sample. If larger volumes are collected, the sample can be split and analyzed for a variety of transmitters (e.g., amino acids using a second HPLC-detection system).
Dialysates are usually analyzed immediately after collection. Therefore, run times must be kept short to keep up with sample collection. If the run time is prolonged and the number of samples is large, an appropriate antioxidant or preservative must be added and the samples must be frozen at −70°C to allow analysis at a later time.
A sensitive assay is required because the basal extracellular concentration of neurotransmitter is low and dialysate levels are even lower because of fractional recovery.
The extracellular concentrations of metabolites are >100× higher than those of dopamine, and in general this complicates simultaneous analysis of dopamine and its metabolites. At a detector sensitivity that is optimal for dopamine, metabolites may exceed the linear range of the detector, and can interfere with the resolution of dopamine. Conversely, a detector sensitivity that is optimal for metabolites is not sufficient for the quantification of dopamine.
Dialysates recovered from the primate brain show several uncharacterized peaks that occur close to that of dopamine.
During routine analysis, it is a constant struggle to prepare a mobile phase that provides good separation of dopamine from its metabolites. In the authors' experience, minimizing organic solvent, manipulating pH, and prolonging the run time improve chromatographic separation of the dopamine peak from other electrochemical signals. Mobile phase and electrochemical detector conditions need to be modified to suit the characteristics of the transmitter of interest. Similarly, protocols for amino acid analysis in rat samples (unit 7.2) may not be suitable for monkey samples (unit 7.3). It is critical that mobile phase and detector conditions be modified for optimum detection of the transmitter of interest.
Mobile phase composition is critical for HPLC/EC detection of dopamine and its metabolites. Retention times are affected by alterations in room temperature as well as by pH, the concentration of ion-pairing reagent, and the inorganic solvent employed. These can be varied to modify analyte resolution. Increasing pH decreases the retention time of acidic metabolites (e.g., DOPAC and HVA). Increasing the concentration of ion-pairing reagents (e.g., octane-sulfonate, octylsulfate, pentanesulfonate, or hexanesulfonate) delays retention of amines, enabling better separation from charged molecules. Increasing solvent concentration results in a decreased retention time of amines and their metabolites. Each of these parameters can be altered to increase analyte resolution.
Additional troubleshooting guidelines can be found in Table 7.4.1.
Table 7.4.1.
Troubleshooting Guide for HPLC/EC Detection System
| Problem | Possible cause | Solution |
|---|---|---|
| Fluctuations in pressure |
Air in check valves | Purge pump with freshly degassed mobile phase to remove air from check valves. In the case of Bioanalytical Systems pump or equivalent, switch inlet check valves between the two heads of the pump; if pressure drop occurs on opposite pump head, clean (e.g., sonicate) or replace check valves. |
| Low pressure | Leaks or bad column, as indicated by mobile phase salt residue that collects at valves or unions as well as by occasional spikes that occur in chromatogram |
Check/replace fittings or seals |
| High pressure | Clogged frits, injection ports, or guard columns; also dirty or too-tightly-packed column |
Open fittings, beginning at detector, and work backwards toward pump. At some point the pressure will drop, pinpointing location of clog. |
| Pump stops | Clog in flow path | Open fittings, beginning at detector, and work backwards toward pump. At some point the pressure will drop, pinpointing location of clog. |
| Wavy baseline (wide irregular, positive and negative fluctuations) |
Bad column or inadequate column equilibration |
Replace or reequilibrate column |
| Baseline drift | Variations in room temperature or chemical contamination |
Passivate system with 6 M nitric acid |
| Peaks that occur in only one direction |
Grounding problems or alterations in the circuit in which the HPLC is connected |
Check and repair electrical connections |
| Very fast spikes that occur in both the positive and negative direction |
Air trapped in detector and/or pump (more periodic spiking) |
Inspect the compartment housing the reference electrode and tap to remove any entrapped bubbles. Inspect reference electrode for air bubbles on its tip and replace if necessary. Loosen cell clamp of working electrode to allow mobile phase to run from the gasket, clearing trapped air. If this does not alleviate the problem, degassing and purging of the system is necessary. A pulse dampener will reduce pulsations from the pump and is highly recommended. |
| Decrease in sensitivity | Mobile phase, electrodes, or column may be old |
Inject fresh standards to see if problem persists. If sensitivity is still low, use new mobile phase and replace columns, reference, or working electrodes and/or repolish the working electrode. |
| High background current |
Can occur immediately after an electrode is polished or replaced. May also be consequence of mobile phase degradation, worn seals, scratched electrodes, and/or chemical contamination. |
Wait 1 to 2 days until reconditioning of the electrode has occurred; prepare freshly degassed mobile phase; replace seals or rods; ensure that detector is operating at the minimum potential for the analyte of interest; and/or passivate the system. If problem persists, check column by connecting pump directly to analytical cell. If background current drops despite high pulsation, clean or replace column. |
| Split peaks | Poor injector alignment or old column | Check injector alignment or age of column |
| Insufficient sensitivity | Use microbore HPLC technique. a Because sample volume collected is substantially less than that of conventional HPLC, the perfusate flow rate used during dialysis can be reduced. This increases probe efficiency, resulting in greater recovery of analyte through the probe. |
Several detailed reviews of the equipment and protocols used for this technique are available (Church and Justice, 1989; Pettit and Justice, 1991).
Acetylcholine (see Basic Protocol 2)
The microbore column is easily clogged, making an inline precolumn filter or guard column useful. Guard columns should be replaced on a regular basis, especially when increased pressure or a loss of performance is observed.
Retention times of acetylcholine and choline primarily depend on the mobile phase ionic strength. A higher ionic strength will result in shorter retention times.
Platinum is a soft material and can scratch easily. If a loss of sensitivity can be traced to the performance of the electrode, avoid unnecessary polishing and try first to activate the electrode electrochemically.
Until recently, detection of dialysate levels of acetylcholine could only be achieved from samples perfused with an aCSF solution containing the acetylcholinesterase inhibitor neostigmine. There is, however, increasing evidence indicating that the micromolar concentrations of neostigmine employed can markedly modify the neurochemical effects of various drugs. Therefore, attempts should be made to use as low a concentration of neostigmine as possible. Several laboratories are now able to measure basal levels of acetylcholine in various brain areas in the absence of neostigmine. Therefore, pilot studies should be conducted to determine the minimum concentration of neostigmine needed for the region examined.
Bacterial contamination of the pump results in the production of enzymes that scavenge hydrogen peroxide. Therefore, the system should be flushed periodically. The addition of a preservative (Proclin) to the mobile phase is also recommended. When setting up the method for the first time, it is critical to passivate the system to ensure a clean system.
Acetylcholine and choline are hygroscopic. Therefore, the solids should be allowed to equilibrate to room temperature before weighing. The solids should be stored in a freezer. Working standards can be refrigerated for up to 2 weeks at 4°C.
Columns should not be in contact with organic solvents such as methanol or acetonitrile. If the system is not to be used for some time, the flow rate of the mobile phase should be lowered or the columns should be washed and stored at 4°C.
A decrease in sensitivity may be caused by bacterial contamination, loss of enzymatic activity in the IMER, a dirty electrode, or insufficient oxygen in the mobile phase.
Amino acids
Deterioration of the OPA reagent can lead to decreased sensitivity. The pH must be basic and 2-mercaptoethanol must be present as the nucleophile for the reaction to yield fluorogenic amino acid isoindole derivatives. Therefore, stock OPA reagent should be prepared weekly. The OPA reaction is also dependent on an aqueous medium. The reaction stops when the OPA/sample mixture is injected into the HPLC system, as a result of the lower pH and presence of methanol in the mobile phase. It is important to dilute the stock OPA reagent with an aqueous solution other than the mobile phase, preferably with the perfusate buffer used in the collection of microdialysates. Un-reacted OPA will elute immediately after aspartate. The presence of this peak can indicate improper mixing of the sample with the OPA reagent, resulting in an underestimation of microdialysate amino acid concentrations. Note that the amino acids asparagine and histidine may appear as peaks directly preceding and following glutamate elution. Detection of amino acids (e.g., serine and glycine) in an OPA-derivatized perfusion buffer blank indicates the presence of bacterial contamination. Amino acids can be detected in microdialysate samples that have been stored frozen at −70°C using this protocol.
The main problems encountered with this method are exogenous contaminants, increases in column pressure, especially when using microbore columns, and high background due to air in the system.
Contrary to other neurotransmitters like catecholamines, indoleamines and acetylcholine, amino acids are very stable at room temperature. They are also ubiquitous in dust, sweat, and organic matter. For this reason, exogenous contamination of samples and solutions is common. It is good practice to wear gloves when handling solutions and to thoroughly clean glassware. It is also necessary to run blanks of the aCSF or Ringer solutions used for preparing standards and running the dialysis experiments.
Increases in HPLC system pressure are also a common problem. The microbore columns are especially sensitive to clogging by particulate matter due to the small inner diameter. Adequate filtering of all the solutions is required. It is advisable to install a precolumn filter with minimal dead volume to trap any particulate matter coming from the injector (even if samples are clean, a faulty valve seal or other moving components in the injector can be the source of particles). Sudden increases in pressure are likely caused by particulate matter trapped in the filter. Sonicating or replacing the filter normally takes care of the problem. Eventually, pressure builds up over time. If replacing the filter does not help, this means that pressure has built up in the column. The repetitive acetonitrile flushes pose a strain on the column packing and eventually it will become loose and pack at the end of the column, increasing the pressure. Sometimes the life of the column can be lengthened by reversing the column and flushing with 40% acetonitrile for several hours (disconnect the column from the detector when attempting this). Finally, slow buildup in pressure may also be due to accumulation of proteins. Since dialysates do not contain proteins, accumulation of proteins in the column likely reflects bacterial contamination in the system upstream from the column. The precolumn filter will not trap proteins in solution. In this case, a guard column may be installed instead of the precolumn filter.
Occasionally, a gradual increase of background may be observed >1 hr, followed by a complete loss of signal. This may be fixed by either preparing a fresh degassed mobile phase or sometimes just degassing the old mobile phase. This problem is related to the high concentration of acetonitrile in the mobile phase since it is normally observed with the GABA mobile phase and it seems related to oxygen in the mobile phase quenching the fluorescent signal.
CE-LIF
Since the CE-LIF technique was developed for the analysis of brain extracellular fluid obtained by microdialysis, the derivatization procedure has to be performed on small volumes and with standards dissolved in the perfusion fluid. The derivatization rate is typically not affected by the higher ionic strength of biological samples when compared to standards in aqueous solution, as evidenced by lack of variation in peak areas when samples are prepared either in water or aCSF (A. Zapata, V.I. Chefer, T.S. Shippenberg, and L. Denoroy, unpub. observ).
The rate of derivatization, stability of the labeled amino acids, and amino acid quantification varies for each amino acid. For example, the reaction of NDA/NaCN with GABA is quicker than with Glu and Asp. This is probably due to the lower steric hindrance in the vicinity of the amine group of GABA relative to other amino acids, similar to that observed after derivatization of histidine and histamine with NDA/NaCN (Zhang and Sun, 2004). In contrast, derivatives of GABA seems less stable that those of Glu and Asp; this may be due to the greater hydrophilic properties of Glu and Asp derivatives.
Implementation of high sampling rate
In vitro response to rapid step changes that occur in the external medium in which the microdialysis probe is placed have shown that rapid fluctuations (<5 sec) of the external medium produce significant responses of the system. Thus, the time spent by the solutes in crossing the membrane is not a limiting factor for monitoring short-lasting events. However, high-sampling-rate microdialysis can be performed only if dead volumes are minimized both at the outlet and at the inlet (if local application of drugs by reverse dialysis is to be carried out) of the dialysis system.
The local application of drugs through the microdialysis probe is performed by switching perfusion fluid from aCSF to an aCSF containing known concentrations of drugs. The control animals do not receive any drug, but switching between two identical perfusion fluids must be performed to take into account short-lasting flow disturbances, which become more apparent with short collection times.
On the other hand, microdialysis experiments on awake animals require long dialysis tubing to allow free movement of the animal. However, the dead volume of the tubing, i.e., between the active dialysis membrane and the outlet of the derivatization system, must be minimized when frequent collection is performed. Indeed, if the interval of time between dialysis and collection is greater than the sampling time, solutes could diffuse significantly between sample plugs. The dead volume can be calculated, but the dead time has to be determined experimentally in order to correlate neurochemical results to physiological events for in vivo studies (e.g., behavior or electroencephalographic recordings).
Reproducibility of migration times
A lack of reproducibility in the migration time of an analyte could be due to excessive variability in the electroosmotic flow. Changing to a new capillary may take care of this problem.
Storage and preservation of samples
Storage time is critical in maintaining the original composition of microdialysis samples. Different analytes have different preservation times.
Contamination in reagent blank
This contamination is likely to be due to the presence of amino acids on the glassware. Purity of water and reagents should also be investigated.
Anticipated Results
Catecholamines
A chromatogram showing retention times and peak heights of monoamines and metabolites in mouse striatal tissue samples using mobile phase I is shown in Figure 7.4.2. Note the use of the internal standard DHBA. A basal dialysate sample obtained from mouse nucleus accumbens using mobile phase II is shown in Figure 7.4.3.
Figure 7.4.2.
Representative chromatogram of catecholamines using mobile phase buffer I. Chromatogram of striatal tissue levels of catecholamines and metabolites. DHBA was included as the internal standard. The oxidation potential was set at +650 mV.
Figure 7.4.3.
Chromatogram for detection of dopamine (DA) in a basal dialysate sample from mouse nucleus accumbens. A 1-mm probe was used. Dialysis flow rate was 0.7 μl/min and 8 μl were injected. Ascorbic acid was included in the aCSF and it elutes as the early, major peak. The number above the DA peak is the dialysate concentration (nM) estimated from the external standard curve. The oxidation potential was set at +700 mV.
Acetylcholine
A chromatogram of a mouse striatal dialysate is shown in Figure 7.4.4. The aCSF contained 30 nM neostigmine and 12 μl were injected into the HPLC system. The use of the precolumn choline oxidase catalyst IMER results in elimination of the choline peak. In combination with the wired peroxidase electrode, this system allows for a detection limit for acetylcholine of 2 to 3 nM.
Figure 7.4.4.
Chromatogram of acetylcholine in mouse ventral striatum. A 2-mm probe was used, dialysis flow rate was 0.7 μl/min. The aCSF included 30 nM neostigmine. The injection volume was 12 μl. The HPLC system included a choline oxidase/catalase precolumn IMER and an acetylcholinesterase/choline oxidase postcolumn IMER. The electrode is a wired peroxidase glassy carbon electrode operating at +100 mV in the reduction mode. The number above the Ach peak is the dialysate concentration (nM) estimated from the external standard curve.
Amino acids (see Basic Protocol 3)
Amino acids with a wide range of polarities can be detected using the HPLC fluorescence detection protocol (see Fig. 7.4.5). Later-eluting peaks such as GABA may broaden with isocratic elution (Fig. 7.4.5A). In general, increasing the methanol concentration of the mobile phase will decrease the retention times of the derivatized sample components. Full resolution of all amino acids, including hydrophobic residues (e.g., valine, isoleucine, and leucine), requires a more complex series of isocratic elution steps coupled with gradient elution displacement (Umagat et al., 1982). The regional distribution of major amino acids in rat-brain microdialysates using OPA derivatization has been published (Tossman et al., 1986). Note that changes in glutamine and taurine evoked by high potassium occur acutely (see Fig. 7.4.5B,C); however, detection of glutamate release in these microdialysates may be offset by rapid uptake via glutamate transporters. Inclusion of the competitive-uptake inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (Massieu et al., 1995) will augment recovery of acutely released glutamate.
Figure 7.4.5.
Chromatograms of OPA-derivatized amino acids. (A) Chromatogram of standard mixture prepared in Ringer's solution at a concentration of 1 ng/ml for each amino acid. A volume of 20 μl of this mixture was loaded onto a C18 column and eluted isocratically (using a 33:67 mixture of methanol and sodium phosphate buffer at a flow rate of 1 ml/min) followed by fluorimetric detection (excitation wavelength, 338 nm; emission wavelength, 450 nm). Components in order of elution are aspartate (Asp), glutamate (Glu), serine (Ser), glutamine (Gln), arginine (Arg), glycine (Gly), threonine (Thr), taurine (Tau), alanine (Ala), and γ-aminobutyrate (GABA). (B,C) Amino acids in microdialysates of murine hippocampus (CMA11 1-mm probe collected at 1 μl/min). Panel B shows results for steady-state microdialysate and panel C shows microdialysate 40 to 60 min after perfusion with Ringer's solution containing 100 mM KCl. Note that this depolarizing stimulus substantially alters the levels of Glu, Gln, and Tau.
A disadvantage of the OPA reaction is its lack of suitability for the detection of thiols in microdialysates. N-(1-pyrenyl)maleimide is an appropriate derivatizing agent (Winters et al., 1995). The preparation time for mobile phase and OPA would be ~30 to 45 min for the novice. These components should be prepared the day before the run; the working OPA diluted from stock works best after overnight storage and the HPLC column requires a mobile phase equilibration period.
The detection of GABA, in particular, suffers from a long retention time, which dramatically increases the time needed to analyze a single microdialysis experiment. In addition, the longer retention time results in decreased sensitivity. If only GABA (or Glu) is of interest, use of the Alternate Protocols will decrease the chromatographic running time and, in the case of GABA, significantly increase assay sensitivity. Chromatograms of mouse nucleus accumbens dialysate are shown in Figure 7.4.6.
Figure 7.4.6.
Chromatograms of Glu (A) and GABA (B) in mouse nucleus accumbens dialysis samples. The system used is described in Basic Protocol 3. A 1-mm probe was used and the dialysis flow rate was 0.7 μl/min. A volume of 4 μl of dialysate was derivatized with 6 μl of OPA working solution, and 12 μl of Borax buffer were added to the final mixture. Total injection volume was 20 μl. The numbers above the Glu and the GABA peaks are the concentrations (μM and nM, respectively) in dialysate estimated from the external standard curve. The big peak eluting just before 7 min is the acetonitrile flushing injection.
Amino acids by CE-LIF (see Basic Protocol 4)
Electropherograms showing separation of Glu and Asp (Fig. 7.4.7A) by CZE and GABA, Glu and Asp (Fig. 7.4.7B) by MEKC are shown in Figure 7.4.7. Note the separation of the internal standards. Both electropherograms correspond to dialysis samples. See figure legend for dialysis and analysis conditions.
Figure 7.4.7.
(A) Electropherogram of Glu and Asp in rat striatum. A 3-mm probe was used and the dialysis rate was 1 μl/min. The sample was analyzed using the system described in Basic Protocol 4. Alpha amino adipic acid (AAd) was included as an internal standard. The numbers above the Glu and Asp peaks are the concentrations (μM) in dialysate estimated from the external standard curve. The big peak migrating just before AAd is phenylethanolamine. (B) Electropherogram of GABA, Glu, and Asp in mouse hippocampus. A 2-mm probe was used and the dialysis flow rate was 1 μl/min. Sample was collected using the system described in Alternate Protocol 3. Cysteic acid was included an internal standard (IS). The numbers above the GABA, Glu and Asp peaks are the concentrations (nM) in estimated from the external standard curve.
Literature Cited
- Abercrombie ED, Keller RW, Jr., Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Anton AH, Sayre DF. A study of the factors affecting the aluminum oxidetrihydroxyindole procedure for the analysis of catecholamines. J. Pharmacol. Exp. Therap. 1962;138:360–375. [PubMed] [Google Scholar]
- Babu CV, Chung BC, Lho DS, Yoo YS. Capillary electrophoretic competitive immunoassay with laser-induced fluorescence detection for methionine-enkephalin. J. Chromatogr. A. 2006;1111:133–138. doi: 10.1016/j.chroma.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT. Capillary liquid chromatography with MS3 for the determination of enkephalins in microdialysis samples from the striatum of anesthetized and freely-moving rats. J. Mass Spectrom. 2005;40:146–153. doi: 10.1002/jms.733. [DOI] [PubMed] [Google Scholar]
- Bowser MT, Kennedy RT. In vivo monitoring of amine neurotransmitters using micro-dialysis with on-line capillary electrophoresis. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Church WH, Justice JB. On-line smallbore chromatography for neurochemical analysis in the brain. In: Giddings JC, Grushka JC, Brown RP, editors. Advances in Chromatography. Marcel Dekker; New York: 1989. pp. 165–194. [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr., Neill DB. Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain Res. 1987;412:397–399. doi: 10.1016/0006-8993(87)91150-4. [DOI] [PubMed] [Google Scholar]
- Damsma G, Westerink BHC, Imperato A, Rollema H, de Vries JB, Horn AS. Automated brain dialysis of acetylcholine in freely moving rats: Detection of basal acetylcholine. Life Sci. 1987;41:873–876. doi: 10.1016/0024-3205(87)90184-6. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Middaugh LD, Becker HC. Voluntary ethanol drinking in mice and ethanol concentrations in the nucleus accumbens. Brain Res. 2007;1138:208–213. doi: 10.1016/j.brainres.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Kehr J. Determination of glutamate and aspartate in microdialysis samples by reversed-phase column liquid chromatography with fluorescence and electrochemical detection. J. Chromatogr. B. 1998a;708:27–38. doi: 10.1016/s0378-4347(97)00677-4. [DOI] [PubMed] [Google Scholar]
- Kehr J. Determination of γ-aminobutyric acid in microdialysis samples by microbore column liquid chromatography and fluorescence detection. J. Chromatogr. B. 1998b;708:49–54. doi: 10.1016/s0378-4347(97)00657-9. [DOI] [PubMed] [Google Scholar]
- Kehr J, Dechent P, Kato T, Ogren SO. Simultaneous determination of acetylcholine, choline and physostigmine in microdialysis samples from rat hippocampus by microbore liquid chromatography/electrochemistry on peroxidase redox polymer coated electrodes. J. Neurosci. Methods. 1998;83:143–150. doi: 10.1016/s0165-0270(98)00074-0. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Watson CJ, Haskins WE, Powell DH, Strecker RE. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr. Opin. Chem. Biol. 2002;6:659–665. doi: 10.1016/s1367-5931(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Kissinger PT, Bruntlett CS, Davis GL, Felice LJ, Riggin RM, Shoup RE. Recent developments in the clinical assessment of the metabolism of aromatics by high-performance, reversed-phase chromatography with amperometric detection. Clin. Chem. 1977;23:1449–1455. [PubMed] [Google Scholar]
- Koslow SH, Racagni G, Costa E. Mass fragmentographic measurement of norepinephrine, dopamine, serotonin and acetylcholine in seven discrete nuclei or rat diencephalon. Neuropharmacology. 1974;13:1123–1130. doi: 10.1016/0028-3908(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Lanckmans K, Van Eeckhaut A, Sarre S, Smolders I, Michotte Y. Capillary and nano-liquid chromatography-tandem mass spectrometry for the quantification of small molecules in microdialysis samples: Comparison with microbore dimensions. J. Chromatogr. A. 2006;1131:166–175. doi: 10.1016/j.chroma.2006.07.090. [DOI] [PubMed] [Google Scholar]
- Lindroth P, Mopper K. High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal. Chem. 1979;51:1667–1674. [Google Scholar]
- Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Microdialysis of extracellular endogenous opioid peptides from rat brain in vivo. Neuroscience. 1989;33:549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- Massieu L, Morales-Villagrán A, Tapia R. Accumulation of extracellular glutamate by inhibition of its uptake is not sufficient for inducing neuronal damage: An in vivo microdialysis study. J. Neurochem. 1995;64:2262–2272. doi: 10.1046/j.1471-4159.1995.64052262.x. [DOI] [PubMed] [Google Scholar]
- Parrot S, Sauvinet V, Riban V, Depaulis A, Renaud B, Denoroy L. High temporal resolution for in vivo monitoring of neurotransmitters in awake epileptic rats using brain microdialysis and capillary electrophoresis with laser-induced fluorescence detection. J. Neurosci. Methods. 2004;140:29–38. doi: 10.1016/j.jneumeth.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Procedures for microdialysis with smallbore HPLC. In: Robinson TE, Justice JB, editors. Microdialysis in the Neurosciences. Elsevier Science Publishers; New York: 1991. pp. 117–154. [Google Scholar]
- Potter PE, Meek JJ, Neff NH. Acetylcholine and choline in neuronal tissue measured by HPLC with electrochemical detection. J. Neurochem. 1983;41:188–194. doi: 10.1111/j.1471-4159.1983.tb13668.x. [DOI] [PubMed] [Google Scholar]
- Powell PR, Ewing AG. Recent advances in the application of capillary electrophoresis to neuroscience. Anal. Bioanal. Chem. 2005;382:581–591. doi: 10.1007/s00216-005-3075-x. [DOI] [PubMed] [Google Scholar]
- Prata C, Bonnafous P, Fraysse N, Treilhou M, Poinsot V, Couderc F. Recent advances in amino acids analysis by capillary electrophoresis. Electrophoresis. 2001;22:4129–4138. doi: 10.1002/1522-2683(200111)22:19<4129::AID-ELPS4129>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Robert F, Bert L, Parrot S, Denoroy L, Stoppini L, Renaud B. Coupling on-line brain microdialysis, precolumn derivatization and capillary electrophoresis for routine minute sampling of O-phosphoethanolamine and excitatory amino acids. J. Chromatogr. A. 1998;817:195–203. doi: 10.1016/s0021-9673(98)00321-5. [DOI] [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal. Chem. 1971;43:880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Sauvinet V, Parrot S, Benturquia N, Bravo-Moraton E, Renaud B, Denoroy L. In vivo simultaneous monitoring of gamma-aminobutyric acid, glutamate, and L-aspartate using brain microdialysis and capillary electrophoresis with laser-induced fluorescence detection: Analytical developments and in vitro/in vivo validations. Electrophoresis. 2003;24:3187–3196. doi: 10.1002/elps.200305565. [DOI] [PubMed] [Google Scholar]
- Tossman U, Jonsson G, Ungerstedt U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol. Scand. 1986;127:533–545. doi: 10.1111/j.1748-1716.1986.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Umagat H, Kucera P, Wen L-F. Total amino acid analysis using pre-column fluorescence derivatization. J. Chromatogr. 1982;239:463–474. [Google Scholar]
- Welch AS, Welch BL. Solvent extraction method for simultaneous determination of norepinephrine, dopamine, serotonin, and 5-hydroxyindoleacetic acid in a single mouse brain. Anal. Biochem. 1969;30:161–179. doi: 10.1016/0003-2697(69)90387-x. [DOI] [PubMed] [Google Scholar]
- Winters RA, Zukowski J, Ercal N, Matthews RH, Spitz DR. Analysis of glutathione, glutathione disulfide, cysteine, homocysteine, and other biological thiols by high-performance liquid chromatography following derivatization by N-(1-pyrenyl)maleimide. Anal. Biochem. 1995;227:14–21. doi: 10.1006/abio.1995.1246. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Sharp T, Marsden CA, Ungerstedt U. In vivo measurement of dopamine and its metabolites by intracerebral dialysis: Changes after D-amphetamine. J. Neurochem. 1983;41:1769–1773. doi: 10.1111/j.1471-4159.1983.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Zhang LY, Sun MX. Determination of histamine and histidine by capillary zone electrophoresis with pre-column napthtalene-2,3-dicarboxaldehyde derivatization and fluorescence detection. J. Chromatogr. A. 2004;1040:133–140. doi: 10.1016/j.chroma.2004.03.051. [DOI] [PubMed] [Google Scholar]