Abstract
Hepcidin expression in vivo is regulated in proportion to iron status (i.e., increased by iron loading and decreased in iron deficiency). However, in vitro studies with hepatoma cell lines often show an inverse relationship between iron status and hepcidin expression. Here, we investigated possible molecular mechanisms responsible for the differences in iron sensing between hepatoma cell lines and human primary hepatocytes. RNA was collected from primary human hepatocytes, and HepG2 and HuH7 hepatoma cells were treated with either transferrin-bound and non-transferrin-bound iron. Expression of hepcidin, transferrin receptor 2, HFE, and hemojuvelin were quantified by real-time PCR. Hepcidin expression was increased in primary human hepatocytes following 24-h exposure to holoferric transferrin. In contrast, hepcidin mRNA levels in hepatoma cells were decreased by transferrin. Hepcidin expression was positively correlated with transferrin receptor 2 mRNA levels in primary human hepatocytes. Compared with primary hepatocytes, transferrin receptor 2 expression was significantly lower in hepatoma cell lines; furthermore, there was no correlation between transferrin receptor 2 and hepcidin mRNA levels in either HepG2 or HuH7 cells. Taken together our data suggest that transferrin receptor 2 is a likely candidate to explain the differences in iron sensing between hepatoma cell lines and primary human hepatocytes.
Keywords: hepcidin, hfe, hemojuvelin
hepcidin, a 25-amino-acid peptide produced predominantly by the hepatocytes, is a key regulator of iron homeostasis. The expression of hepcidin is controlled by several physiological and pathological factors including serum iron levels (6, 24), erythropoietic factors (5, 20), and inflammation (17, 31). Despite these convincing in vivo data, comparable in vitro studies using primary hepatocytes and hepatoma cell lines, with the aim of understanding the molecular mechanisms controlling hepcidin expression, have yielded conflicting results. Transferrin-bound iron treatment of mouse primary hepatocytes resulted in an increase in hepcidin expression (15, 26), whereas hepcidin mRNA levels were not altered in the presence of non-transferrin-bound iron (24). In contrast, one study using human primary hepatocytes found an unexpected decrease in hepcidin following exposure to both transferrin-bound and non-transferrin-bound iron (19). In hepatoma cell lines, iron loading either does not alter (11) or results in a decrease in hepcidin mRNA expression (9). Taken together, these data suggest that there are fundamental differences in the ability of different hepatocyte models to sense changes in transferrin-bound iron levels.
The mechanisms by which the hepatocytes sense changes in iron levels and respond by modulating hepcidin expression remain unclear. Three main candidate genes for iron sensing in hepatocytes have been identified, namely hemojuvelin (HJV), transferrin receptor 2 (TfR2), and the hereditary hemochromatosis gene HFE. A mutation in any one of these genes is associated with inappropriately low expression of hepcidin and as a consequence the iron-loading disorder hemochromatosis (1, 2, 18, 21, 22). In the present study, to address the potential role of these genes as iron sensors, we have measured the expression of HJV, TfR2, and HFE in cultures of primary human hepatocytes and human hepatoma cells and compared this with the ability of the cells to regulate hepcidin following incubation with transferrin-bound iron.
MATERIALS AND METHODS
Cell isolation and culture.
Primary human hepatocytes were isolated from liver resection tissues by a modified two-step collagenase perfusion technique (16). Patients gave informed consent for the use of tissue, and all protocols were approved by the Research Ethics Committee of King's College Hospital. Hepatocytes were purified by low-speed centrifugation at 50 g for 5 min at 4°C, and corresponded to >90% of the isolated cells by morphological analysis. Once isolated, the cells were plated at a density of 1.5 × 106 viable cells/well in collagen-coated six-well plates and cultured in Williams' E medium (Sigma-Aldrich), supplemented with 10% heat-inactivated fetal bovine serum (FCS) (Invitrogen), 10 mM HEPES (Cambrex), 2 mM l-glutamine (Invitrogen), 100 IU/ml penicillin, 100 μg/ml streptomycin, 0.1 μM dexamethasone, and 0.1 μM insulin (Sigma-Aldrich) at 37°C in 95% O2-5% CO2. HepG2 cells and HuH7 cells were maintained in Dulbecco's modified Eagle's medium containing 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine.
Isolated human primary hepatocytes were cultured for at least 16 h before experiments. HepG2 and HuH7 cells were seeded at 1 × 106 cells/well in six-well plates and used 24–48 h postseeding. All cells were treated for 24 h with 100 μM iron supplemented to the growth media in the form of hemin, ferric ammonium citrate, or holo-transferrin (Sigma-Aldrich).
RNA isolation and quantitative real-time PCR.
Following treatment, total RNA was isolated from the cells by using Trizol reagent (Invitrogen) according to the manufacturer protocol. First-strand cDNA synthesis was performed in a total volume of 20 μl with 1 μg RNA primed with anchored oligo(dT)18 by use of a Transcriptor high-fidelity cDNA synthesis kit (Roche).
Quantification of cDNA was performed by real-time PCR by using an ABI Prism 7000HT PCR cycler and a Quanti-Tect SYBR Green PCR kit (Qiagen) according to the manufacturer's protocol. For each gene a standard curve was generated with 10-fold serial dilutions of a pool of cDNA samples. The genes analyzed and the primers used are listed in Table 1. Gene expression levels were normalized to levels of β-actin in the same sample. Data are from at least three independent experiments.
Table 1.
PCR primers used in this study
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACTB | CCAACCGCGAGAAGATGA | CCAGAGGCGTACAGGGATAG |
| HAMP | CTGCAACCCCAGGACAGAG | GGAATAAATAAGGAAGGGAGGGG |
| TFR2 | TATACTCCTGGAGCTGGTGC | GCACGCTGAGGTAGCCCTCTA |
| HFE | AGAACAGGGCCTACCTGGAG | TGTGTCACCTTCACCAAAGG |
| HFE2 | GGAGCTTGGCCTCTACTGGA | ATGGTGAGCTTCCGGGTG |
Western blotting.
Primary hepatocytes were lysed with ice-cold lysis buffer (phosphate-buffered saline, containing 1% sodium dodecyl sulfate, 10 mg/l protease inhibitor cocktail). Lysates were precleared of insoluble membrane material and nuclei by centrifugation (13,000 g for 10 min), and protein samples (40 μg) were solubilized in sample loading buffer and subjected to polyacrylamide gel electrophoresis. Following immobilization on nitrocellulose, the proteins were exposed to commercially available TfR2 antibody (Santa Cruz Biotechnology). Cross-reactivity was observed by use of a horseradish peroxidase-linked secondary antibody (Dako) and ECL Plus (GE Healthcare). Band densities were semiquantified by use of Scion Image software (Scion). At the end of the experiment, the nitrocellulose membranes were stripped (Western Stripping Buffer, Perbio Science) and reprobed with an anti-actin antibody (1:2,000 dilution, Sigma-Aldrich) that acted as a loading control.
Statistical analysis.
Statistical analyses were performed by one-way ANOVA and Dunnett's post hoc test to compare control and treated groups and by linear regression. For all tests a P value <0.05 was considered statistically significant. Descriptive data for continuous variables are reported as means ± SE. Microsoft Excel and SPSS statistical packages were used for statistical analysis.
RESULTS
Basal expression of hepcidin is elevated in primary human hepatocytes compared with hepatoma cell lines.
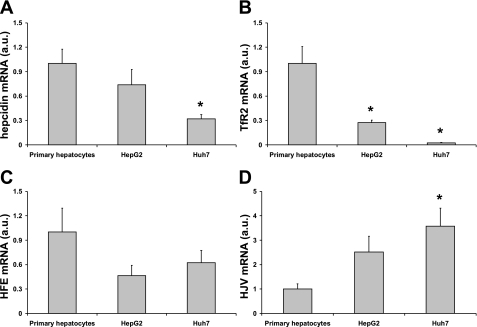
Basal levels of hepcidin mRNA were measured and quantified in primary human hepatocytes and in HepG2 and HuH7 hepatoma cells by real-time PCR (Fig. 1A). Hepcidin expression was some threefold higher in primary hepatocyte cultures compared with HuH7 cells (P < 0.05). HepG2 cell hepcidin levels were not significantly different from those observed in primary hepatocytes.
Fig. 1.
Basal mRNA expression of hepcidin (A), transferrin receptor 2 (TfR2; B), HFE (C), and hemojuvelin (HJV; D) in primary human hepatocytes, HepG2 cells, and HuH7 cells. Data are means ± SE of 3–6 observations in each group. *P < 0.05 (ANOVA and Dunnett's post hoc test), significant difference compared with primary hepatocytes.
TfR2, HFE, and HJV are candidate iron-sensing genes in hepatocytes that might act as regulators of hepcidin expression (1, 2, 18, 21, 22). To determine whether the aberrant hepcidin response of the immortalized cell lines to holo-transferrin could be explained by expression levels of any of these genes, we measured the mRNA levels in primary hepatocytes and HepG2 and HuH7 cells. Candidate iron-sensing genes were also differentially expressed in the three cell lines. TfR2 mRNA was significantly lower in both hepatoma cell lines compared with primary hepatocytes (Fig. 1B). Relative HFE expression was higher in primary hepatocytes compared with hepatoma cells; however, these differences were not statistically significant (Fig. 1C). In contrast, HJV levels were significantly higher in HuH7 cells than in primary hepatocytes (Fig. 1D).
Hepcidin expression correlates with TfR2 levels in primary human hepatocytes.
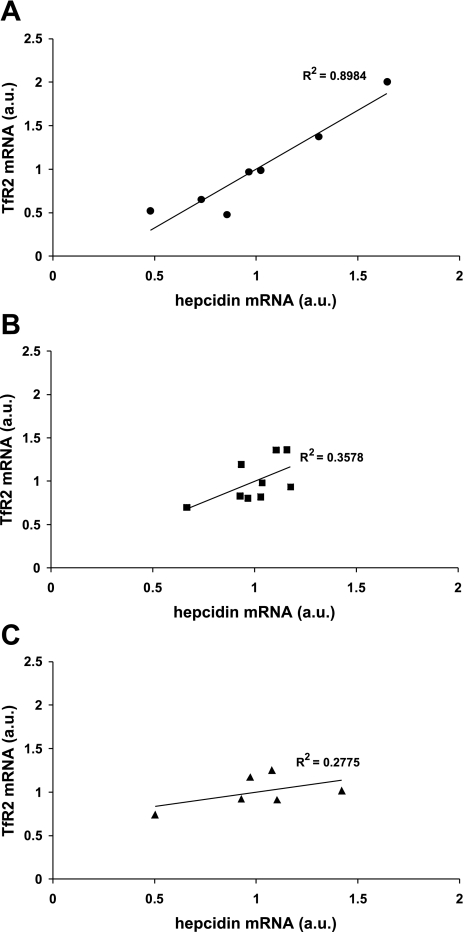
Next we determined whether the relative levels of TfR2 in primary hepatocytes, HepG2 cells and HuH7 cells influenced hepcidin expression. In primary human hepatocytes, preliminary analysis (n = 3 samples) using a multiple linear regression model, incorporating TfR2, HFE, and HJV as predictive variables for hepcidin expression, only TfR2 mRNA significantly correlated with hepcidin mRNA (adjusted R2 = 0.997; P = 0.026). Hepcidin did not correlate significantly with either HFE (R2 = 0.845; P = 0.25) or HJV (R2 = 0.787; P = 0.31) in primary hepatocytes. Analysis of further samples of primary hepatocytes confirmed that there was a significant positive correlation between hepcidin expression and the mRNA levels of TfR2 (Fig. 2A; P = 0.001). In contrast, TfR2 expression did not significantly correlate with hepcidin expression in either HepG2 (Fig. 2B; P = 0.09) or HuH7 cells (Fig. 2C; P = 0.26). There was no significant correlation between hepcidin and either HFE or HJV in hepatoma cell lines (data not shown).
Fig. 2.
Correlation between TfR2 mRNA and hepcidin levels in primary human hepatocytes (n = 7) (A), HepG2 cells (n = 9) (B), and HuH7 cells (n = 6) (C). TfR2 levels positively correlated with hepcidin. This relationship was statistically significantly in primary hepatocytes but not in either of the hepatoma cell lines.
Holo-transferrin increases hepcidin expression in primary human hepatocytes.
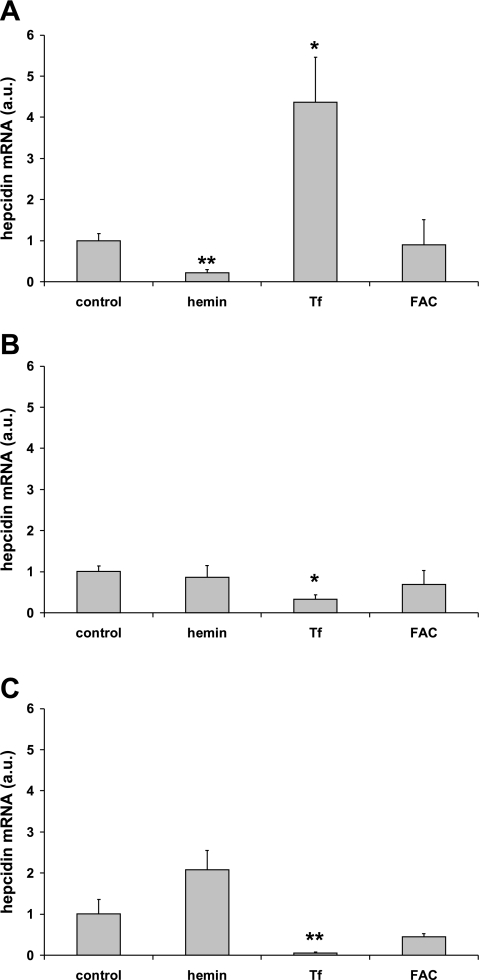
Hepcidin mRNA was significantly increased in cultures of primary human hepatocytes incubated with holo-transferrin (Fig. 3A). In contrast, transferrin-bound iron significantly decreased hepcidin expression in both HepG2 (Fig. 3B) and HuH7 (Fig. 3C) hepatoma cells. Hepcidin expression was not significantly altered by nonheme iron (ferric ammonium citrate) in any of the cell lines, but incubation with hemin significantly decreased hepcidin levels in primary hepatocytes.
Fig. 3.
Effects of iron on hepcidin expression in primary human hepatocytes (A), HepG2 cells (B), and HuH7 cells (C). Hepcidin mRNA levels were significantly increased by transferrin (Tf)-Fe in primary human hepatocytes. In contrast, Tf-Fe resulted in a significant decrease in hepcidin mRNA levels in both HepG2 and HuH7 hepatoma cells. Data are means ± SE of 4–9 observations in each group. *P < 0.001; **P < 0.05 compared with untreated control cells.
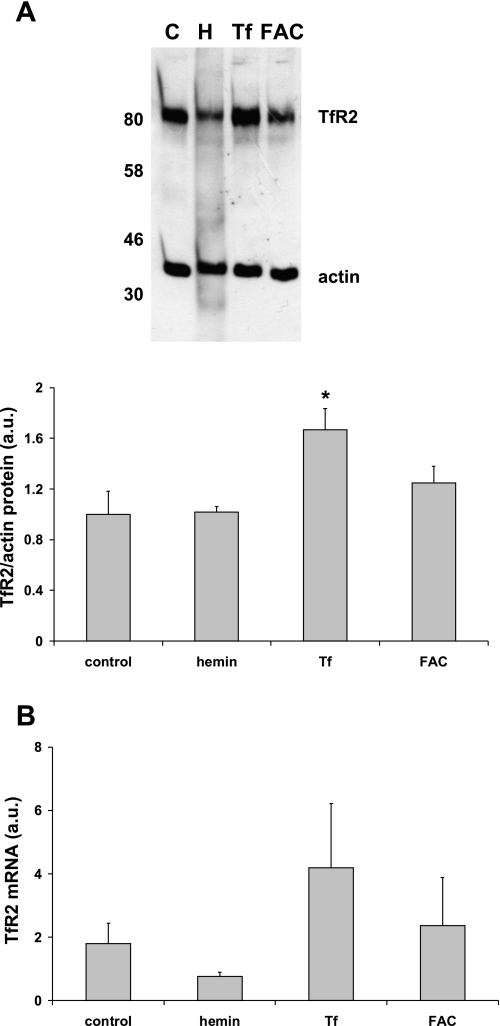
To explore the possibility that iron treatment in primary hepatocytes influenced TfR2 expression and thereby regulated hepcidin, we measured TfR2 protein and mRNA in primary hepatocytes. There was no effect of hemin or ferric ammonium citrate (FAC) on TfR2 protein levels; however, incubation with holo-transferrin significantly increased (P < 0.05) TfR2 expression (Fig. 4A). There was no significant effect of iron treatment of TfR2 mRNA levels in primary hepatocytes (Fig. 4B).
Fig. 4.
Effects of iron on TfR2 protein (A) and mRNA expression (B) in primary human hepatocytes. TfR2 protein levels were significantly increased by Tf-Fe but not by other forms of iron. C, control; H, hemin. There were no significant effects of iron treatment on TfR2 mRNA expression. Data are means ± SE of 3–5 observations in each group. *P < 0.05 compared with untreated control cells.
DISCUSSION
Hepcidin is mainly produced by hepatocytes and is the major circulating regulator of iron homeostasis (14, 23, 24). Hepcidin expression is decreased following prolonged consumption of an iron-deficient diet and is inversely correlated with intestinal iron transporter expression (6). In contrast, feeding a high-iron diet is associated with hepatic iron loading and elevated hepcidin expression (24). However, these findings have not been recapitulated with in vitro cell culture models, in which iron loading is associated with a decrease in hepcidin mRNA (9, 19). In this study we investigated the possibility that there are molecular differences in the iron-sensing pathways in primary hepatocytes and hepatoma cell lines that might explain the anomalous data from in vivo and in vitro studies.
Previous studies have shown that hepcidin mRNA levels in whole human liver are some 140 times higher than HepG2 cells and 160 times higher than HuH7 cells (4). In contrast, hepcidin mRNA in our isolated human primary hepatocyte preparations was only modestly increased (30%) compared with the levels in HepG2 hepatoma cells but was significant greater (3-fold) than in HuH7 cells. Interestingly, low hepcidin expression in HuH7 cells may in part be due to a recently identified mutation in the HFE gene in these cells (29).
A number of putative iron-sensing genes have been described in hepatocytes that might act as regulators of hepcidin expression, including TfR2, HFE, and HJV (1, 2, 18, 21, 22). We investigated whether differential expression of these genes might explain the variations in hepcidin expression in primary hepatocytes and hepatoma cell lines. Compared with levels in primary hepatocytes, it was evident that TfR2 was downregulated in both HepG2 cells and Huh7 cells by almost an order of magnitude. Moreover, in primary hepatocytes there was a positive and significant correlation between TfR2 mRNA levels and hepcidin expression. This correlation was not present in either HepG2 or HuH7 cells. This latter finding is consistent with recent work in HuH7 cells showing that hepcidin mRNA levels are not significantly affected following either overexpression or small interfering RNA-mediated knockdown of TfR2 (11).
Knockout mouse studies have shed some light on the relative roles of TfR2 and HFE in the regulation of hepcidin expression (30). This report demonstrated that liver hepcidin mRNA levels in HFE knockout mice were significantly decreased compared with wild-type controls when normalized for liver iron content. Expression of hepcidin was further decreased in TfR2 knockout mice and was lower still in dual HFE/TfR2 knockout mice. Importantly, these data demonstrate that both HFE and TfR2 are able to sense and signal changes in iron status (30); if that were not the case, hepcidin expression would be expected to be the same in all three knockout models. HFE knockout mice (1) and HFE-related hemochromatosis patients (25) are still able to regulate hepcidin in response to changes in iron stores although the response is impaired (i.e., inappropriately low) compared with control subjects. Nonetheless, recent studies highlight the requirement for the expression of both HFE and TfR2 for full iron-sensing activity (7, 8). In our studies, although HFE levels and hepcidin expression were positively correlated, this association did not reach statistical significance, and this finding is consistent with the data from Wallace et al. (30) suggesting that HFE is lower in the hierarchy of iron sensors than TfR2.
Recent work has revealed that hepcidin mRNA expression is increased in primary mouse hepatocytes in response to holo-transferrin (15, 26). In primary human hepatocytes we also observed a significant increase in hepcidin following exposure to holo-transferrin. This is, to our knowledge, the first demonstration of a positive link between transferrin-bound iron and hepcidin mRNA in human hepatocytes, in contrast with previous work that reported a decrease in hepcidin following exposure to transferrin (19). Isolated hepatocytes are notoriously difficult to work with and there is evidence that cryopreservation (28) or prolonged culture periods significantly alters hepatocyte cell function. Thus the differences between our study and previous work (19) may well reflect the difference in the hepatocyte preparations used.
Iron has been shown to increase the stability of TfR2 protein levels in hepatocytes and hepatoma cells (11, 12, 27). In our study we observed an increase in TfR2 protein in primary human hepatocytes following exposure to holo-transferrin; TfR2 mRNA levels were increased but not significantly. There was no effect of either FAC or hemin on TfR2 expression. The perceived role of TfR2 in iron sensing is complex. Under high-iron conditions, holo-transferrin is thought to displace HFE from TfR1, allowing it to bind to TfR2 (3, 10). It has been suggested that the TfR2/HFE complex interacts with HJV initiating the BMP/SMAD signaling pathway (15), which leads to an increase in hepcidin expression. Recently, direct activation of SMAD signaling in response to an iron challenge has been demonstrated in mouse liver (13). Our data in primary human hepatocytes are consistent with this model.
In conclusion, our present studies suggest that TfR2 is a key gene linking iron sensing and the regulation of hepcidin expression in primary human hepatocytes. Furthermore, low expression of TfR2 in human hepatoma cell lines may contribute to inappropriate downregulation of hepcidin by holo-Tf.
GRANTS
C. Rapisarda was supported by a Medical Research Council Doctoral Training Award. J. Puppi was supported by an Alex Mowat PhD Studentship.
DISCLOSURES
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
We thank the Liver Transplant and Hepatobiliary Surgery Teams and the Liver Pathology Service at King's College Hospital for help in obtaining the liver tissue.
REFERENCES
- 1.Ahmad KA, Ahmann JR, Migas MC, Waheed A, Britton RS, Bacon BR, Sly WS, Fleming RE. Decreased liver hepcidin expression in the Hfe knockout mouse. Blood Cells Mol Dis 29: 361–366, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet 361: 669–673, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Enns CA. The cytoplasmic domain of transferrin receptor 2 dictates its stability and response to holo-transferrin in Hep3B cells. J Biol Chem 282: 6201–6209, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, Gilot D, Boudjema K, Guguen-Guillouzo C, Brissot P, Loreal O, Ilyin G. C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem 277: 41163–41170, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Frazer DM, Inglis HR, Wilkins SJ, Millard KN, Steele TM, McLaren GD, McKie AT, Vulpe CD, Anderson GJ. Delayed hepcidin response explains the lag period in iron absorption following a stimulus to increase erythropoiesis. Gut 53: 1509–1515, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazer DM, Wilkins SJ, Becker EM, Vulpe CD, McKie AT, Trinder D, Anderson GJ. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology 123: 835–844, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Chen J, De Demenico I, Koeller DM, Harding CO, Fleming RE, Koeberl DD, Enns CA. Hepatocyte-targeted HFE and TFR2 control hepcidin expression in mice. Blood 115: 3374–3381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab 9: 217–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gehrke SG, Kulaksiz H, Herrmann T, Riedel HD, Bents K, Veltkamp C, Stremmel W. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood 102: 371–376, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem 281: 28494–28498, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Herbison CE, Thorstensen K, Chua AC, Graham RM, Leedman P, Olynyk JK, Trinder D. The role of transferrin receptor 1 and 2 in transferrin-bound iron uptake in human hepatoma cells. Am J Physiol Cell Physiol 297: C1567–C1575, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood 104: 4287–4293, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 112: 1503–1509, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480: 147–150, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood 110: 2182–2189, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitry RR, Hughes RD, Aw MM, Terry C, Mieli-Vergani G, Girlanda R, Muiesan P, Rela M, Heaton ND, Dhawan A. Human hepatocyte isolation and relationship of cell viability to early graft function. Cell Transplant 12: 69–74, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood 105: 1803–1806, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101: 2461–2463, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110: 1037–1044, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest 115: 2180–2186, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M, Nemeth E, Thompson J, Risler JK, Zaborowska C, Babakaiff R, Radomski CC, Pape TD, Davidas O, Christakis J, Brissot P, Lockitch G, Ganz T, Hayden MR, Goldberg YP. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet 36: 77–82, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276: 7811–7819, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Piperno A, Girelli D, Nemeth E, Trombini P, Bozzini C, Poggiali E, Phung Y, Ganz T, Camaschella C. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood 110: 4096–4100, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica 94: 765–772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood 104: 4294–4299, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Terry C, Mitry RR, Lehec SC, Muiesan P, Rela M, Heaton ND, Hughes RD, Dhawan A. The effects of cryopreservation on human hepatocytes obtained from different sources of liver tissue. Cell Transplant 14: 585–594, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Vecchi C, Montosi G, Pietrangelo A. Huh-7: a human “hemochromatotic” cell line. Hepatology 51: 654–659, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology 50: 1992–2000, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 100: 3776–3781, 2002 [DOI] [PubMed] [Google Scholar]