Abstract
Necrotizing enterocolitis (NEC) is a devastating intestinal disease of premature infants. Epidermal growth factor (EGF) is one of the most promising candidates in NEC prophylaxis. Autophagy regulates cell homeostasis, but uncontrolled activation of autophagy may lead to cellular injury. The aim was to evaluate the effects of EGF on intestinal autophagy in epithelial cells and in the rat NEC model and measure autophagy in NEC patients. Intestinal epithelial cells (IEC-6) and the rat NEC model were used to study the effect of EGF on intestinal autophagy. Protein levels of Beclin 1 and LC3II were measured in the intestinal epithelium in both in vivo and in vitro models. Ultrastructural changes in intestinal epithelium were studied by electron microscopy. Expression of Beclin 1, LC3II, and p62 protein was evaluated in biopsies from NEC patients. Autophagy was induced in IEC-6 cells and inhibited by adding EGF into the culture. In the rat NEC model, EGF treatment of NEC reduced expression of Beclin 1 and LC3II in ileal epithelium. Morphologically, typical signs of autophagy were observed in the epithelium of the NEC group, but not in the EGF group. A strong signal for Beclin 1 and LC3II was detected in the intestine from patients with NEC. Autophagy is activated in the intestinal epithelium of NEC patients and in the ileum of NEC rats. Supplementation of EGF blocks intestinal autophagy in both in vivo and in vitro conditions. Results from this study indicate that EGF-mediated protection against NEC injury is associated with regulation of intestinal autophagy.
Keywords: epithelial homeostasis, enteral nutrition, premature, small intestine
necrotizing enterocolitis (NEC) is a devastating gastrointestinal disease and a leading cause of morbidity and mortality among premature infants. The pathogenesis of NEC is not fully understood, and no effective treatments are available. The major risk factors to develop NEC are prematurity, enteral feeding, and inappropriate bacterial colonization (21). Disturbance of intestinal epithelial integrity and mucosal barrier function plays an important role in pathogenesis of NEC (5).
Autophagy is a highly conserved process of degradation and recycling of cytoplasmic components via delivery of double-membrane-bound vesicle (autophagosome) containing cytoplasm and organelles to lysosomes (24). A basal level of autophagy maintains a balance between anabolism and catabolism to keep normal cellular homeostasis. However, uncontrolled activation of autophagy leads to excessive degradation of cellular content and cell death (12). During the last decade, genetic studies of autophagy in yeast identified 33 autophagy (ATG) genes (18). Among them, ATG5, Beclin 1 (ATG6), ATG7, and microtubule-associated light chain 3 (LC3) (ATG8) are essential proteins in the regulation of autophagosome formation and maturation (45). Beclin 1 is necessary for induction and initiation of autophagosome construction via recruitment of autophagy proteins to the pre-autophagosomal structure (38). Then the formation and maturation of the autophagosome is regulated by the ATG12-ATG5-ATG16 complex and LC3 (31). Finally, the autophagosome fuses with a lysosome and the contents of the autophagosome are degraded. The interaction of the p62 protein with LC3 is essential for autophagosome formation, and degradation of p62 protein is the hallmark of autophagy activation.
Autophagy protects organisms against diverse pathologies, including infections, cancer, neurodegeneration, aging, and heart disease (24). Recently, autophagy has been linked to intestinal pathophysiology (16). Results from several studies indicate that the defect of mammalian ATG16L1 and interferon-regulated GTPase (IRGM) genes leads to increased susceptibility for Crohn's disease (29, 30). However, the role of autophagy in NEC pathogenesis is not known.
Epidermal growth factor (EGF) is a peptide that plays an important role in cell growth and maintenance of epithelial cell homeostasis in the small intestine (7). EGF not only enhances proliferation and differentiation of enterocytes, but also promotes healing of damaged mucosa and intestinal adaptation after injury (7). Clinical studies show that EGF insufficiency plays an important role in the pathogenesis of NEC (41). In the rat NEC model, we reported that oral administration of EGF reduces the incidence and severity of disease (8, 9). We have shown that EGF downregulates the overproduction of proinflammatory cytokines in the injured ileum (13), maintains bile acid homeostasis in the ileum (14), protects intestinal (5) and hepatic (20) cellular integrity and tight junction structure, and regulates intestinal epithelial apoptosis (6). Thus EGF is one of the most promising candidates in NEC prophylaxis (4, 42). However, the effect of EGF on autophagy in the small intestine is not known.
In the present study, we report a novel finding that autophagy is blocked in rat intestinal epithelial cells (IEC-6) treated with EGF. Furthermore, we show that autophagy is activated in the intestinal epithelium during development of NEC in humans and in the animal NEC model. In the rat model of NEC, EGF treatment of NEC reduces the expression of autophagy regulators Beclin 1 and LC3II in enterocytes of the ileum. Our data suggest that EGF-mediated protection against NEC injury is associated with regulation of intestinal autophagy.
MATERIALS AND METHODS
In vitro experiments.
Nontransformed rat small intestinal epithelial IEC-6 cells (passages 18–26) were obtained from ATCC (Manassas, VA; CRL-1592). Cells were cultured in stock Dulbecco's modified Eagle's medium (Sigma, St. Louis, MO) at 37.5°C, 95% air, and 5% CO2 (23). The medium was supplemented with 10% fetal bovine serum (FBS), 40 μg/ml gentamicin, 2 mM glutamine, and 10 mg/l insulin. For experiments, at day 1, the cells were plated at a density of 4.5 × 105/cm2 onto plastic plates (100 mm in diameter). At day 5, the culture medium was replaced. Twenty-four hours later (day 6), the culture medium was removed, each plate was washed with sterile PBS, and replaced with medium containing 10% FBS [serum (S)] or serum-free [serum-free (SF)] medium (23). Lysosomal inhibitors E64d (Peptides International, Louisville, KY) and pepstatin A (Sigma) were added to the above mediums to yield final concentrations of 10 μg/ml (39). Cells were incubated in a series of time points of 3, 6, 12, and 24 h.
In studies with EGF, IEC-6 cells were cultured as described above. At day 6, the culture medium was removed, and each plate was washed with sterile PBS and replaced with either medium containing 10% FBS S or SF medium or SF media containing 10 nM EGF (Harlan Bioproducts, Indianapolis, IN; SF+EGF). Cells were incubated for 12 h.
Experimental NEC model.
The study protocol was approved by the Animal Care and Use Committee at the University of Arizona (A-324801-95081). Neonatal Sprague-Dawley rats (Charles River Laboratories, Pontage, MI) were collected by cesarean section 1 day before scheduled birth. Pups were divided into the following experimental groups: rats hand-fed with a cow's milk-based formula (10) (NEC, n = 36), and rats fed with a formula supplemented with 500 ng/ml of rat EGF (Harlan; EGF, n = 24). Animals were hand-fed six times daily, with a total volume of 850 μl of formula per day. Experimental NEC was induced by asphyxia (breathing 100% nitrogen gas for 60 s) and cold stress (4°C for 10 min) twice daily (5, 14). After 96 h, all surviving animals were terminated via decapitation. Animals that developed signs of distress or imminent death before 96 h were terminated and included in the study. Pathological changes in intestinal architecture were evaluated using our laboratory's previously published NEC scoring system (8, 19).
Human intestinal tissues.
De-identified, archived human intestinal tissues were obtained under appropriate oversight by the Institutional Review Board at University of Alabama. Cases of NEC (n = 10) and appropriate gestational age-matched control healthy margins of tissues resected for indications other than NEC (intestinal obstruction or at ostomy repair, n = 5; gestational age 25–30 wk) were evaluated by a pediatric pathologist.
Western blot of intestinal samples and IEC-6 cells.
Individual frozen ileum samples were homogenized in a homogenization buffer (50 mmol/l Tris·HCl, pH 7.4, 150 mmol/l NaCl, 1 mmol/l EDTA, 0.1% SDS, 1% sodium-deoxycholic acid, 1% Triton X-100, 50 mmol/l DTT, 50 mg/ml aprotinin, 50 mg/ml leupeptin, 5 mmol/l PMSF). IEC-6 cells were washed with PBS and harvested by gentle trypsinization with 0.25% trypsin-0.3% EDTA. Cells were then pelleted by centrifugation at 400 g, resuspended in 100 μl ice-cold PBS containing Complete protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and 2% Triton X-100, and incubated on ice for 30 min. The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected. Total protein concentration was quantified using the Bradford protein assay (2). The samples were run on a 10% or 12% polyacrylamide gels and transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Membranes were incubated with one of the following antibodies: a rabbit polyclonal Beclin 1 antibody (ProSci, Poway, CA), a rabbit polyclonal anti-ATG5 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), or a rabbit polyclonal LC3 antibody (Cell Signaling) overnight at 4°C. A mouse monoclonal β-actin antibody (Santa Cruz Biotechnology) was used to confirm equal protein loading. After washing, the membranes were incubated with horseradish peroxidase-conjugated goat antimouse or antirabbit IgG (Santa Cruz Biotechnology) and visualized with a chemiluminescent system (Pierce, Rockford, IL). Densitometry was performed to compare protein expression between groups with Bio-Rad QuantityOne software (19).
Immunohistology.
Immunostaining of human and rat intestinal samples was performed as previously described (8, 19, 26). Intestinal sections were incubated with either a rabbit polyclonal LC3II antibody (Abgent, San Diego, CA), or a rabbit polyclonal Beclin 1 antibody (ProSci), or a rabbit polyclonal p62 antibody (Abgent), followed by incubation with Alexa-594 conjugated anti-rabbit or anti-mouse secondary antibody (Molecular Probes, Eugene, OR), and mounted with Vectashield Hard Set Mounting Medium containing 4′,6-diamidino-2-phenylindole (DAPI) as a nuclear counterstain (Vector Laboratories). Controls included slides with no primary antibody. Cell nuclei were stained with DAPI (Calbiochem, San Diego, CA) diluted 1:1,000 in PBS. Imaging was performed with a Zeiss Axiovert fluorescence microscope. For quantification of fluorescence signal, digital images were saved in Adobe Photoshop 7 and analyzed using ImageJ software (National Institutes of Health). Statistical comparison of densities in three samples from each experimental group was performed using one-way ANOVA.
GFP-LC3 transfection and autophagy evaluation.
cDNA encoding human LC3 was inserted into the EcoR I site of pEGFP-LC3 (Addgene, Cambridge, MA). pEGFP-LC3 was transfected into the IEC-6 cells using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. After transfection, the medium was replaced with S media (complete growth medium containing 10% FBS), SF, or SF media containing 10 nM EGF (SF+EGF), and incubated for 12 h. Cells were washed with PBS, fixed in methanol, mounted with Prolong Gold Anti-fade Reagent with DAPI, and analyzed by fluorescent microscopy. Autophagy was evaluated by quantification of LC3 dots per cell, as previously described (31).
In separate studies, autophagy was induced in GFP-LC3-transfected IEC-6 cells by incubation in either SF media for 12 h or in the HBSS media for 4 h. Cells were treated with either EGF or AG1478 [a selective EGF receptor (EGFR) inhibitor] or insulin-like growth factor-I (IGF-I) or platelet-derived growth factor (PDGF). After incubation, cells were washed with PBS, fixed in methanol, mounted with Prolong Gold Anti-fade Reagent with DAPI, and analyzed by fluorescent microscopy. The percentage of cells displaying punctuate distribution of GFP-LC3 was determined for at least 300 cells per sample (31).
Electron microscopy.
Transmission electron microscopy was used to detect ultrastructural changes in rat intestinal epithelium and IEC-6 cells. Intestinal tissue and IEC-6 cells were fixed with 3% glutaraldehyde in 0.1 mM cacodylate buffer. Samples were postfixed in 1% osmium tetroxide, dehydrated in a graded series of ethanols, and embedded in epoxy resin. Ultrathin sections were evaluated for morphological changes using a Phillips CM12 transmission electron microscope (Eidenhoven).
Statistical evaluation.
Statistical analyses between experimental groups were performed using ANOVA followed by Fishers paired least significant difference. The χ2 test was utilized to analyze difference in incidence of disease. All numerical data are expressed as means ± SE.
RESULTS
Serum deprivation induces autophagy in IEC-6 cells.
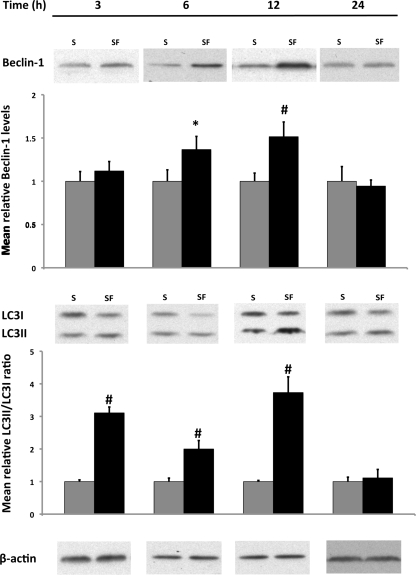
Autophagosome formation is a complex process involving many proteins (31), and Beclin 1 is a key regulator of this process in the early stage of autophagy (24). To determine whether autophagy can be induced in this cell line, IEC-6 cells were cultured in SF media, and a time course for autophagic response was characterized at the following time points: 3, 6, 12, and 24 h. Compared with the S group, protein levels of key autophagic regulators were markedly elevated in the SF group. Beclin 1 protein levels were significantly increased after 6 h, which continued through 12 h (Fig. 1).
Fig. 1.
Effect of serum deprivation on protein expression of essential autophagic regulators in intestinal epithelial cells (IEC-6) following 3, 6, 12, and 24 h of media incubation. IEC-6 cells were incubated in serum (S) media (n = 9) and serum-free (SF) media (n = 9) for time points of varying duration up to 24 h. Samples were collected and analyzed by Western blot for Beclin 1 (top) and light chain 3 (LC3) I and LC3II (bottom). Densitometric values were normalized to β-actin and to S values. Western blot images are representative for each group and time point. #P < 0.01, *P < 0.02 vs. S.
The process of autophagosome maturation can be evaluated by following the phospholipid conjugation of LC3I (cytosolic form) to LC3II (membrane bound form) (31). Detection of LC3II serves as an essential autophagosomal marker, and the ratio between these two LC3 proteins (LC3II/LC3I) correlates with the number of autophagosomes (43). Increased levels of membrane-bound LC3II protein were detected within 3 h of SF media incubation. This trend continued through 12 h. The LC3II-to-LC3I ratio was two to three times higher in the SF group, indicating activation of autophagy in these cells (P ≤ 0.01 vs. S). At 24 h, protein levels of both autophagy regulators returned back to values seen in the S group (Fig. 1).
These results suggest that an autophagic response can be induced in IEC-6 cells within 3 h using SF conditions, followed by peak Beclin 1 and LC3II expression at 12 h. Based on these results, the 12-h time point was selected for further studies with EGF.
EGF reduces autophagy regulators and autophagic vacuoles in IEC-6 cells.
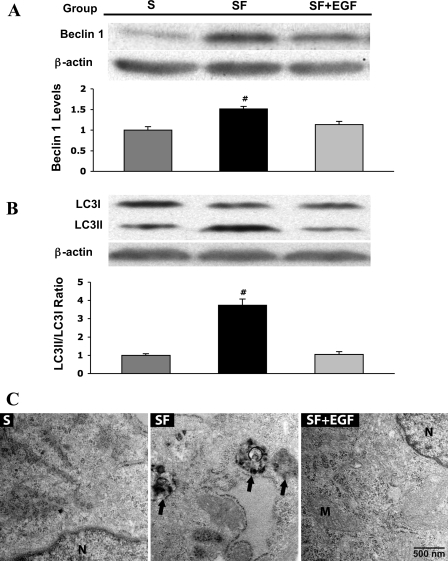
The effect of EGF on autophagic signaling in intestinal epithelial cells is not known. To test whether supplementation of EGF to SF media can protect intestinal epithelial cells against autophagy, we quantified expression of Beclin 1 and LC3I/II proteins (Fig. 2A). Beclin 1 and LC3II levels were significantly reduced in the SF+EGF group compared with the SF group (P ≤ 0.01 vs. S or SF+EGF). Supplementation of EGF decreased the LC3II-to-LC3I ratio, indicating autophagosome membrane reduction and autophagy inhibition (P ≤ 0.01 vs. S or SF+EGF; Fig. 2A).
Fig. 2.
Effect of 10 nM epidermal growth factor (EGF) administration to SF media on protein expression of essential autophagic regulators in IEC-6 cells. A: IEC-6 cells were incubated in S media (n = 9), SF media (n = 9), and SF media + 10 nM EGF (SF+EGF, n = 9) for 12 h. Samples were collected and analyzed by Western blotting for Beclin 1. Densitometric values were normalized to β-actin and expressed as a relative level to S values. Western blot images are representative for each group. #P < 0.01 vs. S or SF+EGF. B: representative picture of Western blot for LC3I and LC3II proteins. Densitometric values were normalized to β-actin and expressed as a relative ratio between LC3II and LC3I isoforms. C: transmission electron microscopy (TEM) images of IEC-6 cells. Normal morphology was observed in IEC-6 cells incubated in medium containing S. IEC-6 cells grown in SF medium show the presence of many autophagic compartments that are electron dense, indicating the presence of degraded cytoplasmic material (black arrows). In contrast, TEM images from the cells treated with SF+EGF show normal morphology. M, mitochondria; N, nucleus.
To examine whether EGF-mediated reduction of Beclin 1 and LC3II proteins had any impact on the formation of autophagosomes in IEC-6 cells, we used transmission electron microscopy, a method frequently utilized to measure the formation of autophagic vacuoles (11). Our results showed striking differences in IEC-6 cell morphology (Fig. 2B). Exposure of IEC-6 cells to the SF media resulted in significant ultrastuctural changes and typical signs of autophagy. Increased number of vacuoles (arrows) with degraded cytoplasmic material (hallmarks for autophagy) and swollen and/or damaged mitochondria were frequently observed in cells from the SF group. The formation of autophagosomes containing cytoplasmic material indicated the late stage of lysosomal degradation in these cells. In contrast, cells in the SF+EGF group displayed normal cellular ultrastructure with healthy cellular constituents and organelles, including mitochondria, similar to structure seen in the S group.
EGF reduces autophagosome formation in IEC-6 cells.
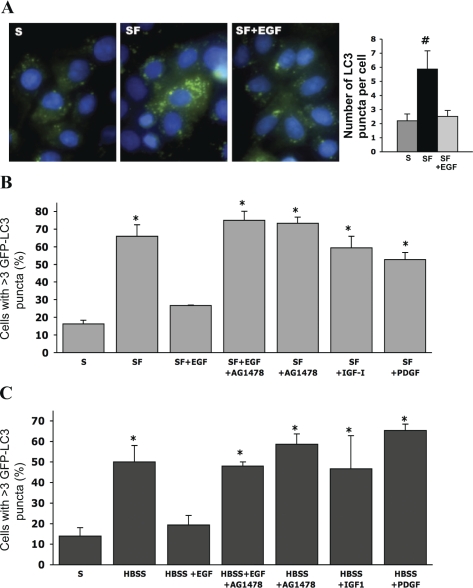
To confirm that EGF reduces autophagy in IEC-6 cells, we utilized a complimentary method that involves the use of an autophagy marker, GFP-LC3. A change in GFP-LC3 localization from diffuse cytoplasmic to punctate (autophagosome-bound LC3 dots) indicates formation of autophagosomes. LC3II protein incorporated into the autophagosome membrane (represented as fluorescent dots in the images) reflects the number of autophagic vacuoles in cells (Fig. 3A). In the SF group, the number of green fluorescent dots was approximately threefold higher compared with the S group. Administration of EGF into media reduced the amount of fluorescent LC3II dots to values observed in the S group (P ≤ 0.01 vs. S or SF+EGF).
Fig. 3.
Effect of EGF on autophagosome formation in IEC-6 cells. A, left: GFP-LC3 transfected IEC-6 incubated in S media, SF media, and SF+EGF conditions for 12 h. LC3II was visualized by fluorescent microscopy and observed as green fluorescent puncta. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). An accumulation of autophagosome incorporated LC3II fluorescent puncta (green) was observed in the SF group. Right: enumeration of LC3II puncta per cell is shown (n = 100). #P < 0.01 vs. S or SF+EGF. B: GFP-LC3 transfected IEC-6 incubated for 12 h in one of the following media: S media (complete growth medium containing 10% FBS), SF media, SF+EGF, SF+EGF + 1 μM AG1478 (SF+EGF+AG1478), SF+AG1478, SF media containing 8 nM insulin-like growth factor-I (SF+IGF-I), and SF media containing 10 nM PDGF (SF+PDGF). C: in separate studies, IEC-6 cells were incubated for 4 h in one of the following media: S media (complete growth medium containing 10% FBS), Hanks′ balanced salt solution (HBSS), HBSS containing 10 nM EGF (HBSS+EGF), HBSS containing 10 nM EGF + 1 μM AG1478 (HBSS+EGF+AG1478), HBSS containing 1 μM AG1478 (HBSS+AG1478), HBSS containing 8 nM IGF-I (HBSS+IGF-I), and HBSS containing 10 nM platelet-derived growth factor (HBSS+PDGF). LC3II was visualized by fluorescent microscopy and observed as green fluorescent dots. Cell nuclei were stained with DAPI (blue). The percentage of cells containing LC3II dots was evaluated in 300 cells per sample. *P < 0.01 vs. S or SF+EGF.
IEC-6 cells cultured in either the SF media (Fig. 3B) or starvation HBSS media (Fig. 3C) exhibited a significant increase in the amount of cells with fluorescent LC3II dots compared with the S group (P ≤ 0.01 vs. S). Administration of EGF into both the SF and HBSS media reduced the number of cells with LC3II dots to values observed in the S group. Inhibition of EGFR signaling with AG1478 completely attenuated the effect of EGF in both SF and HBSS conditions.
To verify if other serum-derived growth factors can induce similar changes as EGF, IEC-6 cells were cultured with either IGF-I or PDGF. Neither IGF-I nor PDGF was able to reduce autophagosome formation. These results clearly indicate that EGF, but not IGF-I or PDGF, is able to block autophagy in IEC-6 cells.
EGF reduces the incidence and severity of injury in experimental NEC.
As shown in our laboratory's previous work, oral administration of EGF reduces ileal damage in the rat model of NEC (5, 8, 20). A blinded evaluator scored histological changes in the ileum. The incidence of NEC was reduced from 62% in the NEC group (n = 36) to 14% in the EGF group (P ≤ 0.01, χ2 analysis; n = 24). The survival rates for these studies were as follows: NEC, 82%, and EGF, 86% (20).
EGF decreases intestinal expression of autophagy regulators in a rat model of NEC.
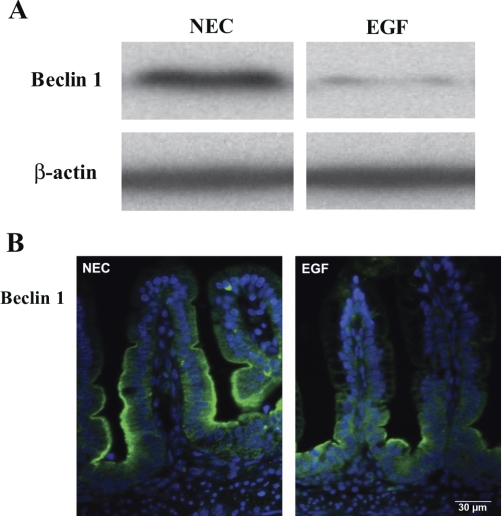
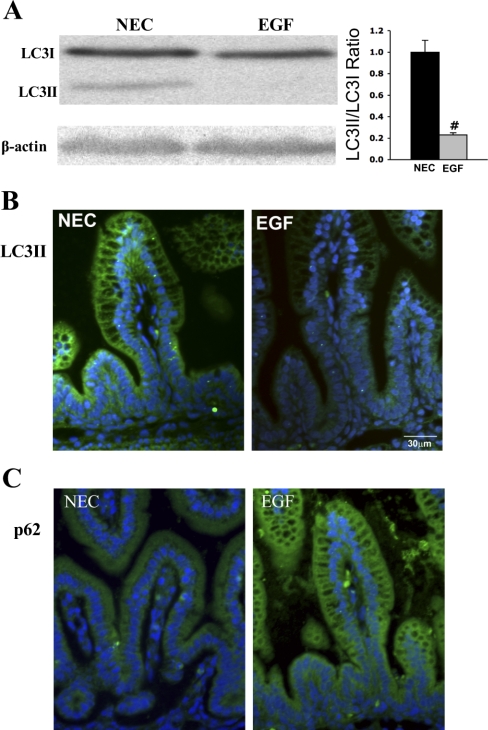
To determine the effect of EGF treatment of NEC on autophagy, intestinal Beclin 1 (Fig. 4), LC3 (Fig. 5A), and p62 (Fig. 5C) protein levels and their histological localization were evaluated in the ileum of rat pups. Ileal Beclin 1 levels were significantly decreased in EGF-treated animals compared with the NEC rats (Fig. 4A). Strong Beclin 1 staining was detected in epithelial cells of crypts and the lower half of villi in NEC rats. In the EGF group, Beclin 1 staining was observed mainly in the crypt enterocytes (Fig. 4B). Intensity of Beclin 1 signal was significantly higher (twofold, P < 0.001) in ileal epithelium of NEC rats compared with the EGF group.
Fig. 4.
Effect of oral administration of EGF on expression and localization of Beclin 1. A: representative Beclin 1 bands from Western blot analyses are shown for the necrotizing enterocolitis (NEC) and EGF groups. B: representative images from NEC and EGF groups stained for Beclin 1 (green). Cell nuclei were stained with DAPI (blue; n = 9 animals/experimental group).
Fig. 5.
Effect of oral administration of EGF on expression and localization of LC3II and p62. A, left: representative LC3 bands from Western blot analyses are shown for the NEC and EGF groups. A lipation from LC3I (17-kDa) into the LC3II (15-kDa), an autophagosomal membrane incorporated isoform, is observed in the NEC group. Right: densitometry quantification of Western blot analysis for LC3 is presented as a ratio of LC3II/LC3I (n = 9). #P < 0.02. B: representative images from NEC and EGF groups stained for LC3II (green). Cell nuclei were stained with DAPI (blue; n = 9 animals/experimental group). C: representative images from NEC and EGF groups stained for p62 protein (green; n = 9 animals/experimental group).
In the ileum of NEC pups, the presence of both LC3I and LC3II isoforms was detected; however, the expression of the LC3II isoform was markedly higher. In EGF-treated pups, a significant decrease of LC3II protein compared with NEC pups was observed (Fig. 5A). The LC3II/LC3I ratio in EGF-treated pups (with the majority of LC3 remaining in its nonlipated LC3I isoform) was significantly decreased, suggesting the reduction of autophagy compared with NEC rats (Fig. 5B; n = 9; P < 0.02). In both experimental groups, the LC3 signal was localized in the majority of epithelial cells of the ileum (Fig. 5C). However, the intensity of LC3II signal was twofold higher in the NEC group compared with the EGF-treated pups (P < 0.001).
The p62 protein recognizes toxic cellular waste, which is then scavenged from cell by autophagy. Direct interaction of p62 with the LC3 complex leads to the degradation of p62 by the autophagy-lysosome system. Thus degradation of p62 protein serves as a unique marker of autophagy activation. Strong p62 staining was observed in ileal epithelium of EGF-treated rats, whereas only a weak and diffuse signal was observed in the NEC group (Fig. 5C).
EGF reduces the presence of autophagic vacuoles in intestinal epithelium of neonatal rats.
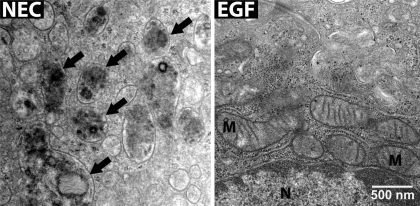
Similar to evaluations of IEC-6 cells, transmission electron microscopy was used to visualize the structure of the ileal epithelium from NEC rats. Typical signs of autophagy, including formation of autophagosomes, autophagolysosomes, and vacuoles, were frequently observed in ileal epithelial cells in the NEC group (Fig. 6). Ultrastructural pictures of epithelial cells from NEC rats showed abnormal cellular structure with numerous autophagic compartments (arrows). In contrast, ileal epithelium from the EGF group displayed minimal structural abnormalities typical of autophagy, and the appearance of these cells indicated healthy cellular structures (Fig. 6).
Fig. 6.
TEM of the ileum from neonatal rats. Normal cellular morphology was observed in the epithelial cells from control animals; many mitochondria with normal structure are present in the cytoplasm. In the NEC rats, epithelial cells show the presence of many autophagic compartments that contain partially degraded cytoplasmic material (black arrows). In contrast, TEM images from the animals treated with EGF show no presence autophagosomes.
Increased expression of autophagy proteins in human NEC.
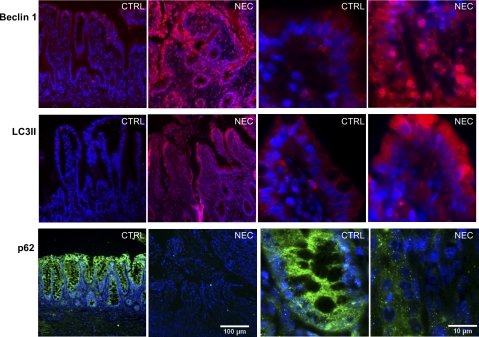
The massive accumulation of essential autophagic regulators (such as Beclin 1 and LC3II) and degradation of p62 protein indicate induction of autophagy in tissue. To further clarify the role of autophagy during NEC pathogenesis, we evaluated expression of these proteins in human tissue. Intestinal samples from prematurely born babies suffering with NEC exhibited a strong staining for both Beclin 1 and LC3II (Fig. 7). The signal was localized predominantly in epithelial cells of crypts and villi from these patients. High magnification pictures showed that Beclin 1 and LC3II were present mainly in the cytoplasm of intestinal epithelium. In contrast, intestinal samples from age-matching controls exhibited only a very low level of both Beclin 1 and LC3II. Expression of p62 protein exhibited the exact opposite pattern. Intestinal epithelium from age-matching controls exhibited strong staining for p62, whereas p62 staining was minimal or absent in tissue from NEC patients (Fig. 7). The degradation of p62 protein and strong upregulation of Beclin 1 and LC3II clearly indicate activation of autophagy in the intestinal epithelium of NEC patients.
Fig. 7.
Intestinal Beclin 1 and LC3II proteins are increased and p62 is decreased in babies with NEC. Beclin 1 (red), LC3II (red), and p62 (green) staining is shown in the intestinal tissue from healthy control (CTRL) and NEC patients. Cell nuclei were stained with DAPI (blue). Images are representative staining from 5 different patients in each group.
DISCUSSION
In this study, for the first time, we report the direct inhibitory effect of EGF on autophagy in the intestinal epithelial cells. This finding is verified in the rat NEC model, where EGF treatment of NEC reduced autophagy in the ileal epithelium. Our laboratory's previous work shows that EGF protects the intestine against NEC injury (8) via regulation of intestinal barrier integrity (5) and maintenance of enterocytes' apoptosis (6). In the present study, we introduce an additional mechanism for EGF-mediated protection against mucosal injury: inhibition of intestinal autophagy in the site of injury. The relevance of results from experimental models is confirmed in human NEC biopsies, showing a strong activation of autophagy in the intestinal epithelium. Results from these studies suggest a novel insight into the mechanism of autophagy regulation and highlight the role of EGF in this process.
Intestinal epithelial homeostasis is (under normal physiological conditions) maintained by balancing the rate of cell proliferation and cell loss. A high turnover rate of intestinal epithelium necessitates rapid removal of cells at the end of their normal life cycle (1). Apoptosis and autophagy are two closely connected cellular mechanisms through which aged, superfluous, or damaged cells are eliminated (27). The importance of apoptosis in preservation of intestinal integrity is well established, whereas the role of autophagy is still not fully understood (16).
In general, autophagy is defined as a cell survival process during periods of metabolic stress, such as nutrient deprivation. It is well known that incubation of cells in medium containing serum blocks autophagy, whereas the use of SF media rapidly activates autophagic signaling (28, 36). However, the specific components of serum responsible for regulation of autophagy are not known. A recent study with bovine mammary cells suggested an inhibitory effect of EGF on autophagy (37). Results from our study show a similar effect in intestinal epithelial cells. The supplementation of EGF into either SF media or HBSS starvation media blocks formation of autophagosomes in IEC-6 cells to values seen in cells cultured in serum-containing medium. Moreover, the effect of EGF on IEC-6 cells is completely blocked by inhibition of EGFR signaling. Finally, a similar anti-autophagic effect cannot be accomplished by supplementation of major serum-derived growth factors (such as IGF-I or PDGF) into starvation media. Thus we conclude that EGF, which is normally present in biologically significant concentrations in serum, is one of the key serum regulators of autophagy.
At a basal level, autophagy maintains normal cell homeostasis(12), while inappropriate activation of autophagy facilitates uncontrolled massive cell death, leading to severe injury (17). Indeed, recent studies show that autophagy is a key element in many disease states, including cancer, neurodegeneration, tuberculosis, and Crohn's disease (16, 40, 44). Similar to Crohn's disease, NEC is an inflammatory disease affecting the small and large intestine. The finding is that polymorphism of two autophagy genes, ATG16L1 and IRGM1, leads to increased susceptibility for Crohn's disease and highlights the importance of autophagic signaling in intestinal pathophysiology (15, 29, 30). However, the role of autophagy in NEC was not examined. Our results show massive activation of intestinal autophagy in babies with NEC. Increased expression of essential autophagy regulators (Beclin 1 and LC3II) and the disappearance of p62 protein are observed in the intestinal epithelium from NEC patients. We speculate that, initially, induction of autophagy is protective for the intestinal mucosa. However, substantial activation of autophagy results in accumulation of autophagic vacuoles, which destroys large proportions of the cytosol and organelles, causing collapse of vital cellular functions and leading to cellular death (25). This impairment of epithelial cell homeostasis makes the intestine more vulnerable to mucosal injury, such as NEC.
In Crohn's disease, mutation of autophagy genes ATG16L1 and IRGM leads to defective Paneth cells function (3, 29, 30) and higher susceptibility to this disease (22). Silencing of ATG16L1 and IRGM expression by short interfering RNA reduces the ability of autophagy to effectively clear bacterial pathogens (29, 35). We speculate that the role of autophagy in NEC pathogenesis is different from Crohn's disease. In NEC, intestinal immaturity is the major risk factor to develop this disease. Both clinical and experimental studies indicate that abnormal function of Paneth cells, which are localized in intestinal crypts, predisposes the premature intestine to NEC injury (32–34). Results from our experimental NEC model show upregulation of essential autophagic regulators Beclin 1 and LC3II in mainly crypt cells of the ileum of NEC rats. In the early stage of NEC, the activation of autophagy provides protection for the intestinal epithelium by removing damaged cytosolic components and organelles. However, continuous exposure of the immature gut to cellular stress may induce uncontrolled autophagy, forcing epithelial cell homeostasis toward cell death, compromising Paneth cells' protective functions, and consequently increasing the susceptibility to mucosal injury, such as NEC.
Previous studies from our laboratory show that EGF reduces experimental NEC (8). The molecular mechanism underlying EGF's protective effects against NEC is complex, including the reduction of inflammation in the ileum (13), maintenance of bile acid transport (14), protection of integrity of intestinal tight junctions (5), and regulation of epithelial apoptosis (6). In the present study, we demonstrate a new role of EGF in the prevention of NEC. We speculate that EGF treatment of experimental NEC downregulates the expression of autophagy proteins and blocks autophagy in the site of injury. This might be an additional molecular mechanism by which EGF downregulates epithelial cell death and thus protects the intestinal epithelium against mucosal injury.
In summary, we are the first to report that autophagy is upregulated in NEC patients, and oral EGF administration blocks autophagy in the neonatal rat NEC model. Utilization of IEC-6 substantiates the role of autophagy under stressed conditions and demonstrates a primary site of autophagy activation by which the sole administration of EGF inhibits autophagy in intestinal epithelial cells. This study presents a novel mechanism by which EGF mediates protection against NEC injury.
GRANTS
National Institute of Child Health and Human Development Grant HD039657 (to B. Dvorak).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Benedetti A, Mancini R, Marucci L, Paolucci F, Jezequel AM, Orlandi F. Quantitative study of apoptosis in normal rat gastroduodenal mucosa. J Gastroenterol Hepatol 5: 369–374, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan M. Is EGF the Holy Grail for NEC? J Pediatr 150: 329–330, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Duh G, Mouri N, Warburton D, Thomas DW. EGF regulates early embryonic mouse gut development in chemically defined organ culture. Pediatr Res 48: 794–802, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Dvorak B, Khailova L, Clark JA, Hosseini DM, Arganbright KM, Reynolds CA, Halpern MD. Comparison of epidermal growth factor and heparin-binding epidermal growth factor-like growth factor for prevention of experimental necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 47: 11–18, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Dvorak B, McWilliam DL, Williams CS, Dominguez JA, Machen NW, McCuskey RS, Philipps AF. Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J Pediatr Gastroenterol Nutr 31: 162–169, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Eskelinen EL. Maturation of autophagic vacuoles in Mammalian cells. Autophagy 1: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez MG, Saka HA, Chinen I, Zoppino FC, Yoshimori T, Bocco JL, Colombo MI. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholera. Proc Natl Acad Sci U S A 104: 1829–1834, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr 36: 126–133, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Gunther S, Prescott NJ, Onnie CM, Hasler R, Sipos B, Folsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 39: 207–211, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Huett A, Xavier RJ. Autophagy at the gut interface: mucosal responses to stress and the consequences for inflammatory bowel diseases. Inflamm Bowel Dis 16: 152–174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaka Y, Suzuki C, Abe T, Okumi M, Ichimaru N, Imamura R, Kakuta Y, Matsui I, Takabatake Y, Rakugi H, Shimizu S, Takahara S. Bcl-2 protects tubular epithelial cells from ischemia/reperfusion injury by dual mechanisms. Transplant Proc 41: 52–54, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kanki T, Wang K, Baba M, Bartholomew CR, Lynch-Day MA, Du Z, Geng J, Mao K, Yang Z, Yen WL, Klionsky DJ. A genomic screen for yeast mutants defective in selective mitochondria autophagy. Mol Biol Cell 20: 4730–4738, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G940–G949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khailova L, Dvorak K, Arganbright KM, Williams CS, Halpern MD, Dvorak B. Changes in hepatic cell junctions structure during experimental necrotizing enterocolitis: effect of EGF treatment. Pediatr Res 66: 140–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliegman RM, Willoughby RE. Prevention of necrotizing enterocolitis with probiotics. Pediatrics 115: 171–172, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Klionsky DJ. Crohn's disease, autophagy, and the Paneth cell. N Engl J Med 360: 1785–1786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolinska J, Lisa V, Clark JA, Kozakova H, Zakostelecka M, Khailova L, Sinkora M, Kitanovicova A, Dvorak B. Constitutive expression of IL-18 and IL-18R in differentiated IEC-6 cells: effect of TNF-a and IFN-g treatment. J Interferon Cytokine Res 28: 287–296, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120: 237–248, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, Zhang W, Dimmitt R, Saeed S, Randolph DA, Aprahamian C, Datta G, Ohls RK. Epithelial cells in fetal intestine produce chemerin to recruit macrophages. Am J Physiol Gastrointest Liver Physiol 297: G1–G10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 28.McElligott MA, Miao P, Dice JF. Lysosomal degradation of ribonuclease A and ribonuclease S-protein microinjected into the cytosol of human fibroblasts. J Biol Chem 260: 11986–11993, 1985 [PubMed] [Google Scholar]
- 29.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, Rotter JI, Duerr RH, Cho JH, Daly MJ, Brant SR. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39: 596–604, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456: 264–268, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, Chang EB. Glutamine increases autophagy under basal and stressed conditions in intestinal epithelial cells. Gastroenterology 136: 924–932, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salzman NH, Polin RA, Harris MC, Ruchelli E, Hebra A, Zirin-Butler S, Jawad A, Martin Porter E, Bevins CL. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res 44: 20–26, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 19: 70–83, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Schaart MW, de Bruijn AC, Bouwman DM, de Krijger RR, van Goudoever JB, Tibboel D, Renes IB. Epithelial functions of the residual bowel after surgery for necrotising enterocolitis in human infants. J Pediatr Gastroenterol Nutr 49: 31–41, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313: 1438–1441, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Slot LA, Lauridsen AM, Hendil KB. Intracellular protein degradation in serum-deprived human fibroblasts. Biochem J 237: 491–498, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobolewska A, Gajewska M, Zarzynska J, Gajkowska B, Motyl T. IGF-I, EGF, and sex steroids regulate autophagy in bovine mammary epithelial cells via the mTOR pathway. Eur J Cell Biol 88: 117–130, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett 581: 2156–2161, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol 445: 77–88, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Van Limbergen J, Stevens C, Nimmo ER, Wilson DC, Satsangi J. Autophagy: from basic science to clinical application. Mucosal Immunol 2: 315–330, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Warner BB, Ryan AL, Seeger K, Leonard AC, Erwin CR, Warner BW. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr 150: 358–363, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Warner BW, Warner BB. Role of epidermal growth factor in the pathogenesis of neonatal necrotizing enterocolitis. Semin Pediatr Surg 14: 175–180, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Dang Y, Su W, Liu C, Ma H, Shan Y, Pei Y, Wan B, Guo J, Yu L. Molecular cloning and characterization of rat LC3A and LC3B–two novel markers of autophagosome. Biochem Biophys Res Commun 339: 437–442, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol 294: F777–F787, 2008 [DOI] [PubMed] [Google Scholar]