Abstract
Eicosapentaenoic acid (EPA) is an ω-3 polyunsaturated fatty acid abundant in fish oil that exerts a wide spectrum of documented beneficial health effects in humans. Because dietary interventions are relatively inexpensive and are widely assumed to be safe, they have broad public appeal. Their endorsement can potentially have a major impact on human health, but hard mechanistic evidence that specifies how these derivatives work at the cellular level is limited. EPA (50 μM) caused a small elevation of cytoplasmic Ca2+ concentration ([Ca2+]) in intact NCM460 human colonic epithelial cells as measured by fura 2 and a profound drop of [Ca2+] within the endoplasmic reticulum (ER) of permeabilized cells as monitored by compartmentalized mag-fura 2. Total internal reflection fluorescence microscopy showed that this loss of ER store [Ca2+] led to translocation of the ER-resident transmembrane Ca2+ sensor STIM1. Using sensitive FRET-based sensors for cAMP in single cells, we further found that EPA caused a substantial increase in cellular cAMP concentration, a large fraction of which was dependent on the drop in ER [Ca2+], but independent of cytosolic Ca2+. An additional component of the EPA-induced cAMP signal was sensitive to the phosphodiesterase inhibitor isobutyl methylxanthine. We conclude that EPA slowly releases ER Ca2+ stores, resulting in the generation of cAMP. The elevated cAMP is apparently independent of classical G protein-coupled receptor activation and is likely the consequence of a newly described “store-operated” cAMP signaling pathway that is mediated by STIM1.
Keywords: stromal interaction molecule 1, polyunsaturated fatty acids, fish oil, mag-fura 2
there is general consensus that diet and lifestyle play a central part in cancer prevention; in fact, it has been estimated that as much as 35% of human cancer mortality could be attributable to diet (51). Inflammation is a precursor to cancer in numerous tissues. ω-3 Polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid [EPA, 20:5(n-3)] and docosahexaenoic acid [22:6(n-3)], which are abundant in fish oils, are considered beneficial in a wide range of human inflammatory disorders, including inflammatory bowel disease (6, 53). Epidemiological (5, 11), clinical (2–4), and animal (52) studies have supported the hypothesis that the anti-inflammatory effects of dietary (n-3) PUFA provide protection against colon cancer.
Given the potential relevance of long-chain ω-3 fatty acids such as EPA in human diseases of the colon, it is surprising that relatively little is known about how these compounds act at the cellular level in colonic epithelial cells. EPA can directly influence inflammation by replacing arachidonic acid as a substrate for enzymes involved in prostaglandin production (48). It is also metabolized to resolvin E1, an anti-inflammatory mediator that exerts potent effects on immune cells (46), including resident immune cells of the colon (21). EPA is known to interact with various intracellular signaling cascades such as mitogen-activated protein kinases (including extracellular-signal-regulated kinases, c-Jun NH2-terminal kinase, and p38), or phosphoinositide 3-kinase (1, 10, 13, 17, 27), culminating in the activation or inactivation of key transcription factors (e.g., NF-κB or the peroxisome proliferator-activated receptors PPARα and PPARγ) (23, 42).
The fundamental intracellular second messengers Ca2+ and cAMP can potentially intersect with the above signaling pathways on multiple levels. These messenger molecules are typically generated intracellularly following activation of cell surface G protein-coupled receptors (GPCRs). Both Ca2+ and cAMP are well documented to participate in the inflammatory response, including long-term transcriptional control. Here we investigated whether EPA could influence Ca2+ or cAMP signals in colonic epithelial cells. Current knowledge on this subject, even in noncolonic cell types, is very limited. EPA has been shown to cause an increase in cytosolic [Ca2+] in cardiomyocytes (22), endothelial cells (34), erythrocytes (49), fibroblasts (NIH-3T3) (35), breast cancer cells (22), and T cells (12). EPA seems to inhibit cAMP formation in adipocytes (54) and MCF-7 human breast cancer cells (45) while apparently increasing cAMP in renal A6 cells (32) and glioma cells (44).
In this work, we have employed traditional fluorescent Ca2+ indicators and newer-generation genetically encoded sensors for cAMP to show that EPA, at physiologically relevant concentrations, causes both Ca2+ and cAMP to increase within the cytoplasm of cultured human colonic epithelial cells. We observed that the ω-3 fatty acid slowly released Ca2+ from internal Ca2+-storing organelles [likely the endoplasmic reticulum (ER)] by an undefined mechanism. This release of Ca2+ proved necessary for most of the ensuing cAMP signal that occurred upon EPA treatment. Interestingly, while cytosolic Ca2+ is well known to influence cAMP production and degradation (7), the EPA-stimulated elevation in cAMP was completely independent of Ca2+ elevation in the cytoplasm.
We recently demonstrated in several cell types (including cultured colonic epithelial cells) that the levels of free Ca2+ within the ER are able to directly influence the activity of the enzymes that generate cAMP [adenylyl cyclases (ACs)] but does not affect the enzymes that degrade cAMP [the phosphodiesterases (PDEs)] (26, 40). This process is absolutely independent of Ca2+ in the cytosol and requires the translocation of a Ca2+-sensing transmembrane ER protein called STIM1 (9). Exciting results from several groups have demonstrated that STIM1 serves as the long-sought sensor that links free intraluminal ER Ca2+ concentration ([Ca2+]) to the activation of plasma membrane Ca2+ entry pathways (9, 29, 41, 56). Because this new signaling pathway depends on the Ca2+ content of the ER Ca2+ store, we have named it “store-operated cAMP signaling” (26). While this phenomenon is manifest to varying extents in different cell types, we have noted that it is particularly prominent in epithelial cells derived from colon. The data presented here indicate that the majority of the EPA-induced cAMP production occurs via this mechanism in NCM460 cells. This study shows how dietary factors that release ER Ca2+ stores can lead to cAMP signaling that is apparently independent of physiological modes of GPCR-dependent activation.
MATERIALS AND METHODS
Cell culture.
Wild-type NCM460 cells (InCell, San Antonio TX), or an NCM460 cell line stably expressing the Epac H30 sensor (26) (see below), were grown in M3:10 medium (INCELL, San Antonio, TX). NCM460 cells are considered to be “normal” (noncancer) of colonic crypt epithelial origin (33). HeLa cells and Caco-2 cells, a heterogeneous cell line derived from human colorectal adenocarcinoma (both from ATCC), were grown in DMEM supplemented with 10% FBS and 0.5% antibiotics (penicillin/streptomycin).
Ca2+ imaging.
Intracellular [Ca2+] was measured using the ratiometric indicator fura 2 (16). Measurements of [Ca2+] within the agonist- and inositol-1,4,5-trisphosphate (InsP3)-sensitive internal store in digitonin-permeabilized cells were obtained using compartmentalized mag-fura 2, a low-affinity Ca2+ indicator, as described previously (19, 20). Cells were loaded in tissue culture medium at 37°C for 35 min with fura 2-AM (2 μM) or for 20 min with mag-fura 2-AM (2–5 μM). When cells were preloaded with 1,2 bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) and fura 2, the chelator was added during the last 20 min of dye loading to ensure cytosolic retention (final concentration 20–40 μM).
Fura 2 and mag-fura 2 ratios were acquired using a fluorescence ratio imaging set-up running Metafluor software (Universal Imaging, West Chester, PA) as described previously (26). Cells were excited alternately at 340 and 380 nm for 80 ms through a ×40 [numeric aperture (NA) 1.4] oil immersion objective. The excitation wavelengths were generated using a microprocessor-controlled filter wheel (Sutter Instruments) placed in the path of a 100-W mercury light source. Pairs of fluorescence images (emission collected above 510 nm) were captured by a Hamamatsu ORCA ER charge-coupled device camera every 4 s, and converted to a ratio image by the Metafluor software.
Measurement of intraluminal ER [Ca2+] in permeabilized cells.
As described previously (20), mag-fura 2-loaded cells were rinsed briefly in a high-K+ solution (in mM: 125 KCl, 25 NaCl, 0.1 MgCl2, and 10 HEPES, pH 7.20) and then exposed for 2–3 min to an “intracellular buffer” (the same solution with free [Ca2+] clamped to 170 nM using Ca2+/EGTA buffers and supplemented with 1 mM Na2ATP) also containing 5 μg/ml digitonin at 37°C. After plasma membrane permeabilization, cells were continuously superfused with intracellular buffer (without digitonin). Ratio measurements of compartmentalized mag-fura 2 fluorescence were performed as above.
Quantification of STIM1 translocation using total internal reflection fluorescence microscopy.
NCM460 cells were transiently transfected with STIM1-YFP (kind gift of Tobias Meyer, Stanford University) (28) as described previously (26). Total internal reflection fluorescence (TIRF) microscopy is an optical technique that permits excitation of fluorophores in an extremely thin (∼100 nm) plane exclusively at the interface of two materials of differing refractive indexes (e.g., the cover glass and the aqueous phase just under the cell membrane). TIRF data were acquired in real time (one data point every 5–10 s) using a “white-light” TIRF accessory (Nikon T-FL-WTIRF) and a ×60 1.45 NA Plan Apo TIRF oil immersion objective mounted on a Nikon TE2000-U microscope (26). Metafluor software (Molecular Devices) was used to control hardware and acquire single wavelength fluorescence data as for other imaging experiments.
Measurement of cAMP with FRET-based probes in colonic cell lines.
Intracellular cAMP concentration ([cAMP]) was imaged in single cells using Epac H30 [CFP-Epac(∂DEP-CD)-YFP] (38) or Epac H90 [CFP(nd)-EPAC(∂DEP/CD)-cp173Venus(d)] (55). These reporters (kindly provided by Kees Jalink, Netherlands Cancer Institute) are soluble monomeric constructs that rely on conformation-dependent FRET between YFP- and CFP-labeled fragments of the Epac protein (38).
An NCM460 cell line stably expressing EpacH30 was used for most cAMP imaging experiments (25, 26). Wild-type NCM460, CaCo-2, and HeLa cells were transiently transfected with EpacH30 or EpacH90 using the Effectene transfection reagent (Qiagen, Valencia, CA) (26). Real-time digital imaging measurements of the 480 nm-to-535 nm FRET emission ratio (reflecting the degree of Epac conformational changes and, hence, intracellular cAMP levels) were carried out on subconfluent cells using a Metafluor-based imaging set-up and the perfusion apparatus described above.
Immunoassay for cAMP.
Subconfluent NCM460 and Caco-2 cells were trypsinized and washed two times with Ringer solution. Cell pellets were divided in half and suspended in equal volumes of Ca2+-free Ringer alone or Ringer complemented with 50 μM EPA. After treatment (10 min), cells were lysed using 0.1 M HCl, and cAMP was measured using the Correlate-EIA Direct cAMP kit (Assay Designs, Ann Arbor, MI) according to the manufacturer's instructions.
Solutions and materials.
Unless otherwise stated, all chemicals were purchased from Sigma (St. Louis, MO). Experiments were performed with a Ringer solution containing (in mM) 121 NaCl, 2.4 K2HPO4, 0.4 KH2PO4. 1.2 CaCl2, 1.2 MgCl2, 5.5 glucose, and 10 HEPES/NaOH, pH 7.40. InsP3, fura 2-AM, mag-fura 2-AM, and BAPTA-AM were obtained from Molecular Probes/Invitrogen (Carlsbad, CA), and EPA was from Cayman (Ann Arbor, MI). The final solvent concentration (DMSO or ethanol) never exceeded 0.1%.
Data analysis and statistics.
Mean values are expressed ± SE of n individual experiments performed. The significance of the observations was evaluated by Student's t-test for paired or unpaired data as appropriate.
RESULTS
EPA releases InsP3-sensitive intracellular Ca2+ stores.
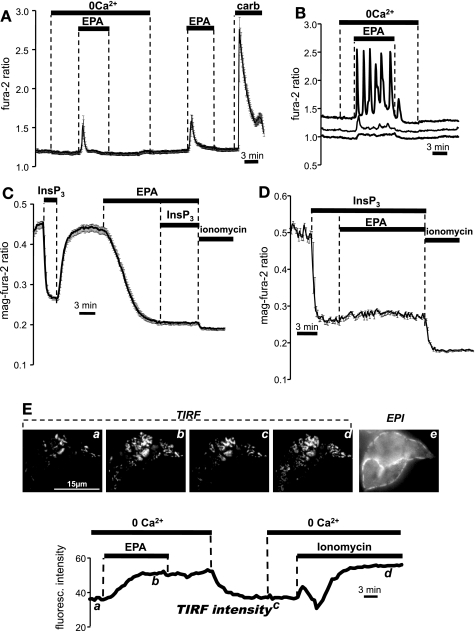
Treatment of NCM460 cells with 50 μM EPA caused intracellular [Ca2+] to increase as measured by fura 2 in both the presence and absence of extracellular Ca2+, indicating that the response was generated predominantly by release of Ca2+ from intracellular stores, and not Ca2+ entry from the extracellular space. Figure 1A depicts the averaged response of 16 cells from a single experiment (typical of n = 129 cells, 6 experiments). As shown in Fig. 1B, the profile of this response was highly variable when examined at the single cell level and included oscillatory signals (∼18% of cells), small Ca2+ transients (∼32%), and responses that were barely detectable (∼50%). Of those cells in which a measurable Ca2+ signal was observed, the increase in the fura 2 ratio was typically much slower and of smaller amplitude (40.02 ± 8.47%; 59 cells, 11 experiments) than the response in the same cells to purinergic stimulation using 100 μM ATP, or to cholinergic stimulation with 100 μM carbachol. Pretreatment for 30 min with 250 nM thapsigargin, a selective and irreversible inhibitor of the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA), a maneuver known to cause passive depletion of Ca2+ from the ER, completely abolished the response of all NCM460 cells to EPA (data not shown; 49 cells, 3 experiments).
Fig. 1.
Effect of eicosapentaenoic acid (EPA) on cytoplasmic and endoplasmic reticulum (ER) Ca2+, and on the translocation of YFP-STIM1 in NCM460 cells. A: change in intracellular Ca2+ in response to 50 μM EPA in the absence and presence of extracellular Ca2+ compared with signal elicited by carbachol (“carb”; 100 μM) as measured with fura 2. Error bars indicate ± SE. B: the response to 50 μM EPA (shown here in Ca2+-free solutions) was highly variable when assessed at the single cell level. C: experiments with the low-affinity Ca2+ indicator mag-fura 2 to measure free Ca2+ in the ER lumen in permeabilized cells. The mag-fura 2 ratio decreased, indicating reduction of free Ca2+ in the lumen of the ER in response to inositol-1,4,5-trisphosphate (InsP3) and EPA. D: as in C, the mag-fura 2 ratio change following InsP3 treatment was not further lowered by subsequent addition of EPA. Ionomycin (5 μM) yielded an additional loss of stored Ca2+. E: bottom, time course of YFP-STIM1 translocation to the cell surface following treatment with 50 μM EPA and 5 μM ionomycin as measured using total internal reflection fluorescence (TIRF) microscopy. Intensity was measured from the regions encompassing the entire two cells; top, a-d: TIRF images of punctae at indicated time points; e: conventional epifluorescence image of same cells showing cellular outline (scale bar = 15 μm). Data typical of results from n = 7 cells in 5 independent experiments.
We previously noted that maneuvers known to cause depletion of ER Ca2+ stores (e.g., SERCA inhibition using tert-butyl hydroquinone or thapsigargin) did not always lead to overt increases in cytosolic Ca2+ in NCM460 cells (possibly reflecting efficient compensation by the Ca2+ homeostatic machinery in this cell type). Therefore, the inconsistent response to EPA as measured by fura 2 is difficult to interpret. We therefore performed direct measurements of ER [Ca2+] using the low-affinity Ca2+ indicator mag-fura 2 (19, 20). The propensity of this indicator to become trapped in organelles allowed us to evaluate the time course, extent, and reversibility of free [Ca2+] changes in the lumen of InsP3-sensitive Ca2+ stores (i.e., the ER). As expected, a supramaximal dose of InsP3 (10 μM) caused a rapid, reversible drop in the 340-to-380 nm mag-fura 2 ratio of digitonin-permeabilized NCM460 cells (Fig. 1C) because of release of Ca2+ from internal stores. Note that this also demonstrates that the permeabilization procedure was successful since InsP3 is membrane impermeant. EPA (50 μM) elicited a large (albeit slower) decrease in the mag-fura 2 ratio in all cells that was not additionally susceptible to release by InsP3, indicating that the PUFA was able to directly release Ca2+ from InsP3-sensitive stores, even in permeabilized cells. Conversely, EPA did not cause significant further reduction in the mag-fura 2 ratio after InsP3 addition (Fig. 1D). The Ca2+ ionophore ionomycin consistently released a small residual component after EPA or InsP3 treatment, indicating the presence of a separate Ca2+-accumulating compartment resistant to both InsP3 and EPA.
In keeping with the above findings, EPA was also able to elicit relocalization and clustering of the ER Ca2+ sensor STIM1 in intact NCM460 cells. It is now well established that STIM proteins are localized within the bulk ER at rest and redistribute to form aggregates just under the plasma membrane following ER Ca2+ store depletion (39). We transiently transfected NCM460 cells with STIM1-YFP and used TIRF microscopy to quantitatively assess the translocation of STIM1 to the plasma membrane following EPA treatment (Fig. 1E). EPA addition in a Ca2+-free medium caused accumulation of STIM1-YFP at the cell surface, reflected as an increase in the TIRF intensity. When EPA was washed out, there was no redistribution of the STIM1-YFP in the bulk ER until Ca2+ was readmitted to the bath. This was expected, since extracellular Ca2+ is needed to refill internal stores (19), and thus reverse the clustering of STIM1. The effect of the Ca2+ ionophore ionomycin is shown for comparison in the same cell. Collectively, the data presented in Fig. 1, A-E, indicate that 50 μM EPA caused the loss of Ca2+ from the ER by an unspecified mechanism.
EPA causes intracellular cAMP to become elevated in NCM460 and CaCo-2 cells.
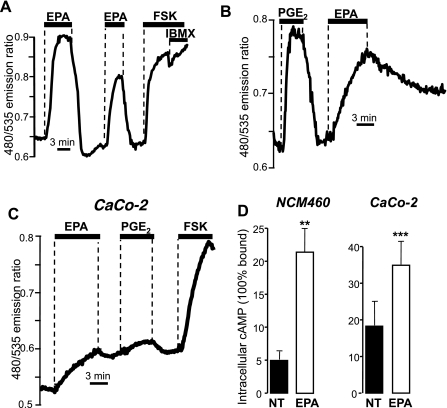
Epac- and FRET-based cAMP sensors (EpacH30 or EpacH90) were used to monitor cAMP in single NCM460 cells. At the end of each experiment, cells were typically challenged with saturating concentrations of the direct AC activator forskolin (50–100 μM) and the PDE inhibitor 3-isobutyl-1-methylxanthine (IBMX, 0.5–1 mM) to establish the maximal 480-to-535 nm FRET emission ratio change. Virtually all NCM460 cells responded to 50 μM EPA with an elevation in cAMP (107 over 108). Figure 2A shows that this increase in [cAMP] was fully reversible upon wash out of EPA, provided Ca2+ was present in the bathing solution. A second, somewhat smaller response (73.0 ± 2.5% of first response, 56 cells, 10 experiments, P < 0.001) was observed when cells were challenged again with EPA after a brief interval. The amplitude of the FRET ratio change following EPA was substantial, being 56.49 ± 7.02% of the maximal response produced by forskolin (100 μM) or forskolin plus IBMX (1 mM). The efficacy of EPA in elevating intracellular cAMP was comparable to that of the endogenous agonist PGE2 (50 nM; Fig. 2B), although the time course was significantly slower. It is possible that EPA could actually become metabolized to prostaglandins via cyclooxygenase (COX) enzymes, directly stimulating cAMP production and/or Ca2+ signaling through various prostanoid receptors. We tested the actions of the nonselective COX inhibitor indomethacin (10 μM) but found no effect of the drug on EPA-induced cAMP signals in NCM460 cells (data not shown; n = 5 experiments, 61 cells).
Fig. 2.
cAMP measurements with the FRET- and Epac-based sensor and immunoassay in response to exposure of colonic cell lines to EPA. A: NCM460 cells stably transfected with the EpacH30 probe. The increase in ratio reflects increases in intracellular cAMP; responses to 50 μM EPA followed by 100 μM forskolin (FSK) + 1 mM 3-isobutyl-1-methylxanthine (IBMX). B: response to EPA and 50 nM PGE2, typical of 28 NCM460 cells, 9 experiments. C: CaCo-2 cells transiently transfected with EpacH30; effect of 50 μM EPA, 50 nM PGE2, and 100 μM forskolin. D: quantitative competitive immunoassay for cAMP measured in NCM460 and Caco-2 cells in the absence of any treatment (“NT”) or after a 10 min treatment with EPA (50 μM) in Ca2+-free solutions (“EPA”). Treatment with EPA yielded a significant increase in cAMP. Data are averages ± SE of n = 3 independent experiments with determinations in duplicate for each condition. **P < 0.02. ***P < 0.003.
EPA was also able to elicit a pronounced increase in cAMP in most CaCo-2 cells (28/33 cells, 19 experiments). The response was 54.5 ± 8.1% (22 cells, 11 experiments) of that of forskolin, but in these cells it was impossible to evaluate the effect of two consecutive EPA additions because of the generally poor reversibility to agonist treatment in this cell type, as shown in Fig. 2C.
EPA induces an increase in total cellular cAMP.
As independent confirmation that the EPA-induced FRET ratio changes depicted in Fig. 2, A-C, reflected actual changes in [cAMP], we measured total cAMP using a commercial quantitative competitive immunoassay kit. Figure 2D shows that treatment with EPA (50 μM for 10 min) yielded a significant increase in cAMP in both NCM460 (P < 0.02) and CaCo-2 (P < 0.003) cells.
EPA-induced elevation of cAMP depends in part on depletion of ER Ca2+ stores.
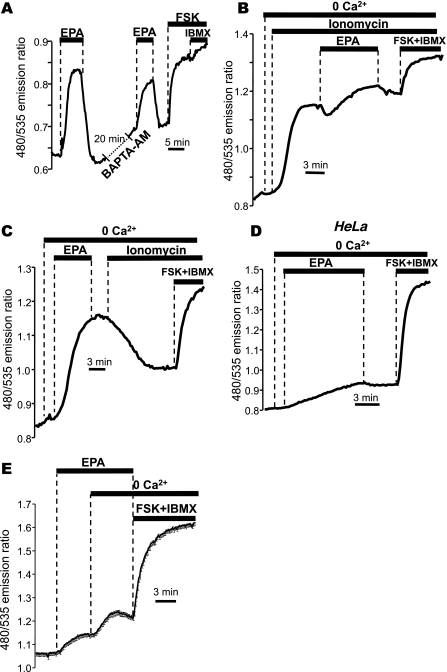
To test whether cytosolic Ca2+ was involved in the EPA-stimulated cAMP changes, we first established the magnitude of a control response to EPA and compared this with the response in the same cell following loading of the Ca2+ chelator BAPTA-AM on the microscope stage (Fig. 3A). Parallel experiments showed that this loading protocol was effective in clamping cytosolic Ca2+ to resting levels following agonist stimulation (data not shown). The cAMP response to EPA was still apparent (52.2 ± 5.3% of first EPA response; n = 26 cells, 5 experiments, compared with control in Fig. 2A) after BAPTA-AM treatment, indicating that this effect did not rely entirely on the elevation of cytosolic [Ca2+].
Fig. 3.
EPA acts via a store-operated cAMP signaling process in NCM460 cells. A: effect of the Ca2+ chelator 1,2 bis (2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM, 40 μM) on the response to EPA as measured with EpacH30. After a first control response, cells were incubated for 20 min with BAPTA-AM on the microscope stage and then challenged again with 50 μM EPA. B: the response to 50 μM EPA was significantly reduced following exposure to ionomycin, and, conversely, the response to ionomycin was abolished when cells were first challenged with 50 μM EPA (C). D: response of HeLa cells to exposure to 50 μM EPA. E: effect of EPA was larger in the absence of extracellular Ca2+ (average of 15 cells ± SE). B-D: representative of experiments performed using EpacH90 sensor.
Because we have shown previously that depletion of ER Ca2+ with ionomycin causes cAMP to become elevated in NCM460 cells via a STIM1-dependent, store-operated cAMP signaling process, we considered that the effect of EPA might occur through this mechanism. When we first released the internal stores using ionomycin in a Ca2+-free solution, we observed, as expected, an increase in the FRET ratio (Fig. 3B). We have shown previously that this response to ionomycin does not depend on the elevation in cytosolic Ca2+, but rather, relies exclusively on the lowering of [Ca2+] within the ER lumen. Subsequent treatment with EPA caused only a minimal increase in the ratio (24.3 ± 6.7% of the maximal ratio change vs. 80.3 ± 3.8% under control conditions in the absence of ionomycin; data from n = 4 experiments/38 cells and 4 experiments/31 cells, respectively; P < 0.001). This suggests that most of the EPA-induced cAMP elevation required an intact ER Ca2+ store. In contrast, when cells were challenged first with EPA in Ca2+-free solutions (producing a large increase in cAMP as measured by the FRET ratio), the large response usually observed with ionomycin was completely abolished (Fig. 3C), pointing to a common mechanism of action for EPA and ionomycin (typical of n = 4 experiments/38 cells).
Taken together, the most likely explanation for the data from Fig. 3, A–C, is that the majority of the cAMP generated upon EPA treatment derives from the store-operated cAMP pathway. Consistent with this interpretation, EPA only modestly affected cAMP production in noncolonic HeLa cells (Fig. 3D), a cell type that we have shown previously to lack this ER- and STIM-dependent cAMP signaling mechanism (26). Moreover, we previously reported that this pathway is suppressed when intracellular [Ca2+] is high (26). It is therefore noteworthy that the elevation in the FRET ratio stimulated by EPA was consistently augmented by removal of Ca2+ from the bath (typical of 83 cells in 5 experiments; Fig. 3E).
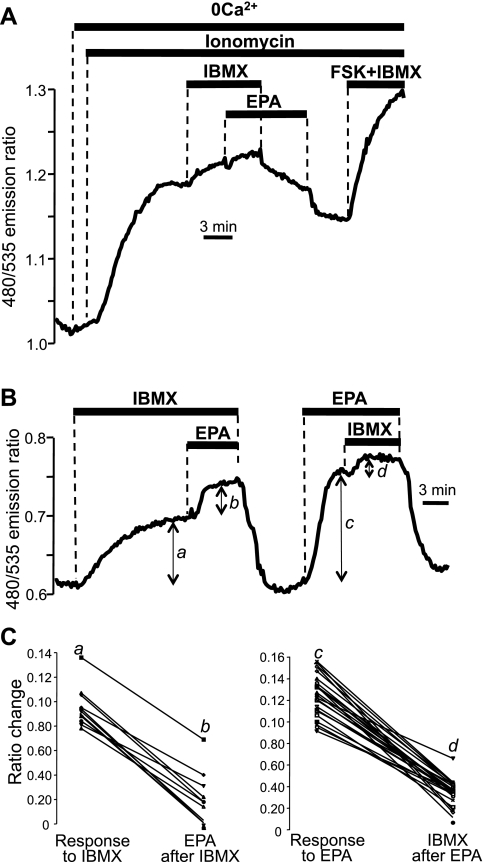
Although the collective data of Fig. 3 support the idea that EPA acts through a store-operated mechanism, Fig. 3B indicates that there is a residual effect of EPA that is independent of ER Ca2+ stores. In light of data from Dubois and colleagues (15), we considered the possibility that EPA might additionally elevate cAMP through effects on PDEs in NCM460 cells. We therefore tested the action of the nonspecific PDE inhibitor IBMX (1–2 mM) in NCM460 cells. As shown in Fig. 4A, when the store-operated component of EPA was eliminated by pretreatment of cells with ionomycin, EPA was no longer able to generate an increase in intracellular cAMP in the presence of IBMX (trace typical of n = 6 independent experiments, 38 cells).
Fig. 4.
Effect of the phosphodiesterase (PDE) inhibitor IBMX on the cAMP response to EPA. A: EPA-induced cAMP response as measured using EpacH90 was eliminated in NCM460 cells after exposure to ionomycin and IBMX. B: EpacH30 response to 50 μM EPA in the presence of the PDE inhibitor IBMX (2 mM) and response to IBMX in the presence of EPA in the same NCM460 cell. C: summary of data expressed as line scatter plots for individual cells. a, b, c, and d are the delta values taken as shown in B. Response to IBMX (a to b): 24 cells, 3 experiments; response to EPA (c to d): 11 cells, 2 experiments.
Figure 4B shows a typical experiment in which the response to EPA was tested in the presence or absence of IBMX and vice versa in the same cell. As shown in Fig. 4B and summarized in Fig. 4C, the response to EPA was significantly suppressed by IBMX (P < 0.0001, 24 cells, 3 experiments), and, conversely, the response to IBMX was significantly inhibited by the ω-3 fatty acid (P < 0.0001, 11 cells, 2 experiments). The reciprocal inhibition of EPA and IBMX is a strong indication that the two compounds act on the same target (PDE). From these data we can infer that the increase in cAMP observed in response to EPA was at least partially due to an inhibitory action of the ω-3 fatty acid on PDE and that this effect was not mediated by ER Ca2+.
DISCUSSION
Diseases of the colon such as inflammatory bowel diseases and colorectal cancer are particularly prevalent in those developed countries consuming rich Western diets. That dietary habits influence cancer susceptibility is incontrovertible; however, maximizing the potential benefits of “healthy” foods requires an understanding of how specific bioactive components contained within those foodstuffs work at the cellular level. At the same time, it must be recognized that “nutraceuticals,” such as EPA, although not widely appreciated as such, are in effect potent pharmacological agents. Indiscriminate use of refined and concentrated preparations of these unregulated compounds may present potential public health hazards.
PUFAs in the diet are mostly absorbed in the upper gastrointestinal tract such that only a small percentage is ultimately delivered to the colonic lumen (36). However, recent studies have shown that supplementation with 2 g/day of fish oil for 4 wk resulted in plasma concentrations of EPA in excess of 100 μM (31). In this work, we demonstrated that acute treatment with 50 μM EPA led to a gradual release of intracellular Ca2+ stores in colonic cells, which in turn activated the clustering of the ER-resident protein STIM1 in “punctae” at ER-plasma membrane junctions (Fig. 1). This store depletion was responsible for the large majority of cAMP produced upon EPA treatment in NCM460 cells, working through a previously described “store-operated” cAMP signaling mechanism in colonic cells that links STIM1 translocation to AC activation (26). This action was independent of cytosolic Ca2+, since it was not eliminated by loading cells with BAPTA-AM (Fig. 3A), but it was very much reduced when ER stores had been emptied by pretreatment with the Ca2+ ionophore ionomycin (Fig. 3B).
Although our study did not address how EPA caused the emptying of internal Ca2+ stores, our experiments in digitonin-permeabilized cells (Fig. 1C) suggest that this effect occurs directly at the level of the ER membrane. Modifications of the lipid environment (such as would occur following incorporation of n-3 PUFAs in the ER membrane) are well known to affect the activity of membrane transporters, so it is possible that EPA or a metabolite thereof directly activates Ca2+ release channels in the ER membrane (48). These data emphasize how xenobiotic agents that release intracellular Ca2+ stores, even with a slow kinetic that results in minimal elevation of cytosolic [Ca2+], can elicit cAMP generation in the absence of physiological hormonal activation of Gsα-coupled GPCRs in colon-derived cells.
A small fraction of the cAMP generated in response to EPA was dependent neither on cytosolic nor ER Ca2+ in NCM460 cells. This residual effect was due to the apparent inhibition of PDEs, the enzymes that degrade cAMP, as judged by the sensitivity of this phenomenon to the broad-spectrum PDE inhibitor IBMX (Fig. 4). These results are consistent with previous reports in rat cardiomyocytes suggesting that PUFAs can inhibit cAMP and cGMP PDE activity (15, 37, 48).
Widespread anecdotal reports and clinical evidence have documented diarrhea to be a major unwanted side effect of supplemental fish oil ingestion (6, 8). In the human colon, both Ca2+- and cAMP-generating agonists activate electrogenic Cl− movements that underlie fluid transport (24, 43). Cl− secretion occurs via apical membrane Cl− channels acting in conjunction with the basolateral membrane Na+-K+-2Cl− cotransporter. Parallel activation of K+ channels can further enhance secretion. Efficient anion secretion in the colon is generally believed to require functional cystic fibrosis transmembrane conductance regulator, which is cAMP and protein kinase A dependent (18). Our results showing that EPA can potently elevate cAMP (and presumably anion and fluid transport) in colonic cells provides a plausible mechanistic explanation linking ingestion of high doses of fish oil with diarrhea.
Much recent work has emphasized the link between PGE2 signaling via EP2/EP4 receptors during inflammation and adenoma formation in the colon (14, 30, 47, 50). These two PGE2 receptor subtypes are coupled to cAMP signaling in colonic epithelial cells. EPA likely influences the activity of multiple cell types within the complex landscape of the colonic mucosa, including resident immune cells that mediate the inflammatory response; however, the direct effects of EPA on second messenger signaling in colonocytes may also figure in the progression of these cells down the pathway to tumor formation. Chronic elevation of cAMP might be predicted to be deleterious to the cell; however, the actions of long-term dietary supplementation with ω-3 fatty acids on cAMP signaling may differ from the short-term actions observed in the present study. It remains to be seen whether these cells are direct targets of the anti-tumor actions of EPA or its metabolites in vivo.
GRANTS
This study was supported by a Merit Review award from the Department of Veteran's Affairs (A. M. Hofer.) and by a National Institutes of Health Center grant from the Harvard Digestive Diseases Center (to A. M. Hofer). K. Lefkimmiatis is the recipient of an American Heart Association Postdoctoral Fellowship award.
DISCLOSURES
M. P. Moyer holds partial ownership of INCELL, which sells M3:10 medium. The other authors declare no competing financial interests.
REFERENCES
- 1.Ait-Said F, Elalamy I, Werts C, Gomard MT, Jacquemin C, Couetil JP, Hatmi M. Inhibition by eicosapentaenoic acid of IL-1beta-induced PGHS-2 expression in human microvascular endothelial cells: involvement of lipoxygenase-derived metabolites and p38 MAPK pathway. Biochim Biophys Acta 1631: 77–84, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, Parrella P, Canetta C, Gentiloni N, De Vitis I. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology 107: 1709–1718, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology 103: 883–891, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, Richter F, Richter A, Kasper H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology 105: 1317–1322, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Belluzzi A, Boschi S, Brignola C, Munarini A, Cariani G, Miglio F. Polyunsaturated fatty acids and inflammatory bowel disease. Am J Clin Nutr 71: 339S–342S, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Belluzzi A, Brignola C, Campieri M, Pera A, Boschi S, Miglioli M. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med 334: 1557–1560, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Bruce JI, Straub SV, Yule DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium 34: 431–444, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Burns CP, Halabi S, Clamon G, Kaplan E, Hohl RJ, Atkins JN, Schwartz MA, Wagner BA, Paskett E. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia. Cancer 101: 370–378, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol 11: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calviello G, Di Nicuolo F, Gragnoli S, Piccioni E, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, Palozza P. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and -2 and HIF-1alpha induction pathway. Carcinogenesis 25: 2303–2310, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer 74: 159–164, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow SC, Jondal M. Polyunsaturated free fatty acids stimulate an increase in cytosolic Ca2+ by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in T cells through a mechanism independent of phosphoinositide turnover. J Biol Chem 265: 902–907, 1990 [PubMed] [Google Scholar]
- 13.Denys A, Hichami A, Khan NA. Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase enzyme activity in human T-cells. Mol Cell Biochem 232: 143–148, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Doherty GA, Byrne SM, Molloy ES, Malhotra V, Austin SC, Kay EW, Murray FE, Fitzgerald DJ. Proneoplastic effects of PGE2 mediated by EP4 receptor in colorectal cancer (Abstract). BMC Cancer 9: 207, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois M, Picq M, Nemoz G, Lagarde M, Prigent AF. Inhibition of the different phosphodiesterase isoforms of rat heart cytosol by free fatty acids. J Cardiovasc Pharmacol 21: 522–529, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 17.Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, Kaneko S, Itoh T, Gohda T, Horikoshi S, Tomino Y. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant 21: 605–615, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hirtz S, Gonska T, Seydewitz HH, Thomas J, Greiner P, Kuehr J, Brandis M, Eichler I, Rocha H, Lopes AI, Barreto C, Ramalho A, Amaral MD, Kunzelmann K, Mall M. CFTR Cl- channel function in native human colon correlates with the genotype and phenotype in cystic fibrosis. Gastroenterology 127: 1085–1095, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hofer AM, Landolfi B, Debellis L, Pozzan T, Curci S. Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. Embo J 17: 1986–1995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofer AM, Machen TE. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci USA 90: 2598–2602, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm Bowel Dis 16: 87–95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jude' S, Roger S, Martel E, Besson P, Richard S, Bougnoux P, Champeroux P, Le Guennec J-Y. Dietary long-chain omega-3 fatty acid of marine origin: A comparison of their protective effects on coronary hearth disease and breast cancers. Progr Biophys Molec Biol e-public: 1–20, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 41: 41–78, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev 82: 245–289, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. “cAMP sponge”: a buffer for cyclic adenosine 3′,5′-monophosphate. PLoS One 4: e7649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol 11: 433–442, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int 67: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA 104: 9301–9306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loffler I, Grun M, Bohmer FD, Rubio I. Role of cAMP in the promotion of colorectal cancer cell growth by prostaglandin E2 (Abstract). BMC Cancer 8: 380, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki KC, Reeves MS, Farmer M, Griinari M, Berge K, Vik H, Hubacher R, Rains TM. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res 29: 609–615, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Mies F, Shlyonsky V, Goolaerts A, Sariban-Sohraby S. Modulation of epithelial Na+ channel activity by long-chain n-3 fatty acids. Am J Physiol Renal Physiol 287: F850–F855, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Moyer MP, Manzano LA, Merriman RL, Stauffer JS, Tanzer LR. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim 32: 315–317, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun 232: 487–491, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Palakurthi SS, Fluckiger R, Aktas H, Changolkar AK, Shahsafaei A, Harneit S, Kilic E, Halperin JA. Inhibition of translation initiation mediates the anticancer effect of the n-3 polyunsaturated fatty acid eicosapentaenoic acid. Cancer Res 60: 2919–2925, 2000 [PubMed] [Google Scholar]
- 36.Pawlosky RJ, Hibbeln JR, Lin Y, Goodson S, Riggs P, Sebring N, Brown GL, Salem N., Jr Effects of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. Am J Clin Nutr 77: 565–572, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Picq M, Dubois M, Grynberg A, Lagarde M, Prigent AF. Specific effects of n-3 fatty acids and 8-bromo-cGMP on the cyclic nucleotide phosphodiesterase activity in neonatal rat cardiac myocytes. J Mol Cell Cardiol 28: 2151–2161, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 5: 1176–1180, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 42: 103–110, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Putney JW., Jr SOC: now also store-operated cyclase. Nat Cell Biol 11: 381–382, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy N, Barnett M, Knoch B, Dommels Y, McNabb W. Nutrigenomics applied to an animal model of inflammatory bowel diseases: transcriptomic analysis of the effects of eicosapentaenoic acid- and arachidonic acid-enriched diets. Mutat Res 622: 103–116, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Sahi J, Nataraja SG, Layden TJ, Goldstein JL, Moyer MP, Rao MC. Cl- transport in an immortalized human epithelial cell line (NCM460) derived from the normal transverse colon. Am J Physiol Cell Physiol 275: C1048–C1057, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Salvati S, Natali F, Attorri L, Raggi C, Di Biase A, Sanchez M. Stimulation of myelin proteolipid protein gene expression by eicosapentaenoic acid in C6 glioma cells. Neurochem Int 44: 331–338, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Sauer LA, Dauchy RT, Blask DE, Krause JA, Davidson LK, Dauchy EM. Eicosapentaenoic acid suppresses cell proliferation in MCF-7 human breast cancer xenografts in nude rats via a pertussis toxin-sensitive signal transduction pathway. J Nutr 135: 2124–2129, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. J Biol Chem 280: 26565–26572, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Siddiqui RA, Harvey KA, Zaloga GP. Modulation of enzymatic activities by n-3 polyunsaturated fatty acids to support cardiovascular health. J Nutr Biochem 19: 417–437, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Soldati L, Lombardi C, Adamo D, Terranegra A, Bianchin C, Bianchi G, Vezzoli G. Arachidonic acid increases intracellular calcium in erythrocytes. Biochem Biophys Res Commun 293: 974–978, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med 7: 1048–1051, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3: 768–780, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother 56: 215–222, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Teitelbaum JE, Allan Walker W. Review: the role of omega 3 fatty acids in intestinal inflammation. J Nutr Biochem 12: 21–32, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Tisdale MJ. Mechanism of lipid mobilization associated with cancer cachexia: interaction between the polyunsaturated fatty acid, eicosapentaenoic acid, and inhibitory guanine nucleotide-regulatory protein. Prostaglandins Leukot Essent Fatty Acids 48: 105–109, 1993 [DOI] [PubMed] [Google Scholar]
- 55.van der Krogt GN, Ogink J, Ponsioen B, Jalink K. A comparison of donor-acceptor pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example. PLoS One 3: e1916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]