Abstract
Biological rhythms coordinate the timing of our internal bodily functions. Colonic motility follows a rhythm as well: most people will have a bowel movement in the morning and rarely during the night. Recent work provides a potential mechanism for this observation: the mouse colon possesses a functional circadian clock as well as a subset of rhythmically expressed genes that may directly impact on colonic motility. Furthermore, measures of colonic motility such as the colonic tissue contractile response to acetylcholine, stool output, and intracolonic pressure changes vary as a function of the time of day, but these variations are attenuated in mice with disrupted clock function. These laboratory findings are supported by clinical observations. Gastrointestinal symptoms such as diarrhea or constipation are prevalent among shift workers and time-zone travelers, both of which are conditions associated with disruptions in biological rhythms. This review will discuss new insights into the role of clock genes in colonic motility and their potential clinical relevance.
Keywords: colon, biological rhythm, gastrointestinal symptoms, shift work
“keep the balance that nature intended,” “regulate the rhythm of your bowel,” “restore your body's natural digestive rhythm” are some of the commercial slogans with which the industry targets the millions of individuals that suffer from irregular bowel habits. Such slogans appeal to the general recognition that bowel habits follow a rhythm. Healthy individuals will have bowel movements during the day, but seldom at night. Furthermore, it is well recognized that disruption of daily rhythms, such as occurs with shift work or time zone traveling, can lead to gastrointestinal symptoms including bloating, abdominal pain, diarrhea, or constipation. These observations suggest a functional correlation between daily rhythms and gastrointestinal physiology.
The terms “biological rhythm,” “diurnal rhythm,” and “circadian rhythm” are frequently used interchangeably. However, it should be noted that there is a fundamental difference between them. Biological rhythms encompass both diurnal and circadian rhythms and generally refer to periodic fluctuations in physiology and/or behavior. When biological rhythms are solely driven by cyclic events in the environment such as the light-dark cycle, they are referred to as diurnal rhythms. Biological rhythms can also be generated by so-called “clock genes,” a group of genes that govern 24-h, circadian rhythms. The key requirement for the description of a rhythm as circadian is to show that the rhythm persists under constant conditions (i.e., the rhythm must continue within an approximate 24-h period when external time cues such as light are removed from the environment), thus demonstrating the endogenous existence of a time-keeping mechanism. Other chronobiological terms relevant to this review are summarized in Table 1.
Table 1.
Chronobiology terminology
| Period | Duration of 1 complete cycle in a rhythmic variation |
| Phase | Time at which a rhythmic activity/process occurs |
| Acrophase | Measure of the timing of the peak value recurring in each cycle relative to a phase reference, such as the time of lights on or, in the case of research subjects in constant environmental conditions, the time of activity onset |
| Zeitgeber time (ZT) | Periodic environmental signal (cue) that synchronizes or sets the time of a circadian rhythm i.e., ZT0 corresponds to the time of lights on, ZT1 is 1 h later, etc., until ZT 24 |
| Circadian time (CT) | Time of the circadian clock under constant environmental conditions, i.e., CT0 corresponds to the beginning of the subjective day |
| Phase shift | A change in the timing of environmental cues will result in a shift in phase of the rhythm |
| Entrainment | The process of synchronization to environmental cues |
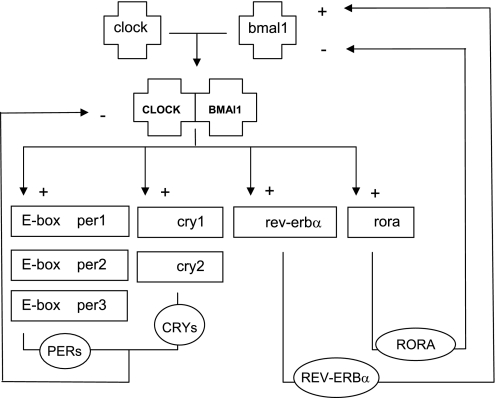
The molecular basis for biological rhythms is formed by so-called “clock genes” and their products. Clock genes participate in two interlocked transcription-translation feedback loops (Fig. 1). In the first loop, CLOCK and BMAL1 start the cycle by forming a heterodimer that drives the expression of the period (per1, per2, and per3) and cryptochrome (cry1 and cry2) genes, whose protein products then inhibit CLOCK/BMAL1 activity. In the second loop, CLOCK/BMAL1 will drive the expression of Rev-erbα and rora, a class of nuclear hormone receptors, whose mutually opposing actions on the Bmal1 promoter produce a 24-h rhythm in Bmal1 transcription (4). The resulting oscillations in CLOCK/BMAL1 activity give rise to circadian rhythmicity.
Fig. 1.
Simplified presentation of the circadian-feedback loops. The cycle starts with the formation of a CLOCK/BMAL heterodimer that initiates the transcription of the per-, cry-, rora-, and rev-Erbα genes. PER and CRY proteins subsequently inhibit CLOCK/BMAL1 transcription (loop 1), REV-ERBα inhibits Bmal transcription, whereas RORA activates Bmal transcription (loop 2).
The most direct mechanism by which clock genes drive circadian gene expression is through regulation of promoter activity of clock-controlled genes. Clock genes can also indirectly drive circadian gene expression through regulation of promoter activity of clock-controlled genes, which in turn regulate the transcription of other downstream genes at specific times of the day (7). Thus, while regulating their own activity, clock gene proteins can also regulate the transcription of downstream genes.
A central clock or “pacemaker” is located in the hypothalamic suprachiasmatic nucleus (SCN), which receives photic input via the retinohypothalamic tract. However, it has now become clear that the majority of peripheral tissues contain functional clock genes as well. Since these peripheral clock genes cannot perceive light, the SCN communicates with peripheral tissues via neuronal and humoral pathways, although the precise mechanism of interaction through which the central clock entrains peripheral clocks is unknown (7). Peripheral clocks can be entrained by other stimuli such as restricted feeding, independent of the central clock. Lesions of the SCN abolish all behavioral, physiological, and biochemical rhythms. However, in the absence of the SCN, anticipatory food-associated behavior as well as rhythms in peripheral clock genes can be entrained by timed feeding (47).
The purpose of this review is to provide an overview of the clinical data that first demonstrated the presence of biological rhythms in colonic motility and to review data from animal studies that demonstrate that such biological rhythms may be driven by clock genes. It will also discuss the potential role of biological rhythms in gastrointestinal diseases that are characterized by alterations in bowel habits, such as irritable bowel syndrome. Finally, it will review remaining questions and challenges in this novel area of research.
Rhythmic Changes in Measures of Gastrointestinal Motility: Clinical Observations
Stomach.
Goo et al. (17) measured gastric emptying rates in 16 healthy male subjects at the start of the day (8 AM) and at the start of the evening (8 PM) and found gastric emptying half-times for the evening meal to be significantly longer for solids but not for liquids compared with morning emptying half-times.
Small bowel.
Kumar et al. (28) used twin intraluminal pressure-sensitive radiotelemetric capsules for prolonged monitoring of proximal small bowel motility in healthy volunteers and observed a significant variation between daytime and nocturnal propagation velocities of the migrating motor complex.
Colon.
At least two studies in healthy human volunteers have demonstrated rhythmic changes in measures of colonic motility. In 1987, Narducci et al. (36) demonstrated that colonic motor activity was low before meals and minimal during sleep and that colonic motor activity increased significantly after meals and at morning awakening. Rao et al. (41) described similar findings in ambulatory colonic pressure recordings from 25 healthy individuals and showed that a threefold increase in colonic pressure activity occurs immediately after awakening as well as following a meal.
Rectum.
Auwerda et al. (2) studied rectal motor complexes, which are defined as distinct patterns of regular pressure fluctuations with a frequency of either three or six cycles per minute, in healthy human, fully ambulant volunteers. The number of rectal motor complexes was significantly lower during sleep and the duration and peak amplitude of these nocturnal rectal motor complexes were significantly reduced, suggesting that such complexes are following a biological rhythm. Meals provided a stimulus for increased rectal motor activity as well.
Thus rhythmic changes in motility appear to occur throughout the entire gastrointestinal tract of healthy humans. Whether these rhythmic changes are truly circadian remains to be determined because none of the studies were completed under constant conditions.
Colonic Clock Gene Expression: From Rhythmic Phenomenology to Physiological Relevance
Over the past two decades, many components of the molecular clock have been identified. However, prior to the identification of the molecular clock, various rhythmic processes were already characterized within the gastrointestinal tract including gastrointestinal motility (17), absorption rates (12), mucosal enzyme activities (31), cell proliferation rhythms (8), and gastric acid secretion (34), as reviewed elsewhere in greater detail (21, 44). The identification of multiple clock genes led to an initial surge in chronobiological studies that focused on the role and regulation of the individual clock genes within the transcription-translation feedback loops. However, more recently researchers are focusing on the role of clock genes in organ physiology and pathophysiology. One such focus has been on the potential role of clock genes in colonic motility.
Clock Genes Are Expressed Within the Epithelial Cells and Myenteric Plexus of the Colon
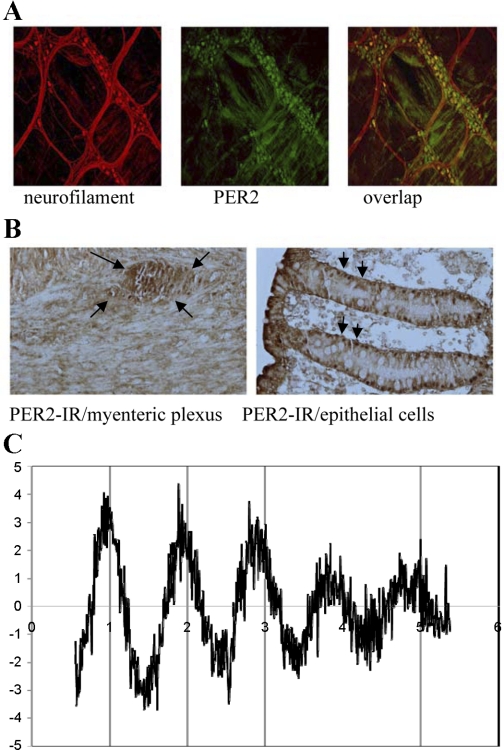
In 2007, two independent groups presented a comprehensive overview of clock gene expression within the rodent colon in parallel publications (23, 46). Using in situ hybridization and immunohistochemistry, Sladek et al. (46) demonstrated per1 and bmal1 gene expression within epithelial cells of the rat distal colon, more specifically within the crypts of the colonic epithelium. Hoogerwerf et al. (23) demonstrated PER2 and BMAL1 protein expression within colonic epithelial cells as well as within the myenteric plexus, an important site of neurotransmitter synthesis and coordination of colonic motility. To more definitively demonstrate the expression of clock genes within individual neurons of the mouse colon, additional immunohistochemical studies were performed on longitudinal muscle myenteric plexus (LMMP) preparations of the mouse colon (Fig. 2A). These LMMP preparations firmly establish the expression of clock genes within the neurons of the myenteric plexus. In addition, immunohistochemical studies were performed on human colonic tissue, which confirmed the expression of clock genes within the colonic epithelial cells as well as within the ganglia of the myenteric plexus (Fig. 2B). This observation demonstrates the potential clinical relevance and translatability of the rodent studies to humans. In addition, Pardini et al. (38) showed that it is technically feasible to examine clock gene expression at both the RNA and protein level in single intact colonic crypts isolated by chelation from biopsies obtained from healthy humans during colonoscopy.
Fig. 2.
Expression and function of colonic clock genes. A: longitudinal muscle myenteric plexus (LMMP) of the mouse colon using double-labeling immunohistochemical staining of neurofilament (red) with PER2 (green) in the mouse colon. Overlap represents superimposition of fluorescent images. B: cross section of human colon showing PER2 immunoreactivity (IR) within ganglia of the myenteric plexus and within the epithelial cells. C: rhythmic per2-luciferase activity in a LMMP preparations from mouse colon maintained in constant darkness for a total of 5 days without medium changes. Although the oscillations dampened after 4 days, this dampening is more likely to represent a dampening due to decreased availability of the substrate luciferin.
Clock Genes Are Rhythmically Expressed Within the Colon
Patterns of clock gene expression within individual tissues are most commonly assessed by polymerase chain reaction on whole tissues obtained at regular time intervals over a 24-h period. By using this technical approach, rhythmic expression of clock genes within the rodent colon was confirmed (23, 46). However, clock genes are expressed within the neurons of the myenteric plexus as well as within the epithelial cells, and by using whole tissue the relative contribution of the different cell types to the measured rhythmic expression of clock genes could not be determined. Nonetheless, the presence of a distinct single peak in oscillation suggested that the clock genes in the different cell types of the gut are in phase with each other (23). This assumption has since then been more definitively confirmed through in vitro studies of mice in which the per2 gene is linked to a luciferase reporter. Thanks to its relatively short half-life, luciferase is ideal for expression analysis that follows time-course changes, as in the analysis of circadian rhythms. When luciferase bioluminescence was measured from mucosal/submucosal and LMMP preparations from the proximal colon, there was no difference in the phase of PER2 expression (W. A. Hoogerwerf and V. M. Cassone, unpublished observations). And although it is technically more difficult to obtain neurons from the myenteric plexus through colonic biopsies from humans, the finding that the phase of clock gene expression within epithelial cells parallels that of the phase of clock gene expression within the neurons of the myenteric plexus suggests that phase determinations of the clock genes within the epithelial cells can serve as a surrogate marker for the phase of neuronal clock gene expression. Such an approach would facilitate studies on the role of clock genes in human tissues. In addition, LMMP preparations showed robust oscillations in PER2 expression, which results from PER2-expressing neurons within the myenteric plexus. Oscillations within SCN neurons have been shown to persist in vitro for over a year following ongoing medium/substrate changes (52). It is therefore conceivable that LMMP cultures can be maintained in vitro for prolonged periods of time, thereby presenting a unique opportunity for future mechanistic studies on the role of clock genes within the neurons of the myenteric plexus.
Colonic Clock Genes Display Circadian Rhythm Properties
Attributes of circadian rhythms include their ability to persist under constant conditions (i.e., in the absence of light) and their ability to be synchronized or reset by environmental cues such as time of feeding. Thus, in examining the strict attributes of circadian rhythms within the colon, they theoretically ought to be examined under conditions in which neither light or food availability can serve as a cue. When mice were placed under constant darkness with ad libitum access to food, colonic clock gene expression remained rhythmic (23). When mice were placed in constant darkness without access to food (but with ad libitum access to water), rhythmicity persisted although the amplitude of clock gene oscillations decreased dramatically (25).
The colonic clock can become uncoupled and function independent of the central clock. Restricted feeding has been established as a major synchronizer of clock gene expression in tissues within and outside of the gastrointestinal tract. When determining the role of restricted feeding in the synchronization or entrainment of clock genes, one should be aware that rodents are nocturnal animals. They will eat most of their food, ∼75%, within the first few hours after the onset of the dark cycle. Thus their food intake follows a naturally rhythmic pattern (5). To examine the effect of restricted feeding (food only provided during the first 4 h of the light cycle) on the phase of colonic and central clock gene expression, colonic tissue and brains were collected following ad libitum feeding, restricted feeding for 48 h, and restricted feeding for 1 wk. The phases of all clock gene mRNA accumulation profiles in the colon differed 7–12 h between mice fed during the daytime and mice fed ad libitum (23). Following 1 wk of restricted feeding, no additional phase shifting occurred (23), suggesting that shifts in colonic clock gene expression occur within the first 48 h of restricted feeding. In contrast, no statistically significant difference in either acrophase or amplitude was found among the three different conditions for either per2 or bmal in the brain (23). Thus colonic clock gene expression in response to restricted feeding occurs independent of the central clock. This finding is in line with findings by other investigators (14, 19, 48). However, it has been suggested that central clock gene expression may shift when feeding is not only restricted to daytime hours but also hypocaloric in its contents (32). The latter suggests that the phase of clock genes expression within the SCN may be modulated by metabolic cues.
A Subset of Genes in the Mouse Colon Follows a Rhythmic Expression Pattern
By use of microarray analysis, rhythmic gene expression profiles were detected in ∼3.7% of distal colonic genes. Both vasoactive intestinal peptide (VIP) and neuronal nitric oxide synthase (nNOS), two genes that play important roles in the modulation of colonic motility, were identified as rhythmically expressed, supporting a potential role for clock genes in their transcriptional regulation (23). A large number of genes were found to be involved in cell signaling, differentiation, proliferation, and cell death. This is not surprising because clock genes are expressed within the epithelial cells of the colon. However, gene arrays may underestimate the number of rhythmically expressed gene because of an underestimation of the amount of gene product (3, 25). It is therefore conceivable that an even larger number of colonic genes are under circadian clock control.
Measures of Colonic Motility Are Rhythmic in WT but not in per1per2 Double-Knockout Mice
To investigate the physiological relevance of clock genes in colonic motility, measures of colonic motility were assessed in wild-type (WT) mice and in per1per2 double-knockout mice. These measures consisted of a combination of in vivo (stool output, intracolonic pressure changes) and ex vivo (colonic circular muscle contractility) methods. First, stool output was rhythmic in WT mice with most stools being passed during the dark cycle. In contrast to WT mice, per1per2 double-knockout mice did not demonstrate rhythmicity in stool output (24). Second, colonic pressure activity was assessed through the use of a telemetry-based system that allows recordings of intracolonic pressure changes as a measure of colonic contractile activity on a continuous basis in freely eating and moving mice. Colonic pressure activity was greatest during the dark phase in WT mice but there was no rhythmicity in colonic pressure activity in per1per2 double-knockout mice (24). Third, the acetylcholine-evoked colonic circular muscle contractile response was greater at the beginning of the dark cycle when compared with the beginning of the light cycle in WT mice but not in per1per2 double-knockout mice (24). Persistence of rhythmicity in stool output, intracolonic pressure changes, and tissue contractility in WT mice under constant darkness and the absence of this rhythmicity in per1per2 double-knockout mice confirmed that these measures of colonic motility are indeed circadian and controlled by an endogenous clock-driven process.
Rhythmic Changes in Colonic Contractility Are in Part Mediated by nNOS
Under normal conditions the colonic circular smooth muscle contractile response to acetylcholine is greatest at the beginning of the dark cycle. However, in the presence of the neuronal inhibitor tetrodotoxin (TTX), there was no longer a difference in the contractile response at the beginning of the light cycle when compared with the contractile response at the beginning of the dark cycle (24). This difference was lost because the contractile response at the start of the light cycle is much greater in the presence of TTX, suggesting that TTX abrogated an inhibitory response that attenuates the response to acetylcholine under normal conditions (24). Additional organ bath studies in both WT and mice genetically deficient in nNOS demonstrated that this neuronally mediated inhibitory response is mediated through nNOS.
These observations are in line with the general concept that smooth muscle cells are kept under tonic inhibition in the resting state through the basal release of inhibitory neurotransmitters and that muscle contractions only occur only when the inhibitory neurons are “switched off” (51). It is conceivable that this tonic state of inhibition is mediated in part through a nNOS-dependent clock-controlled mechanism.
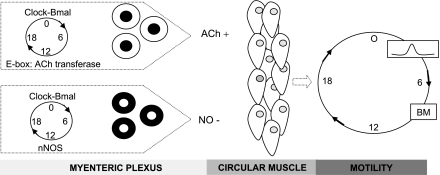
In summary, the findings described above suggest that clock genes within the neurons of the myenteric plexus directly or indirectly control the transcription of a subset of colonic genes (e.g., nNOS and VIP) that, in turn, modulate colonic motility. Rhythmic changes in the expression of nNOS may lead to a predominant inhibitory effect on colonic smooth muscle during the inactive phase and a decrease in inhibition during the active phase of colonic motor activity. A proposed model for clock-controlled regulation of colonic motility is presented in Fig. 3.
Fig. 3.
Conceptual model for circadian regulation of colonic motility. The rhythmic expression of clock genes within the neurons of the myenteric plexus modulate colonic motility through direct or indirect clock-controlled transcription of genes such as acetylcholine (ACh) transferase and neuronal nitrix oxide synthase (nNOS). Direct clock-controlled transcription can be mediated through an E-box element (the consensus sequence for the E-box element is CANNTG) through which clock genes can enhance transcription of downstream gene. Transcription of ACh and nNOS will lead to the rhythmic release of ACh and nitric oxide (NO), which will initiate diverse biochemical, cellular, and physiological processes within the colonic circular muscle, which may in turn, through a cascade of second order messengers and various signaling pathways lead to enhanced colonic motility and, eventually, a bowel movement (BM) at one time of the day and decreased colonic motility at another time of day.
Clinical Relevance of a Biological Clock in Colonic Motility
What is the clinical relevance of the presence of a colonic clock that may be involved in colonic motility? To answer this question, one should first consider the relevance of molecular clocks in general. Circadian clocks have been highly conserved throughout evolution and their main role may have been to present living beings with a survival advantage by allowing them to adapt to environmental changes and anticipate the physiological needs for the time of day. The most well-recognized biological rhythm in humans, namely the sleep-wake cycle, dictates that we should be sleeping at night and awake during the day. Therefore many of our biological functions are primed to function optimally during the day, whereas others are set to function optimally during the night. The importance of adherence to a regular sleep/wake cycle is demonstrated by epidemiological studies of individuals forced to disrupt their biological rhythms through participation in shift work. Participation in shift work has been associated with a plethora of diseases that carry a tremendous health care burden. For example, epidemiological studies have demonstrated that shift workers are at increased risk for the development of obesity, diabetes, and cardiovascular disease (26, 35). In addition, participation in shift work has been associated with an increased risk for the development of breast and colon cancer (42, 43) and most recently with an increased risk for irritable bowel syndrome (37). The association between participation in shift work and obesity is particularly interesting. In the seventies, Halberg et al. (18) demonstrated that the timing of food intake can determine whether an individual will gain, maintain, or lose weight. Consumption of a 2,000-kcal meal within 1 h of first awakening can lead to weight loss whereas the same meal can lead to weight gain when consumed 12 h after first awakening (20). These observations are particularly relevant in this day and age in which obesity has reached epidemic proportions. The role of biological rhythms in the pathogenesis of obesity, a modern epidemic, has been reviewed in greater detail elsewhere but illustrates the point that life styles that force a continuous shift in our biological rhythms, including the timing of our food intake, may have adverse health consequences (22).
The clinical data as well as the data obtained from rodent studies suggest that there may be an optimal time for colonic motility. As discussed earlier, colonic contractile activity in humans increases significantly following awakening and following meals. These observations suggest that increases in colonic motor activity may occur through two independent mechanisms, one that is food intake independent and one that is food intake dependent. The latter has been well characterized as a physiological reflex in response to the presence of food in the stomach and is referred to as the gastrocolic reflex, which may prompt an urge to defecate (49). The former has been less well characterized but its daily recurrence at the time of awakening suggests that the human colon is naturally primed to empty early in the morning. And it is the colonic clock that may be responsible for the priming or sensitization of this process, as suggested by the rodent studies discussed above.
IBS: a Manifestation of Circadian Rhythm Dysfunction?
Disruption of biological rhythms secondary to shift work, travel across different time zones, or space flights has been associated with gastrointestinal symptoms such as abdominal discomfort, constipation, or diarrhea (10, 11, 13, 27, 29, 50). These changes may relate to alterations in colonic motility that may occur due to disruption of the molecular clock in those settings. In addition, these symptoms overlap with those reported by patients with irritable bowel syndrome (IBS). A recent cross-sectional observational study that aimed to determine the prevalence of IBS amongst nurses participating in shift work, found an association between participation in shift work and IBS (37). In particular, those working rotating shifts were found to have a significantly higher prevalence of IBS compared with persons working a standard daytime schedule. This association was independent of the quality of sleep. These observations suggest that IBS may be a manifestation of an underlying circadian rhythm disorder. This is supported by reports that the administration of melatonin, a known regulator of circadian rhythms, can improve IBS symptoms (30). It has also been shown that the urinary excretion of the main melatonin metabolite, 6-sulfatoxymelatonin, differs between IBS patients and healthy controls (40), further supporting a potential role for circadian rhythm dysfunction in the pathogenesis of IBS.
Remaining Questions and Challenges
Mechanism of peripheral clock gene entrainment.
One of the most intriguing questions is how entrainment of the colonic clock, and of clocks in a variety of other peripheral organs, is regulated. The role of the central clock may be limited in the entrainment of peripheral clock gene expression. First, peripheral clock gene expression (including gastrointestinal clock gene expression) can become uncoupled from the central clock in response to restricted feeding (23). Second, entrainment of peripheral clock gene expression can be induced in response to restricted feeding in mice in which the central clock has been ablated. These observations have suggested the presence of a putative “food-entrainable oscillator” (FEO). However, the location of this FEO and the signals responsible for its entrainment remain elusive despite extensive research by multiple investigators over the past two decades, which have been reviewed extensively elsewhere (6, 9, 15, 16). The vagal nerve is unlikely to play a significant role in the modulation of gastrointestinal clock gene expression as vagotomy did not alter gastric clock gene expression (23). Miki et al. (33) demonstrated that daytime administration of total parenteral nutrition significantly shifted per2 expression in the rat liver. It is therefore unlikely that the direct contact of food with the lining of the gastrointestinal tract mediates clock gene expression. However, it is conceivable that the entrainment of gastrointestinal clock genes is humorally mediated. Future studies should focus on those peptides that are known to follow a circadian rhythm and that may alter gastrointestinal motility as well. For example, uroguanylin and guanylin are intestinal ligands for guanylyl cyclase that are rhythmically expressed in the rodent intestine and that modulate secretion and motility (45). Other candidate peptides include the cortisol-releasing factor (CRF) as well as neuropeptide Y, glucagon-like peptide 1 (GLP-1), cocaine- and amphetamine-regulated transcript (CART), and ghrelin. These rhythmically expressed peptides modulate food intake but they also act in the brain to stimulate colonic motility through the modulation of CRF signaling pathways. It is therefore conceivable that rhythmic changes in colonic motility over the time of day result from the effect of these rhythmically expressed modulators of colonic motility via CRF receptor-dependent mechanisms.
Role of the central clock in modulation of colonic motility.
To fully characterize the role of the central clock in the regulation of rhythmic changes in colonic motility, measures of colonic motility will need to be examined in SCN-ablated mice. A loss of rhythmicity in measures of colonic motility in SCN-ablated mice would support a role for the central clock in the regulation of colonic motility.
Mechanisms of regulation of circadian rhythmicity within the colon.
Current data suggest that rhythms in colonic motility are regulated by both clock genes and a nNOS-mediated inhibitory process and suggest a connection between these two mechanisms. However, the mechanism through which clock genes may regulate nNOS expression and function within the neurons of the myenteric plexus remains to be determined. It is likely that a variety of other modulators of colonic motility, including stimulatory neurotransmitters, are clock controlled as well.
Special attention should be directed to the role of microRNA (miRNA) in the clock-modulated regulation of colonic motility. miRNA are small RNA molecules that regulate gene and protein expression by binding to the 3′-untranslated regions of specific mRNAs. miRNAs may mediate circadian rhythmicity through posttranscriptional and posttranslational mechanisms (39).
Conclusion.
Further research is needed to determine the underlying mechanism through which rhythmic changes in colonic motility occur under normal circumstances and to determine the consequences of disturbed clock function on colonic motility. Based on a better understanding of the mechanisms that underlie rhythmic changes in colonic motility, drugs can be designed that target tissue specific clock genes. At the present time, high-output genomic screening has already led to the identification of compounds that can delay or advance molecular clocks in individual cells. Eventually, such compounds may be used to enhance the synchronization of colonic clock genes to changes in the environment, improvement in colonic motility, and resolution or prevention of gastrointestinal symptoms. In addition, new drugs may be specifically designed to alter the phase of the gastrointestinal clock. A recent article describing the potential of sildenafil to speed up the adjustment of the central clock to changes in the light-dark cycle demonstrates that such medical approaches may be feasible in the near future (1). In addition, behavioral therapies could be designed, based on optimization of meal times and sleep.
Finally, researchers should be encouraged to define the actual timing of experiments in relation to the light-dark cycle. After all, one can only guess what the implications are of a biological database that is built almost entirely on work with postprandial mice that were just roused out of their sleep. Although this argument has been made by others in the past, it has not been implemented outside of the chronobiological field. However, with the growing recognition of the role of biological rhythms in human health and disease, this recommendation is becoming increasingly more important and might benefit from reinforcement by scientific journals.
GRANTS
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R21 DK074477.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
Slides of human colonic tissue were provided as a courtesy by Shanthi Srinivasan, MD, Emory University, Atlanta, GA.
REFERENCES
- 1.Agostino PV, Plano SA, Golombek DA. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc Natl Acad Sci USA 104: 9834–9839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auwerda JJ, Bac DJ, Schouten WR. Circadian rhythm of rectal motor complexes. Dis Colon Rectum 44: 1328–1332, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL, Cassone VM. Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol 17: 2084–2095, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544–556, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostwick J, Nguyen D, Cornelissen G, Halberg F, Hoogerwerf WA. Effects of acute and chronic STZ-induced diabetes on clock gene expression and feeding in the gastrointestinal tract. Mol Cell Biochem 338: 203–213, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Bron R, Furness JB. The rhythm of digestion: keeping time in the gastro-intestinal tract. Clin Exp Pharmacol Physiol 36: 1041–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Brown SA, Schibler U. The ins and outs of circadian timekeeping. Curr Opin Genet Dev 9: 588–594, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Buchi KN, Moore JG, Hrushesky WJ, Sothern RB, Rubin NH. Circadian rhythm of cellular proliferation in the human rectal mucosa. Gastroenterology 101: 410–415, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Carneiro BT, Araujo JF. The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals? Chronobiol Int 26: 1273–1289, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Caruso CC, Lusk SL, Gillespie BW. Relationship of work schedules to gastrointestinal diagnoses, symptoms, and medication use in auto factory workers. Am J Ind Med 46: 586–598, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cassone VM, Stephan FK. Central and peripheral regulation of feeding and nutrition by the mammalian circadian clock: implications for nutrition during manned space flight. Nutrition 18: 814–819, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Clench J, Reinberg A, Dziewanowska Z, Ghata J, Smolensky M. Circadian changes in the bioavailability and effects of indomethacin in healthy subjects. Eur J Clin Pharmacol 20: 359–369, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Costa G. The impact of shift and night work on health. Appl Ergon 27: 9–16, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson AJ. Search for the feeding-entrainable circadian oscillator: a complex proposition. Am J Physiol Regul Integr Comp Physiol 290: R1524–R1526, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2: 32–39, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Goo RH, Moore JG, Greenberg E, Alazraki NP. Circadian variation in gastric emptying of meals in humans. Gastroenterology 93: 515–518, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Halberg F, Haus E, Cornelissen G. From biologic rhythms to chronomes relevant for nutrition. In: Not Eating Enough: Overcoming Underconsumption of Military Operational Rations, edited by Marriott B. Washington, DC: National Academy Press, 1995, p. 361–372 [PubMed] [Google Scholar]
- 19.Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6: 269–278, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hirsch E, Halberg E, Halberg F, Goetz FC, Cressey D, Wendt H, Sothern R, Haus E, Stoney P, Minors D, Rosen G, Hill B, Hilleren M, Garett K. Body weight change during 1 week on a single daily 2,000-calorie meal consumed as breakfast (B) or dinner (D). Chronobiologia 2: 31–32, 1975 [Google Scholar]
- 21.Hoogerwerf WA. Biologic clocks and the gut. Curr Gastroenterol Rep 8: 353–359, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord 10: 293–300, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Hoogerwerf WA, Hellmich HL, Cornelissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133: 1250–1260, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Hoogerwerf WA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Bartell PA, Cassone VM. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol 298: G143–G150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornelissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology 135: 2019–2029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58: 747–752, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knutsson A. Health disorders of shift workers. Occup Med (Oxf) 53: 103–108, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Kumar D, Wingate D, Ruckebusch Y. Circadian variation in the propagation velocity of the migrating motor complex. Gastroenterology 91: 926–930, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Lu WZ, Gwee KA, Ho KY. Functional bowel disorders in rotating shift nurses may be related to sleep disturbances. Eur J Gastroenterol Hepatol 18: 623–627, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lu WZ, Gwee KA, Moochhalla S, Ho KY. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther 22: 927–934, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Markiewicz A, Kaminski M, Chocilowski W, Gomoluch T, Boldys H, Skrzypek B. Circadian rhythms of four marker enzymes activity of the jejunal villi in man. Acta Histochem 72: 91–99, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci 25: 1514–1522, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miki H, Yano M, Iwanaga H, Tsujinaka T, Nakayama M, Kobayashi M, Oishi K, Shiozaki H, Ishida N, Nagai K, Monden M. Total parenteral nutrition entrains the central and peripheral circadian clocks. Neuroreport 14: 1457–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Moore JG, Englert E., Jr Circadian rhythm of gastric acid secretion in man. Nature 226: 1261–1262, 1970 [DOI] [PubMed] [Google Scholar]
- 35.Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Effect of shift work on body mass index and metabolic parameters. Scand J Work Environ Health 33: 45–50, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Narducci F, Bassotti G, Gaburri M, Morelli A. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut 28: 17–25, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nojkov B, Rubenstein JH, Chey WD, Hoogerwerf WA. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol 105: 842–847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardini L, Kaeffer B, Trubuil A, Bourreille A, Galmiche JP. Human intestinal circadian clock: expression of clock genes in colonocytes lining the crypt. Chronobiol Int 22: 951–961, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Pegoraro M, Tauber E. The role of microRNAs (miRNA) in circadian rhythmicity. J Genet 87: 505–511, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Radwan P, Skrzydlo-Radomanska B, Radwan-Kwiatek K, Burak-Czapiuk B, Strzemecka J. Is melatonin involved in the irritable bowel syndrome? J Physiol Pharmacol 60, Suppl 3: 67–70, 2009 [PubMed] [Google Scholar]
- 41.Rao SS, Sadeghi P, Beaty J, Kavlock R, Ackerson K. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol 280: G629–G639, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst 93: 1563–1568, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst 95: 825–828, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Scheving LA. Biological clocks and the digestive system. Gastroenterology 119: 536–549, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Scheving LA, Jin WH. Circadian regulation of uroguanylin and guanylin in the rat intestine. Am J Physiol Cell Physiol 277: C1177–C1183, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Sladek M, Rybova M, Jindrakova Z, Zemanova Z, Polidarova L, Mrnka L, O'Neill J, Pacha J, Sumova A. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology 133: 1240–1249, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms 17: 284–292, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Tansy MF, Kendall FM. Experimental and clinical aspects of gastrocolic reflexes. Am J Dig Dis 18: 521–531, 1973 [DOI] [PubMed] [Google Scholar]
- 50.Vener KJ, Szabo S, Moore JG. The effect of shift work on gastrointestinal (GI) function: a review. Chronobiologia 16: 421–439, 1989 [PubMed] [Google Scholar]
- 51.Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut 45, Suppl 2: II6–II16, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol 393: 288–301, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]