Abstract
Studies have demonstrated that angiotensin II (Ang II) can regulate intestinal fluid and electrolyte transport and control intestinal wall muscular activity. Ang II is also a proinflammatory mediator that participates in inflammatory responses such as apoptosis, angiogenesis, and vascular remodeling; accumulating evidence suggests that this hormone may be involved in gastrointestinal (GI) inflammation and carcinogenesis. Ang II binds to two distinct G protein-coupled receptor subtypes, the AT1R and AT2R, which are widely expressed in the GI system. Together these studies suggest that Ang II-AT1R/-AT2R actions may play an important role in GI tract physiology and pathophysiology. Currently it is not known whether miRNAs can regulate the expression of the human AT1R (hAT1R) in the GI system. PCR and in situ hybridization experiments demonstrated that miR-802 was abundantly expressed in human colon and intestine. Luciferase reporter assays demonstrated that miR-802 could directly interact with the bioinformatics-predicted target site harbored within the 3′-untranslated region of the hAT1R mRNA. To validate that the levels of miR-802 were physiologically relevant in the GI system, we demonstrated that miR-802 “loss-of-function” experiments resulted in augmented hAT1R levels and enhanced Ang II-induced signaling in a human intestinal epithelial cell line. These results suggest that miR-802 can modulate the expression of the hAT1R in the GI tract and ultimately play a role in regulating the biological efficacy of Ang II in this system.
Keywords: gastrointestinal system, miRNAs
angiotensin II (Ang II), an octapeptide hormone, is the biologically active component of the renin-angiotensin system (RAS) (13, 47). Ang II has emerged as a critical hormone that affects the function of virtually all organs, including heart, kidney, vasculature, brain, adrenal, liver, reproductive organs, and the gastrointestinal (GI) system (13, 47). Pharmacological and morphological studies have demonstrated that Ang II plays an important role in GI system epithelial transport processes (11, 33, 34). For example, in the GI tract, Ang II has been shown to mediate epithelial sodium and water absorption in the jejunum, ileum, and distal colon (33). More recent studies have demonstrated that in the jejunum, the effect of Ang II on sodium and water transport is dose dependent (5, 25, 35, 36). Finally, it has been demonstrated that luminal Ang II is a negative regulator of intestinal glucose transport (63).
Ang II is also a potent constrictive agent in vascular smooth muscle and, although less investigated, influences intestinal smooth muscle function as well (3, 17, 39, 51, 58, 61). For example, Fishlock and Gunn (17) reported that Ang II elicited dose-dependent contractions in the human colon. Ludtke et al. (39) investigated Ang II effects on both gastric and duodenal smooth muscle preparations and also found a dose-dependent contraction. Schinke et al. (51) reported that Ang II, in a dose-dependent fashion, elicited contractions in rat duodenal and ileal longitudinal muscular strips. Additionally, several recent studies demonstrated that Ang II elicits concentration-dependent contractions in human duodenal and jejunal as well as ileal isolated muscle preparations (16, 56). Finally, it has been suggested that Ang II participates in the physiological control of human esophageal motor activity (9).
In mammalian cells, Ang II binds to two distinct high-affinity plasma membrane receptors designated AT1R and AT2R (13, 47). Both receptor subtypes, which are seven-transmembrane-spanning G protein-coupled receptors, have been cloned and pharmacologically characterized (13, 47). The current view is that the AT2R counteracts the “classical” Ang II responses in the cardiovascular and renal systems mediated via the AT1R (4). Radioligand binding studies have demonstrated the existence of functional AT1R in the rat ileum and duodenum (51). In the ileum these receptors were mainly located on the longitudinal smooth muscle and coupled to contraction (51). Autoradiographic characterization of Ang II receptor subtypes in rat intestine demonstrated that the AT1R was moderately abundant in the mucosa and the muscularis of both jejunum and ileum, whereas its presence was undetected in the submucosa and the serosa (52). A small population of AT2R was also observed in the rat intestine (52). In the colon, AT1R binding was significantly more abundant in the muscularis than in the mucosa (52). Finally, immunohistochemical localization demonstrated that in normal human colon, AT1Rs were localized in vessel walls, myofibroblasts, and macrophages in lamina propria, crypt bases, and surface epithelium (22). AT2Rs were found in mesenchymal cells and weakly in parts of surface epithelium (22).
miRNAs are a family of small, ∼21-nucleotide long, nonprotein-coding RNAs that have emerged as key posttranscriptional regulators of gene expression (reviewed in Refs. 2, 6). miRNAs are processed from precursor molecules (pri-miRNAs), which either are transcribed from independent miRNA genes or are portions of introns of protein-coding RNA polymerase II transcripts. Following their processing, miRNAs are assembled into ribonucleoprotein complexes called microribonucleoproteins (miRNPs) or miRNA-induced silencing complexes (miRISC). The miRNA acts as an adaptor for miRNA-induced silencing complex to specifically recognize and regulate particular mRNAs. Mature miRNAs recognize their target mRNAs by base pairing interactions between nucleotides 2 and 8 of the miRNA (the seed region) and complementary nucleotides in the 3′-untranslated region (3′-UTR) of mRNAs. miRISCs subsequently inhibit gene expression by targeting mRNAs for translational repression or destabilization (2, 6). miRISCs subsequently inhibit gene expression by targeting mRNAs for translational repression or destabilization, depending upon the overall degree of complementarity of the miRNA binding site, the number of miRNA binding sites, and the accessibility of those miRNA binding sites (2, 6).
Given that the expression levels of the AT1R and AT2R define the biological efficacy of Ang II, it is important to understand the mechanisms by which receptor density is regulated. Currently it is not known whether or not miRNAs can regulate the expression of the human AT1R (hAT1R) in the GI system. Therefore, in this study, we have examined the hypothesis that hAT1R expression can be regulated by miRNAs in the GI tract. Experimental analyses demonstrate, for the first time, that miR-802 is predominantly expressed in the human fetal colon submucosa, lamina propria, and the outer muscularis layer and regulates the expression of the hAT1R in the human colorectal adenocarcinoma cell line, C2BBe1.
MATERIALS AND METHODS
hAT1R mRNA/miRNA bioinformatic analyses.
To predict putative miRNA recognition sites harbored in the 3′-untranslated region (3′-UTR) of hAT1R mRNAs, multiple computational algorithms were utilized (TargetScan 5.1: http://www.targetscan.org, Refs. 18, 21, 38; miRBase: http://www.mirbase.org/, Ref. 20; PITA http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html, Ref. 28). These computational analyses demonstrated that the hAT1R 3′-UTR theoretically harbors hundreds of miRNA recognition sites and, unfortunately, many of the identified sites did not overlap between analyses (data not shown). Given that the combinations of computational analyses often perform worse than the prediction of a single algorithm (1), we chose to focus on TargetScan-predicted miRNA targets since this algorithm has a precision rate of ∼50% with a sensitivity of ∼12% (1).
Cell culture.
Chinese hamster ovary cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1× antibiotic-antimycotic (Invitrogen), and 0.0175 mg/ml l-proline (Sigma). The C2BBe1 cell line, which was subcloned from human colon adenocarcinoma Caco-2 cells, demonstrate features of enterocytic differentiation and form polarized monolayers with an apical brush border morphology comparable with that of human intestine (48) were purchased from ATCC and maintained in DMEM medium (Invitrogen) supplemented with 10% FBS (Atlanta Biologicals), 1% insulin-transferrin-selenium (ITS, Becton Dickinson) and 1× antibiotic-antimycotic (Invitrogen).
Luciferase reporter constructs.
An 883-bp fragment encompassing the entire hAT1R 3′-UTR was PCR amplified by utilizing sense (5′-CATGTTCGAAACCTGTCCATAAAG-3′) and antisense (5′-ATAAAATTATTTTATTTTAAAGTAAAT-3′) primers by using standard procedures and a proofreading polymerase (Platinum Pfu, Invitrogen). A full-length hAT1R cDNA clone (40) was used as template. Following the manufacturer's protocol, the PCR product was treated for 10 min with Taq polymerase. The PCR product was subsequently subcloned into the pCR2.1 vector according to the manufacturer's protocol (Invitrogen). Plasmid DNA was isolated from recombinant colonies and sequenced to ensure authenticity. The hAT1R 3′-UTR inserts were removed from the pCR2.1 plasmid by EcoRI digestion. The fragments were gel purified, filled in, and blunt-end ligated into a filled-in XhoI site downstream of the Renilla luciferase (r-luc) reporter gene (psiCHECK-2, Promega). The authenticity and orientation of the inserts relative to the Renilla luciferase gene were confirmed by dideoxy sequencing. The resulting recombinant plasmid was designated psiCHECK/hAT1R. The mutant reporter construct, psiCHECK/mut-802, was generated by utilizing the psiCHECK/hAT1R plasmid as template and mutating the miR-802 (located at 610–616 bp) seed sequence recognition site harbored in the hAT1R 3′-UTR by use of the QuikChange site-directed mutagenesis kit (Stratagene). Briefly, a forward miR-802 mutagenic primer (5′-GCT TAT TTG TAT AAT GGA CAA TGA TAA GTC ACA TAT AAA AGT-3′) and/or a complementary reverse miR-802 mutagenic primer (5′-ACT TTT ATA TGT GAC TTA TCA TTG TCC ATT ATA CAA ATA AGC-3′) were synthesized and utilized in a PCR experiment as described by the manufacturer. The nucleotides to be mutated are shown in bold print. The amplification reactions were treated with DpnI restriction enzyme to eliminate the parental template, and the remaining DNA was used for transformation. The mutation of the miR-802 seed binding site was confirmed by dideoxy chain termination sequencing. Finally, transformed bacterial cultures were grown and each reporter construct was purified by use of a PureLink Hipure Plasmid Maxiprep Kit (Invitrogen).
Transfection and luciferase assay.
miR-802, and scrambled sequence negative control mimics (partially double-stranded RNAs that mimic the Dicer cleavage product and are subsequently processed into their respective mature miRNAs) were obtained from Dharmacon (Lafayette, CO). miR-802, and scrambled sequence negative control peptide nucleic acid (PNA) miRNA inhibitors (antisense single-stranded chemically enhanced oligonucleotides, ASO) were obtained from Panagene (Daejeon, Korea). Transfection of CHO and C2BBe1 cells with small RNAs was optimized utilizing Lipofectamine 2000 (Invitrogen) and a fluorescein-labeled double-stranded RNA oligomer designated BLOCKiT (Invitrogen). Once conditions were optimized, CHO cells (approaching 100% transfection efficiency) were transfected with the luciferase reporter constructs described above and the appropriate miRNA precursor as indicated. After 24 h, CHO cells were washed and lysed with passive lysis buffer (Promega), and firefly and Renilla luciferase activities were determined by using the Dual-Luciferase Reporter Assay System (Promega) and a luminometer. Renilla luciferase expression in the psiCHECK vector is generated via an SV40 promoter. Additionally, the psiCHECK-2 vector possesses a secondary firefly reporter expression cassette which is under the control of the HSV-TK promoter. This firefly reporter cassette has been specifically designed to be an intraplasmid transfection normalization reporter; thus, with use of the psiCHECK-2 vector, the Renilla luciferase signal is normalized to the firefly luciferase signal. C2BBe1 cells (∼70% confluent) were transiently transfected with the miRNA reagents utilizing Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol. At 48–72 h after transfection, total RNA isolated and miR-802 and hAT1R mRNA levels were quantitated by RT-PCR as described below. Additionally, C2BBe1 miRNA transfected cells were subjected to radioreceptor binding, phospho-ERK1/2, transepithelial electrical resistance (TEpR), and paracellular macromolecule flux assays.
Real-time PCR.
Total RNA was isolated from transfected cells with Trizol (Invitrogen). The RNA was subsequently treated with RNase-free DNase I, and mature human miR-802 was quantified by utilizing TaqMan microRNA assay kits specific for each miRNA (Applied Biosystems, Foster City, CA) as previously described (30, 31, 40–42). Briefly, 100 ng of total RNA was heated for 5 min at 80°C with 2.5 μM of the miR-802 and RNU48 antisense primers, followed by 5 min at 60°C then cooling to room temperature. The resulting solution was added to a cocktail and reverse transcription was performed in a 20-μl reaction according to the manufacturer's recommendations (Applied Biosystems). Quantitative real-time PCR (20 μl total reaction) was performed by using 5 μl of a 1:5 dilution of cDNA. Gene expression was calculated relative to RNU48 and threshold cycle (Ct) values were normalized to “1” for control samples to simplify data presentation.
Total RNA samples isolated from miRNA transfected cells were utilized to measure hAT1R steady-state mRNA levels using a hAT1R TaqMan Gene Expression Assay (Hs01096942_m1, Applied Biosystems). Reverse transcription was performed using Superscript III reverse transcriptase (Invitrogen). Briefly, 500 ng of RNA was heated for 5 min at 65°C with random hexamer primers followed by cooling on ice for 1 min. The resulting solution was added to a cocktail and reverse transcription was performed in a 20-μl reaction according to the manufacturer's recommendations. Quantitative real-time PCR (20 μl total reaction) was performed using 5 μl of a 1:5 dilution of cDNA. Gene expression was calculated relative to 18S rRNA and Ct values were normalized to “1” for control samples to simplify data presentation.
Finally, a miRNA-certified FirstChoice human total RNA survey panel was purchased from Ambion. The RNA was subsequently treated with RNase-free DNase I, and cDNA was synthesized from 100 ng of total RNA using gene-specific primers to miR-802 precursor and RNU48 as described above (30, 31, 40–42). Quantitative real-time PCR (20 μl total reaction) was performed by using 5 μl of a 1:10 dilution of cDNA. Gene expression was calculated relative to RNU48, as calculated by 2−(Ct miR-802−Ct RNU48). Relative gene expression was multiplied by 1,000 to simplify data presentation.
Radioligand binding studies.
C2BBe1 cell AT1R binding was measured as previously described (40–42). Briefly, 72 h after transfection with the denoted miRNA mimics or inhibitors, the cell medium was aspirated and replaced with monoiodinated 125I-[Sar1,Ile8]Ang II (2–3 × 105 cpm; Peptide Radioiodination Service, Oxford, MS) in Hanks' balanced salt solution, 20 mM HEPES, 0.1% bovine serum albumin. Following incubation at room temperature for 30 min, unbound ligand was removed by washing each well twice with 1 ml of ice-cold phosphate-buffered saline. Bound ligand was recovered by dissolving the protein in each well with 1 ml 0.5 M NaOH-0.01% SDS. Nonspecific binding was determined by performing the binding assay in the presence of 1 μM unlabeled Ang II. The quantity of 125I-[Sar1,Ile8]Ang II present in each sample was determined using a Cobra γ-spectrophotometer (Packard Bell, Palo Alto, CA). Protein content in wells was measured using the Bio-Rad protein assay dye reagent (Bio-Rad). Values represent specific (total minus nonspecific) binding.
Immunoassay for ERK.
Ang II/AT1R-mediated phosphorylation of extracellular signal-related kinase (ERK) in C2BBe1 cells was measured as previously described (40, 41). Briefly, 48 h after transfection with the denoted miRNA mimics or inhibitors, the cells were washed and serum-starved for an additional 24 h. Serum-starved cells were stimulated with Ang II (1 μM) for 5 min, washed with phosphate-buffered saline, and lysed with a concentrated buffer solution containing 250 mM Tris, pH 6.8, 8% SDS, 40% glycerol, 200 mM dithiothreitol, and 0.04% bromphenol blue (300 μl/1 × 106 cells). An aliquot of the supernatant was separated by 10% SDS-PAGE. Following transfer to nitrocellulose membrane and blocking with 5% nonfat milk, the blot was incubated with an antibody (1:2,000) specific for phospho-ERK1/2 (Cell Signaling, Beverly, MA). The immunoblot was then incubated with a secondary antibody conjugated with horseradish peroxidase, visualized with ECL, and the autoradiograph was quantitated by densitometric analysis. The blots were subsequently stripped and reprobed with an ERK1/2-specific antibody (Cell Signaling) to normalize the level of phosphorylated ERK to total ERK.
miRNA in situ hybridization.
Locked nucleic acid probes complementary to human mature miR-802 (5′-ACAAGGAUGAAUCUUUGUUACUG-3′) and scrambled negative control (5′-UUCACAAUGCGUUAUCGGAUGU-3′) digoxigenin-labeled at the 5′ and 3′ position were purchased from Exiqon (Vedbaek, Denmark). Detection of mature miRNAs by in situ hybridization utilizing oligonucleotide probes was performed as previously described (30–32, 41, 46). Food + Drug Administration (FDA) normal human organ tissue arrays were purchased (FDA995, 99 cores, 33 sites, 75 cases; US Biomax, Rockville, MD). Human fetal colon and small intestine were obtained (after institutional review board approval) from the Brain and Tissue Bank for Developmental Disorders, University of Maryland at Baltimore, in contract with the National Institute of Child Health and Human Development. The formalin-fixed human fetal colon and small intestine specimens were embedded in wax and 5-μm sections were cut. The human tissue slides were subsequently deparaffinized and treated with pepsin at 2 mg/ml in RNase-free water for 2 h. Hybridization was performed at 37°C overnight followed by a low-stringency wash in 0.2× SSC and 2% bovine serum albumin at 4°C for 10 min. The probe-target complex was visualized by utilizing a digoxigenin antibody conjugated to alkaline phosphatase acting on the chromogen nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. Therefore, positive-expressing miRNA cells stain dark blue. Nuclear fast red served as the counterstain. Identical control experiments were performed utilizing a digoxigenin-labeled scrambled miRNA and control experiments were conducted in which the miRNA probe was omitted. All specimens were sequentially viewed in their entirety under a ×100 objective and number of positively stained cells per field was scored.
Immunohistochemistry.
Immunohistochemical testing was performed by using the Ventana Benchmark System (Ventana Medical Systems, Tucson, AZ) as previously described (31). FDA normal organ tissue arrays were dewaxed in a 60°C oven for 20 min, transferred to xylene, and rinsed in 100% ethanol before being either stained histologically (with hematoxylin and eosin) or immunohistochemically. The primary antibody, AT1R (1:100 dilution, Abcam), was added after antigen retrieval for 30 min. The antigen-antibody complex was detected by using the Ventana ultrasensitive universal fast red system. The counterstain was dilute hematoxylin. The sections were placed under a glass coverslip with mounting medium (Permount). The cell type(s) that exhibited positive immunostaining were determined by morphological and cytological criteria. Negative controls included omission of the primary antibody as well as the antigen retrieval step plus the internal negative controls.
Measurement of TEpR in vitro.
TEpR serves as an index of the integrity of tight junctions and the extent of paracellular transport across the epithelial cell monolayer in vitro. C2BBel cells, as the intestinal epithelial cell model, were plated in complete medium on gold electrodes at 37°C in a humidified atmosphere of 5% CO2-95% air. Seventy-two hours after transfection with the denoted miRNA mimics or inhibitors (i.e., to ensure that the cells were confluent), C2BBel cell monolayers on gold TEpR of the electrodes were measured on an electric cell substrate impedance sensing system (ECIS; Applied Biophysics, Troy, NY) following the treatment of cell monolayers with MEM only or MEM containing different concentrations of Ang II in a humidified atmosphere of 5% CO2-95% air at 37°C as previously published (55). The total epithelial electrical resistance, as measured across the C2BBel epithelial cell monolayer on gold electrodes, was determined by the combined resistance between the basal and/or cell matrix adhesion. TEpR measurements on both the control untreated and Ang II-treated monolayers were performed in triplicate and expressed as normalized resistance values.
Assay of paracellular macromolecule flux (leak) across intestinal epithelial cell monolayer in vitro.
To establish the tight junction assembly and function of the intestinal epithelium in response to Ang II action and AT1R function, we utilized the previously published procedure of FITC-dextran paracellular flux (leak) (27) across the C2BBel cell (Caco-2 cell-derived) monolayer as the intestinal epithelium model (49). Briefly, C2BBel cells were cultured on 12-well sterile inserts (0.4 μM size) and when cells reached 90% confluence they were transfected as described above. Seventy-two hours after transfection with the denoted miRNA inhibitors, confluence was verified by observing the monolayer of cells grown on the inserts under a light microscope before conducting studies. The method of determining the FITC-dextran paracellular flux across the cell monolayer was validated by subjecting the cells to hydrogen peroxide, an oxidant, which is known to increase the paracellular flux of macromolecules by oxidative stress-mediated tight junction alterations (59). Subsequently, the transfected C2BBel cells were treated with MEM alone or MEM containing Ang II (5 μM) for 30 min. Culture and treatment of cells were performed in a humidified atmosphere of 5% CO2-95% air at 37°C. An insert without cells was included as a positive control. Following the treatment of cells, the medium was removed by gentle aspiration and 0.9 ml of basal MEM was added to the well. The inserts with cells were replaced into the well, followed by the addition of 0.2 ml of basal MEM into the inserts. This procedure allowed the medium to equilibrate in both the insert and well for 30 min. Medium was then removed from the inserts by gentle aspiration and 0.2 ml of prewarmed basal MEM containing FITC-dextran (molecular weight 70,000; 1 mg/ml concentration) was added into the inserts. Medium (50 μl) was collected from the well (outside of the insert) after 60 min of addition of FITC-dextran-containing MEM to the well, and the fluorescence of the medium was determined on a fluorescence microplate reader (Biotek Synergy HT Plate Reader) at 480 nm excitation and 530 nm emission. Paracellular flux (leak) of macromolecules across the C2BBel epithelial cell monolayer was calculated from the fluorescence (arbitrary units) of FITC-dextran in the medium of the well underneath the cell monolayer in triplicate and expressed as % leak of FITC-dextran normalized to the untreated control cells (set at 100%).
Statistical analysis.
Data are reported as means ± SE or SD for each data point and were calculated from triplicate determinations. Data were subjected to one-way analysis of variance, and pairwise multiple comparisons were performed by Dunnett's method with P < 0.05 indicating significance.
RESULTS
miR-802 is expressed in the GI system.
miRNA-mediated regulation of gene expression results when a miRNA interacts with a specific recognition element within the 3′-UTR of a target mRNA and suppresses its translation or initiates its degradation (2, 6). Bioinformatic approaches have been successfully used to identify potential miRNA/mRNA target pairs (2, 6). In this study, the computational algorithm, TargetScan (http://www.targetscan.org; Refs. 18, 21, 38), was utilized to predict putative miRNA recognition sequences located in the hAT1R 3′-UTR. TargetScan analysis predicted that the hAT1R 3′-UTR harbors 58 putative miRNA binding sites, none of which are conserved across species (data not shown). To begin to prioritize the functional importance of these potential miRNA binding sites in regulating the expression of the hAT1R in the GI system, they were initially ranked based on total context score and site-type of the predicted complimentary “seed” sequence (8mer, 7mer-m8, or 7merA1). The context score accounts for contribution from site-type, 3′ pairing, local AU effect, and position in the 3′-UTR (18, 21, 38). A total of 11 predicted hAT1R 3′-UTR miRNA binding sites showed a context score greater than −0.30 (Fig. 1A), indicating a higher probability of in vivo functionality. Additional, preference was also given to binding sites predicted as 8mers, since previous studies have demonstrated that this type of miRNA binding site results in more robust gene repression, presumably due to the increased complementarity of the binding site (2). Seven of the 11 predicted binding sites with the robust context score were predicted as 8mers (miR-34, -455, -548, -802, -891b, -1248, and -1303, Fig. 1A).
Fig. 1.
A: schematic representation of the location of the prioritized putative miRNA binding sites harbored in the human AT1R (hAT1R) 3′-untranslated region (3′-UTR). TargetScan bioinformatic analysis predicted that a total of 58 miRNA binding sites were harbored in the hAT1R 3′-UTR. Only those predicted miRNAs with a context score greater than −0.30 are shown. The miRNAs shown in bold print denote those predicted miRNAs with an 8-mer complementary “seed” sequence site-type (18, 21, 38). The UGA represents the beginning of the 3′-UTR, which is 883 bp in length. B: relative expression of mature miR-802 in human tissues. Total RNA samples isolated from a number of human tissues were quantified by utilizing miR-802 and RNU48 TaqMan microRNA assays (Applied Biosystems, Foster City, CA) as previously described (30, 31, 40–42). The relative expression of the mature miR-802 gene was normalized to RNU48 expression in each tissue. Relative gene expression was calculated as 2−(Ct miR-802−Ct RNU48). Relative gene expression was multiplied by 105 to simplify data presentation. The mean activities ± SE from 3 independent quantitative PCR (qPCR) experiments are shown.
To begin to investigate whether or not any of the seven prioritized miRNAs were expressed in the GI system, quantitative PCR (qPCR) experiments were performed utilizing total RNA samples isolated from a number of human tissues, including colon, esophagus, and small intestine. Importantly, of the miRNAs investigated, miR-802 was shown to be highly expressed in the colon and small intestine (Fig. 1B). In comparison, considerably lower levels of miR-802 were detected in the remaining 18 tissues (Fig. 1B). In contrast, the expression of miR-34, -455, -548, -891b, -1248, and -1303 was not as robust as miR-802 in the GI system (data not shown). These data indicate that although miR-802 was expressed in low levels in many human tissues, it may be of particular importance in the intestinal tract.
To further validate that miR-802 was expressed in the GI system, miRNA in situ hybridization experiments were performed utilizing several paraffin-embedded, formalin-fixed human fetal colon specimens as previously described by our laboratory (31, 41, 46). In situ hybridization experiments demonstrated that miR-802 (blue signal) was expressed in the epithelial (Fig. 2, A, B, and D, blue arrow) and stromal cells of the lamina propria (Fig. 2, A, B, and D, orange arrow) and in the submucosa (Fig. 2, A–D, yellow arrow). A miR-802 signal was also visualized in the outer muscularis layer (Fig. 2, A and C, green arrow). The hybridization signal was not evident in tissue samples hybridized with a scrambled control probe (data not shown). Additionally, in situ hybridization experiments demonstrated that miR-802 was also expressed in human adult colon epithelial cells (Fig. 2, E and F, blue arrow); however, the signal was not as robust as the signal observed in fetal samples.
Fig. 2.
In situ detection of miR-802 in paraffin-embedded, formalin-fixed human fetal colon. Representative example of the distribution of miR-802 after in situ hybridization analysis with a locked nucleic acid (LNA) miR-802-specific probe in a section of unremarkable human fetal colon (n = 3) at ×100 (A), ×200 (B), ×400 (C), and ×400 (D). The probe-target complex (blue signal) was visualized by utilizing a streptavidin-alkaline phosphatase conjugate acting on the chromogen nitroblue tetrazdium and bromochloroindolyl phosphate (NBT/BCIP). Nuclear fast red served as counterstain. miR-802 signal was evident in the epithelial (blue arrows) and stromal cells of the lamina propria (orange arrows) and in the submucosa (yellow arrows). A miR-802 signal was also visualized in the outer muscularis layer (green arrows). The signal was not evident in the serial section (4 μm away) when the scrambled LNA miRNA probe was utilized (data not shown). Representative example of the distribution of miR-802 after in situ hybridization analysis with a LNA miR-802-specific probe in a section of unremarkable human adult colon at ×400 (E) and ×1,000 (F). miR-802 signal was evident in the epithelial cells (blue arrows).
Given that a miRNA and its respective target mRNA must be coexpressed in order for the miRNA to repress the expression of its biological target, hAT1R immunohistochemistry experiments were also performed on human fetal colon samples. These results demonstrated that the hAT1R is expressed (pink signal) in colon epithelial (orange arrow) and endothelial cells (yellow arrow) in the lamina propria and submucosa (Fig. 3, A–D). Staining for the hAT1R was also visualized in the muscularis layer (Fig. 3, A–D, green arrow). Since miR-802 and the hAT1R protein are expressed in similar colon cell types, this suggests that miR-802 may play a physiological role in regulating the expression of the hAT1R in the GI system.
Fig. 3.
hAT1R is expressed in human fetal colon specimens. Representative photomicrographs of hAT1R expression in fetal colon (A, ×20; B, ×40; C, ×1,000; D, ×1,000). Immunoreactivity was visualized with fast red chromogen (positive staining deep pink color). hAT1R is expressed in colon epithelial (orange arrows) and endothelial cells (yellow arrows) and in the muscularis (green arrows). Hematoxylin (blue) was the counterstain. All hAT1R staining was lost if the primary antibody was omitted (data not shown).
miR-802 interacts with the 3′-UTR of the hAT1R mRNA.
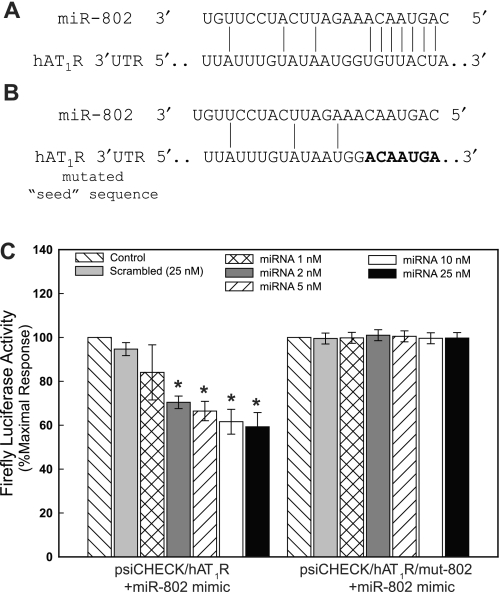
To begin to investigate whether or not miR-802 can regulate hAT1R expression, a luciferase reporter assay system was utilized. The rationale for employing this assay is that the binding of a given miRNA to its specific mRNA target site will repress reporter protein production thereby reducing luciferase activity. Therefore, the entire hAT1R 3′-UTR (i.e., 883-bp region) was subcloned immediately downstream from the r-luc open reading frame, and this construct was designated psiCHECK/hAT1R. This construct contains one predicted 8-mer miR-802 binding site at position 610–616 of the hAT1R 3′-UTR (Fig. 4A). To assess the effectiveness of miR-802 in regulating luciferase expression, psiCHECK/hAT1R was transfected into CHO cells with increasing concentrations of miR-802 mimic or control nontargeting miRNA mimics, and luciferase activities were measured (Fig. 4C). These experiments demonstrated that miR-802 can regulate luciferase activity in a concentration-dependent manner (maximal inhibition 10 nM), with a significant decrease in r-luc activity as low as 2 nM miR-802. In contrast, transfection with control nontargeting miRNA (i.e., scramble) had no effect on luciferase activity.
Fig. 4.
miR-802-mediated inhibition of luciferase/hAT1R 3′-UTR activity. A: complementarity between miR-802 and the putative hAT1R 3′-UTR binding site (616 base pairs downstream from the hAT1R stop codon). B: the miR-802 “seed sequence” was mutated by using PCR and the resulting construct was designated psiCHECK/hAT1R/mut-802. C: CHO cells were transfected with psiCHECK, psiCHECK/hAT1R, or psiCHECK/mut-802 luciferase reporter constructs and miR-802 or scrambled miRNA at the concentrations indicated. Twenty-four hours following transfection, luciferase activities were measured. Renilla luciferase activity was normalized to firefly luciferase activity, and mean activities ± SE from 5 independent experiments are shown (*P < 0.01 psiCHECK/hAT1R + miR-802 vs. psiCHECK/hAT1R alone).
To validate that miR-802 interacted with a specific target sequence localized within the hAT1R 3′-UTR, an additional luciferase reporter construct was generated in which the 7-bp “seed” sequence (i.e., UGUUACU), which is complementary to the 5′-end of miR-802 (Fig. 4B), was mutated (i.e., ACAAUGA). The mutant construct, psiCHECK/hAT1R/mut-802, was transfected into CHO cells with increasing concentrations of miR-802 or control nontargeting miRNA, and luciferase activity was compared with that of the nonaltered construct (Fig. 4C). miR-802 could no longer diminish r-luc activity in the altered construct, even at a concentration of 25 nM. Transfection of control nontargeting miRNA (i.e., scramble) with either construct had no effect on luciferase activity. These results indicate that miR-802 can interfere with luciferase mRNA translation by direct interaction with the hAT1R 3′-UTR at the predicted target site.
hAT1R is an in vivo target of mir-802.
If hAT1R mRNA is a true target of miR-802, manipulation of the endogenous expression of miR-802 should correspond to predictable changes in hAT1R protein levels. For example, overexpression of a given miRNA should result in decreased target protein expression due to increased mature miRNA levels (i.e., gain-of-function). In contrast, overexpression of a specific miRNA inhibitor (antisense oligoribonucleotide, ASO) should result in augmented target protein expression as a result of decreased translation repression or mRNA degradation due to a reduction in mature miRNA levels (i.e., loss-of-function). Given that it is necessary to validate that hAT1R expression is regulated by miR-802 in the GI system, a human intestinal epithelial, C2BBe1, cell line (48, 49) was utilized to perform gain- and loss-of-function experiments. C2BBe1 cells provide an excellent experimental model system since both hAT1Rs (44, 45) and miR-802 (data not shown) are endogenously expressed in these cells.
Gain-of-function experiments were performed in C2BBe1 cells by transfecting these cells with 100 nM miR-802 mimic, and hAT1R levels were subsequently quantitated by radioreceptor binding assays. hAT1R levels in cells transfected with miR-802 showed a small but significant decrease (18.1 ± 3.2%) compared with control (Fig. 5A). The mechanism by which miR-802 caused the reduction in receptor levels was investigated by real-time qPCR. RNA isolated from transfected C2BBe1 cells was assayed for hAT1R mRNA levels. These results demonstrated that transfection with miR-802 did not significantly decrease hAT1R steady-state mRNA levels in these cells (Fig. 5B). To determine whether the reduction in hAT1R density also resulted in decreased Ang II-induced signal transduction, C2BBe1 cells were transfected with 100 nM miR-802 mimic and subsequently activated with 0.1 μM Ang II for 5 min and phospho-ERK1/2 levels were determined. These results demonstrated that cells transfected with miR-802 exhibited decreased phospho-ERK1/2 levels (24.5% ± 8.6%, P < 0.001) compared with controls (Fig. 5, C and D). Taken together, gain-of-function experiments suggest that miR-802 can decrease hAT1R expression by inhibiting translation of the mRNA, rather than targeting for degradation. Importantly, decreased hAT1R expression also resulted in a significant decrease in Ang II-induced signaling events.
Fig. 5.
miR-802 gain- and loss-of-function experiments inversely regulates hAT1R expression. A: human intestinal epithelial C2BBe1 cells were either mock transfected or transfected with miR-802 mimic or anti-miRNA-802 and 72 h after transfection the cells were utilized as follows. AT1R radioreceptor binding assays were performed as described in materials and methods. Data have been normalized for protein and transfection differences and represent specific binding. Values are shown as percent of maximal specific binding of mock-transfected C2BBe1 cells and represent means ± SE from 4 independent experiments (*P < 0.01 vs. mock-transfected cells). B: qPCR experiments were performed as described in materials and methods utilizing total RNA isolated from transfected C2BBe1 cells. The relative gene expression of hAT1R mRNA was normalized to 18S rRNA expression and is expressed in arbitrary units. The mean of hAT1R steady-state mRNA levels from 4 independent transfection experiments are shown. C: Ang II-induced phospho-ERK1/2 experiments were performed utilizing serum-starved, transiently transfected C2BBe1 cells as described in materials and methods. A representative immunoblot is shown. Results are representative of 4 independent experiments. D: quantitation of Ang II-(1 μM for 5 min) induced ERK1/2 phosphorylation was determined by densitometry. Values are expressed as a percent of the maximal phosphorylation of ERK1/2 in response to Ang II in mock-transfected cells and represent means ± SE from 4 independent transfection experiments (*P < 0.01).
To further investigate whether miR-802 expression levels in C2BBe1 cells was physiologically relevant, these cells were transfected with an antisense PNA oligonucleotide complementary to miR-802 (i.e., anti-miR-802 at 100 nM) and hAT1R receptor levels were measured by radioreceptor binding assays. Importantly, loss-of-function experiments demonstrated that hAT1R levels were increased by 26.2 ± 9.1% (Fig. 5A). qPCR experiments utilizing RNA isolated from transfected C2BBe1 cells again demonstrated that transfection with anti-miR-802 did not significantly increase hAT1R steady-state mRNA levels in these cells (Fig. 5B). To examine whether anti-miR-802 also modulated Ang II-induced signaling events, phospho-ERK1/2 levels were determined. These experiments demonstrated that cotransfection of anti-miR-802 not only increased hAT1R expression but also enhanced Ang II-induced signaling via the hAT1R (14.2 ± 7.1%, P < 0.01, Fig. 5, C and D). Taken together, these results suggest the inhibition of the endogenously expressed miR-802 by anti-miR-802 resulted in enhanced levels of hAT1R, indicating that miR-802 plays a physiological role in regulating the expression of hAT1Rs in C2BBe1 cells.
Ang II mediates increased TEpR.
If hAT1R mRNA is a true target of miR-802 in the GI then modulation of endogenous miR-802 levels should also equate to changes in Ang II/AT1R-mediated signal transduction pathways in C2BBel cells. Therefore, we initially investigated whether Ang II would have any effect on the TEpR due to the modulation in tight junction assembly and function in C2BBel cell monolayer grown on gold electrodes. Specifically an increase in transcellular electrical resistance including that of epithelial cells in monolayer in vitro reflects not only the cell-to-cell tightening/adherence due to enhanced tight junction assembly but also the decrease or attenuation of paracellular flux of macromolecules across the cell monolayer (55). Control cell monolayers treated with culture media alone exhibited a steady basal TEpR up to 6 h of incubation without any major alterations (Figs. 6A). In contrast, C2BBel cell monolayers treated with Ang II (1, 5, and 10 μM) for 1–6 h showed a gradual and significant increase in TEpR in a time- and dose-dependent fashion (Figs. 6A). Taken together, these results clearly demonstrate that C2BBel cells express hAT1Rs and that these receptors when activated by Ang II lead to a functional response (i.e., enhanced tight junction assembly and therefore less paracellular leak because of preserved barrier properties). Based on these observations, miR-802 loss-of-function experiments (i.e., transfection of anti-miR-802) were performed and TEpR experiments were repeated. Unfortunately, transfected C2BBel cells did not exhibit consistent TEpR (data not shown), possibly because of the transfection reagent-electrode interactions or cell damage or death due to transfection. Therefore, this assay cannot be utilized to demonstrate that miR-802 can modify Ang II/hAT1R-mediated signaling.
Fig. 6.
Antisense miR-802-mediated increase of hAT1R expression levels attenuates Ang II-induced paracellular flux of macromolecules across intestinal epithelial cell monolayers. A: C2BBel cells were cultured as monolayer in complete medium on gold electrodes at 37°C and they were subsequently treated with culture media alone or culture media containing Ang II (1, 5, and 10 μM) up to 6 h. and transepithelial electrical resistance of the epithelial cell monolayer was measured on electric cell substrate impedance sensing system as described in materials and methods. The normalized resistance values obtained from 3 independent measurements at selected time points of treatment of C2BBel cell monolayers treated as described above are shown. Each histogram represents means ± SD from 3 independent determinations (*P < 0.05 culture media-treated vs. Ang II-treated cells). B: C2BBel cells (untransfected or transfected with anti-miR-802) were cultured as monolayers on 12-well sterile inserts (0.4 μM size) and FITC-dextran (molecular weight 70,000) paracellular flux (leak) across the epithelial cell monolayers 60 min following the treatment with culture media alone or culture media containing Ang II (5 μM) for 30 min, was assayed as described in materials and methods. Data are expressed as % transport of FITC-dextran normalized to the Ang II-untreated control cell monolayers set at 100%. Each histogram represents means ± SD from 3 independent determinations (*P < 0.05 culture media-treated vs. Ang II-treated cells or #P < 0.01 nontransfected Ang II-treated cells vs. anti-miR-802-transfected Ang II-treated cells).
Antisense mir-802-mediated increase of hAT1R expression levels attenuates Ang II-induced paracellular flux of macromolecules across intestinal epithelial cell monolayer.
To overcome the experimental limitations described above, anti-miR-802 (i.e., loss-of-function)-transfected C2BBel cells were subjected to FITC-dextran paracellular transport assays (27). As shown in Fig. 6B, Ang II (5 μM), at 30 min of treatment, resulted in a significant decrease (43 ± 3%) in paracellular flux (i.e., decreased leak) of FITC-dextran compared with control mock transfected Ang II-untreated monolayer of cells. In contrast, in the anti-miR-802-transfected C2BBel cell monolayers, Ang II (5 μM) at 30 min of treatment, caused a more robust and almost complete attenuation (94 ± 0.9%) of paracellular flux of FITC-dextran compared with Ang II-treated mock transfected cells in the Ang II-untreated control counterparts (Fig. 6B). Furthermore, these results suggest that Ang II/hAT1R mediated paracellular flux is inversely related to hAT1R expression. Taken together, these results indicate that miR-802 can regulate the expression of the hAT1R in the GI tract and that the modulation of receptor expression can influence the efficacy of Ang II-mediated changes in intestinal epithelial cell tight junction assembly and barrier properties.
DISCUSSION
The major findings in the present study are that miR-802 is predominantly expressed in the GI tract and that this miRNA interacts with a specific binding site harbored in the hAT1R 3′-UTR. To validate that the levels of miR-802 were physiologically relevant in human intestinal epithelial cells, we demonstrated that miR-802 “loss-of-function” experiments resulted in augmented hAT1R levels and enhanced Ang II-induced signaling (i.e., increased phospho-ERK1/2 levels, increased electrical resistance, and decreased paracellular flux). Taken together, these results suggest that miR-802 can modulate the expression of the hAT1R in the GI tract and ultimately play a role in regulating the biological efficacy of Ang II in this system.
Given that a major function of Ang II is to regulate extracellular fluid volume and electrolyte homeostasis, the importance of this peptide hormone in regulating these processes in the GI tract has been investigated. Early experiments demonstrated that mucosal net fluid and buffer transport were clearly influenced by Ang II (5, 25, 35, 36). More recent studies have demonstrated that both receptor subtypes play a role in mediating Ang II regulation of intestinal fluid and electrolyte transport. For example, Jin et al. (25) demonstrated that Ang II mediates jejunal sodium and water absorption by an action at the AT2R involving cGMP formation. Their data also show that Ang II inhibits absorption via the AT1R by a mechanism that is both negatively coupled to cAMP and increases jejunal PGE2 production (25). Furthermore, Wong et al. (63) demonstrated that Ang II binding to the brush border membrane initiates cellular responses that culminate in the rapid inhibition of sodium-dependent glucose transporter (SGLT1)-mediated glucose uptake. Additionally, in human esophageal epithelium hAT2R stimulation increases epithelial ion transport, whereas the hAT1R inhibits ion transport and increases epithelial resistance (10).
Since Ang II also regulates vascular tone (13, 47) several studies have investigated whether Ang II controls intestinal wall muscular activity. Early experiments demonstrated that Ang II elicited dose-dependent contractions in human colon and gastric and duodenal smooth muscle preparations and in rat duodenal and ileal longitudinal muscular strips (3, 17, 39, 51, 58, 61). More recent studies have shown that Ang II-elicited concentration-dependent contractions in muscle strips from the human duodenum, jejunum, and ileum (16). This study also showed that, in rats, these Ang II effects were resistant to both atropine and guanethidine, suggesting a site of action either directly on the musculature or through noncholinergic, nonadrenergic enteric neurons (16). The latter hypothesis was supported by Wang et al. (60), who discovered that AT1R, but not AT2R, can be found on neurons of the myenteric plexus in human and guinea pig. Further pharmacological analysis now indicates that the contractile action elicited by Ang II on human jejunal wall musculature is primarily through the hAT1R located on the musculature (56). Interestingly, Casselbrant et al. (9) demonstrated that the hAT1R-mediated contractions of the human esophagus and lower esophageal sphincter in vitro and showed that there was a hAT1R-dependent component of the peristaltic contraction force upon swallowing in vivo.
Importantly, several recent studies suggest that Ang II/AT1R may also play a role in GI tract pathophysiology. For example, it has been demonstrated that RAS plays an important role not only in the development of experimental colitis but also in the pathogenesis of inflammatory bowel disease (IBD), such as ulcerative colitis and Crohn's disease (CD) in humans (24). Specifically, it was shown that mucosal levels of Ang I and II were elevated in CD patients compared with healthy subjects (24). In parallel, elevation in Ang II expression in colon mucosa was also demonstrated in an animal model of trinitrobenzene sulfonic acid-induced experimental colitis. These changes were prevented by interfering with angiotensinogen gene expression (angiotensinogen-deficient mice) (23) or pharmacological blockade of Ang II or its receptor by ACE inhibitor and angiotensin II type1 receptor blocker, respectively (7, 57, 62). The importance of AT1R for colitis was recently established in a colitis model using AT1R-knockout mice (26). Katada et al. (26) demonstrated that dextran sulfate sodium (DSS)-induced acute colitis resulted in upregulation of Ang II and the AT1R and the induction of colonic inflammation as evidenced by the morphological changes in the colonic mucosa of wild-type (WT) mice. Moreover, DSS administration in WT mice caused severe body weight loss, severe shortening of colon length, and an increase in disease activity index score. These changes were accompanied by an increase in mucosal proinflammatory cytokine (TNF-α) expression, neutrophil accumulation, and lipid peroxidation (26). Importantly, the end points mentioned above were significantly ameliorated in DSS-challenged AT1R-deficient mice (26). Mizushima et al. (43) reported that AT1R regulates the expression of mucosal addressin cell adhesion molecule-1 under colonic inflammatory conditions through regulation of the translocation of NF-κB into the nucleus. Furthermore, they demonstrated that inhibition of the AT1R ameliorated colitis in a mouse colitis model (43).
Finally, there is abundant evidence that Ang II/AT1R signaling is involved in tumor biology, including colorectal and gastric cancer. For example, inhibitors of cyclooxygenase-2 (COX-2) activity, such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and specific COX-2 antagonists, significantly reduce the occurrence of colorectal carcinoma in both animal and human studies (14, 19). Studies on the mechanism by which NSAIDs decrease the risk of colorectal cancer suggest that inhibition of prostaglandin synthesis by COX-2 is the major mechanism in this chemoprevention (15, 53). Importantly, Ang II-dependent increase of prostaglandin production has been shown to be mediated through the AT1R via the stimulation of COX-2 induction in intestinal cells (54). Moreover, a recent study demonstrated that specific COX-2 and ACE inhibitors synergistically reduce tumor growth in an in vivo mouse model for colon cancer (37). These data suggest that Ang II/AT1R could play a significant role in colon carcinogenesis possibly by induction of COX-2 expression. Furthermore, Röcken et al. (50) demonstrated that the expression of AT1R in gastric cancers, particularly those with intestinal type differentiation, correlated with the presence and mean number of lymph node metastases, the N category, as well as the International Union Against Cancer tumor stage. It was also demonstrated that Ang II mediates invasion in gastric cancer cell lines and that this invasion can be attenuated by ACE inhibitors, as well as AT1R and AT2R antagonists (8). Kinoshita et al. (29) have obtained in vivo evidence of an intrinsic Ang II-generating system in gastric cancer and indicate locally formed Ang II is involved in cellular proliferation and survival.
In conclusion, many studies now suggest that Ang II/AT1R actions may play an important role in GI tract physiology and pathophysiology. Given that the expression levels of the AT1R define the biological efficacy of Ang II, it is important to understand the mechanisms by which receptor density is regulated. Experimental analyses demonstrate, for the first time, that miR-802 is predominantly expressed in the human fetal colon muscularis layer and the epithelial and stromal cells and regulates the expression of the hAT1R in the human colorectal adenocarcinoma cell line, C2BBe1. Since AT1R activation initiates proliferative, proinflammatory, proangiogenic, and antiapoptotic effects in the GI tract, aberrant expression of the AT1R could lead to IBD or colorectal or gastric cancer. As a result of our present study, one possible scenario that would lead to abnormally high levels of AT1R would be if miR-802 levels were atypically low. Recently, it has been demonstrated that miRNAs are involved in the pathogenesis of solid tumors and support their function in either dominant or recessive fashion, by controlling the expression of protein-coding tumor suppressors and oncogenes (reviewed in Ref. 12). Therefore, future studies will begin to investigate the potential role of miRNAs in mediating diseases of the GI system.
GRANTS
This work was supported by National Institutes of Health Grants HL048848 and HD058997 (T. S. Elton) and Fondation Jerome Lejeune Research Grant (T. S. Elton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Alexiou P, Maragkakis M, Papadopoulos GL, Reczko M, Hatzigeorgiou AG. Lost in translation: an assessment and perspective for computational microRNA target identification. Bioinformatics 25: 3049–3055, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beleslin DB. The effect of angiotensin on the peristaltic reflex of the isolated guinea-pig ileum. Br J Pharmacol 32: 583–590, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk BC. Angiotensin type 2 receptor (AT2R): a challenging twin. Sci STKE 181: PE16, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bolton JE, Munday KA, Parsons BJ, York BG. Effects of angiotensin II on fluid transport, transmural potential difference and blood flow by rat jejunum in vivo. J Physiol 253: 411–428, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 23: 175–205, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Byrnes JJ, Gross S, Ellard C, Connolly K, Donahue S, Picarella D. Effects of the ACE2 inhibitor GL1001 on acute dextran sodium sulfate-induced colitis in mice. Inflamm Res 58: 819–827, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Carl-McGrath S, Ebert MP, Lendeckel U, Röcken C. Expression of the local angiotensin II system in gastric cancer may facilitate lymphatic invasion and nodal spread. Cancer Biol Ther 6: e1–e9, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Casselbrant A, Edebo A, Wennerblom J, Lönroth H, Helander HF, Vieth M, Lundell L, Fändriks L. Actions by angiotensin II on esophageal contractility in humans. Gastroenterology 132: 249–260, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Casselbrant A, Edebo A, Hallersund P, Spak E, Helander HF, Jönson C, Fändriks L. Angiotensin II receptors are expressed and functional in human esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 297: G1019–G1027, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Cox HM, Cuthbert AW, Munday KA. The effect of angiotensin II upon electrogenic ion transport in rat intestinal epithelia. Br J Pharmacol 90: 393–401, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Ann NY Acad Sci 1183: 183–194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 14.DuBois RN. Cyclooxygenase-2 and colorectal cancer. Prog Exp Tumor Res 37: 124–137, 2003 [DOI] [PubMed] [Google Scholar]
- 15.DuBois RN, Shao J, Tsujii M, Sheng H, Beauchamp RD. G1 delay in cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res 56: 733–737, 1996 [PubMed] [Google Scholar]
- 16.Ewert S, Spak E, Olbers T, Johnsson E, Edebo A, Fändriks L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol (Oxf) 188: 33–40, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fishlock DJ, Gunn A. The action of angiotensin on the human colon in vitro. Br J Pharmacol 39: 34–39, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimura T, Ohta T, Oyama K, Miyashita T, Miwa K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience. World J Gastroenterol 12: 1336–1345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36 (Database issue): D154–D158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirasawa K, Sato Y, Hosoda Y, Yamamoto T, Hanai H. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem 50: 275–282, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Inokuchi Y, Morohashi T, Kawana I, Nagashima Y, Kihara M, Umemura S. Amelioration of 2,4,6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut 54: 349–356, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaszewski R, Tolia V, Ehrinpreis MN, Bodzin JH, Peleman RR, Korlipara R, Weinstock JV. Increased colonic mucosal angiotensin I and II concentrations in Crohn's colitis. Gastroenterology 98: 1543–1548, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Am J Physiol Regul Integr Comp Physiol 275: R515–R523, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Katada K, Yoshida N, Suzuki T, Okuda T, Mizushima K, Takagi T, Ichikawa H, Naito Y, Cepinskas G, Yoshikawa T. Dextran sulfate sodium-induced acute colonic inflammation in angiotensin II type 1a receptor deficient mice. Inflamm Res 57: 84–91, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Kelly JJ, Moore TM, Babel P, Diwan H, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 274: L810–L819, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet 39: 1278–1284, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita J, Fushida S, Harada S, Yagi Y, Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M, Hirakawa K, Ohta T. Local angiotensin II-generation in human gastric cancer: correlation with tumor progression through the activation of ERK1/2, NF-kappaB and survivin. Int J Oncol 34: 1573–1582, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Kuhn DE, Nuovo GJ, Martin MM, Malana GE, Pleister AP, Jiang J, Schmittgen TD, Terry AV, Jr, Gardiner K, Head E, Feldman DS, Elton TS. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts. Biochem Biophys Res Commun 370: 473–477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Kuhn DE, Nuovo GJ, Terry AV, Jr, Martin MM, Malana GE, Sansom SE, Pleister AP, Beck WD, Head E, Feldman DS, Elton TS. Chromosome 21-derived microRNAs provide an etiological basis for aberrant protein expression in human Down syndrome brains. J Biol Chem 285: 1529–1543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Kuhn DE, Martin MM, Feldman DS, Terry AV, Jr, Nuovo GJ, Elton TS. Experimental validation of miRNA targets. Methods 44: 47–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levens NR. Control of intestinal absorption by the renin angiotensin system. Am J Physiol Gastrointest Liver Physiol 249: G3–G15, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Levens NR. Response of rat jejunum to changes in sodium and volume balance. Am J Physiol Gastrointest Liver Physiol 251: G413–G420, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Levens NR, Peach MJ, Carey RM. Interactions between angiotensin peptides and the sympathetic nervous system mediating intestinal sodium and water absorption in the rat. J Clin Invest 67: 1197–1207, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levens NR, Peach MJ, Carey RM, Poat JA, Munday KA. Response of rat jejunum to angiotensin II: role of norepinephrine and prostaglandins. Am J Physiol Gastrointest Liver Physiol 240: G17–G24, 1981 [DOI] [PubMed] [Google Scholar]
- 37.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 352: 179–184, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Ludtke FE, Golenhofen K, Schubert F. Angiotensin II stimulates human gastric smooth muscle in vitro. J Auton Pharmacol 9: 139–147, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Martin MM, Lee EJ, Buckenberger JA, Schmittgen TD, Elton TS. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J Biol Chem 281: 18277–18284, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD, Elton TS. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microrna-155 binding. J Biol Chem 282: 24262–24269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Martin MM, Buckenberger JA, Jiang J, Malana GE, Knoell DL, Feldman DS, Elton TS. TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways. Am J Physiol Lung Cell Mol Physiol 293: L790–L799, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Mizushima T, Sasaki M, Ando T, Wada T, Tanaka M, Okamoto Y, Ebi M, Hirata Y, Murakami K, Mizoshita T, Shimura T, Kubota E, Ogasawara N, Tanida S, Kataoka H, Kamiya T, Alexander JS, Joh T. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol 298: G255–G266, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Musch MW, Li YC, Chang EB. Angiotensin II directly regulates intestinal epithelial NHE3 in Caco2BBE cells. BMC Physiol 9: 5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura K, Matsui T, Adachi H, Yamagishi SI. Involvement of angiotensin II in intestinal cholesterol absorption. Pharmacol Res 61: 460–465, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Nuovo GJ, Elton TS, Nana-Sinkam P, Volinia S, Croce CM, Schmittgen TD. A methodology for the combined in situ analyses of the precursor and mature forms of microRNAs and correlation with their putative targets. Nat Protoc 4: 107–115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Ray EC, Avissar NE, Salloum R, Sax HC. Growth hormone and epidermal growth factor upregulate specific sodium-dependent glutamine uptake systems in human intestinal C2BBe1 cells. J Nutr 135: 14–18, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Röcken C, Röhl FW, Diebler E, Lendeckel U, Pross M, Carl-McGrath S, Ebert MP. The angiotensin II/angiotensin II receptor system correlates with nodal spread in intestinal type gastric cancer. Cancer Epidemiol Biomarkers Prev 16: 1206–1212, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Schinke M, Doods HN, Ganten D, Wienen W, Entzeroth M. Characterization of rat intestinal angiotensin II receptors. Eur J Pharmacol 204: 165–170, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Sechi LA, Valentin JP, Griffin CA, Schambelan M. Autoradiographic characterization of angiotensin II receptor subtypes in rat intestine. Am J Physiol Gastrointest Liver Physiol 265: G21–G27, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 99: 2254–2259, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slice LW, Chiu T, Rozengurt E. Angiotensin II and epidermal growth factor induce cyclooxygenase-2 expression in intestinal epithelial cells through small GTPases using distinct signaling pathways. J Biol Chem 280: 1582–1593, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Sliman SM, Eubank TD, Kotha SR, Kuppusamy ML, Sherwani SI, O'Connor Butler ES, Kuppusamy P, Roy S, Marsh CB, Stern DM, Parinandi NL. Hyperglycemic oxoaldehyde, glyoxal, causes barrier dysfunction, cytoskeletal alterations, and inhibition of angiogenesis in vascular endothelial cells: aminoguanidine protection. Mol Cell Biochem 333: 9–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spak E, Casselbrant A, Olbers T, Lönroth H, Fändriks L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf) 193: 181–190, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Spencer AU, Yang H, Haxhija EQ, Wildhaber BE, Greenson JK, Teitelbaum DH. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci 52: 1060–1070, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turker RK, Kayaalp SO. Inhibitory effect of angiotensin on the intestinal motility of the cat and its relation to sympathetic nervous system. Arch Int Physiol Biochim Biophys 75: 735–744, 1967 [DOI] [PubMed] [Google Scholar]
- 59.Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal 5: 723–730, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Wang GD, Wang XY, Hu HZ, Fang XC, Liu S, Gao N, Xia Y, Wood JD. Angiotensin receptors and actions in guinea pig enteric nervous system. Am J Physiol Gastrointest Liver Physiol 289: G614–G626, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Weekley LB. Rat duodenal smooth muscle contractile responses to angiotensin II are dependent on calmodulin. Clin Exp Pharmacol Physiol 17: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- 62.Wengrower D, Zanninelli G, Pappo O, Latella G, Sestieri M, Villanova A, Faitelson Y, Pines M, Goldin E. Prevention of fibrosis in experimental colitis by captopril: the role of TGF-beta1. Inflamm Bowel Dis 10: 536–545, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Wong TP, Debnam ES, Leung PS. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol 584: 613–623, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]