Abstract
Background
Kisspeptins bind to the G protein coupled receptor (GPR54), to activate hypothalamic luteinizing hormone releasing hormone (LHRH) secretion at the time of puberty. Alcohol (ALC) causes depressed prepubertal LHRH release, resulting in depressed luteinizing hormone (LH) secretion and delayed puberty. Because KiSS-1 and GPR54 are important to the onset of puberty, we assessed the effects of chronic ALC administration on basal expression of these puberty related genes within the reproductive hypothalamus, as well as hormones and transduction signaling pathways contributing to their activity.
Methods
Immature female rats were fed a liquid-diet containing ALC for 6 days beginning when 27 days old. Controls received either companion isocaloric liquid-diet or rat chow and water. Animals were decapitated on day 33, in the late juvenile stage of development. Blood was collected for assessment of serum hormone levels. Brain tissues containing the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei were obtained for assessing expression of specific puberty related genes and proteins.
Results
KiSS-1 mRNA levels in the AVPV and ARC nuclei were suppressed (p<0.01) in the ALC-treated rats. GPR54 gene and protein expressions were both modestly increased (p<0.05) in AVPV nucleus, but not in ARC nucleus. ALC exposure also resulted in suppressed serum levels of insulin-like growth factor −1 (IGF-1), LH and estradiol (E2). Since IGF-1, in the presence of E2, can induce expression of the KiSS-1 gene, we assessed the potential for ALC to alter IGF-1 signaling in the reproductive hypothalamus. IGF-1 receptor gene and protein expressions were not altered. However, protein expression of phosphorylated Akt, a transduction signal used by IGF-1, was suppressed in the AVPV (p<0.05) and ARC (p<0.01) nuclei.
Conclusions
ALC causes suppressed KiSS-1 gene expression in the reproductive hypothalamus; hence, contributing to this drug’s ability to cause suppressed LHRH secretion and disruption of the pubertal process. We suggest that this action, at least in part, is through altered IGF-1 signaling.
Keywords: Alcohol, KiSS-1 gene, Hypothalamus, Puberty
INTRODUCTION
The age at the time when puberty is initiated is variable and depends on a complex series of centrally mediated events resulting in the increased pulsatile secretion of luteinizing hormone releasing hormone (LHRH) from the hypothalamus. This increase in LHRH release at puberty appears to utilize an interactive participation of neuronal circuits and glial cells (Ojeda et al., 2006). Furthermore, it is well known that neuronal and glial functions can be further influenced by metabolic signals, genetic and environmental influences, as well as drugs of abuse.
Alcohol (ALC) is a drug of abuse that is capable of altering functions within the preoptic area (POA) and hypothalamus, the areas of the brain that play a pivotal role in synchronizing events leading to the onset of mammalian puberty. This is important since there has been a growing trend indicating that ALC use and abuse often occurs during early adolescence (Johnson et al., 2006). The increasing incidence of ALC use by adolescents is noteworthy because it represents a potentially vulnerable time for these youth, since their tolerance to the drug is likely low. While endocrine related case reports involving ALC use by adolescent humans are limited in number and scope, it has been revealed that ALC can cause altered puberty related hormones in adolescent boys and girls (Block et al., 1993; Diamond et al., 1986). Both rats and rhesus monkeys have been used as animal models to more closely assess the effects of ALC on puberty related events. Studies using immature rats have shown that chronic ALC exposure causes suppressed circulating levels of reproductive hormones (Dees and Skelley, 1990; Srivastava et al., 1995; Srivastava et al., 2002a; Steiner et al.,1997), and can delay both female (Bo et al., 1982; Dees and Skelley, 1990; Emanuele et al., 2002) and male (Anderson et al., 1981; Ramaley, 1982) puberty. Using developing female rhesus monkeys, we showed that chronic ALC administration inhibits secretion of puberty related hormones, including LH and E2, and delayed the development of a normal pattern of menstruation (Dees et al., 2000). Studies using both rats (Ching et al., 1988; Dees et al., 2005; Hiney and Dees, 1991; Hiney et al., 2003; Ogilvie and Rivier, 1997) and monkeys (Dissen et al., 2004) have provided evidence showing that the ALC-induced suppression in LH secretion is due to an action within the hypothalamus to inhibit LHRH secretion. Whether ALC only acts directly on LHRH neurons and/or their nerve terminals, or whether the drug acts first to alter the action of a specific signal or upstream gene that is known to contribute to the central activation of the LHRH releasing system at puberty is not known.
KiSS-1, a metastasis suppressor gene, is responsible for the synthesis of kisspeptin. This family of peptides are ligands of the G-protein-coupled receptor 54 (GPR54), and play a key role in the timing of mammalian puberty. The importance of the KiSS-1/GPR54 system to reproduction was revealed when a mutation of GPR54 in human (de Roux et al., 2003) and a deletion of the GRP54 locus in mice (Seminara et al., 2003) resulted in hypogonadotropic hypogonadism and delayed pubertal development. Soon thereafter, it was shown that the expression of both KiSS-1 and GPR54 genes increase at puberty (Navarro et al., 2004a), and that the KiSS-1 products, kisspeptin 54 and kisspeptin 10, act within the hypothalamus to stimulate LH secretion in rats and rhesus monkeys (Navarro et al., 2004a; Shahab et al., 2005; Thompson et al., 2004), and advance vaginal opening in rats (Navarro et al., 2004b). Because of the involvement of the KiSS-1/GPR54 system at the time of puberty, we assessed in the present study whether short-term ALC administration would alter the expression of these puberty related genes in the reproductive hypothalamus. Furthermore, we determined whether their responses were associated with alterations in the circulating levels of important puberty related hormones, and then began to assess potential upstream actions.
MATERIALS AND METHODS
Animals and Surgery
Eighteen-day pregnant female rats of the Sprague-Dawley line were purchased from Charles River (Boston, MA) and allowed to deliver pups normally in the Texas A&M University lab animal facility. Female pups were weaned at twenty-one days of age and housed three per cage under controlled conditions of light (lights on, 0600h; lights off, 1800h) and temperature (23 C), with ad libitum access to food and water. All procedures performed on the animals were approved by the University Animal Care and Use Committee and in accordance with the NAS-NRC Guidelines for the Care and Use of Laboratory Animals. Each animal was anesthetized at 23 days of age with 2.5% tribromoethanol (0.5ml/60g body weight) and was surgically implanted with a permanent intragastric cannula by a procedure that has been described previously (Dees et al., 1984). All animals were allowed to recover from surgery for 4 days prior to the beginning of the experiments.
Experimental Procedure
When the rats were 26 days old, they were weighed and divided into three groups, with each group being assigned one pup from a given litter. Female littermates not used were assigned to another study. Group 1 consisted of 50 animals that received a 5% ALC liquid-diet, and group 2 consisted of 25 animals that received the companion isocaloric control liquid diet (Bioserve, Inc., Frenchtown, NJ). Group 3 served as an additional set of controls that consisted of 28 animals that were cannulated and maintained on rat chow and water, ad libitum, throughout the experiment. Since we have never detected differences between the two control groups in any of the parameters assessed over the last twenty years of using the following dosing regimen, the number of animals used in the two control groups together totaled approximately the same number of animals as used in group 1. Each group received their respective diet by a regimen described previously (Dees and Skelley, 1990; Srivastava et al., 1995) and modified only slightly (Srivastava et al., 2002a). Briefly, on day 27, the liquid-diets were administered in such a manner that 6 ml of the respective diet was injected via the intragastric cannula (four injections of 1.5 ml each) equally dispersed over the lights-on period, and then 30 mls of diet was available ad libitum (bottle) during the lights-off period. To provide an adequate food supply for these immature growing animals, beginning on day 28 and again on days 30 and 32, the amount of diet injected via the cannula was increased by 0.5 ml per injection. Also, at these same times, the amount of diet made available each night in the bottle was increased to 35, 40, and 50 ml, respectively. Thus, by day 33, each animal was receiving the maximum of four intragastric injections (3.0 ml each) of diet during the lights-on period and 50 ml of diet, ad libitum, during the lights-off period. A tail tip blood sample was drawn at 1500 hours on day 5 to assure the blood ALC concentrations (BACs) were similar to levels observed in our previous studies at this point in the experiment. During the study, if an animal did not consume the full lights-off portion of the diet the difference was made up via gastric infusion the following day, and on occasion, if a given animal appeared intoxicated during the afternoon hours it was infused with the control liquid-diet in place of the ALC liquid-diet. We have reported previously that this procedure enabled the liquid-diet control group to grow at exactly the same rate as the chow-fed control group (Dees and Skelley, 1990; Srivastava et al., 2002a). Additionally, this method ensures not only that all liquid diet-fed animals received approximately the same number of calories per day, but also that all of the ALC-treated animals received the same amount of ALC per day.
Tissue Collection
On the morning of day 33, all animals were weighed and killed by decapitation 1.5 hours after their last gastric infusion. The animals were confirmed to be in the late juvenile stage of pubertal development as assessed by criteria described previously (Dees and Skelley, 1990; Srivastava et al, 1995). Since none of the ALC-treated rats had entered the peripubertal period, it was important that only late juvenile controls be used for comparisons with the ALC-treated animals; hence, the few control animals that had entered the peripubertal period by day 33 were not utilized. Serum was stored at −80 C until assayed for BAC, LH, IGF-1 and E2. The brains were removed and the preoptic - hypothalamic region was dissected out and then divided into two blocks of tissue as recently detailed (Hiney et al., 2009). Briefly, one block contained the anteroventral periventricular (AVPV) nucleus and the other the arcuate (ARC) nucleus. The tissue block containing the AVPV nucleus was cut approximately 1 mm rostral to the optic chiasm (OC), then again at the caudal border of the OC. The block was then formed by making cuts along the lateral borders of the OC and the dorsal border of the anterior commissure. The block containing the ARC nucleus extended from the previous cut to the mammillary bodies, caudally. This block was formed by making cuts along the borders of the hypothalamic sulci laterally, and along the dorsal border of the thalamus. These two specific nuclei were assessed because they are the ones that contain the KiSS-1 neurons contributing to the regulation of LHRH secretion. While the two tissue blocks are slightly larger than the respective nuclei which they contain, the immediate surrounding tissue is devoid of KiSS-1 neurons. Tissues were frozen on dry ice at −80 C until analyzed by Real-time PCR or Western blot analysis.
Isolation of total RNA
Total RNA was initially isolated from the brain tissues by homogenizing in TRIzol Reagent (Invitrogen, CA). The homogenates were further extracted for RNA using QIAGEN RNeasy kit and treated with RNase-free DNase I according to the manufacturer’s instructions (Qiagen Inc., Valencia, CA). The integrity of the RNA was checked by the visualization of the ethidium bromide-stained 28S and 18S ribosomal RNA bands. Total RNA was quantitated spectrophotometrically by absorbance at 260 nm in a model Smartspec 3000 spectrophotometer (Bio-Rad Laboratories, Hercules, CA)
Reverse Transcription and real-time quantitative PCR
Total RNA (1µg) from each sample was denatured at 65 C for 5 min and was used for reverse transcription with oligo (dT) using SuperScript III First-strand Synthesis System (Invitrogen Life Tech., CA). Real-time PCR was performed using ABI PRISM 5700 sequence detection system as described previously (Hiney et al., 2009). Briefly, PCR reactions were performed in 25 µl reactions containing 2 µl cDNA, 500nM primer pairs and 1X SYBR green PCR master mix in 96-well plates. PCR primers for the analysis were designed according to the guidelines of Applied Biosystems with the help of Primer Express 2.0 software (Applied Biosystems, Foster City, CA). Each primer was checked for the absence of cross-reactivity by BLAST search. Primers, specific for the house keeping gene, β-actin, were also run in all reactions separately under the same experimental conditions to normalize for the amount of RNA in the initial reverse transcription reaction. A reaction without reverse transcriptase was also performed to rule out the possibility of any undesired amplicons. The primers for the PCR reactions are as follows: rat KiSS-1[GeneBank accession AY196983], forward, 5’-GCTGCTGCTTCTCCTCTGTGT-3’, reverse, 5’-CTGTTGGCCTGTGGGTTCA-3’(product size 88 bp); rat GPR54 [GeneBank accession AF115516], forward, 5’-GCGGCCACAGATGTCACTTT-3’, reverse, 5’-AGGTGGGCAGCGGATAGAG-3’(product size 70 bp); rat IGF-1R [GeneBank accession NM_052807 ], forward, 5’-ATTGCCTCGGAATTGGAGAA-3’, reverse, 5’-ATGGGAATGGCGGATCTT-3’(product size 75 bp); rat β-actin [GenBank accession NM_031144], forward, 5’-TCTGTGTGGATTGGTGGCTCTA-3’, reverse, 5’-CTGCTTGCTGATCCACATCTG-3’(product size 69 bp). The PCR cycling conditions were 95 C for 10 min, followed by 40 cycles at 95 C for 15seconds and 60 C for 1 minute. PCR product purity was confirmed by dissociation curve analysis for each gene at the end of the PCR reaction. In this regard, each amplicon yielded a single peak and did not show any peak when the template was not included in the PCR reaction. Additionally, each PCR-generated DNA product was electrophoresed onto 2% agarose gel containing ethidium bromide, which showed a single band of the expected size. This confirms the specificity of the primers in which there was no formation of primer-dimers. The relative levels of expression for each gene were determined from the raw data as described by Hettinger et al. (2001) using delta-delta CT method.
Immunoblotting
Brain tissues were homogenized in 1X PBS, 1% Igepal CA 630, 0.5% sodium deoxycholate, 0.1% SDS, 1mM PMSF, 10 µg/ml aprotinin, 10µg/ml leupeptin, 1 mM sodium orthovanadate at 4 C. The homogenates were incubated on ice for 30 minutes and centrifuged at 12,000Xg for 15 min. The concentration of total protein in the resulting supernatants was then determined by the Bradford protein assay (Bio-Rad Laboratories, Richmond, CA) using bovine serum albumin as standard. Immunoblot analysis was performed by solubilizing the proteins (100µg) in a sample buffer containing 25 mM Tris Cl, pH 6.8, 1% SDS, 5% β-mercaptoethanol, 1mM EDTA, 4% glycerol, and 0.01% bromophenol blue and electrophores through a 12% SDS-PAGE under reducing conditions. The separated proteins were electrophoretically transblotted onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% nonfat dried milk/0.05% Tween 20 in PBS (pH 7.4) for 3 hr and subsequently incubated at 4 C overnight with goat anti- GPR54 (1:200; Santa Cruze Biotech, CA) or rabbit anti-IGF-1R, anti-phospho-Akt (Ser 473, 1;300; Abcam Inc, Cambridge, MA) or anti-total Akt (1µg/ml; Abcam Inc, Cambridge, MA). Following incubation, membranes were washed in PBS buffer containing 0.05% Tween-20 and then incubated with horseradish peroxidase-labeled donkey anti-goat IgG (1;10000; Santa Cruze Biotech, CA) for GPR54 and goat anti-rabbit IgG (1:12000; Abcam Inc., Cambridge, MA) for phospho-Akt or total Akt for 2 hr at room temperature. After washing, the specific signals were detected with the enhanced chemiluminiscence method (Western Blot Chemiluminescence Reagent Plus, NEN) and quantified with scanning densitometry using Quantity one software (Bio-Rad, Hercules, CA). Subsequently, membranes were stripped with 100mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris HCl, pH 6.7 and reprobed with a goat anti-mouse polyclonal antibody to the β-actin and anti-goat horseradish peroxide –conjugated secondary antibody, to normalize for the amount of sample loading. Following washing, the detection of proteins and quantitation was done as described above.
Radioimmunoassays
The serum levels of IGF-1, LH and E2 were measured as described previously (Hiney et al., 1996). Serum IGF-1 was measured by a specific rat IGF-1 radioimmunoassay kit purchased from Diagnostic System lab, Inc. (Webster, TX). Values were expressed as nanograms per milliliter of serum. The sensitivity of the assay was 150 ng/ml, and the intra-assay variation was less than 10%. Serum LH levels were measured by a kit purchased from the National Hormone and Pituitary Program, using the rLH-S-11 antiserum. The sensitivity of the assay was 0.07 ng/ml and the intra-assay variation was less than 5%. The E2 assay kit was purchased from Diagnostic Products Corp. (Los Angeles, CA). The sensitivity of this assay was 5pg/ml and the intra-assay variation was 5%.
Statistical Analysis
Initially, multiple comparisons were performed using ANOVA with post hoc testing using the Student-Newman-Keul’s multiple range test. Because no differences were detected in any of the assessments between the chow-fed and liquid diet-fed control groups, their data were subsequently combined. Final comparisons between the control and ALC-treated group were analyzed using Student’s t-test. These statistical tests were conducted with INSTAT software (GraphPad Software, San Diego, CA). Probability values less than 0.05 were considered significantly different.
Serum Alcohol Analysis
Blood alcohol concentrations (BACs) were assessed from tail tip blood samples collected on day 5 and from the trunk blood collected at the end of the experiment. Serum was transferred to microcentrifuge tubes for analysis of serum alcohol concentrations as described previously (Dees et al., 2005) by an enzymatic method using a diagnostic kit purchased from Genzyme, Oxford, CT.
RESULTS
There were no differences detected between the chow-fed and liquid diet-fed control animals with regard to body weight or daily weight gain during this 6 day study. Furthermore, no differences were detected between these groups regarding gene or hormonal measurements, which is in agreement with previous reports using this feeding regimen applied to prepubertal, growing animals (Dees and Skelley, 1990; Srivastava et al., 1995). Thus, control results were again combined and presented together in the following figures to simplify comparative descriptions. On the final day of the study, the mean ± SEM body weights of the ALC-treated animals were less (p<0.01) than the control animals (ALC: 74.6 ± 7.1 vs CON: 94.3 ± 9.8g), an effect associated with well-known ALC-induced suppressions in serum IGF-1 and growth hormone. The mean ± SEM BAC was 165 ± 9 mg/dl on day 5 at 1500 hours in the afternoon and 204 ± 15 mg/dl at the time the animals were killed 1.5 hours after their last gastric infusion of liquid-diet on day 6.
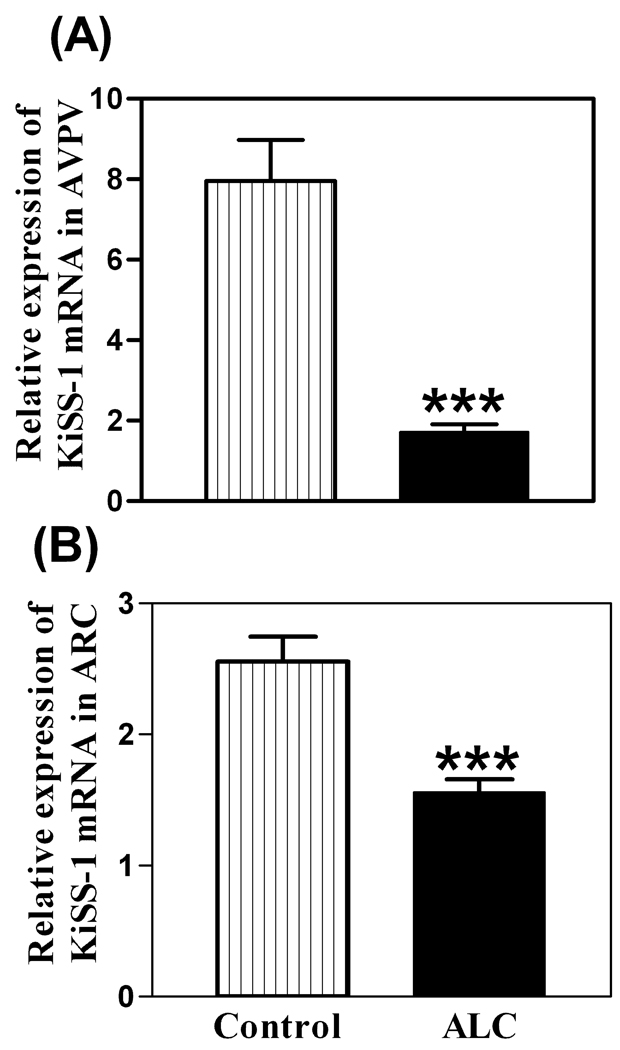
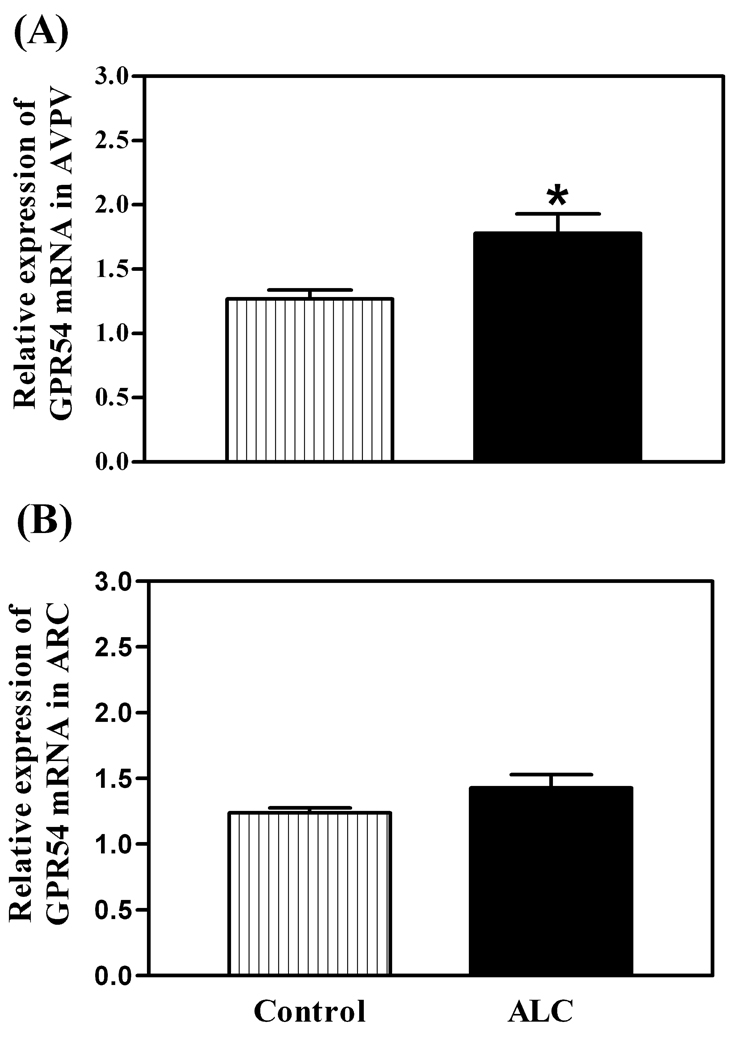
As shown in figure 1, the basal levels of KiSS-1 mRNA were decreased in both the AVPV (p<0.01) and ARC (p<0.01) nuclei of ALC-treated animals compared to control animals. The ALC caused a modest increase (p<0.05) in the basal expression of the GPR54 gene in the AVPV nucleus (figure 2A), but not in the ARC nucleus (figure 2B). Western blot analysis revealed that GPR54 protein expression following ALC administration was also modestly increased (p<0.05) in the AVPV nucleus (figure 3A and B), with no changes noted in the ARC nucleus (figure 3C and D). Table 1 demonstrates that these effects were associated with altered concentrations of specific puberty related hormones. In this regard, the ALC-treated animals showed suppressed serum levels of IGF-1 (p<0.001), LH (p<0.01) and E2 (p<0.001) compared to control animals.
Fig. 1.
Effect of chronic ALC exposure on basal KiSS-1 gene expression in AVPV (A) and ARC (B) nuclei of prepubertal female rats as determined by real-time PCR. Note that ALC markedly reduced expression of the KiSS-1 gene in both nuclei compared with control animals. The respective bars illustrate the mean (±SEM) of an N of 12 per group. ***p<0.001 versus control.
Fig. 2.
Effect of chronic ALC exposure on basal GPR54 gene expression in AVPV (A) and ARC (B) nuclei of prepubertal female rats as determined by real-time PCR. Note that the GPR54 mRNA levels, were increased in the AVPV nucleus compared with control animals. The respective bars illustrate the mean (±SEM) of an N of 12 per group. *p<0.05 versus control.
Fig. 3.
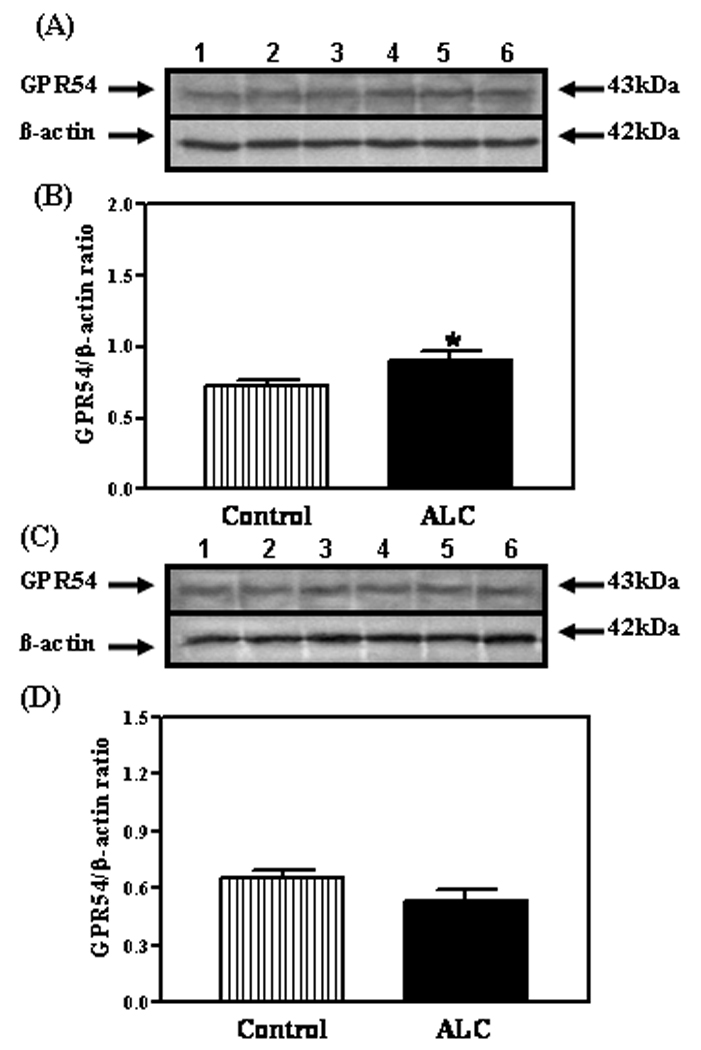
Effect of chronic ALC exposure on GPR54 protein expression in prepubertal female rats. (A) Representative Western immunoblot of GPR54 and β-actin proteins in the AVPV nucleus isolated from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing GPR54 protein expression in the AVPV nucleus. (C) Representative Western immunoblot of the GPR54 and β-actin proteins in the ARC nucleus isolated from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. (D) Densitometric quantitation of all of the bands from two blots assessing GPR54 protein expression in the ARC nucleus. These data were normalized to the internal control β-actin protein, and the densitometric units represent the GPR54/β-actin ratio. Note that GPR54 protein expression was modestly increased in the AVPV nucleus of the ALC-treated animals. The respective bars illustrate the mean (±SEM) of an N of 7 per group. *p<0.05 versus control.
Table 1.
Effects of ALC on mean (±SEM) concentrations of serum IGF-1, LH and E2.
| Hormone | Control (N=22) | ALC (N=21) |
|---|---|---|
| IGF-1 | 1009 ± 28 | 349 ± 38 *** |
| LH | 0.52 ± .06 | 0.25 ± .04 ** |
| E2 | 19.9 ± 1.2 | 11.9 ± 1.25 *** |
p<0.001;
p<0.01
Because we recently showed that IGF-1, in the presence of E2, can induce expression of the KiSS-1 gene (Hiney et al., 2009), we assessed the potential for ALC to alter IGF-1 signaling in the reproductive hypothalamus. IGF-1R gene (figure 4A and B) and protein (figure 4C–F) expressions were not altered following ALC administration. Subsequently, we assessed the effect of ALC on Akt, a transduction signal that is synergistically activated by IGF-1 and E2. We have shown the presence of phosphorylated Akt protein in the AVPV nucleus (figure 5A) and that ALC administration caused a decrease (figure 5B; p<0.05) in its expression in this region. Total Akt protein was also expressed in the AVPV nucleus (figure 5C), but was not affected by the ALC (figure 5D). Similarly, phosphorylated Akt protein was detected in the ARC nucleus (figure 6A), where the levels of its expression were suppressed (figure 6B; p<0.01) following ALC exposure. Total Akt was also observed in the ARC nucleus (figure 6C), with no ALC-induced changes noted (figure 6D).
Fig. 4.
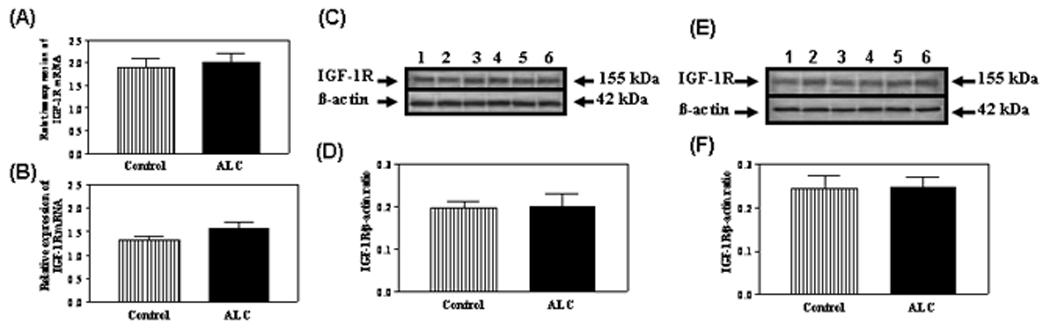
Effect of chronic ALC exposure on basal IGF-1R gene and protein expressions in prepubertal female rats. A and B) Real-time PCR analysis of IGF-1R gene expressions in AVPV and ARC nuclei, respectively. C) Representative Western blot of IGF-1R and β- actin proteins in the AVPV nucleus from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. D) Densitometric quantitation of all bands from two blots assessing IGF-1R protein in the AVPV nucleus. E) Representative Western blot of IGF-1R and β-actin proteins in the ARC nucleus from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. F) Densitometric quantification of all bands from two blots assessing IGF-1R protein in the ARC nucleus. Note that ALC did not alter IGF-1R gene or protein expressions in either region compared with control animals. The respective bars illustrate the mean (±SEM) of an N of 6–8 per group.
Fig. 5.
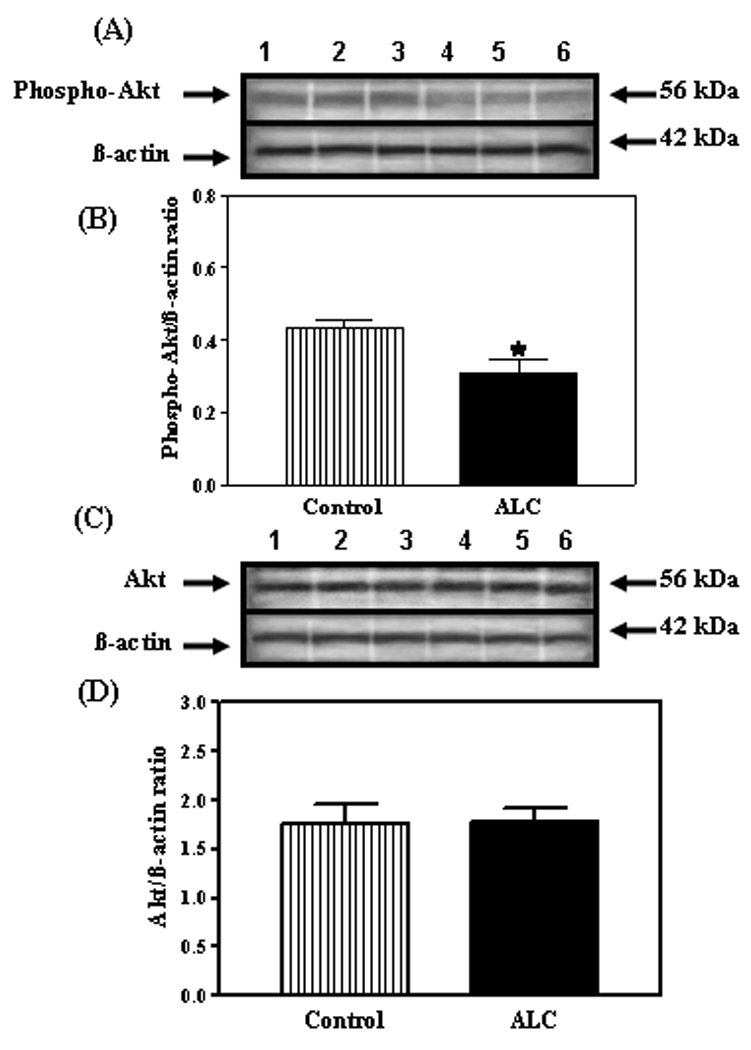
Effect of chronic ALC exposure on phosphorylated and total Akt protein expression in the AVPV nucleus of prepubertal female rats. (A) Representative Western immunoblot of phosphorylated Akt and β-actin proteins isolated from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing phosphorylated Akt protein. These data were normalized to the internal control β-actin protein, and the densitometric units represent the phosphorylated Akt/β-actin ratio. Note that the ALC-treated animals showed a marked decrease in phosphorylated Akt protein expression compared with control animals. (C) Representative Western immunoblot of total, non-phosphorylated Akt and β-actin proteins isolated from control (lanes 1–3)and ALC-treated (lanes 4–6) animals. (D) Densitometric quantitation of all of the bands from two blots assessing the total, non-phosphorylated Akt protein. These data were normalized to the internal control β -actin protein, and the densitometric units represent the total Akt/β -actin ratio. Note that chronic ALC-exposure did not affect total Akt protein expression. The respective bars illustrate the mean (±SEM) of an N of 7 per group. *p<0.05 versus control.
Fig. 6.
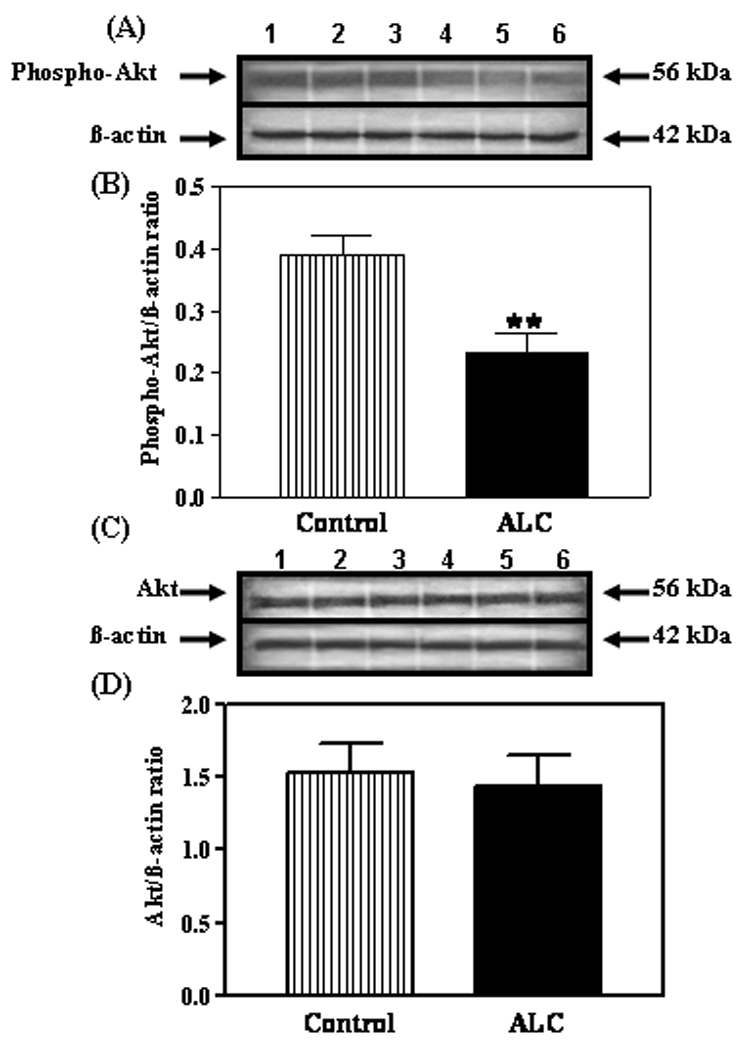
Effect of chronic ALC exposure on phosphorylated and total Akt protein expression in the ARC nucleus of prepubertal female rats. (A) Representative Western immunoblot of phosphorylated Akt and β- actin proteins isolated from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. (B) Densitometric quantitation of all of the bands from two blots assessing the phosphorylated Akt protein. These data were normalized to the internal control β -actin protein, and the densitometric units represent the phosphorylated Akt/β-actin ratio. Note that chronic ALC-treated animals showed a marked decrease in phosphorylated Akt protein expression compared with control animals. (C) Representative Western immunoblot of total, non-phosphorylated Akt β-actin proteins isolated from control (lanes 1–3) and ALC-treated (lanes 4–6) animals. (D) Densitometric quantitation of all of the bands from two blots corresponding to the total, non-phosphorylated Akt protein. These data were normalized to the internal control β-actin protein, and the densitometric units represent the total Akt/β -actin ratio. Note that chronic ALC-exposure did not affect total Akt protein expression. The respective bars illustrate the mean (±SEM) of an N of 7 per group. **p<0.01 versus control.
DISCUSSION
The KiSS-1/GPR54 system contained within the AVPV and ARC nuclei is considered an important component of early LHRH signaling processes that lead to the onset of mammalian puberty. Expression of KiSS-1, as well as GPR54, increase during pubertal development (Navarro et al., 2004a), and the kisspeptins have been shown to act within the reproductive hypothalamus to stimulate LHRH/LH release and drive the pubertal process in immature rats and rhesus monkeys (Navarro et al., 2004a; Navarro et al., 2004b; Shahab et al., 2005; Thompson et al., 2004). Recently, we began a series of studies in order to gain a better understanding of potential substances that may control or alter the prepubertal expression of KISS-1 in developing females. One such substance is ALC, a drug of abuse that is known to act at the hypothalamic level to interfere with parameters associated with pubertal development. ALC has been shown to cause suppressed puberty related hormones in adolescent humans (Block et al., 1993; Diamond et al., 1986), as well as immature female rhesus monkeys (Dees et al., 2000; Dissen et al., 2004) and rats (Dees and Skelley, 1990; Srivastava et al., 1995; Srivastava et al., 2002a). Furthermore, chronic ALC exposure is associated with delayed vaginal opening in rats (Bo et al., 1982: Dees and Skelley, 1990) and delayed development of a normal pattern of menstruation in rhesus monkeys (Dees et al., 2000). Thus, because of the critical role of KiSS-1 and GPR54 at puberty, and because of the detrimental effects of ALC on the pubertal process, we felt it important to discern whether ALC administration would alter the basal expression of these puberty related genes in the reproductive hypothalamus, and whether any changes were associated with suppressions in the serum levels of puberty related hormones.
In the present study we demonstrated that short-term ALC administration caused a marked suppression in KiSS-1 gene expression in the AVPV and ARC nuclei, the two principal areas of the reproductive hypothalamus that express KiSS-1/kisspeptin. The suppressed expression of KiSS-1 was associated with decreased circulating levels of LH and E2. The suppressed circulating levels of these two hormones following ALC administration was not surprising, and is in agreement with the primate and rodent studies mentioned earlier. Importantly, this suppression in their levels would be expected following a reduced KiSS-1/kisspeptin influence over LHRH secretion. The fact that KiSS-1 gene expression was suppressed in both the AVPV and ARC in response to the decreased levels of serum E2 is interesting. Previous studies have shown that there is a regional, differential response of the KiSS-1 gene to changes in sex steroid levels. In this regard, ovariectomy caused a decreased expression in the AVPV nucleus, but an increased expression in the ARC, whereas the opposite effects were observed following steroid replacement (Smith et al., 2005; Smith et al., 2007). In the present study, the suppressed levels of serum E2 along with suppressed KiSS-1 expression in the AVPV nucleus seem plausible; however, the lack of the normal responsiveness to suppressed E2 with increased KiSS-1 expression in the ARC suggests that the drug has an additional, central effect on these KiSS-1 containing neurons independent of altered E2. In addition to the ALC-induced effects noted in KiSS-1 expression, we have also demonstrated that ALC exposure was associated with a modest increase GPR54 gene and protein expressions within the AVPV nucleus, but did not elicit changes within in the ARC. The increased expression within the AVPV nucleus may have been because ALC did not negatively affect this gene and there was a compensatory rise due to suppressed KiSS-1/kisspeptin. Also, since the duration of ALC exposure was only six days, it is possible that down regulation of this receptor had not yet occurred.
Whether ALC alters KiSS-1 expression directly or indirectly by first affecting something upstream that controls this gene is an important consideration. In order to address this it is necessary to identify factors that are capable of activating the prepubertal expression of this gene. Insulin-like growth factor-1 (IGF-1) is a peptide that we have recently considered a candidate for such an action. This potential for IGF-1 is supported by several lines of evidence. The serum levels of the peptide increase before puberty in rodents (Handelsman et al., 1987; Hiney et al., 1996), as well as subhuman primates (Copeland et al., 1982) and humans (Anders et al., 1994; Tam et al., 2006). IGF-1 can cross the blood brain barrier and bind to type 1 receptors located throughout the reproductive hypothalamus (Bondy et al., 1992; Daftary and Gore, 2004; Lesniak et al., 1988). Other studies suggest that centrally derived IGF-1 may also play a role at puberty (Daftary and Gore, 2003). The central administration of IGF-1 has been shown to activate LHRH / LH secretion and advance puberty in female rats (Hiney et al., 1996). Normal puberty was restored in GH-deficient mice that received IGF-1 replacement (Danilovich et al, 1999), and IGF-1 administration advanced the time of first ovulation in rhesus monkeys (Wilson, 1998). Because some of these actions of IGF-1 are similar to those of the KiSS-1/GPR54 system at puberty, we recently assessed the potential for IGF-1 to induce the KiSS-1 gene. Importantly, both central and systemic administration of IGF-1 caused a precocious increase in KiSS-1 gene expression in the AVPV nucleus of juvenile female rats, and E2 appeared to play an important, yet permissive role in this effect, since IGF-1 was ineffective in stimulating the gene when E2 levels were too low (Hiney et al., 2009).
Since IGF-1 is a regulator of the KiSS-1 gene, it is possible that the suppressed circulating levels of this peptide may affect its central action to induce KiSS-1 expression at puberty. We showed previously, using the same diet regimen described here, that ALC did not alter the gene expression of IGF-1 in the hypothalamus (Hiney et al., 1996). In the present study, however, the ALC-induced suppression in KiSS-1 gene expression was associated with decreased serum levels of IGF-1 available to the hypothalamus. This action of ALC to suppress circulating IGF-1 is in line with previous reports using both rats and rhesus monkeys (Dees et al., 2000; Soszynski and Frohman, 1992; Srivastava et al., 1995). Interestingly, not only can ALC cause suppressed serum IGF-1 levels in prepubertal animals by decreasing the serum levels of growth hormone (Dees and Skelley, 1990; Dees et al., 2000; Sonntag and Boyd, 1988; Soszynski and Frohman, 1992), but it can also act directly within the liver to inhibit synthesis of the peptide by hepatocytes (Srivastava et al., 2002b). Thus, it appears that ALC interferes with the interrelationship between IGF-1 and KiSS-1 during pubertal development, an action that could be due to reduced circulating levels of IGF-1 entering the brain, and/or to a direct central action to alter specific functions of the peptide.
It is now well recognized that IGF-1 and E2, through their cross-regulated receptors, work together in the hypothalamus to regulate neuronal development, plasticity and neuroendocrine function (Cardona-Gomez et al., 2002, 2003; Etgen et al., 2006; Hiney et al., 2004, 2009). Importantly, the hypothalamic actions of IGF-1 to stimulate KiSS-1 gene expression and induce LH secretion both require the presence of sufficient circulating levels of E2 (Hiney et al., 2004, 2009). Therefore, because IGF-1/E2 can activate the KiSS-1 gene at puberty, and because ALC causes suppressed hypothalamic KiSS-1 gene expression along with decreased serum levels of IGF-1 and E2, we began addressing potential hypothalamic actions of ALC by assessing whether the drug affected the expression IGF-1Rs within the AVPV and ARC nuclei. Our results indicated that neither gene nor protein expression was altered by the ALC in either region. Since both central administration of IGF-1 and E2 have been shown to independently and synergistically activate the Akt (protein kinase B) transduction signaling pathway in the rodent female hypothalamus (Cardona-Gomez et al., 2002), we assessed whether ALC could affect the activation of this protein downstream of the IGF-1R. Our results showed that the ALC caused suppressed basal levels of phosphorylated Akt in both the AVPV and ARC nuclei. ALC-induced suppressions in the phosphorylation of Akt have been shown previously in other brain regions (Li et al., 2004; Tsuji et al., 2008) and in the liver (He et al., 2006).
The action of ALC described in the present study to impair phosphorylation of Akt is likely due to altered IGF-1 signaling, which may occur in several ways. The IGF-1 signal could be low due to the suppressed serum levels of the peptide entering the brain. A loss of the facilitative effects of E2 on IGF-1 could have resulted from the suppressed serum levels of the steroid following ALC. Importantly, it is conceivable that ALC altered the balance between IGF-1 and E2 needed for activation of the signaling pathways of the IGF-1R, like Akt for example (Cardona-Gomez et al., 2002). While ALC did not appear to affect IGF-1R synthesis, we can not rule out, however, that it may have altered some pharmacological properties contributing to IGF-1R function. Finally, ALC may have exerted a post-receptor action upstream from Akt, such as interfering with translocation of Akt from the cytosol to the plasma membrane and binding to phosphatidylinositol-3, 4, 5-triphosphate, which is critical for phosphorylation on Thr by phosphoinositide-dependent kinase 1 (Andjelkovic et al.,1977; Hill and Hemmings, 2002).
While the ALC exposure period in this study is short compared to most chronic studies using rodents, it does relate to a chronic situation in that the exposure time covers the peripubertal period of development in this species. It is clear that ALC exposure alters the pubertal influences of both IGF-1 (Srivastava et al., 1995; Dees et al., 2000) and KiSS-1 (present report). Furthermore, we recently showed that IGF-1 can activate the KiSS-1 gene within 6 hours after the central injection of the peptide (Hiney et al., 2009), and that this action can be blocked acutely by ALC (Srivastava et al., 2009). This acute response raises the question as to whether the suppressed KiSS-1 gene expression observed in the present study was due to an acute effect or to an accumulation of daily acute effects. The suppressed basal KiSS-1 expression noted in the present study was not apparently due to a single acute effect, since we have evidence that gastric ALC administration over one day did not alter basal KiSS-1 expression, although it did block the ability of IGF-1 to induce the expression of this gene (Srivastava et al., 2009). At this time, however, we can not rule out the possibility that a series of acute, daily influences of ALC may have contributed to the suppressed expression of KiSS-1 detected in the present six day study.
The possibility of a series of daily acute effects of ALC may not be unexpected, since our rodent model typically reveals BACs of 40–70 mg/dl in the mornings, indicating reduced feeding for several hours before the lights-on time begins. As the ALC is administered gastrically during the day the levels rise to between 150 and 200 mg/dl during the late morning to afternoon hours. Using rhesus monkeys, we have showed that daily late-morning gastric ALC infusion for 1 year resulted in similar afternoon BACs, and that there was little to no ALC remaining in blood by the following morning. Importantly, we reported that there was an acute, daily hypothalamic effect of ALC to alter LH secretion in those monkeys, which was superimposed over the established chronic effect (Dissen et al., 2004). This action could be important with regard to patterns of ALC use by adolescents and their overall endocrine effects. Thus, further assessments to define the additive acute effects of ALC over time and under different BACs will be of potential importance, and may indeed help determine when a specific acute or short-term endocrine effect of ALC develops into a chronic problem. This may be especially important during adolescence, since more information is needed to better understand how certain patterns of use alter endocrine function at this vulnerable time of development when individuals are more sensitive to the drug and less tolerant to its detrimental effects than adults.
In conclusion, we have reported here for the first time that short-term ALC exposure causes suppressed KiSS-1 expression in the reproductive hypothalamus of prepubertal female rats; thus, indicating that this is a major factor contributing to the ability of this drug of abuse to cause diminished LHRH/LH secretion and disruption of the pubertal process. Furthermore, we suggest that this effect on the KiSS-1 gene is due, at least in part, to alter IGF-1 signaling.
Acknowledgments
This work was supported by the NIH grant AA07216 (to WLD).
REFERENCES
- Anders J, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-1 in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size and body mass index. J Clin Endocrinol Metab. 1994;78:744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Willis BR, Oswald C, Gupta A, Zaneveld L. Delayed male sexual maturation induced by chronic ethanol ingestion. Fed Proc. 1981;40:825–829. [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, French M, Cron P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1977;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Block GD, Yamamoto ME, Mallick A, Styche AJ. Effects on pubertal hormones by ethanol abuse in adolescents. Alc Clin Exp Res. 1993;17:505. [Google Scholar]
- Bo WJ, Krueger WA, Rudeen PK, Symmes SK. Ethanol-induced alterations in the morphology and function of the rat ovary. Anat Rec. 1982;202:255–260. doi: 10.1002/ar.1092020210. [DOI] [PubMed] [Google Scholar]
- Bondy C, Werner H, Roberts CT, LeRoith D. Cellular pattern of type-1 insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors 1 and 11. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-1 in the brain: molecular mechanisms and functional implications. J Steroid Biochem and Mol Biol. 2003;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-1 in the activation of P13K/Akt signaling in the adult rat hypothalamus. Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Ching M, Valencia M, Negro-Villar A. Acute ethanol treatment lowers hypophyseal portal plasma LHRH and systemic plasma LH levels in orchadectomized rats. Brain Res. 1988;443:325–328. doi: 10.1016/0006-8993(88)91627-7. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Kuehl TJ, Castracane VD. Pubertal endocrinology of the baboon: elevated somatomedin-C/insulin-like growth factor 1 at puberty. J Clin Endocrinol Metab. 1982;55:1198–1201. doi: 10.1210/jcem-55-6-1198. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. Developmental changes in hypothalamic insulin-like growth factor-1: Relationship to gonadotropin-releasing hormone neurons. Endocrinology. 2003;144:2034–2045. doi: 10.1210/en.2002-221025. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormone neurons during postnatal development. J Neuroendocrinol. 2004;16:160–169. doi: 10.1111/j.0953-8194.2004.01149.x. [DOI] [PubMed] [Google Scholar]
- Danilovich V, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role for IGF-1. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- Dees WL, Dissen GA, Hiney JK, Lara F, Ojeda SR. Alcohol ingestion inhibits the increased secretion of puberty-related hormones in the developing female rhesus monkey. Endocrinology. 2000;141:1325–1331. doi: 10.1210/endo.141.4.7413. [DOI] [PubMed] [Google Scholar]
- Dees WL, Skelley CW. The effects of ethanol during the onset of female puberty. Neurondocrinology. 1990;51:64–69. doi: 10.1159/000125317. [DOI] [PubMed] [Google Scholar]
- Dees Wl, Skelley CW, Kozlowski GP. Intragastric cannulation as a method of ethanol administration for neruoendocrine studies. Alcohol. 1984;1:177–180. doi: 10.1016/0741-8329(84)90094-6. [DOI] [PubMed] [Google Scholar]
- Dees WL, Srivastava VK, Hiney JK. Alcohol alters insulin-like growth factor-1 activated Oct-2 POU gene expression in the immature female hypothalamus. J Studies on Alcohol. 2005;66:35–45. doi: 10.15288/jsa.2005.66.35. [DOI] [PubMed] [Google Scholar]
- De Roux N, Genen E, Carel J, Matsuda F, Chaussin J, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GRP54. Proc Natl Acad Sci. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond F, Ringenberg L, MacDonald D, Barnes J, Shi Hu C, Ducket G, Sweetland M, Root A. Effects of drug and alcohol abuse upon pituitary-testicular function in adolescent males. J Alc Hlth Care. 1986;7:28–33. doi: 10.1016/s0197-0070(86)80091-2. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Dearth RK, Scott HM, Ojeda SR, Dees WL. Alcohol Alters prepubertal luteinizing hormone secretion in immature female rhesus monkeys by a hypothalamic action. Endocrinology. 2004;145:4558–4564. doi: 10.1210/en.2004-0517. [DOI] [PubMed] [Google Scholar]
- Emanuele N, Ren J, LaPaglia N, Steiner J. Ethanol disrupts female mammalian puberty: age and dependence. Endocrine. 2002;18:247–254. doi: 10.1385/ENDO:18:3:247. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Gonzalez-Flores O, Todd BJ. The role of insulin-like growth factor-1 and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Fronteirs in Neuroendocrinol. 2006;27:363–375. doi: 10.1016/j.yfrne.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Spaliviero JA, Scott CD, Baxter RC. Hormonal regulation of the preripubertal stage of insulin-like growth factor-1 in the rat. Endocrinology. 1987;120:491–496. doi: 10.1210/endo-120-2-491. [DOI] [PubMed] [Google Scholar]
- He L, Simmen FA, Mehendale HM, Ronis MJJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with cell membrane. J Biol Chem. 2006;281:11126–11134. doi: 10.1074/jbc.M510724200. [DOI] [PubMed] [Google Scholar]
- Hettinger AM, Allen MR, Zhang BR, Goad DW, Malayer JR, Geisert RD. Presence of the acute phase protein, bikunin, in the endometrium of gilts during estrous cycle and early pregnancy. Biol Reprod. 2001;65:507–513. doi: 10.1095/biolreprod65.2.507. [DOI] [PubMed] [Google Scholar]
- Hill MM, Hemmings BA. Inhibition of protein kinase B/Akt. Implications for cancer therapy. Pharm. Ther. 2002;93:243–251. doi: 10.1016/s0163-7258(02)00193-6. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Dearth RK, Srivastava VK, Rettori V, Dees WL. Actions of ethanol on epidermal growth factor receptor activated luteinizing hormone secretion. J. Stud. Alcohol. 2003;64:809–816. doi: 10.15288/jsa.2003.64.809. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Dees WL. Ethanol inhibits LHRH release from the median eminence of prepubertal female rats in vitro: Investigation of its actions on norepinephrine and prostaglandin E2. Endocrinology. 1991;128:1404–1408. doi: 10.1210/endo-128-3-1404. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Dearth RK, Dees WL. Influence of estradiol on IGF-1 induced luteinizing hormone secretion. Brain Res. 2004;1013:91–97. doi: 10.1016/j.brainres.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor-1 (IGF-1) of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3727. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- Hiney JK, Srivastava VK, Pine MD, Dees WL. Insulin-like growth factor-1 activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology. 2009;150:376–384. doi: 10.1210/en.2008-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LD, O’Malley PM, Backman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975-1005: Vol. 1 Secondary school students. Bethesda MD: NIDA; 2006. (NIH Publication no. 06-5883) [Google Scholar]
- Lesniak MA, Hill JM, Kiess W, Rojeski M, Candace BP, Roth J. Receptors for insulin-like growth factors 1 and 11: autoradiographic localization in rat brain and comparison to receptor for insulin. Endocrinology. 1988;123:2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- Li Z, Ding M, Thiele CJ, Luo J. Ethanol inhibits brain-derived neurotrophic factor-mediated intracellular signaling and activator protei-1 activation in cerebellar granule neurons. Neuroscience. 2004;126:149–162. doi: 10.1016/j.neuroscience.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barriero ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated mRNA expression of KiSS-1 and its putitive receptor, GPR54, in rat hypothalamus and potent LH-releasing activity of KiSS-1 peptide. Endocrinology. 2004a;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004b;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Effect of alcohol on the proestrus surge of luteinizing hormone (LH) and the activation of LH-releasing hormone (LHRH) neurons in the female rat. J. Neuroscience. 1997;17:2595–2604. doi: 10.1523/JNEUROSCI.17-07-02595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent A, Matagne V, Mungenast AE. The neuroendocrine regulation of puberty: Is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ramaley JA. The regulation of gonadotropin secretion in immature ethanol-treated male rats. J Androl. 1982;3:248–252. [Google Scholar]
- Seminara SB, Messager S, Chatizidaki E, Thresher A, Acierno J, Shagoury J, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick A, Zahn D, Dixon J, Kaiser U, Slaugenhaupt S, Gusella J, O’Rahilly S, Carlton M, Crowley W, Aparicio S, Colledge W. The GPR54 gene as a regulator of puberty. New Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara S, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of KiSS-1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Boyd RL. Chronic ethanol feeding inhibits plasma levels of insulin-like growth factor-1. Life Sci. 1988:1325–1330. doi: 10.1016/0024-3205(88)90588-7. [DOI] [PubMed] [Google Scholar]
- Soszynski PA, Frohman LA. Inhibitory effects of ethanol on the growth hormone (GH)-releasing hormone-GH-insulin-like growth factor-1 axis in the rat. Endocrinology. 1992;131:2603–2608. doi: 10.1210/endo.131.6.1359962. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Nyberg CL, Dees WL. Effect of ethanol on the synthesis of insulin-like growth factor-1 (IGF-1) and the IGF-1 receptor in late prepubertal female rats: A correlation with serum IGF-1. Alc Clin Exp Res. 1995;19:1467–1473. doi: 10.1111/j.1530-0277.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dees WL. Effects of chronic alcohol administration on KiSS-1 gene expression in the reproductive hypothalamus of prepubertal female rats. RSA Scientific Conference; San Diego, CA. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Dearth RK, Dees WL. Chronic effects of prepubertal ethanol administration on steroidogenic acute regulatory protein in the rat ovary. Alc Clin Exp Res. 2002a;26:107–113. [PubMed] [Google Scholar]
- Srivastava VK, Dearth RK, Hiney JK, Chandrashekar V, Mattison LA, Bartke A, Dees WL. Alcohol suppresses insulin-like growth factor-1 gene expression in prepubertal transgenic female mice overexpressing the bovine growth hormone gene. Alc Clin Exp Res. 2002b;26:1697–1702. doi: 10.1097/01.ALC.0000036922.18456.EF. [DOI] [PubMed] [Google Scholar]
- Steiner JC, LaPaglia N, Hansen M, Emanuele NV, Emanuele MA. Effect of chronic ethanol on reproductive and growth hormones in the peripubertal male rat. J. Endocrinology. 1997;154:363–370. doi: 10.1677/joe.0.1540363. [DOI] [PubMed] [Google Scholar]
- Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J. Clin. Endocrinol Metab. 2006;91:4369–4373. doi: 10.1210/jc.2006-0953. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- Tsuji R, Fattori V, Abe S, Costa LG. Kobayashi Effects of postnatal ethanol exposure at different developmental phases on neurotrophic factors and phosphorylated proteins on signal transductions in rat brain. Neurotoxicology and Teratology. 2008;30:228–236. doi: 10.1016/j.ntt.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Premature elevation in serum insulin-like growth factor-1 advances first ovulation in monkeys. J Endocrinology. 1998;158:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]