Abstract
Dietary calcium is believed to reduce colon cancer risk, but the mechanism by which this occurs is poorly understood. Employing the Citrobacter rodentium-induced transmissible murine colonic hyperplasia (TMCH) model, we previously showed that a high-calcium diet (hCa) significantly abrogated hyperplasia in the distal colons of NIH-Swiss mice. Here, we explored the mechanism of dietary protection by hCa by analyzing the expression of genes involved in the regulation of Ca uptake/flux in the intestinal epithelium, including the Ca-sensing receptor, vitamin D receptor, Ca binding protein, and transient receptor potential cation channels, subfamily V, members 5 and 6 (TRPV5/6). Interestingly, while TRPV6 expression increased significantly during TMCH, the expression of the other gene products was unchanged. This elevated TRPV6 expression was significantly abrogated by a hCa diet. Immunofluorescence revealed apical membrane localization of TRPV6 in the normal colon, whereas during TMCH we observed intense apical pole and cytoplasmic staining along the entire longitudinal crypt axis, including the expanded proliferating zone. The hCa diet reversed this effect. In humans, overexpression of TRPV6 was associated with early-stage colon cancer, and in colon carcinoma cells, inhibition of TRPV6 expression by small interfering RNA inhibited their proliferation and induced apoptosis. TRPV6 small interfering RNA also diminished the transcriptional activity of the calcium-dependent nuclear factors in activated T cells. Thus the aberrant overexpression of TRPV6 contributes to colonic crypt hyperplasia in mice and to colon cancer cell proliferation in humans. Therefore, it is likely that suppression of TRPV6 by a hCa diet is required for its protective effects in the colon.
Keywords: crypt hyperplasia, TRPV6, cancer prevention
epidemiological studies suggest that a Western lifestyle that includes diets high in fat and low in fiber, calcium, and vitamin D increases the incidence of colon cancer (10, 15, 34). Indeed, studies in animal models demonstrate that lifelong exposure to a Western diet increases the incidence of adenomas and restoring calcium and vitamin D to the diet prevents this increase (28, 41). However, these studies raised the following questions: can changing dietary habits later in life still be protective, and what are the optimal amounts of these protective nutrients and supplements? These questions are difficult to address because the mechanism of action of these dietary ingredients is not fully understood.
To identify the signals that promote colonic epithelial cell proliferation and to determine how they are affected by diet, we have used an in vivo mouse model called transmissible murine colonic hyperplasia (TMCH) (24). In this model, sustained proliferation of the distal colonic epithelium is induced by infection of mice with the attaching and effacing bacterium Citrobacter rodentium (CR). The cellular and molecular events that occur in this model are similar to those that occur in colon adenomas and carcinomas (29). Furthermore, this bacterium-induced hyperplasia increases the susceptibility of the mouse colon to adenomatous tumors (neoplasia) (1, 27). The cellular changes include a two- to threefold increase in the length of the crypts, an eight- to ninefold increase in mitotic activity in the crypts, and goblet cell depletion (3, 24). The molecular changes that occur include, but are not limited to, activation of phospholipase C with an associated increase in the influx of inositol triphosphate and calcium and a profound and sustained activation of established epithelial cell modulators, such as β-catenin and NF-κB (19, 32, 38). This model is attractive, not only because these cellular and molecular events occur rapidly but also because it is very sensitive to dietary manipulations. For example, it was shown that if the infected mice were fed either a high-fiber (6% pectin) or high-calcium (hCa 1% instead of 0.5%) diet, the hyperplasia was diminished without changing the amount of CR colonization in the colon (36). Additional studies found that the pectin-rich diet diminished β-catenin activation (36) and downregulated NF-κB activation (S. Umar, unpublished data), suggesting that pectin prevents hyperplasia by suppressing these signals. Surprisingly, a hCa diet did not have any detectable effect on either the β-catenin or NF-κB pathway (Ref. 36, and S. Umar, unpublished data), suggesting that the hCa diet must target another epithelial cell modifier in the colon. Because one of the earliest events in TMCH is a change in intracellular calcium homeostasis through phospoholipase C activation (19), we hypothesized that gene regulatory events associated with cellular and/or extracellular calcium homeostasis could contribute to epithelial hyperplasia and be targets for the growth inhibitory effect of a hCa diet. Here, we show that the transient receptor potential cation channel, subfamily V, member 6 (TRPV6), which regulates the physiological process of active calcium absorption in the proximal small intestine (4, 35), promotes colonic cell proliferation and is a likely target for the growth inhibitory effect of a hCa diet.
MATERIALS AND METHODS
Materials.
Purified diets (TD.94045, TD.97200, and TD.97202) were purchased from Harlan Teklad. Calcitriol [1,25-dihydroxyvitamin D3; 1,25(OH)2D3] was obtained from Roche Pharmaceuticals. Sheep anti-murine CYP27B1 antibodies were purchased from The Binding Site, San Diego, CA. Chicken anti-mouse TRPV6 antibodies were a generous gift from Dr. Michael Freeman (Harvard University). For detection of TRPV6 mRNA, a commercial cDNA array of unidentifiable human colon samples was purchased from OriGene Technologies. Tri-reagent, PMA, ionomycin, and cyclosporine A were purchased from Sigma-Aldrich. The WST-1 assay reagent was purchased from Roche Diagnostics. The homogenous caspase 3/7 assay kit, the dual luciferase assay kit, and the nuclear factor of activated T cells (NFAT)-luciferase plasmid were purchased from Promega. Lipofectamine 2000 and optiMEM-1 were purchased from Invitrogen. Silencer negative control no. 1 small interfering RNA (siRNA) and TRPV6 siRNA duplex sets (ID: s30899 and s30900) were purchased from Applied Biosystems.
TMCH mouse model.
The Institutional Animal Care and Use Committees of The University of Texas Medical Branch (Galveston) and The University of Texas M. D. Anderson Cancer Center approved all of the animal studies. Male NIH-Swiss mice (20–25 g) were housed in a conventional specific pathogen-free facility and were maintained on unpurified Teklad 7012 diet unless otherwise indicated. Prior to CR infection, mice were transferred to a biohazard facility in microisolator cages. To induce TMCH they were given an overnight culture of CR diluted with drinking water, as described previously (32, 38). Mice in the control group were given water-diluted sterile Luria broth as a mock infection. When indicated, purified diets were provided to uninfected and infected mice on the third day following either infection or mock infection. Animals were euthanized at 6 and 12 days postinfection, and 4 cm of the distal colons was removed and used for further analyses.
Isolation of intact colonic crypts and Western blotting.
Distal colonic crypts were isolated for length measurement and processed for biochemical assays as described previously (32, 38). Briefly, distal colons were attached to a paddle and immersed in Ca2+-free standard Krebs-buffered saline (in mM: 107 NaCl, 4.5 KCl, 0.2 NaH2PO4, 1.8 Na2HPO4, 10 glucose, and 10 EDTA) at 37°C for 10–20 min, gassed with 5% CO2-95% O2. Individual crypt units were then separated from the submucosa and musculature by intermittent (30 s) vibration into ice-cold potassium gluconate-HEPES saline (in mM: 100 potassium gluconate, 20 NaCl, 1.25 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, and 5 sodium pyruvate) and 0.1% BSA.
For Western blotting, crude cellular extracts were prepared from either isolated crypts or whole distal colons of normal and CR-infected mice by homogenization in buffer containing (in mM) 50 Tris·HCl, 250 sucrose, 2 EDTA, 1 EGTA (pH 7.5), and 10 β-mercaptoethanol with 0.5% Triton X-100, plus protease and phosphatase inhibitors. After a low-speed spin (15,000 g for 15 min), the clear supernatant was saved as total solubilized protein cell extract. Protein concentrations were determined, and extracts were frozen in liquid nitrogen and stored at −70°C for further analyses.
The colon or crypt extracts (25–100 μg protein/lane) were subjected to SDS-PAGE and electrotransferred to nitrocellulose membrane. The membranes were blocked with 5% nonfat dried milk in TBS [20 mM Tris·HCl and 137 mM NaCl (pH 7.5)] for 1 h at room temperature and then overnight at 4°C. The antigen was detected by incubating the membranes for 1–2 h with the relevant antibodies (0.5–1.0 μg/ml in TBS containing 0.1% Tween 20). After washing, membranes were incubated with horseradish peroxidase-conjugated rabbit anti-sheep IgG and developed using the ECL detection system (Amersham, Arlington Heights, IL) according to the manufacturer's instructions.
Immunohistochemistry.
Immunohistochemistry for bromodeoxyuridine (BrdU) and immunofluorescence for PCNA and for TRPV6 were performed on 5-μm-thick frozen sections from distal colons of normal and TMCH mice (day 6 and day 12 postinfection) utilizing the horseradish peroxidase (HRP)-labeled polymer conjugated to secondary antibody using Envision + System-HRP (DAB; DakoCytomation, Carpinteria, CA) with microwave accentuation as described (38). Antibody controls were either omission of the primary antibody or substituting it with preimmune IgG. The visualization was carried out either via light microscope (immunohistochemistry) or confocal microscopy.
RNA extraction and RT-PCR.
Total RNA was extracted from isolated colonic crypts or from whole kidney homogenates by using TRI Reagent. Total cDNA was synthesized by using Superscript II and random primers. Specific gene products were identified by performing semiquantitative PCR using 1/20 dilution of the cDNA, For each gene product, the amplification cycle number was chosen empirically within the linear range. Primer sequences and each PCR product size are provided in Supplemental Table S1.
The PCR products were separated by polyacrylamide gel electrophoresis and visualized by ethidium bromide staining of the gels under UV light. Gel data were recorded with the Bio-Rad FluorS Imaging System, and relative densities of the bands were determined with Quantity One software (Bio-Rad, Hercules, CA). Gene expression was normalized with GAPDH expression.
Cell culture and transfections.
The human colon carcinoma cell line Caco-2 was obtained from ATCC and maintained in DMEM and 10% fetal bovine serum. To assess expression of TRPV6 mRNA, the cells were seeded in six-well plates at 600,000 cells/well. Forty-eight hours later, the medium was replaced by opti-MEM (Invitrogen) and a Lipofectamine-siRNA duplex mixture was added (using 1 μl of Lipofectamine per 100 nM of siRNA duplex per 1 ml of culture medium). Six hours later, the medium containing the transfection mixture was replaced by DMEM containing 10% FBS, without antibiotics. RNA was extracted 48 h later by using the Tri reagent, and TRPV6 mRNA was assessed by semiquantitative RT-PCR, using β-actin mRNA as a loading control.
Reporter gene expression.
To determine the effect of drugs (PMA, ionomycin) or siRNA on NFAT-luciferase activity, Caco-2 cells were seeded in 24-well plates (150,000 cells/well). After 48 h, the medium was replaced by opti-MEM and transfection mixtures containing Lipofectamine 2000 (1 μl per well), siRNA duplex (100 nM), NFAT-luciferase (firefly) plasmid (250 ng/well), and the reference Renilla luciferase plasmid (50 ng/well) were added. After 6 h, the transfection mixture was replaced by DMEM and 10% serum. After 24 h, either vehicle (DMSO) or drugs (1 μM ionomycin, 50 ng/ml PMA, 1 μg/ml cyclosporine A) were added. Forty-eight hours after transfection, the medium was removed and the cells were lysed in passive lysis buffer, according to the manufacturer's instruction. The dual luciferase assay was performed by using 20 μl of cell lysate, and Renilla luciferase activity was used to normalize NFAT-luciferase (firefly) activity.
Assessment of cell proliferation and apoptosis.
To assess cell proliferation, Caco-2 cells were seeded in 96-well plates at a density of 7,500 cells/well. Forty-eight hours later, the medium was changed to opti-MEM, and a transfection mixture containing 0.8 μl of Lipofectamine and 100 nM of siRNA duplex was added for 6 h. Next, the medium was changed to DMEM + 10% fetal bovine serum without antibiotics, and the cells were incubated for up to 72 h. Cell proliferation was assessed by measuring the cleavage of the stable tetrazolium salt WST-1 to a soluble formazan dye, which causes a colorimetric reaction. This was done by measuring the absorbance of the sample between 450 and 620 nm. These measurements were performed immediately after transfection (day 0) and 3 days later by use of an ELISA plate reader. All measurements were performed four times. Similar plating and transfection conditions were used to assess apoptosis by using the homogeneous caspase 3/7 assay kit, which measures the enzymatic activities of caspases 3 and 7 in cell lysates using the profluorescent caspase 3/7 consensus substrate, Z-DEVD-R110. In this assay, when the DEVD peptide is cleaved on the COOH-terminal side of the aspartate residue by the caspase-3/7 enzymes, rhodamine 110 becomes fluorescent when excited at a wavelength of 498 nm with an emission maximum at 521 nm. The assay was performed 3 days after transfection, and fluorescence was recorded with an ELISA plate reader.
Statistical analysis.
Data are presented as means ± SD of values obtained from three to five experiments (2–4 mice per treatment group). To test for significant differences between means, nonparametric Mann-Whitney test was employed using Statview 4.1 (Abacus Concepts, Berkeley, CA); P values less than 0.05 were considered to be statistically significant.
RESULTS
Expression of calcium-regulating genes in the colons of CR-infected mice.
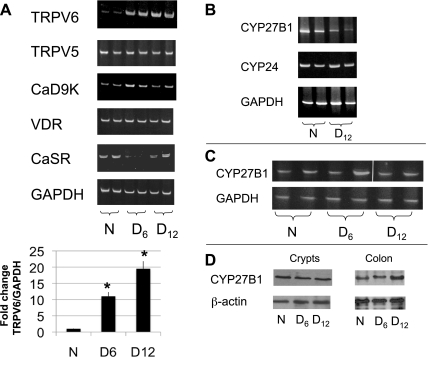
To determine whether TMCH is associated with changes in the expression of genes that regulate cellular and extracellular calcium homeostasis in the gut (35), we measured mRNA levels of vitamin D receptor (VDR), the calcium-sensing receptor (CaSR), calbindin D9K, and the calcium channels TRPV5 and TRPV6 using RNA extracted from colonic crypts isolated from uninfected and CR-infected mice. Figure 1A shows a significant and sustained change in TRPV6 expression only, which was elevated 10-fold by day 6 and 20-fold by day 12 postinfection.
Fig. 1.
Expression of calcium-regulating genes and vitamin D-metabolizing enzymes during transmissible murine colonic hyperplasia (TMCH). A: total RNA was extracted from colonic crypts isolated from either uninfected (N) or Citrobacter rodentium (CR)-infected mice 6 (D6) and 12 (D12) days postinfection. The indicated gene products were amplified by semiquantitative RT-PCR (with primers shown in Supplemental Table S1); relative changes in TRPV6 mRNA are shown at bottom. Total RNA was extracted from either kidneys (B) or colonic crypts (C) of uninfected or infected mice; GAPDH mRNA was used as a loading control. D: CYP27B1 protein was assessed in tissue extracts from isolated crypts or whole distal colon homogenates by Western blotting and β-actin was used as loading control. Results shown are representative of 3 experiments each performed with 2–4 mice per treatment group. A white line was inserted in C to indicate the joining of separate fields from a single gel image.
In the proximal small intestine, 90% of TRPV6 expression is dependent on VDR and its ligand, 1,25(OH)2D3. In addition, TRPV6 mRNA is induced by 1,25(OH)2D3 in a VDR-dependent manner in the normal mouse colon (26, 35, 40). Therefore, we measured whether the elevated TRPV6 expression in the infected colon was associated with a systemic increase in 1,25(OH)2D3 synthesis through renal CYP27B1 (12) or with a local increase caused by the upregulation of CYP27B1 expression by the inflammatory response in the colon (43). We assessed the expression of CYP27B1 in both the kidneys and the colons of uninfected and infected mice. We also assessed the expression of CYP24 to determine whether a reduction in the catabolism of 1,25(OH)2D3 could contribute to the elevated expression of TRPV6. We found that renal expression of CYP27B1 mRNA, but not CYP24 mRNA, was reduced by the infection (Fig. 1B). CYP24 (data not shown) and CYP27B1 mRNA levels in the crypts isolated from normal or infected mice were unchanged (Fig. 1C). In addition, the CYP27B1 protein levels in the isolated crypts did not increase during TMCH. In whole colon extracts, the CYP27B1 protein level was unchanged 6 days postinfection, even though the TRPV6 level at that time was already very high, and the CYP27B1 level was only modestly (2-fold) upregulated by day 12 postinfection (Fig. 1D). These studies suggest that upregulation of TRPV6 expression in the colonic crypts is probably not mediated by an overproduction of 1,25(OH)2D3.
Differential regulation of TRPV6 expression in response to diet and 1,25(OH)2D3.
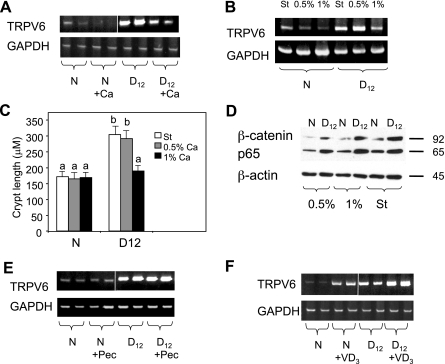
To determine whether a hCa diet had any effect on TRPV6 expression, mice were fed either a standard diet (Teklad 7012) or a semipurified hCa diet (TD.97200, 1% calcium), and we assessed TRPV6 mRNA levels in colonic crypts from uninfected or infected mice 6 and 12 days postinfection. The TRPV6 mRNA levels were reduced in crypts from both normal and infected mice fed the semipurified hCa diet (Fig. 2A).
Fig. 2.
Regulation of TRPV6 expression in colonic crypts by diet and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. A: TRPV6 expression in crypts from mice on either a standard diet or high-calcium (hCa) diet. B: TRPV6 expression in crypts from mice on different diets as indicated. C: bar graph of crypt length in uninfected (N) and infected (D12) colons from mice on different diets as indicated. Bars with different letters are significantly different (P < 0.05). D: β-catenin and p65 protein levels in whole cell extracts isolated from colonic crypts from mice on different diets as indicated. E: TRPV6 expression in crypts from mice on either a standard or high-pectin (Pec) (6%) diet. F: effect of 1,25(OH)2D3 (3 ng/g, 3 days a week) on TRPV6 expression in crypts from uninfected and infected mice. The results shown are representative of 3–5 experiments each performed with 2–4 mice per treatment group. A white line was inserted in A, E, and F to indicate the joining of separate fields from a single gel image. ST, standard diet; 0.54%, 1%, semipurified diets containing 0.5% or 1% calcium, respectively.
Because the estimated calcium content in the standard Teklad 7012 diet is similar (0.95%) to that of the semipurified hCa diet (1%), we wished to ascertain that the ingredient causing the inhibition of TRPV6 expression in the semipurified diet was indeed Ca. Therefore, uninfected and infected mice were fed a standard diet (Teklad 7012), a semipurified diet containing 0.5% Ca (TD.94045), or a semipurified diet containing 1% Ca (TD.97200) for 10 days (starting 2 days after either mock or CR infection), and we assessed TRPV6 expression in isolated crypts. The TRPV6 expression increased in the crypts of mice fed either the standard or semipurified diet containing 0.5% Ca but was suppressed in the mice fed the semipurified diet containing 1% Ca (Fig. 2B). The changes in TRPV6 expression correlated with the anticipated cellular responses to these diets: the crypt lengths increased twofold in infected mice fed either the standard or the semipurified diet containing 0.5% Ca, whereas the crypt lengths were near normal in the mice fed the hCa diet (Fig. 2C). We also confirmed that the semipurified hCa diet did not prevent the accumulation of β-catenin and the p65 subunit of NF-κB, which are established indicators of activation of these two signaling pathways in TMCH (Fig. 2D and Refs. 32, 37, 38).
Because our earlier studies suggested that pectin-rich and hCa diets inhibit colon hyperplasia by suppressing distinct growth-promoting signals (36), we next determined whether TRPV6 expression was preferentially responsive to the semipurified hCa diet. Interestingly, feeding the infected mice a semipurified pectin-rich diet (TD.97202 containing 6% pectin and 0.5% Ca) had no effect on TRPV6 mRNA levels (Fig. 2E).
These results indicate that CR infection upregulates and Ca diminishes TRPV6 expression in colonic crypts. These experiments also suggest that Ca availability is significantly greater in the semipurified hCa diet than in the standard diet, as been demonstrated by others (17).
Because both dietary Ca and 1,25(OH)2D3 are physiological modulators of TRPV6 expression (16), we next examined the effect of 1,25(OH)2D3 on TRPV6 expression in the infected colon. Total colonic crypt RNA was extracted from either uninfected or infected mice 6 h after an injection with 3 ng/g 1,25(OH)2D3. TRPV6 was remarkably responsive to 1,25(OH)2D3 in the normal colon, and its elevated expression in crypts from infected mice was further upregulated by 1,25(OH)2D3 (Fig. 2F).
Effect of diet and TMCH on TRPV6 localization in the colon.
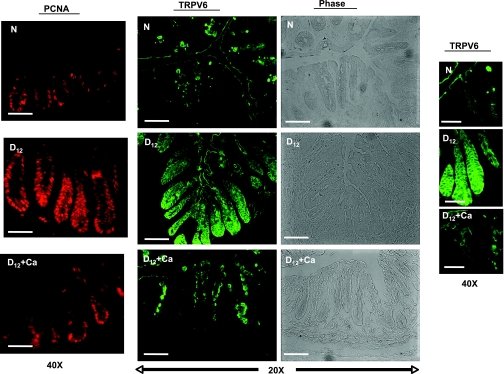
Because TRPV6 regulates transcellular calcium absorption in the intestine, its normal localization is in the apical membrane of absorptive enterocytes (4). To determine how either CR infection or the hCa diet affect the cellular distribution and abundance of TRPV6, we performed immunofluorescence studies by using frozen sections of distal colon from uninfected and infected mice fed either a standard or semipurified hCa diet (Fig. 3).
Fig. 3.
Effect of CR infection and a hCa diet on TRPV6 protein localization. Immunofluorescence was used to detect TRPV6 in frozen sections of colons from uninfected (N) and infected (D12) mice on either a standard or purified hCa diet (D12+Ca). Phase-contrast images of the same fields were used for orientation and immunostaining, and PCNA was used to define the location of the proliferating zone in the crypts. PCNA bars = 100 μm; TRPV6 and phase-contrast bars = 200 μm.
In the normal colon, TRPV6 was localized primarily in the apical membrane at the absorptive surface of the colon, and there was no detectable TRPV6 in the proliferating zone of the normal crypts (Fig. 3). In contrast, in the crypts from infected mice, there was greatly more TRPV6 protein than in the uninfected control mice, and although it was still detected at the partially damaged absorptive surface of the colon, it was also found in the basolateral membrane and the perinuclear area of many epithelial cells, extending from the crypt base to the apex. This localization pattern overlapped with the expanded proliferating zone, as reflected by PCNA immunofluorescence (Fig. 3). As expected, a hCa diet diminished the amount of TRPV6 and restored its localization to the apical pole, which coincided with an abrogation of hyperplasia (Fig. 3).
Taken together, the dramatic increases in TRPV6 mRNA and protein levels and its localization during TMCH imply a change in TRPV6's function from regulating calcium absorption to modulating epithelial cell growth.
Colonic crypt epithelia have opposite cellular responses to a hCa diet and 1,25(OH)2D3.
Since a hCa diet suppressed both hyperplasia and TRPV6 expression, we wished to determine whether 1,25(OH)2D3, which upregulates TRPV6 expression, would induce the opposite cellular response in both the normal and hyperplastic colon and whether these responses would counteract the protective effect of the hCa diet in TMCH. Therefore, we examined the colon weights, crypt lengths, and proliferative activity in crypts from uninfected and infected mice subjected to the following treatments for 10 days (starting 2 days postinfection in mice that received CR inoculation): 1) a standard diet; 2) a purified hCa diet; 3) a standard diet and 1,25(OH)2D3 injections; and 4) a purified hCa diet and 1,25(OH)2D3 injections. The hCa diet restored the distal colon weights in the infected mice to normal and reduced the crypt length significantly (Supplemental Fig. S1, A and B). Unexpectedly, we found that the intermittent injections of 1,25(OH)2D3 increased the colon weights and the length and thickness of the crypts in the colons from uninfected mice. Furthermore, 1,25(OH)2D3 increased the thickness of the colonic crypts from the infected mice substantially (Supplement Figs. S1B and 2A) and attenuated the growth-inhibitory effect on colon hyperplasia of the purified hCa diet (Supplemental Fig. S1, B and C).
Some of these changes in cellular mass were underscored by evaluating DNA synthesis and cell proliferation in the crypts by using BrdU and PCNA antibodies (Supplemental Figs. 2B and Table 1). These analyses showed the expected expansion of the proliferative zone from the base to the apex in the infected crypts. They also showed that the semipurified hCa diet reduced cell proliferation in the infected crypts and restored the proliferative zone to the crypt base. In contrast to the hCa diet, the changes in cellular mass induced by 1,25(OH)2D3 did not correlate significantly with the proliferation indexes; there were small increases in BrdU and PCNA labeling of crypts from uninfected mice treated with 1,25(OH)2D3 but no change in these parameters in crypts from 1,25(OH)2D3-treated infected mice. These results suggest that 1,25(OH)2D3 increases the cellular mass in the crypts by attenuating apoptosis rather than by increasing mitotic activity. Interestingly, we found that the hCa diet inhibited cell proliferation in the crypts from uninfected mice treated with 1,25(OH)2D3 but failed to inhibit cell proliferation in the crypts from infected mice treated with this calcitropic hormone (Supplemental Fig. S2B and Table 1). These results suggest that 1,25(OH)2D3 treatment preferentially desensitizes the crypts in TMCH mice to the antiproliferative effects of a hCa diet. Thus we have demonstrated a direct correlation between TRPV6 expression levels and colonic crypt cell proliferation and provide evidence that 1,25(OH)2D3 adversely affects the cellular dynamics in the colonic crypts.
Table 1.
Effect of diet and 1,25(OH)2D3 on colonic crypt cell proliferation
| Treatment | *BrdU+ Cells/Crypt | †PCNA+ Cells/Crypt |
|---|---|---|
| N | 7 ± 2a | 12 ± 3a |
| N + Ca | 3 ± 1b | 8 ± 2a |
| N +1,25D3 | 9 ± 4a | 17 ± 4b |
| N + Ca +1,25D3 | 3 ± 1b | 9 ± 3a |
| D12 | 30 ± 5c | 28 ± 5c |
| D12 + Ca | 8 ± 3a | 11 ± 3a |
| D12 + 1,25D3 | 23 ± 7c | 33 ± 6c |
| D12 + Ca +1,25D3 | 19 ± 4c | 22 ± 4b,c |
N, uninfected mice fed a standard diet (Teklad 7012); N + Ca, uninfected mice fed a semipurified high-calcium (hCa) diet (TD.97200); N +1,25D3, uninfected mice fed a standard diet and injected with 3 ng/g 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] 3 times per week; N + Ca +1,25D3, uninfected mice fed a hCa diet and injected with 1,25(OH)2D3; D12, infected mice fed a standard diet and euthanized 12 days postinfection. D12 + Ca, infected mice fed a hCa diet; D12 + 1,25D3, infected mice fed a standard diet and injected with 1,25(OH)2D3; D12 + Ca +1,25D3, infected mice fed a hCa diet and injected with 1,25(OH)2D3.
Mice were injected with bromodeoxyuridine (BrdU; 160 mg/kg) 90 min before euthanasia. DNA synthesis in the crypts was assessed in the distal colon sections after immunohistochemical analysis using BrdU antibodies. Values are means ± SD of positively stained nuclei/crypt (20 crypts per treatment group). P < 0.05 for values with different letters in this column.
Cell proliferation in distal colonic crypts was assessed by immunofluorescence using antibodies to proliferative cell nuclear antigen (PCNA). Values are means ± SD of positively stained nuclei/crypt (20 crypts per treatment group). P < 0.05 for values with different letters in this column.
TRPV6 expression and function in colon cancer.
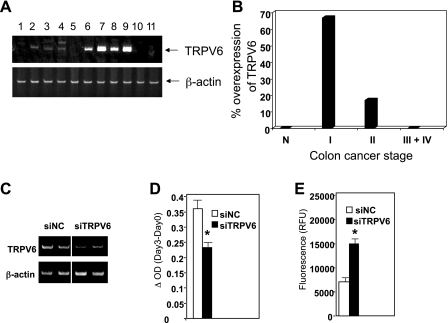
Although our experiments imply that overexpression of TRPV6 is associated with aberrant epithelial cell proliferation in the mouse colon, they do not provide proof of a similar association in the human colon. Therefore, we assessed the expression of TRPV6 during colon cancer progression using a commercial cDNA array prepared from 48 human colon specimens including both normal mucosa and tumors from stage I to IV (Fig. 4A). A semiquantitative RT-PCR analysis of these samples revealed that TRPV6 was overexpressed in 66% of stage I tumors (Fig. 4A) and 17% of stage II tumors but was barely detectable in the stage III and IV tumors (Fig. 4B). These results suggest that aberrant overexpression of TRPV6 could be associated with early colon carcinogenesis but not with frank malignancy.
Fig. 4.
Expression and function of TRPV6 in human colon cancer and colon carcinoma cells. A: a commercial cDNA array prepared from 48 samples of human colons was used to assess TRPV6 expression by semiquantitative PCR. β-Actin was used as loading control. The PCR products were separated by polyacrylamide gel electrophoresis and detected by SYBR green staining. Shown are TRPV6 expression in normal mucosa (lanes 1-5) and stage I colon cancer (lanes 6-11). B: bar graph summarizing the incidence of TRPV6 overexpression in all 48 samples (normal mucosa, n = 5; stage I, n = 6; stage II, n = 12; stages III and IV, n = 25). C–E: Caco-2 cells were transfected with either TRPV6 small interfering RNA (siRNA) duplexes (siTRPV6) or negative control siRNA (siNC). C: semiquantitative RT-PCR of TRPV6 mRNA from siRNA-transfected cells. A representative gel from 4 experiments is shown. D: cell proliferation was determined by colorimetric reaction with WST-1. Data were obtained from quadruplicate wells on the day of transfection (day 0) and after 72 h (day 3). Results are expressed as net cell growth over 3 days. E: apoptosis was determined by using the caspase 3/7 assay kit. Data were obtained in quadruplicates, 3 days after transfection. Bar graphs in D and E represent the means ± SE from 4 experiments. *Significantly different from negative control siRNA (P < 0.05).
To establish a direct role for TRPV6 in regulating colonic epithelial cell proliferation, we used the colon carcinoma cell line Caco-2, which expresses constitutively high levels of TRPV6. Caco-2 cells were treated either with TRPV6-specific siRNA or with a control siRNA, and their proliferation and apoptosis were assessed by the WST-1 and homogeneous caspase 3/7 assays, respectively. TRPV6 siRNA reduced the TRPV6 mRNA levels by 50–60% (Fig. 4C), leading to a 40% reduction in Caco-2 cell proliferation and a more than twofold increase in apoptosis (Fig. 4, D and E). These results demonstrate that, in addition to its role in intestinal calcium absorption, TRPV6 has the potential to promote the proliferation of colonic epithelial cells and protect them from apoptosis.
A potential downstream target for inward calcium currents induced by TRPV6 is the calcium/calcineurin pathway (23). The calcium-dependent activation of the phosphatase calcineurin induces the transcriptional activity of nuclear factors in activated T cells (NFAT), contributing to the hypertrophy of many cell types. This process leads to elevated expression of genes, such as COX-2, c-myc, and vascular endothelial growth factor (VEGF), that are implicated in colon cancer progression (2, 8, 23). To determine the relationship between TRPV6 and NFAT in colon carcinoma cells, we cotransfected Caco-2 cells with an NFAT-luciferase reporter gene and either negative-control or TRPV6-specific siRNA. The luciferase activity was tested after treatment with either a control vehicle or a drug combination that fully activates NFAT (PMA and ionomycin). TRPV6 siRNA inhibited the baseline activity of this transgene by 40% and the drug-stimulated NFAT promoter activity by 90% (Fig. 5A). This inhibition was as effective as that induced by the calcineurin inhibitor cyclosporine A (33) (Fig. 5A). It is important to note that suppression of NFAT activity in these cells by cyclosporine A is associated with inhibition of their proliferation and inhibition of c-Myc expression (25).
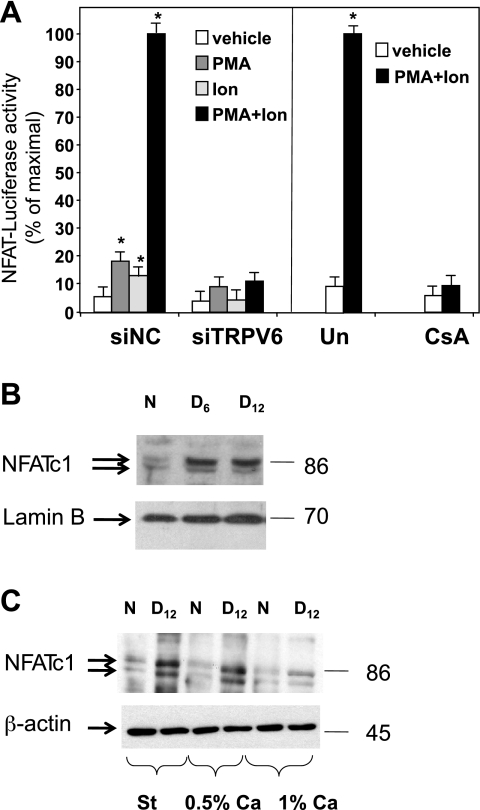
Fig. 5.
Regulation of nuclear factor of activated T cells (NFAT) transcriptional activity in vitro and in TMCH. A: Caco-2 cells were transfected with either siTRPV6 or siNC. NFAT transcriptional activity was assessed by measuring luciferase activity in extracts from Caco-2 cells transfected with the NFAT-luciferase reporter gene (50 ng/well) and Renilla luciferase control plasmid (10 ng/well), either alone or with the indicated siRNA duplex. After 24 h, the cells were treated with PMA (50 ng/ml), ionomycin (Ion, 1 μM), or a combination of the 2 drugs. Solvent (Un) or cyclosporine A (CsA, 1 μg/ml) was added to the transfected cells 30 min before PMA and ionomycin. Firefly luciferase activity was measured 48 h after transfection and normalized to the Renilla luciferase activity. Values in the bar graphs represent the mean ± SE of 3 experiments each performed in quadruplicates. *Significantly different from vehicle control (P < 0.05). B: Western blot determined abundance of NFATc1 in nuclear extracts from colonic crypts of uninfected (N) and infected mice (D6 and D12 postinfection). C: Western blots determined the effect of infection and diets (St, 0.5% and 1% Ca) on cellular abundance of NFATc1 protein. Lamin B and β-actin were used as loading controls in B and C.
These findings led us to examine changes in NFAT activation in TMCH, and the effect of diets on its abundance in colonic crypts in vivo. There was a profound increase in nuclear NFATc1 protein in colonic crypts of mice during progression of CR-induced hyperplasia (day 6 and day 12 postinfection) (Fig. 5B). Furthermore, the infection increased total cellular NFATc1 protein abundance, in crypts from mice maintained on either standard or semipurified 0.5% Ca diet, but this increase in NFATc1 was abrogated in infected mice fed a semipurified hCa diet (Fig. 5C). These findings imply that tight functional relationship exist between TRPV6 and the calcineurin/NFAT pathway in colonic cells in vitro and in vivo.
DISCUSSION
In this study, we show for the first time the profound and sustained overexpression of TRPV6 in TMCH, a mouse model for colonic crypt hyperplasia. This overexpression is not associated with an increase in 1,25(OH)2D3 synthesis or a dietary calcium deficiency, but it is induced by infection with the attaching/effacing bacterium CR. TRPV6 overexpression in TMCH appears to be part of a repertoire of epithelial signals, including wnt/β-catenin and NF-κB, that are activated in response to bacterial injury and contribute to the wound healing process through compensating proliferation and antiapoptotic responses of the colonic epithelium (24, 32, 37, 38). This overexpression of TRPV6 is probably not caused by the increased activity of NF-κB or wnt/β-catenin because its levels remained elevated (Fig. 2E) when infected mice were fed a high-pectin diet that blocks these signals (Ref. 36; data not shown). These findings suggest the presence of an alternative and powerful mode of TRPV6 regulation in colon hyperplasia. For instance, CR colonization of the colon could change the profile of fermentation products in favor of compounds that induce TRPV6 expression (i.e., short-chain fatty acids) (9).
We provide circumstantial evidence that TRPV6 promotes colonic epithelial cell proliferation in vivo by showing a direct correlation between TRPV6 expression and the mitotic activity and/or cellular mass of colonic crypts in both uninfected and infected mice. Furthermore, our in vitro studies using the colon carcinoma cells Caco-2 provide direct evidence for the regulation of cell growth by TRPV6; partially silenced TRPV6 expression reduced their proliferation and increased their apoptosis. These findings concur with the possibility that the cellular actions of TRPV6 in vivo are required for the repair process of colonic epithelium in CR-infected mice but might also lead to an aberrant cell proliferation and malignant transformation colon carcinogenesis. These in vitro findings also corroborate two recent studies that demonstrated similar growth-inhibitory responses to TRPV6 siRNA in breast and prostate cancer cell lines (5, 21). However, it is important to note that the cellular activities of TRPV6 can be harnessed to yield different outcomes, depending on the cell type and context. For example, in gastric carcinoma cells, TRPV6 is essential for the induction of apoptosis by the drug capsaicin (7), whereas in primary keratinocytes its expression is necessary for the calcium-dependent transition of the cells from proliferation to differentiation (22). Therefore, the actual role of TRPV6 in the colon should be determined by manipulating its expression and function in the normal or aberrant crypts in vivo, since these structures are regulated by a gradient of cellular signals (including a calcium gradient) that tightly orchestrates cell proliferation, differentiation, and apoptosis along the crypts' longitudinal axis (6, 31).
The dietary manipulations in our study revealed that overall diet composition is extremely important for the protective action of Ca: TMCH was robust in infected mice when they were fed either standard diet containing 0.98% Ca or semipurified diet containing 0.5% Ca, but a semipurified diet containing 1% Ca was sufficient to suppress the hyperplasia and TRPV6 expression. Therefore, it seems that availability of Ca is substantially different in standard diets and semipurified diets. This was proven by other investigators (17) who fed either standard and or semipurified diets to severely hypocalcemic VDR-ablated mice. In that study, a semipurified diet containing 1% Ca restored serum Ca to normal levels whereas unpurified diet containing the same amount of Ca did not. There are several reasons for these differences in Ca absorbability and availability. For instance, AIN-93-based semipurified diets but not standard diets (i.e., Teklad 7012) contain 20% casein (g/kg), and casein phosphopeptides enhance Ca absorption (13). In addition, the standard diet, but not the semipurified diet, is based on grains and legumes (wheat, oats, and soybean), which contribute to the formation of nonsoluble, nonabsorbable Ca-phytate complexes (13).
Another interesting finding of our studies is that the CR-injured mouse colonic epithelium is highly sensitive to a modest increase in Ca content of the semipurified diet (0.5% to 1%). This is in contrast to other dietary studies in rodents that demonstrated cellular changes only by comparing colon responses to Ca-sufficient and extremely Ca-deficient diets. (18, 42). We speculate that the colon in TMCH acquires sensitivity to extracellular calcium that is greater than that of the normal colon, and that the CaSR is the most likely target of this change (30). We propose that in TMCH the threshold for CaSR activation by extracellular calcium increases substantially due to the overwhelming production of type II CaSR agonists (polyamines) in the CR-infected colon (11). Polyamines such as spermine have been shown to increase the sensitivity of colonocytes to extracellular calcium, and together they induce a sustained elevation in the intracellular calcium concentration (14). Since elevated intracellular calcium inactivates TRPV6 and elevated extracellular Ca suppresses TRPV6 expression (39), we propose that the TRPV6-overexpressing epithelial cells in TMCH are poised to respond significantly to even modest increases in dietary calcium through CaSR signaling.
In this study we also identified potential downstream molecular targets of TRPV6. In Caco-2 cells, TRPV6 regulated 90% of the drug-induced transcriptional activity of NFATs. Our studies did not determine whether the activity of NFATs is responsible for the effects of TRPV6 expression on Caco-2 cells growth, but the involvement of NFATs in gene regulatory events that contribute to carcinogenesis in general, and to colon cancer in particular, is well documented and includes transcriptional upregulation of COX-2 and c-myc (8, 23). It is important to note that similar relationship between TRPV6 expression and NFAT activation has been reported for another cancer cell line (LnCaP; Ref. 21). However, our studies have provided evidence that this relationship may also exist in vivo, because NFATc1 increased in both abundance and activity (as is implied from the substantial elevation in its nuclear localization) in TRPV6-overexpressing colonic crypts in TMCH, and this abundance decreased to baseline level when TRPV6 expression was suppressed by hCa diet.
Although the initial goal of this study was to identify molecular targets for the protective action of dietary Ca in the colon, we believed, initially, that 1,25(OH)2D3, the active metabolite of vitamin D, would improve the response to a hCa diet. Unexpectedly, our experiments provided strong evidence that an intermittent large dose of 1,25(OH)2D3 increased the cellular mass in the normal colonic crypts and clearly antagonized the growth-inhibitory effect of a hCa diet in the CR-infected colon. Our findings are supported by a recent study showing that crypt hyperplasia associated with extensive mitotic activity of an injured colon in a mouse model of colitis is strongly dependent on the vitamin D receptor (20). Since TRPV6 expression is extremely responsive to VDR and 1,25(OH)2D3 in the large intestine, we propose that in conditions of severe epithelial damage (i.e., colitis), the wound healing response requires VDR and 1,25(OH)2D3 and their molecular targets, including TRPV6. However, the administration of excessive amounts of the active vitamin D metabolite could also lead to undesirable overexpression of TRPV6, leading to tumor promotion and antagonizing the protective actions of dietary calcium.
GRANTS
Financial support was provided by National Institute of Health grants CA131936 (to S. Peleg) and CA131413 (to S. Umar) and by the M. D. Anderson Institutional Research Grant (to S. Peleg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. J. C. Fleet for insightful comments regarding this manuscript, and C. V. Nguyen for excellent technical assistance.
Present address for S. Umar: Department of Internal Medicine-Gastroenterology, University of Oklahoma Health Sciences Center, 975 NE 10th St., BRC West 1268B, Oklahoma City, OK 73104 (e-mail: shahid-umar@ouhsc.edu).
REFERENCES
- 1.Barthold SW, Jonas AM. Morphogenesis of early 1,2-dimethylhydrazine-induced lesions and latent period reduction of colon carcinogenesis in mice by a variant of Citrobacter freundii. Cancer Res 37: 4352–4360, 1977 [PubMed] [Google Scholar]
- 2.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1933, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom KS, Guttman JA, Rumi M, Ma C, Bouzari S, Khan MA, Gibson DL, Vogl AW, Vallance BA. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect Immun 76: 796–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA. Marked disturbance of calcium homeostasis in mice with targeted disruption of the trpv6 calcium channel gene. J Bone Miner Res 22: 274–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolanz KA, Hediger MA, Landowski CP. The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther 7: 271–279, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Russell N, Albrecht S, Davies RJ. The effect of dietary vitamin D3 on the intracellular calcium gradient in mammalian colonic crypts. Cancer Lett 127: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Chow J, Norng M, Zhang J, Chai J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells—Mechanisms behind a possible new “hot” cancer treatment. Biochim Biophys Acta 1773: 565–576, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Duque J, Fresno M, Iñiguez MA. Expression and function of the nuclear factor of activated T cells in colon carcinoma cells: involvement in the regulation of cyclooxygenase-2. J Biol Chem 280: 8686–8693, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fukushima A, Aizaki Y, Sakuma K. Short-chain fatty acids induce intestinal transient receptor potential vanilloid type 6 expression in rats and Caco-2 cells. J Nutr 139: 20–25, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E. The epidemiology of vitamin D and colorectal cancer: recent findings. Curr Opin Gastroenterol 22: 24–29, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Gobert AP, Cheng Y, Akhtar M, Mersey BD, Blumberg DR, Cross RK, Chaturvedi R, Drachenberg CB, Boucher JL, Hacker A, Casero RA, Jr, Wilson KT. Protective role of arginase in a mouse model of colitis. J Immunol 173: 2109–2117, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Goltzman D, Miao D, Panda DK, Hendy GN. Effects of calcium and of the Vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol 89–90: 485–489, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr 19: 119S–136S, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Hebert SC, Cheng S, Geibel J. Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium 35: 239–247, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Holt PR, Bresalier RS, Ma CK, Liu KF, Lipkin M, Byrd JC, Yang K. Calcium plus vitamin D alters preneoplastic features of colorectal adenomas and rectal mucosa. Cancer 106: 287–296, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hoenderop JG, Nilius B, Bindels RJ. Epithelial calcium channels: from identification to function and regulation. Pflügers Arch 446: 304–308, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr 131: 1787–1791, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kállay E, Bises G, Bajna E, Bieglmayer C, Gerdenitsch W, Steffan I, Kato S, Armbrecht HJ, Cross HS. Colon-specific regulation of vitamin D hydroxylases—a possible approach for tumor prevention. Carcinogenesis 26: 1581–1589, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kenny B, Finlay BB. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma1. Infect Immun 65: 2528–2536, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Lehen'kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene 26: 7380–7385, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lehen'kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, Prevarskaya N. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem 282: 22582–22591, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Huan C. Transcription factor NFAT, its role in cancer development, and as a potential target for chemoprevention. Curr Cancer Drug Targets 7: 343–353, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Luperchio SA, Schauer DB. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect 3: 333–340, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Masuo T, Okamura S, Zhang Y, Mori M. Cyclosporine A inhibits colorectal cancer proliferation probably by regulating expression levels of c-Myc, p21(WAF1/CIP1) and proliferating cell nuclear antigen. Cancer Lett 285: 66–72, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW. The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20: 1447–1461, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Newman JV, Kosaka T, Sheppard BJ, Fox JG, Schauer DB. Bacterial infection promotes colon tumorigenesis in Apc(Min/+) mice. J Infect Dis 184: 227–230, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis 22: 1871–1875, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, Groden J. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res 65: 166–176, 2005 [PubMed] [Google Scholar]
- 30.Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev 30: 178–195, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Sellin JH, Umar S, Xiao J, Morris AP. Increased beta-catenin expression and nuclear translocation accompany cellular hyperproliferation in vivo. Cancer Res 61: 2899–2906, 2001 [PubMed] [Google Scholar]
- 33.Sieber M, Baumgrass R. Novel inhibitors of the calcineurin/NFATc hub-alternatives to CsA and FK506? Cell Commun Signal 7: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slattery ML. Diet, lifestyle, and colon cancer. Semin Gastrointest Dis 11: 142–146, 2000 [PubMed] [Google Scholar]
- 35.Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr 133: 374–380, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Umar S, Morris AP, Kourouma F, Sellin JH. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif 36: 361–375, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umar S, Wang Y, Morris AP, Sellin JH. Dual alterations in casein kinase I-epsilon and GSK-3beta modulate beta-catenin stability in hyperproliferating colonic epithelia. Am J Physiol Gastrointest Liver Physiol 292: G599–G607, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Xiang GS, Kourouma F, Umar S. Citrobacter rodentium-induced NF-kappaB activation in hyperproliferating colonic epithelia: role of p65 (Ser536) phosphorylation. Br J Pharmacol 148: 814–824, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wissenbach U, Niemeyer BA. TRPV6. Handb Exp Pharmacol 179: 221–234, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 136: 1317–1327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K, Yang W, Mariadason J, Velcich A, Lipkin M, Augenlicht L. Dietary components modify gene expression: implications for carcinogenesis. J Nutr 135: 2710–2714, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res 68: 7803–7810, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13: 621–629, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.