Case Report
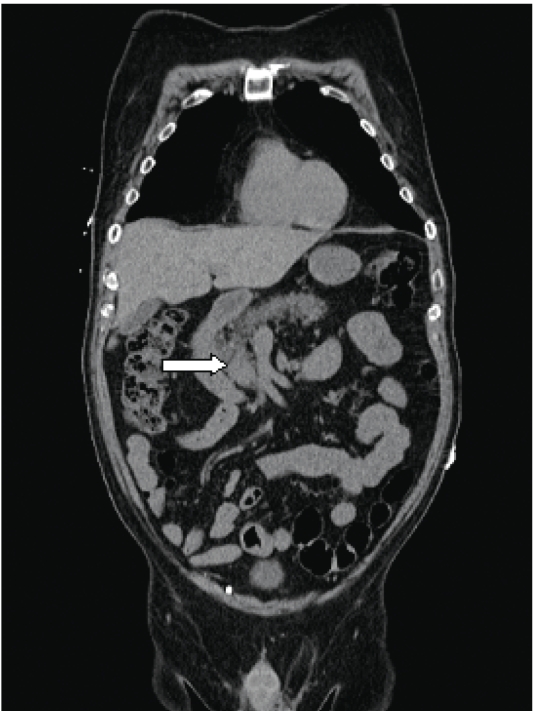
A 66-year-old man was suspected of having acute Cushing syndrome based upon the following symptoms: hyperglycemia; hypokalemic alkalosis; an elevated serum total cortisol level of 41.0 μg/dL (normal, 2.9–19.4 μg/dL), which did not respond to corticotrophin-releasing hormone stimulation; and adrenocorticotropic hormone (ACTH) levels of 121–146 pg/mL (normal, 20–80 pg/mL), which were not suppressed by either low- or high-dose dexamethasone. Magnetic resonance imaging of the patient's pituitary was negative; however, computed tomography (CT) imaging of the chest, abdomen, and pelvis suggested a vague 1.7-cm × 2.4-cm mass-like abnormality in the uncinate process and bilateral adrenal thickening (Figures 1).
Figure 1.
Coronal computed tomography image of the chest, abdomen, and pelvis. The arrow reveals a 1.7-cm × 2.4-cm mass at the uncinate process.
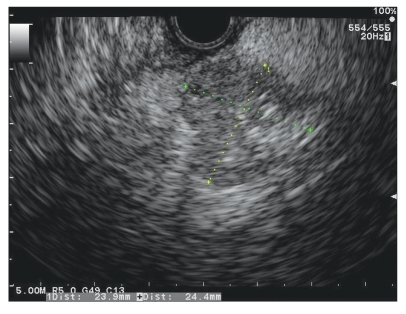
The patient was referred for endoscopic ultrasound (EUS) and fine-needle aspiration (FNA) evaluation of the pancreas. A linear EUS examination clearly revealed a 2.4-cm × 2.4-cm hypoechoic heterogenic uncinate mass with regular borders and without cystic features (Figures 2), which was consistent with a T2N0MX neuroendocrine tumor based upon EUS criteria. Tissue evaluation was performed using 2 passes each of 25- and 22-gauge FNA needles as well as a core biopsy. Pathologic evaluation of both the fine-needle aspirates and the core biopsies demonstrated uniform neoplastic cells, small to medium in size, with scant cytoplasm, oval nuclei with coarse chromatin, and a solid nesting pattern (Figures 3). Immunoperoxidase stains showed strong cytoplasmic staining within the tumor cells for antibodies directed against ACTH (Figures 4).
Figure 2.
Linear endoscopic ultrasound of a 2.4-cm × 2.4-cm hypoechoic heterogenic uncinate mass.
Figure 3.
Uniform neoplastic cells, small to medium in size, with scant cytoplasm, oval nuclei with coarse chromatin, and a solid nesting pattern (hematoxylin & eosin stain, 40×).
Figure 4.
Strong cytoplasmic staining within the tumor cells for antibodies directed against adrenocorticotropic hormone (immunohistochemical stain, 40×).
The patient underwent a successful pancreaticoduo-denectomy. Postoperatively, his ACTH level decreased from 121 pg/mL to 21 pg/mL, confirming the diagnosis of an ectopic ACTH-secreting tumor (Cushing syndrome).
Discussion
Approximately two thirds of patients with symptoms consistent with Cushing syndrome actually have Cushing disease, an ACTH-secreting pituitary tumor.1 Cushing syndrome secondary to ectopic ACTH production is a relatively uncommon clinical condition that accounts for up to 16% of patients with ACTH-dependent hypercorti-solism.1,2 These patients have elevated cortisol and ACTH levels and frequently have CT scans that show bilateral adrenal enlargement.3 The most common ectopic ACTH-secreting tumors are bronchial and thymic carcinoids, with pancreatic islet cell tumors being responsible for less than 1% of all causes of Cushing syndrome.1
Although the chronic syndrome is often clinically indistinguishable from pituitary-dependent hypercorti-solism presenting with plethora, truncal obesity, buffalo hump, and red striae, the typical Cushing habitus is absent in many acute cases.4 Acute Cushing syndrome, which is how our patient presented, is more often associated with the rapid onset of hypertension, weakness, edema, hypokalemia, glucose intolerance, anorexia, and weight loss.4
Dynamic testing based upon differential sensitivity to glucocorticoid feedback or ACTH stimulation in response to corticotropin-releasing hormone or cortisol reduction is a reliable way to discern between an ectopic versus a pituitary source of excess ACTH.5 High-dose dexamethasone suppresses morning serum cortisol levels in approximately 80% of pituitary ACTH-producing adenomas, though it fails to suppress ACTH in approximately 90% of ectopic cases. However, bronchial and other carcinoids are well-documented exceptions to these general guidelines, as these ectopic sources of ACTH may exhibit feedback regulation indistinguishable from pituitary adenomas.5
Although approximately 90% of patients with ectopic ACTH-producing tumors can be cured by surgical resection, pancreatic tumors are virulent neoplasms associated with a rapidly progressive clinical course.1 In a retrospective analysis of patients with pancreatic islet cell tumors associated with Cushing syndrome, 60% of patients died within 2 years, and the 5-year survival rate was reported to be 16%.6 Patients with ectopic ACTH secretion may be at risk of death from metastasis and infections caused by the immunosuppressive effects of excess adrenocortical steroid.6 Therefore, functioning pancreatic tumors should be operated early on in the course of the disease to provide a cure.7 The optimal treatment for patients with ectopic ACTH production is localization and surgical excision of the source of the ACTH secretion.1 Successful surgical excision depends upon the ability to identify the site of the tumor and the resectability and metastatic capacity of the neoplasm before operation.1
References
- 1.Amikura K, Alexander HR, Norton JA, et al. Role of surgery in management of adrenocorticotropic hormone-producing islet cell tumors of the pancreas. Surgery. 1995;118:1125–1130. doi: 10.1016/s0039-6060(05)80123-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee T, Karl M, Solorzano CC. Adrenocorticotropic hormone-secreting pancreatic islet cell carcinoma. J Am Coll Surg. 2004;199:336–337. doi: 10.1016/j.jamcollsurg.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Uecker JM, Janzow MT. A case of Cushing syndrome secondary to ectopic adrenocorticotropic hormone producing carcinoid of the duodenum. Am Surg. 2005;71:445–446. [PubMed] [Google Scholar]
- 4.Miehle K, Tannapfel A, Lamesch P, et al. Pancreatic neuroendocrine tumor with ectopic adrenocorticotropin production upon second recurrence. J Clin Endocrinol Metab. 2004;89:3731–3736. doi: 10.1210/jc.2003-032164. [DOI] [PubMed] [Google Scholar]
- 5.Jameson JL, Johnson BE. Paraneoplastic syndromes: endocrinologic/hematologic. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison's Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Professional; 2008. pp. 617–623. [Google Scholar]
- 6.Clark ES, Carney JA. Pancreatic islet cell tumor associated with Cushing syndrome. Am J Surg Pathol. 1984;8:917–924. doi: 10.1097/00000478-198412000-00004. [DOI] [PubMed] [Google Scholar]
- 7.PlÖckinger U, Wiedenmann B. Neuroendocrine tumors of the gastro-enteropancreatic system: the role of early diagnosis, genetic testing and preventive surgery. Dig Dis. 2002;20:49–60. doi: 10.1159/000063164. [DOI] [PubMed] [Google Scholar]